Abstract
PURPOSE
Nusinersen is a drug approved in December 2016 for treatment of spinal muscular atrophy (SMA). We want to share our initial experience with image-guided, non-image-guided, and port-delivered nusinersen injections in a large single-center SMA patient cohort, treating both pediatric and adult patients with focus on technical considerations and other patient concerns from a combined perspective of patient, neurologist, and radiologist.
METHODS
All nusinersen injections between February 2017 and September 2018 were retrospectively reviewed. We obtained age, sex, SMA type and technical details of the injections and postprocedure complications for each procedure.
RESULTS
A total of 52 patients (24 women [46%]; 4 patients with SMA-1 [7.6%]; 30 patients with SMA-2 [57.8%]; 18 patients with SMA-3 [34.6%]; mean age, 25.5 years [7 months to 62 years]) with a total of 265 injections were included. Of the 265 injections, 206 (77.9%) were performed with local anesthetic, 25 (9.4%) with moderate sedation, and 23 (8.6%) under general anesthesia. We performed 65 CT-guided transforaminal injections in 13 patients, 106 fluoroscopy-guided lumbar punctures in 24 patients and 83 lumbar punctures in 16 patients using conventional technique. Only 6 of 265 injections (2.2%) ended up with a post-lumbar puncture headache (PLPH) requiring medical treatment. None required an epidural blood patch. Fourteen PLPH (5.2%) occurred and resolved at the same day without any treatment. After 6 of 265 injections (2.2%), patients reported soreness at the injection site which resolved spontaneously. Three elected to have an intrathecal reservoir placement (2 lumbar, 1 intraventricular) with a total of 11 injections. One patient with lumbar catheter developed infection after surgery with subsequent meningitis and treatment delay. After the resolution of meningitis, a new intraventricular reservoir was placed without any complication in the following injections.
CONCLUSION
With the introduction of nusinersen treatment, neurologists and radiologists play an important role in treatment of SMA patients and therefore should be familiar with different techniques and complications of drug administration. Using good technique, it is possible to have very low complication rates even in this complex patient population, and various image-guided procedures can be a safe alternative to surgical approach, even in the most difficult cases.
Spinal muscular atrophy (SMA) is an autosomal recessive neuromuscular disorder. The hallmark of SMA is progressive muscle weakness and atrophy from the degeneration and death of anterior horn cells due to homozygous mutation in the SMN1 gene, located on the long arm of fifth chromosome. This gene encodes survival motor neuron (SMN) protein, which is vital for motor neurons (1). SMA has three types presenting with different clinical presentation due to severity of the disease. Type 1 (SMA-1) is the most severe and common form (60%), presenting in early infancy. These infants develop symptoms during the first six months of life and never achieve the ability to sit independently. Majority of them do not survive beyond the second year of life without support due progressive muscle weakness and respiratory failure (2). In type 2 SMA (SMA-2), symptoms develop between 6 and 18 months of life. SMA-2 patients are able to develop the ability to sit independently, but can never achieve the ability to stand or ambulate without assistance. They require extensive supportive treatments including respiratory and nutritional support. They develop serious orthopedic complications including scoliosis, contractures, hip dysplasia, and others due to progressive muscle weakness. Type 3 (SMA-3) is a less severe form, presenting later in life with muscle weakness; however, these patients can walk independently and may have a relatively normal life span (1).
Until recently, no therapeutic treatment option was available in SMA, and patients were only receiving supportive treatment to manage comorbidities/complications. In December 2016, the FDA approved the first therapeutic drug, nusinersen (Spinraza, Biogen), and we have been performing nusinersen injections in SMA patients at our institution since February 2017. Nusinersen is an antisense oligonucleotide drug that alters the splicing of SMN2 messenger RNA. The SMN2 gene is paralogous to SMN1 and is usually present in SMA patients. While both genes can produce SMN protein, the SMN encoded by SMN2 gene can produce only 10% of stable and functional SMN protein. Therefore, SMN2 gene serves as a modifier for the SMA course, with the number of copies inversely correlated with the severity of the disease. Nusinersen modifies altered splicing of SMN2 which leads to increased production of fully functional SMN protein (1, 3, 4).
In our institution, indications approved by the FDA are followed. Nusinersen is prescribed to pediatric and adult patients for treatment of SMA. All patients treated with nusinersen undergo genetic testing prior to treatment to confirm zero copies of SMN1 gene and two or more copies of SMN2 gene. All patients are required to have normal safety laboratory tests including prothrombin time, international normalized ratio (INR), platelets, urine protein at baseline and before each injection.
Single dose of nusinersen (12 mg /5 cc) is administered into the subarachnoid space to achieve the highest concentration of the drug in the central nervous system. The treatment course consists of loading phase with three injections given every two weeks and the fourth injection 4 weeks after the third. Maintenance therapy consists of repeat injections at every 4-month intervals.
