Abstract
Lymphedema is an important medical issue around the world, caused by an anomalous collection of fluid in soft tissue due to congenital malformations or stenosis or obstruction of lymphatic vessels. Magnetic resonance lymphangiography (MRL) is an emerging technique focused on noninvasive or minimally invasive imaging of lymphatics with the goal to diagnose and treat lymphedema. This review will briefly discuss lymphatic imaging starting with lymphography and radionuclide lymphoscintigraphy up to the newest methods, focusing on MRL, a rising technique, and highlighting the technical aspects fundamental for achieving high-resolution MRL.
Lymphedema is an important medical issue around the world. According to the literature, 180–250 million people are affected by lymphedema (1); however, prevalence is frequently underrated due to delayed clinical identification and insufficient follow-up (1, 2). It is estimated that 1–2 million people are affected by primary lymphedema and 2–3 million by secondary lymphedema, in the United States only (3).
Lymphatic vessels transport lymph back to the venous system, allowing the elimination of excessive fluid from the interstitium. Lymphedema is represented by an anomalous collection of fluid in soft tissue (4), caused by primary (congenital abnormalities) or secondary lymphatic system disorders; the latter is related to stenosis or obstruction of lymphatic vessels, which often arise as a result of oncological conditions such as lymph node dissection or radiotherapy (4–8). By the accumulation of subcutaneous protein rich fluid, lymphedema determines a pro-inflammatory state, adipose tissue hypertrophy, and progressive interstitial space fibrosis (6), with an increased risk of recurrent cellulitis (8). Lymphedema diagnosis and assessment is mainly based on clinical evaluation and on circumferential measurement of the limbs, taken at fixed anatomical points (9). Decongestive physiotherapy, with a mix of skin care, lymphatic massage and compressive therapy, is considered the treatment of choice (10). However, some patients are not sufficiently responsive to nonsurgical treatments (11); thus, currently different surgical techniques are being investigated for improving lymphatic peripheral circulation in order to reduce limb diameter (12). Surgical options include reconstructive techniques such as lymphaticovenular anastomosis (LVA) and vascularized lymph node transfer (VLNT) or reductive techniques such as debulking/excision and liposuction (13). VLNT promote lymphangiogenesis throughout a microvascular transplantation of functional lymph nodes into an extremity. It generally consists of transferring both deep inferior epigastric artery perforator and superficial inguinal lymph node flaps; however, several other VLNT options are available today (14). LVA represents a microsurgical technique aimed to establish an anastomosis between distal functioning lymphatic collectors and adjacent small subdermal venules less than 0.8 mm in diameter. Microsurgical sites are identified by superficial veins mapping; then, under local anesthesia, skin incision and a subcutaneous tissue dissection are performed under a high magnification microscope, so vessels are dissected and endoluminal sutures are performed (15). LVA represents a valid technique for the resolution of obstructive lymphedema, as demonstrated by Campisi et al. (16), with an 87% reduction in the incidence of cellulitis after the procedure and increased softness of the limbs and decreased peripheral edema at 7 years follow-up in patients with chronic obstructive lymphedema (16). Local anesthesia is sufficient to perform LVA, making it a suitable option for patients with comorbidities or elderly patients (17).
Currently, there are different imaging techniques suitable for first evaluation and follow-up of lymphedema; however, screening and identification require specialized equipment (18, 19). For many years radionuclide lymphoscintigraphy has been the gold standard in cases of lymphedema, followed by indocyanine green fluorescence lymphangiography (ICGL), with several limitations ranging from long acquisition time and ionizing radiation exposure to limited anatomic coverage of ICGL (20). Recently, magnetic resonance imaging (MRI)-based new study protocols have been developed focusing on lymphedema screening and follow-up, creating imaging sequences with and without contrast medium (21, 22). There are no objective measurements to show that surgical management schemes (VLNT/LVA) are better than conventional treatments, and surgical management is frequently accompanied by conservative treatment. In this context, magnetic resonance lymphangiography (MRL) represents a noninvasive imaging technique, helpful in lymphatic follow-up after conservative or surgical management or during lymphatic assessment. Noninvasive MRL (NIMRL), through heavily T2-weighted sequences with very long TR/TE, has the possibility to highlight lymphatic vessels without the use of an exogenous contrast material (22). On the other hand, MRL performed with injections of gadolinium-based contrast agents in the interdigital web spaces or with intravenous injection of the same gadolinium-based contrast can be used to suppress the venous signal for better evaluation of lymphatic vessels, suitable for performing surgical treatments (23, 24).