There are several challenges in therapy. First, the list price of each dose is 125 000 USD. Additional fees (e.g., transport, hospital service, lumbar puncture [LP], imaging, physician, therapist) increase to a significant level and insurance coverage is a significant issue standing in front of the treatment (5). Second, successful delivery of nusinersen into the intrathecal space is imperative. While conventional LP is feasible for many children and older patients without prior spine surgery, the anatomy is often challenging in patients with scoliosis with and without spinal fusion. These fusion procedures often result in extensive interlaminar ankylosis, which can render interlaminar LP difficult or impossible. In those, a preprocedural computed tomography (CT) is an effective means to evaluate for small openings in otherwise fused interlaminar spaces and to plan the approach. Imaging guidance is usually necessary to safely achieve intrathecal access in complex patients (6). Ultrasound can be used in children and adults with body habitus preventing easy identification of landmarks that allows adequate visualization of the site of injection. Fluoroscopy-guided lumbar puncture (FGLP) is useful in larger patients in whom diagnostic lumbar CT has revealed a small aperture in an otherwise solid interlaminar ankylosis (7, 8). To solve this problem, intrathecal reservoir placement and cervical punctures (CP) are recommended (9–11). However, these contain possible risks for severe complications and CP may not be a feasible alternative in many patients due to challenging anatomy. This led us to an alternative approach, CT-guided transforaminal lumbar puncture (CT-TFLP) (6).
We describe our experience with image-guided, non-image-guided, and port-delivered nusinersen injections in a large single-center SMA patient cohort, treating both pediatric and adult patients. We focus on technical considerations and other patient concerns from a combined perspective of the patient, neurologist, and radiologist.
Methods
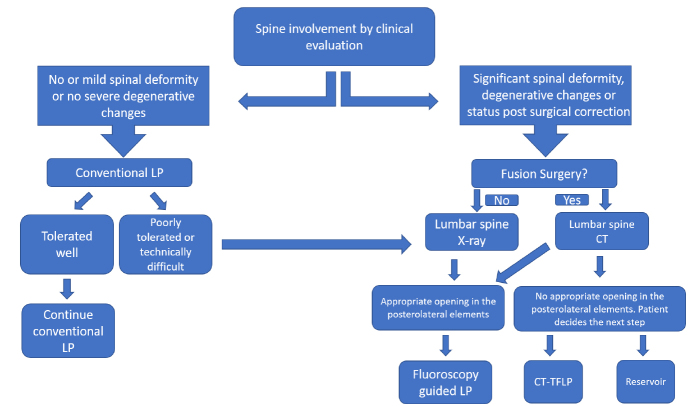
This retrospective cross-sectional study was approved by our institutional review board (IRB study number: STUDY00001458). All intrathecal nusinersen injections performed at our institution between February 2017 and September 2018 were reviewed via electronic medical records (EMR) and PACS. This included patients treated with conventional LPs and intrathecal reservoir injections performed by neurology team along with FGLPs and CT-TFLPs performed by neuroradiology team. Whether to treat with nusinersen was decided by the neurology team based on the abovementioned indications, and route of delivery was a joint decision between the neurology and neuroradiology departments based on patient’s history, age, anatomy, and spinal imaging (Fig. 1). Procedural informed consent was obtained from either the patient or patient’s legal guardian. All LPs using conventional technique were performed in outpatient setting by the neurology team, only ultrasonography guidance was occasionally used as necessary to define the injection site.
Figure 1.
Flow chart used in our institution to select the most appropriate injection technique. LP, lumbar puncture; CT-TFLP, CT-guided transforaminal lumbar puncture.
All CT-TFLP and FGLP injections were performed in outpatient setting by an experienced pediatric neuroradiologist or a pediatric neuroradiology fellow in the lateral decubitus position. For the CT-TFLP technique, patients were positioned in the lateral decubitus with the convex side pointing superiorly. A low dose scout CT was obtained with grid placement to plan the needle entrance side and shortest needle course. Conus medullaris level, curvature of the scoliosis, caliber of the neural foramina and relationship to the adjacent organs were evaluated. A 22 G or 25 G Quincke type spinal needle was advanced, targeting the most posterior aspect of the neural foramen under CT fluoroscopy guidance. Once the needle hit the lamina, using different needle direction techniques such as bevel orientation or curving the needle, the needle was advanced through the most posterior aspect of the neural foramen into thecal sac in order to avoid any nerve damage. For more advanced details of the technique, we refer interested readers to the paper by Nascene et al. (6).
All CT-TFLPs were performed with local anesthesia. Conventional LPs and FGLPs were also generally performed via local anesthesia, unless moderate sedation or general anesthesia was necessary.
Post-LP bedrest was 30 minutes for the first 8 months and was later extended to at least 60 minutes in an attempt to decrease the incidence of postprocedural headaches. If general anesthesia was used, patients were discharged per anesthesia guidelines. Following each procedure, every patient/legal guardian was contacted by a clinic coordinator to assess treatment complications within two days after the procedure.
For each patient, age, sex, body mass index (BMI), SMA type were recorded. For each injection, injection method (LP with conventional technique, FGLP, CT-TFLP, reservoir), injection level, needle size/length/type, and postprocedural complications were obtained from the EMR. For each CT-TFLP, radiation dose was recorded using total dose length product (DLP), average kVp and mAs. Fluoroscopy time was recorded to assess radiation exposure in FGLP cases, and it was a surrogate for procedural difficulty. For each FGLP and conventional LP, the difficulty of the procedure and applied sedation was also recorded. The procedural difficulty was assessed by the radiologist based on imaging and procedure note, using a scale from 1 to 3 (1: easy, 2: moderate, 3: difficult). In patients with intrathecal reservoir, hospitalization after surgery and additional complications regarding the surgery and reservoir were recorded, if present.
Results
A total of 265 injections were performed in 52 patients (24 women [46%]; 28 men [54%]; 4 patients with SMA-1 [7.6%]; 30 patients with SMA-2 [57.8%]; 18 patients with SMA-3 [34.6%]; mean age, 25.5±17 years [7 months to 62 years]). Twenty-one patients were less than 18 years of age, and 117 of 265 injections (44.2%) were performed in these cases. Of 265 injections, 65 (24.5%) were performed in 13 patients using CT-TFLP, 106 (40%) were performed in 24 patients using FGLP, 83 (31.3%) were performed in 16 patients using conventional technique and 11 (4.2%) were performed in 3 patients using intrathecal reservoir. Results are summarized in the Table. Some examples are shown in Figs. 2 and 3.
Table.
Summary of results
| Patients n | Female/male n (%) | Age mean±SD (min–max) | BMI mean±SD (min–max) | SMA type (1/2/3), n | Injection n (%) | Level n (%) | Needle size n (%) | Needle length n (%) | Anesthesia n (%) | Radiation | Complication, n | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CT-TFLP | 13 | 9 (69.2) /4 (30.8) | 35.4±7 (23–51) | 21.9±5.8 (12.6–30.8) | 0/12/1 | 65 (24.5) | L2–L3: 23 (35.3) L3–L4: 34 (52.3) L4–L5: 8 (6.4) |
22 G: 64 (98) 25G: 1 (2) |
3.5″: 24 (40.6) 5″: 32 (54.2) 7″: 3 (5.2) |
All local | Total DLP (mGy·cm) Mean 52.1 (22–158) |
PLPH >1 day: 1 PLPH spontaneous resolution: 7 Hot flashes: 1 Radicular pain: 2 Soreness: 2 |
| FGLP | 24 | 9 (37.5) /15 (62.5) | 28.7±17.2 (15–62) | 24.5±8.9 (10.9–39.7) | 1/11/12 | 106 (40) | L1–L2:1 (0.9) L2–L3: 23 (21.7) L3–L4: 16 (15) L4–L5: 26 (24.5) L5-S1:26 (24.5) S1–S2:14 (13.2) |
22 G: 102 (96.2) 25 G: 4 (3.8) |
1.5″: 1 (0.9) 3.5″: 78 (73.6) 5″: 25 (23.6) 7″: 2 (1.9) |
Local: 101 (96) Moderate: 2 (2) General anesthesia: 3 (2) |
Fluoroscopy time (min) Median: 0.5 (0.1–7.2) |
PLPH > 1 day: 4 PLPH spontaneous resolution: 3 Soreness: 2 |
| Conventional LP | 16 | 7 (43.7) /9 (54.3) | 12±17.1 (<1–52) | 19.4±7 (13.5–38.1) | 3/8/5 | 83 (31.3) | L2–L3: 5 (6) L3–L4: 66 (79.5) L4–L5: 12 (14.5) |
All 22 G | 1.5″: 48 (57.8) 3.5″: 34 (40.9) 5″: 1 (1.3) |
Local: 29 (35) Moderate: 23 (28) General anesthesia: 20 (24) Unknown: 11 (13) |
NA | PLPH >1 day: 1 PLPH spontaneous resolution: 3 Soreness: 2 |
| Reservoir | 3 | 1 (33.4) /2 (66.6) | 23.3±10.5 (13–34) | 28.3±4.5 (23.2–31.9) | 0/1/2 | 11 (4.2) | 1 lumbar 2 cranial |
NA | NA | All local | NA | Reservoir infection: 1 Vagal syncope: 1 Headache with spontaneous resolution: 1 |
| Total | 52 | 24 (46.2) /28 (53.8) | 25.5±17 (<1–62) | 22.2±7.3 (10.9–39.7) | 4 /30 /18 | 265 | Local: 206 (77.9) Moderate: 25 (9.4) General anesthesia: 23 (8.6) Unknown: 11 (4.1) |
NA | PLPH >1 day: 6 PLPH spontaneous resolution: 14 Soreness: 6 Radicular pain: 2 Hot flashes: 1 Vagal syncope: 1 |
BMI, body mass index; SMA, spinal muscular atrophy; CT-TFLP, computed tomography-guided transforaminal lumbar puncture; DLP, dose length product; PLPH, post-lumbar puncture headache; FLGP, fluoroscopy-guided lumbar puncture; NA, not available.