The aim of this review article is to describe scientific progression in lymphatic vessels evaluation leading up to the MRL technique, and to investigate the differences between the two most commonly used techniques in the literature (MRI with and without contrast agent) in order to provide a complete statement on the best method to achieve high-quality MRL images according to the clinical scenario.
Imaging of lymphatics
Lymphography
Lymphography was the first imaging modality to study lymphatic vessels (25). Similar to angiography, the direct injection of a contrast agent into a vessel allows the visualization of the vascular system. The contrast (Direct Blue or Patent Blue) is injected into the dermis where it is absorbed by initial lymphatics and fills the lymphatic vessels that drain the injection site, highlighting only the lymphatic vessels draining that position (26). This technique was developed for the first time by Kinmonth in 1952 and allows the identification of lymphatic channels that can be cannulated and injected with an opaque contrast agent for radiographic imaging (27, 28). Lipiodol (oil-soluble nature), the usual contrast material, is no longer available, because it was observed that this oil-based contrast causes obliteration of the vessels through inflammatory process or direct blockage by the oily material itself (29). Contrast agent was then modified according to clinical and experimental applications (30), and the technique was eventually superseded by newer methods.
Indocyanine green fluorescence lymphangiography
Indocyanine green lymphography (ICGL) has been widely used in the field of lymphedema as a complementary technique to lymphoscintigraphy (28). Currently, ICG fluorescence lymphangiography allows a direct real-time evaluation of subdermal lymphatics and it is considered an essential diagnostic imaging technique for the evaluation of lymphatic system, because it is minimally invasive and highly sensitive (31). ICG (0.2–1 mL, ICG 0.5%) is administered through an intradermal injection, at II interdigital space on the dorsum of the foot and at level of the lateral edge of Achilles tendon, in cases of lower limb lymphedema or at II interdigital space of the dorsum of the hand and on the ulnar ventral surface of the wrist, in cases of upper limb lymphedema. Images of lymphatic system are then obtained using an infrared camera (28). It seems to be an effective technique; however, it is not able to detect lymphatic vessels deeper than 1.5–2 cm under the skin. This condition represents an important limitation for the preoperative evaluation of peripheral lymphedema (31), which can be overcome by the use of other techniques, such as MRL. Moreover, even though it is a safe and minimally invasive technique that can be performed repeatedly, the patient is exposed to radiation dose (28–31).
Radionuclide lymphoscintigraphy
For many years, radionuclide lymphoscintigraphy has been the gold standard in cases of lymphedema (32). This imaging modality includes the injection of intradermal or subcutaneous radiolabeled tracers with subsequent gamma camera monitoring. It largely depends on the choice of radiotracer: 99m Tc-filtered sulfur colloid (particle size 100 nm) is a radiotracer frequently used, because it is cheap, safe and effective; however, it has the disadvantages of minimal absorption from the injection site with a slow transport rate; moreover, this slow transit increases the acquisitions times.
The type and site of injection also play a crucial role in lymphoscintigraphy; in fact, subcutaneous injection of colloidal agents seems to produce more reliable results compared with intradermal injection (32, 33). The colloid is administered through a 26-gauge needle injection for each site (the space between the 1st and 2nd and the 2nd and 3rd digits of the hands or feet) and generally both limbs are prepared (one is used as control). A dual-detector instrument is used to follow the progression of the tracer and to record the images. A transmission scan permits anatomic localization of the visualized areas (34). Data is collected within 10 minutes from the injection, at 1–2 h, and 4–6 h after tracer administration (35–37).
However, the poor spatial and temporal resolution of this technique, in addition to ionizing radiation exposure for both patients and clinicians, limited its use (35). For these reasons, other techniques emerged to replace lymphoscintigraphy for many applications.
Magnetic resonance lymphangiography
MRL, with or without the use of gadolinium-based contrast agent, is a widely described technique in recent literature (21, 22), as a valid support to diagnose lymphedema and to map lymphatic vessels. This approach is helpful to follow the disease development and to plan the appropriate surgical treatment, especially LVA. In order to plan physiological reconstructive techniques, and in particular LVA, MRL is essential to assess the lymphatic channels’ status (23), and to find the small subdermal lymphatic channels and venules necessary to carry out the anastomoses. As nuclear lymphoscintigraphy is not able to discern singular lymphatic channels, venules and ICGL has a limited penetration depth of about 2 cm, MRL is usually used as the preferred preoperative imaging modality. When MRL does not detect functioning superficial lymphatic channels, there is no indication for performing LVA bypass (21). The major advantage of MRL compared to radionuclide lymphoscintigraphy are better spatial and temporal resolution without ionizing dose exposure to the patient (21, 31, 36, 37).
MRL techniques
Magnetic resonance equipment
MRL can be executed on a 1.5 T or 3.0 T unit (22, 38, 39). According to recent studies, a quadrature detection phased-array coil should be used to study the thoracic duct, while a six-channel phased array body coil should be used for imaging the retroperitoneal lymphatic vessels, lower or upper limbs (40, 41); a phased-array 36-channel peripheral angiography coil and an 8-channel body coil can be also used for both upper and lower limb studies (42).
Patient preparation and positioning
Patients are asked to wear elastic stockings or bandages for 1 day to reduce lymphatic drainage. Moreover, to study retroperitoneal lymphatic vessels, patients are asked to drink pineapple juice 30 min before the examination to reduce bowel content signal intensity (43). It is very important to instruct the patient about the duration of the examination, and the correct position that should be maintained during the entire period. Positioning varies depending on the site of investigation. For upper limb evaluation, the patient is placed in prone position, head first, and fasting is recommended during the examination to reduce motion artifacts. Direct contact of the coil with the skin should be avoided to reduce the hyperintensity artifacts. Anatomical sites evaluated are the following: hand, wrist and forearm; elbow, arm, shoulder; axillary lymph nodes. In case of lower limb evaluation, the patient is placed in the supine position, feet first, reaching parallelism of lower extremity and the main magnetic field and near the most homogeneous area of B0. The toes emerge from the holes of the coil and are easily accessible for the injection of the contrast agent. The acquisition is usually performed in 3 or 4 runs to cover all the anatomical sites of the extremities, according to the height of the patient. Anatomical areas evaluated are the following: feet, ankle, lower leg; knee, upper leg, lower thigh; medial and proximal thigh; inguinal and pelvic region, root of the thigh.
A 24- to 28-gauge thin needle is generally used to perform subcutaneous injection in the dorsal aspect of interdigital web spaces (foot or hand according to the site of interest). Generally, a volume of 1 mL (2 mL max) for each space is sufficient.
A mixture of the standard dose (0.1 mmol/kg body weight) of a paramagnetic contrast medium and 0.5 mL of lidocaine 1% for local anesthesia is injected subcutaneously/intradermally; furthermore a topical local anesthetic cream (mixture of lidocaine 2.5% and prilocaine 2.5%, EMLA 5%, AstraZeneca) is placed to each interdigital web space 30 min before the injection. Most authors use gadobenate dimeglumine (Gd-BOPTA, Multihance, Bracco Imaging) (23, 44) or gadobutrol (Gadovist 1.0 M, Bayer) (45); however, some other contrast agents have also been safely used for MRL: gadoterate meglumine (Gd-DOTA, Dotarem, Guerbet), gadoteridol or (Gd-HPDO3A, Prohance, Bracco Imaging) (46). The low molecular weight of extracellular gadolinium-based contrast agents allows these agents to be taken up by the lymphatic circulation after intracutaneous injection (47). The injection sites are normally massaged for 60 s after contrast material injection to facilitate lymphatic uptake (31).