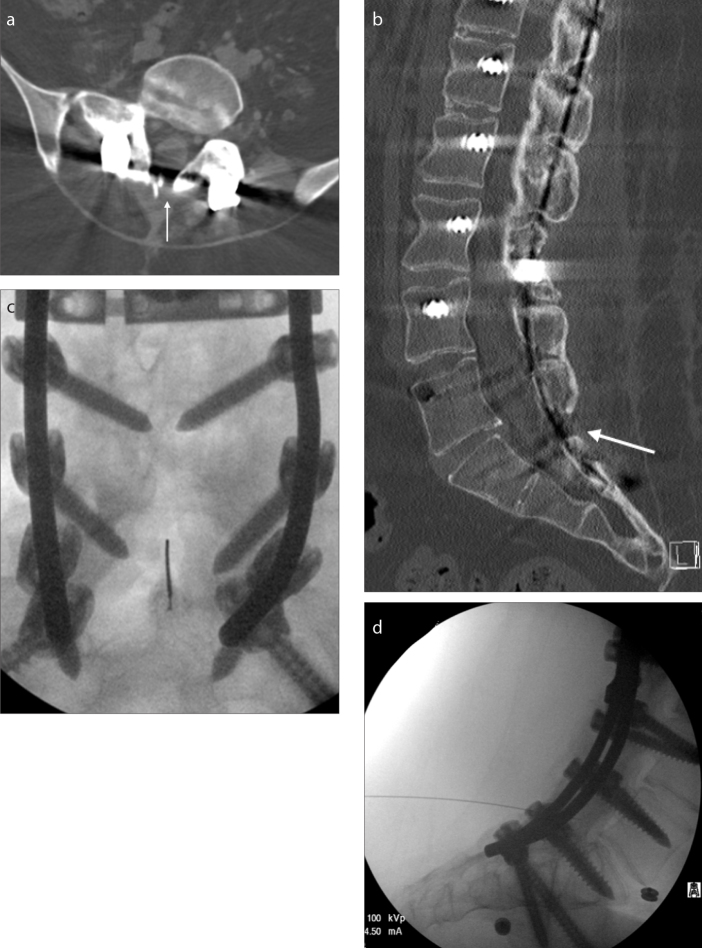
Figure 2. a–d.
A 21-year-old male SMA-3 patient with near complete fusion of posterior elements. Only a tiny nonfused area (white arrow) was present at L5-S1 on axial (a) and sagittal (b) CT reconstructions. This area could be visualized with fluoroscopy (c, d) when correlated with CT; however, patient’s sacral soft tissues were significantly thick, which required a 7-inch long needle. Initially, the first two injections lasted 7.2 and 4.6 min to complete. However, after the initial experience and familiarity with patient’s anatomy, the subsequent procedures were completed in less than 30 s.
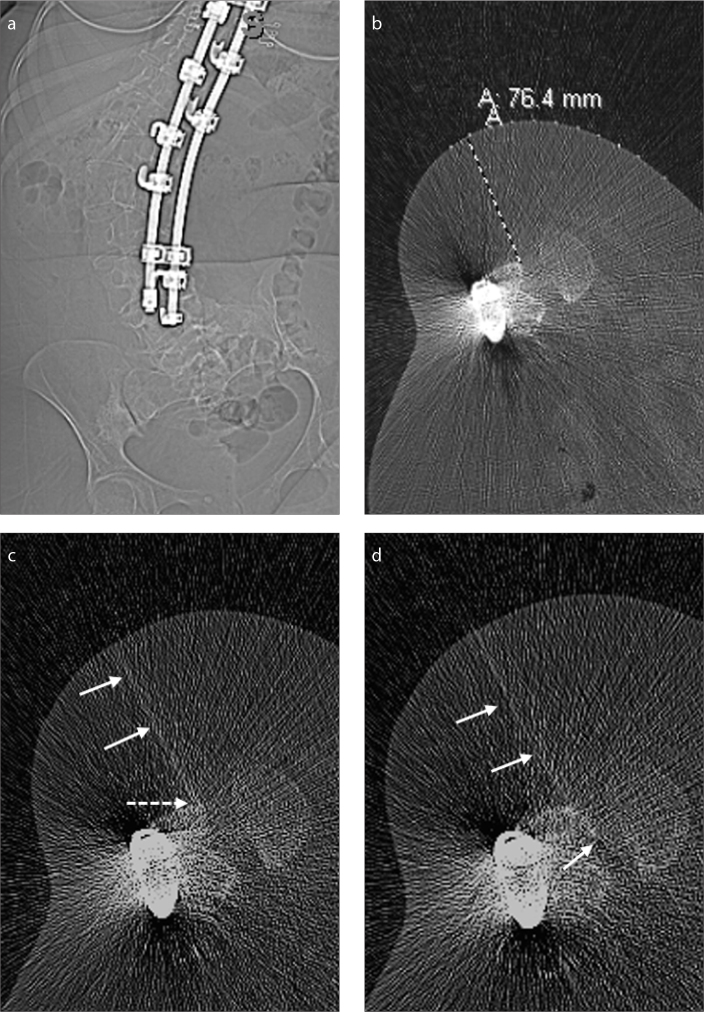
Figure 3. a–d.