For intravenous contrast administration, relatively novel contrast agents such as Ultrasmall superparamagnetic iron oxide particles (USPIO) were specifically created for MRL; after intravenous administration, USPIO are taken up by the macrophages of the lymph nodes and lymphatics, where they accumulate (48). Mitsumori et al. (46) used a gadolinium-based contrast agent for intravenous injection before the introduction of an iron-oxide blood-pool contrast agent (Ferumoxytol; Feraheme, Advanced Magnetics, carbohydrate-coated ultrasmall iron-oxide particle, with an intravascular half-life of 15 hours), recently introduced to remove venous signal secondary to T2* suppression (46, 49). Ferumoxytol can also be used as an off-label agent for magnetic resonance angiography in patients with contraindications to gadolinium-based contrast, even if there is currently limited access to it worldwide (50).
Noncontrast method (NIMRL)
Recently some articles reported the possibility to make noninvasive MRL (NIMRL), for both upper and lower extremities (43, 44). The major advantage is the absence of an exogenous contrast; however, many disadvantages have been reported, such as the slower velocity of lymph vs. blood and the increased field heterogeneity and radiofrequency labeling inefficiency in the extremities.
Imaging protocol
At first a survey and a calibration for all the anatomical sites are performed, followed by axial and coronal True FISP sequences to delineate the volumes to be covered. Some authors perform an axial T2 TSE to calculate lymph node number and size (42, 51, 52). Then, a T2-lymphangiography sequence is acquired (heavily T2-weighted 3D sequence, with a very long TR/TE). Other sequences can be associated and are reported in Table 1.
Table 1.
NIMRL-associated sequences according to the literature
| Sequence | Plane | TR | TE | Matrix size | FOV (mm) | Flip angle | Slice thickness (mm) | |
|---|---|---|---|---|---|---|---|---|
| Arrivé (53) | HASTE without fat suppression | Axial | 1200 | 114 | 176Å~256 | NS | 180° | 6 |
| Arrivé (42) | IDEAL | Axial | 4233 | 76 | 320Å~192 | 380Å~380 | NS | 6 |
NIMRL, noninvasive magnetic resonance lymphangiography; TR, repetition time; TE, echo time; FOV, field of view; HASTE, half-Fourier acquisition single-shot turbo spin-echo; NS, not specified; IDEAL, iterative decomposition of water and fat with echo asymmetry and least-squares estimation.
The acquisition parameters of NIMRL according to the studies reported in the literature are summarized in Table 2. Moreover, this technique can be extended to abdominal organs (53).
Table 2.
Technical parameters of NIMRL according to the studies reported in the literature
| MRI unit | Sequence | Plane | TR | TE | Matrix size | FOV (mm) | Flip angle | Slice thickness (mm) | |
|---|---|---|---|---|---|---|---|---|---|
| Cellina (22) | Magnetom Avanto, Siemens Healthineers | T2 space COR FS TSE | Coronal | 2870 | 797 | 358×384 | 380×380 | 140° | 1 |
| Takahashi (41) | VISART EX, Toshiba | 3D Half-Fourier TSE | Coronal | 7000 | 500 | 320×320 | 360×360 | NS | 2 |
| Arrivé (53) | Magnetom Symphony, Siemens | 3D TSE | Coronal | 1400 | 800 | 256×256 | NS | 180° | 1 |
| Arrivé (43) | Signa HDxt, GE | 3D TSE | Coronal | 4000 | 884 | 512×288 | 400×400 | 90° | 0.8–1.4 |
| Liu (44) | Magnetom Vision Plus, Siemens | TSE | Coronal | 2800 | 1100 | 256×256 | NS | 150° | 3 |
NIMRL, noninvasive magnetic resonance lymphangiography; TR, repetition time; TE, echo time; FOV, field of view; TSE, turbo spin-echo; 3D, three-dimensional; NS, not specified.