A 35-year-old female SMA-3 patient with complete fusion of posterior elements, precluding a standard LP (a). Patient decided the TFLP method for drug administration. Panel (b) shows planning scout CT image with grids, allowing to decide the needle length and needle approach. In panel (c), the needle (arrows) is purposefully oriented to the most posterior border of the neural foramen, to hit the anterior margin of the lamina (dotted arrow). In panel (d), after hitting the bone, using simple needle manipulations, the needle (arrows) was successfully advanced into the thecal sac, without causing any radicular pain and damaging the exiting nerve roots. The patient had PLPH, which spontaneously resolved in the same day without any treatment.
Regarding complications, 6 of 265 injections (2.2%) ended up with a postlumbar puncture headache (PLPH) lasting more than 1 day and required medical treatment. All were resolved within 5 days without need of epidural blood patch (EBP). Fourteen injections (5.2%) resulted in PLPH, which resolved spontaneously within the same day without any medical intervention. On 6 injections (2.2%) patients reported notable soreness at injection site with spontaneous resolution. In one case with lumbar reservoir, the catheter got infected after the surgery as described below. No drug-related complication was identified.
Using CT-TFLP, 65 injections in 13 patients were performed with 100% technical success. Of these, 24 injections in 7 patients was previously reported in order to describe this alternative technique (6). Although exact procedure time was not recorded, it was never longer than 60 minutes starting from patients’ entrance into CT room until exit. Procedure time has significantly decreased with technologist and radiologist experience. Each procedure started with a planning scout CT and a low-dose planning CT in order to assess needle entrance point, needle size, needle angle and adjacent critical structures. Milliampere-second was always kept at 50 mAs. Peak kilovoltage was initially selected as 80 kVp, but was increased to 100 kVp in 9 and to 120 kVp in 3 procedures to increase imaging quality secondary to high BMI. Mean total DLP was 52.1±26.5 mGy·cm (22–158 mGy·cm). L3–4 level was punctured in 34 of 65 (52.3%) and a left side puncture was performed in 40 of 65 (61.5%).
Using FGLP, 106 injections in 24 patients were performed with a 99% technical success. One failed fluoroscopy attempt was transferred to CT where LP was successfully performed. Median fluoroscopy time was 0.5 min (0.1–7.2 min) and 62 of 106 (58.4%) were under 0.5 min. In two patients, after completion of initial injection under fluoroscopy guidance, remaining injections were performed at the bedside using conventional technique due to scheduling availability. Of 106 injections, 47 (44.3%) were rated easy, 27 (25.5%) moderate and 32 (30.2%) difficult.
Using the conventional LP technique, 83 injections were performed in 16 patients. Three of these were infants when they started the treatment (7–12 months) and portable ultrasonography was used for guidance. In two patients, the first injections were difficult; therefore, these patients were referred to radiology for further FGLPs. One case was moderately difficult requiring multiple attempts. The remaining 80 injections (96.3%) were graded as easy and performed without any difficulty.
Three patients elected to have an intrathecal reservoir placement for nusinersen treatment due to complete fusion of posterior elements. It should be noted that this is an off-label use of nusinersen, which is only approved for administration via LP. These patients were admitted by neurosurgery and reservoirs were placed under general anesthesia which required 1-day or 2-day hospitalization. In two cases, reservoirs were placed into the lumbar spine and in one case cranially. However, in one case with lumbar reservoir, the catheter got infected immediately after the surgery. After resolution of infection, a second catheter was placed cranially, which delayed the nusinersen therapy. Reservoir injections were performed by an experienced nurse. The port was accessed by port needle using sterile technique. After checking the patency via cerebrospinal fluid aspiration, nusinersen was injected and flushed with 5 cc of saline to clear the residue. In one occasion, post-injection headache occurred, which resolved spontaneously within hours and in one occasion, patient developed vagal syncope.
Discussion
Nusinersen treatment has opened a new chapter in the lives of SMA patients and their families. This treatment requires a multidisciplinary approach including neurologist, nurse, radiologist, radiology technologist, orthopedist /neurosurgeon, anesthesia personnel, finance specialist, and pharmacist. We have started to observe positive results with this therapeutic option, based on feedbacks from the patients and objective measurements of endpoints. To our knowledge, this is the largest study regarding the procedural perspective.
In our series, most patients had SMA-2, and imaging guidance was necessary in about 65% of injections. Therefore, initial assessment required a thorough evaluation of the spine for deformities, degenerative changes, and postsurgical changes. In 30% of patients, the treatment could be performed with conventional LP. In severe scoliosis without fusion surgery, no further imaging is warranted, and procedure can be performed under fluoroscopy guidance. However, in patients after spinal fusion surgery a noncontrast lumbar spine CT must be obtained to assess if there is any available nonosseous interlaminar space allowing FGLP. If there is no available window for FGLP, CT-TFLP can be considered (6). When we first started applying CT-guided TFLP and our experience was limited, in one of our very first patients, we decided to perform the subsequent procedure under fluoroscopy guidance. Patient was positioned in the lateral decubitus position similar to how we perform the CT-TFLP. However, while advancing the needle to the target, patient developed significant flank pain which raised suspicion for renal puncture. Therefore, we moved the patient to the CT room and continued the procedure under CT guidance. CT revealed perinephric stranding consistent with renal puncture. In the close follow-up, patient’s symptoms resolved without any treatment. However, after this experience we decided to perform all TFLPs under CT guidance.
Regarding complications, only 2.2% developed a clinically significant PLPH requiring medical treatment and 5.5% had PLPH which resolved spontaneously within the same day. No EBP was required. In normal populations, clinically significant PLPH rates vary between 5.5% and 32% in non-image-guided LPs, while the lowest PLPH rate is reported as 2.2% with imaging guidance and EBP rates following FGLP vary between 0.8% and 1.8% ( 7, 12, 13). In an initial study including 73 nusinersen injections in SMA patients, Haché et al. (4) reported a PLPH incidence of 23%, in which 10% required medical treatment. Recently, Stolte et al. (14) reported PLPH in 26.7% of 122 injections while Mousa et al. (15) did not report any PLPH in 104 injections. In our study, we called every patient following injections and all complications were meticulously recorded. Therefore, our results should not reflect any documentation or recall bias. Our PLPH complication rate is lower than previously reported studies performed solely in SMA patients, close to what is reported in normal patient populations. In 2.2% of injections, soreness occurred at the injection site, which did not require any treatment. Vagal syncope was seen in one case, likely from a combination of positioning and injection-related pain. One patient reported postinjection facial flushing that resolved spontaneously. This adverse event was not considered as a medication side effect. Specific to CT-TFLP, on two occasions (3%), patients developed significant radicular pain. In one patient this resolved without intervention. The other patient was admitted to emergency department and had diagnostic imaging without evidence of epidural hemorrhage or other findings. Symptoms resolved with narcotic pain management. The exiting lumbar nerves can easily be punctured or irritated during the procedure due to small neural foraminal size. Therefore, we believe the needle should be directed to the most posterior aspect of the foramen, preferentially aiming for the posterior osseous border of the foramen with subsequent anterior sliding into the spinal canal.
Regarding the radiation dose of CT-TFLP, the mean total DLP was 52.1 mGy·cm (22–158 mGy·cm). Total DLP is a value calculated by the CT machine and it is a predictor of effective radiation dose (16). In our center, depending on patient size, volume of interest and scanning parameters, total DLP of a routine spine CT usually varies between 200 and 800 mGy·cm, which is approximately equal to 2 years of natural background radiation (17). Even with conservative methods, the mean total DLP values reported in CT-guided lumbar spinal pain injections, mean total DLP values were reported as 94.2 mGy·cm (18). Recently, Wurster et al. (19) reported a mean of 89 mGy·cm in their series with CT-guided procedures in patients with SMA and concluded that the additional risk of cancer due to radiation from CT-guided LP was 0.06%–0.2%. While not statistically analyzed for this project, we noted a trend toward lower procedure time and radiation doses as our experience increased and this was also reported by other authors as well (16). Considering the mean total DLP of 52.1 mGy·cm in our series, it is possible to reduce this risk even more.
Regarding FGLP, about 60% was performed at traditional L2–L5 levels while approximately 40% was completed using an unusually patent level below L5. In many SMA patients, the spinal canal and lumbar neural foramina are relatively wider than non-SMA patients, making FGLP feasible in certain cases where levels above L5 are completely fused. Severe osteoporosis, frequent in more severely affected SMA patients, can make identification of poorly mineralized osseous margins difficult, and correlation with prior CT is extremely important. In about 30%, cases were deemed extremely hard, although FGLP was possible with planning, even in cases where there was a 1–2 mm gap between the posterior osseous elements. The radiation dose in FGLP is largely affected by operator experience. Using pulsed fluoroscopy, limiting continuous radiation and optimal collimation are keys to obtaining low fluoroscopy time/dose. In normal patient population, mean effective radiation dose is reported as 2.9 mSv, which is roughly equivalent to two spine radiographs, one intravenous pyelogram, or one year of natural background radiation (17, 20).
Sedation or general anesthesia was rarely necessary in the procedures with imaging guidance while nearly 50% of the LPs with conventional technique required either moderate sedation or general anesthesia. This is partly due to the non-image-guided procedures being performed more often in young children. However, moderate sedation and general anesthesia increases the procedure time, recovery period, and costs. In addition, anesthesia could be considered relatively high risk in this patient population due to restrictive lung disease and potentially difficult intubation in the setting of patient positioning and cervical contractures, especially in adult patients. On the other hand, in the study by Bielsky et al. (21), 8 SMA-2 children (mean age: 4 years) underwent general anesthesia using sevoflurane and propofol in 61 procedures and they reported no anesthesia-related complications. We have had the same experience and encountered no complications related to conscious sedation or anesthesia.
Intrathecal nusinersen injection utilizing thoracic spinal catheter is recently reported, which is an off-label use of nusinersen (9, 22). In our cohort, we had three patients with reservoir placement. While two had no complications and tolerated both the operation and subsequent nusinersen injections well, one patient developed a postoperative catheter infection and associated meningitis which required additional treatment, hospitalization, repeat operation and delayed treatment of nusinersen injections. In theory, once the catheter is placed, subsequent injections are relatively easier compared to other methods. However, knowledge in the literature is limited and catheter infection is always a risk. In our experience, even the most difficult cases with posterior fusion are feasible using either FGLP or CT-TFLP methods, although reservoir can still be considered as an option for interested patients.
We would like to share some practical points in nusinersen injections based on our experience. According to product information, the medication company recommends and insurance requires to check platelet counts, coagulation factors and urine proteins prior to each treatment, based on the data obtained from initial clinical trials, which showed that few patients developed lower platelet count and increased urine protein levels compared to normal controls (23). On several occasions, procedures had to be delayed and we had scheduling problems within the same day since getting a urine can be problematic in this patient group. In order to avoid this problem, we started to inform each patient to urinate at home prior to the procedure and bring the sample within a provided cup. In addition, scheduling should be organized according to every institute’s unique blood collection and laboratory workup routine. Finally, we want to emphasize that we did not observe any adverse effect attributed to nusinersen.
With the introduction of intrathecal nusinersen treatment, neurologists and neuroradiologists play an important role in the treatment of SMA patients, and therefore, should be familiar with different techniques and complications of drug administration. Using good technique, it is possible to have very low complication rates even in this complex patient population, and various image-guided procedures can be a safe alternative to surgical approach, even in the most difficult cases.
Main points.
A total of 265 nusinersen injections were performed in 52 spinal muscular atrophy patients. Of these, 24.5% were performed with CT-guided transforaminal lumbar puncture, 40% with fluoroscopy-guided lumbar puncture, 31.3% with conventional technique, and 4.2% via reservoir.
Six injections (2.2%) ended up with a post-lumbar puncture headache (PLPH) lasting more than 1 day and required medical treatment. All were resolved within 5 days without need of epidural blood patch.
Fourteen cases of PLPH (5.2%) occurred within the same day and resolved spontaneously without any medical intervention.
Three patients elected to have an intrathecal reservoir placement. However, in one case with lumbar reservoir, the catheter got infected immediately after the surgery.
Using good technique, it is possible to have very low procedure-related complication rates in complex SMA patient population, and various image-guided procedures can be a safe alternative to surgical approach, even in the most difficult cases.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Kolb SJ, Kissel JT. Spinal muscular atrophy. Neurol Clin. 2015;33:831–846. doi: 10.1016/j.ncl.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verhaart IEC, Robertson A, Wilson IJ, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy - a literature review. Orphanet J Rare Dis. 2017;12:124. doi: 10.1186/s13023-017-0671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corey DR. Nusinersen, an antisense oligonucleotide drug for spinal muscular atrophy. Nat Neurosci. 2017;20:497–499. doi: 10.1038/nn.4508. [DOI] [PubMed] [Google Scholar]
- 4.Hache M, Swoboda KJ, Sethna N, et al. Intrathecal injections in children with spinal muscular atrophy: Nusinersen clinical trial experience. J Child Neurol. 2016;31:899–906. doi: 10.1177/0883073815627882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michelson D, Ciafaloni E, Ashwal S, et al. Evidence in focus: nusinersen use in spinal muscular atrophy: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;91:923–933. doi: 10.1212/WNL.0000000000006502. [DOI] [PubMed] [Google Scholar]
- 6.Nascene DR, Ozutemiz C, Estby H, McKinney AM, Rykken JB. Transforaminal lumbar puncture: an alternative technique in patients with challenging access. AJNR Am J Neuroradiol. 2018;39:986–991. doi: 10.3174/ajnr.A5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozutemiz C, Koksel YK, Huang H, Rubin N, Rykken JB. The efficacy of fluoroscopy-guided epidural blood patch in the treatment of spontaneous and iatrogenic cerebrospinal fluid leakage. Eur Radiol. 2019;29:4088–4095. doi: 10.1007/s00330-018-5828-x. [DOI] [PubMed] [Google Scholar]
- 8.Ozutemiz C, Rykken JB. Lumbar puncture under fluoroscopy guidance: a technical review for radiologists. Diagn Interv Radiol. 2019;25:144–156. doi: 10.5152/dir.2019.18291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakhotia A, Bhalla S, Doll E, Gump W. Use of ommaya reservoir with a thoracic spinal catheter for intrathecal delivery of nusinersen in a patient with spinal muscular atrophy type 2. Neurology. 2018;90(Suppl 15):p4.464. [Google Scholar]
- 10.Veerapandiyan A, Pal R, D’Ambrosio S, Young I, et al. Cervical puncture to deliver nusinersen in patients with spinal muscular atrophy. Neurology. 2018;91:e620–e624. doi: 10.1212/WNL.0000000000006006. [DOI] [PubMed] [Google Scholar]
- 11.Ortiz CB, Kukreja KU, Lotze TE, Chau A. Ultrasound-guided cervical puncture for nusinersen administration in adolescents. Pediatr Radiol. 2019;49:136–140. doi: 10.1007/s00247-018-4240-7. [DOI] [PubMed] [Google Scholar]
- 12.Armon C, Evans RW. Therapeutics, technology assessment subcommittee of the American Academy of Neurology. Addendum to assessment: prevention of post-lumbar puncture headaches: report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology. 2005;65:510–512. doi: 10.1212/01.wnl.0000173034.96211.1b. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez D, Branstetter BF, Agarwal V, et al. Journal club: incidence of complications following fluoroscopically guided lumbar punctures and myelograms. AJR Am J Roentgenol. 2016;206:20–25. doi: 10.2214/AJR.15.14664. [DOI] [PubMed] [Google Scholar]
- 14.Stolte B, Totzeck A, Kizina K, et al. Feasibility and safety of intrathecal treatment with nusinersen in adult patients with spinal muscular atrophy. Ther Adv Neurol Disord. 2018;11:1756286418803246. doi: 10.1177/1756286418803246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mousa MA, Aria DJ, Schaefer CM, et al. A comprehensive institutional overview of intrathecal nusinersen injections for spinal muscular atrophy. Pediatr Radiol. 2018;48:1797–1805. doi: 10.1007/s00247-018-4206-9. [DOI] [PubMed] [Google Scholar]
- 16.Huda W, Mettler FA. Volume CT dose index and dose-length product displayed during CT: what good are they? Radiology. 2011;258:236–242. doi: 10.1148/radiol.10100297. [DOI] [PubMed] [Google Scholar]
- 17.Radiation dose in x-ray and CT exams. [Accessed on 11/13/2016]. Available from: http://www.radiologyinfo.org/en/info.cfm?pg=safety-xray.
- 18.Amrhein TJ, Schauberger JS, Kranz PG, Hoang JK. Reducing patient radiation exposure from CT fluoroscopy-guided lumbar spine pain injections by targeting the planning CT. AJR Am J Roentgenol. 2016;206:390–394. doi: 10.2214/AJR.15.14436. [DOI] [PubMed] [Google Scholar]
- 19.Wurster CD, Winter B, Wollinsky K, et al. Intrathecal administration of nusinersen in adolescent and adult SMA type 2 and 3 patients. J Neurol. 2019;266:183–194. doi: 10.1007/s00415-018-9124-0. [DOI] [PubMed] [Google Scholar]
- 20.Brook AD, Burns J, Dauer E, Schoendfeld AH, Miller TS. Comparison of CT and fluoroscopic guidance for lumbar puncture in an obese population with prior failed unguided attempt. J Neurointerv Surg. 2014;6:324–328. doi: 10.1136/neurintsurg-2013-010745. [DOI] [PubMed] [Google Scholar]
- 21.Bielsky AR, Fuhr PG, Parsons JA, Yaster M. A retrospective cohort study of children with spinal muscular atrophy type 2 receiving anesthesia for intrathecal administration of nusinersen. Paediatr Anaesth. 2018;28:1105–1108. doi: 10.1111/pan.13500. [DOI] [PubMed] [Google Scholar]
- 22.Strauss KA, Carson VJ, Brigatti KW, et al. Preliminary safety and tolerability of a novel subcutaneous intrathecal catheter system for repeated outpatient dosing of nusinersen to children and adults with spinal muscular atrophy. J Pediatr Orthop. 2018;38:e610–e617. doi: 10.1097/BPO.0000000000001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Product monograph including patient medication information spinraza (nusinersen injection) [accessed on 12.21.2018]. Available from: https://www.biogen.ca/content/dam/corporate/en_CA/pdfs/products/SPINRAZA/SPINRAZA_PM_EN_Feb2018.pdf.