MRL with intradermal injection of gadolinium-based contrast agent
Recently, MRL performed with injections of gadolinium-based contrast agents in the interdigital web spaces has been widely used. Intradermal injections enable rapid access of gadolinium into the initial lymphatics and is limited to 0.1 cc of volume. Sometimes subcutaneous injections are performed to study for larger volumes. The major advantage of intradermal injection is the possibility to clearly identify pathological lymphatic vessels suitable for performing LVA treatment. This imaging modality is also widely used to assess and define lymphatic pathology in pediatric patients with good results (54).
Imaging protocol
At first a survey and calibration for all anatomical sites are done as in NIMRL; the basic sequence is a post-contrast T1-weighted 3D with fat saturation; however, sequences can vary, depending on different MRI units as described in Table 3: fast low angle shot (FLASH), volume interpolated breath-hold examination (VIBE), 3D spoiled gradient-recalled echo T1-weighted sequence with SPECtral inversion at lipid (FSPGR with SPECIAL) sequences can be used (55). Usually an acquisition (mask) before contrast agent injection is performed and then subtracted from post-contrast acquisitions, in order to highlight contrast uptake in the vessels (23). Most authors acquired the post-contrast sequences at 10 minutes after contrast media injection (32, 45); Mazzei et al. (21) reported using an interval time of 15 minutes, in particular, acquiring the first site and repeating it at 5, 20 and 35 minutes after the injection of the contrast medium; the other sites were acquired in sequence after the first one at 5, 20 and 35 minutes (the total average examination time is 1 hour and 15 minutes for the lower limb (3 min Å~3/4 anatomical sites and 3 min 50 s Å~3/4 anatomical sites Å~4 times [at 0, 5, 20, and 35 min]) and 50 minutes for the upper limb (21). The mean acquisition time was 15 and 25 minutes for lower leg, and 35 and 45 min for thigh and pelvis (45).
Table 3.
Technical parameters of contrast-enhanced MRL according to the studies reported in the literature
| MRI unit | Sequence | Plane | TR | TE | Matrix size | FOV (mm) | Slice thickness (mm) | NEX | Bandwidth (kHz) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mazzei (21) | Signa TwinSpeed HDxt GE | 3D spoiled GRE T1-weighted with SPECtral inversion at lipid balanced | Coronal | 5 | 2.1 | 448×320 | 44×44 | 2.8/1.4 | 1 | ±111.1 |
| Bae (35) | Magnetom Trio; Siemens | 3D T1-weighted GRE | Coronal | 3.5 | 1.3 | 288 Å~ 202 | 279 | 1.2 | NS | 511 |
| Mitsumori (46) | NS | 3D T1-weighted GRE | Sagittal | 7.2 | 3.3 | NS | 485×162×100 | NS | NS | NS |
| White (45) | Magnetom Avanto; Siemens | Volume interpolated breath-hold examination (VIBE) | Coronal | 3.74 | 1.51 | 448×448 | 500×375 | 1.5 | NS | NS |
| White (45) | Magnetom Trio; Siemens | Fast low angle shot (FLASH) | Coronal | 2.8 | 1.06 | 512×512 | 500×312 | 1.5 | NS | NS |
| Jeon (55) | Ingenia, Philips Medical Systems | 3D fat-suppressed T1-weighed FSE | Coronal | 350 | 17 | NS | 350×450 | 1 | NS | 355 |
| Jeon (55) | Ingenia, Philips Medical Systems | 3D fat-suppressed intermediate-weighed FSE | Coronal | 1400 | 40 | NS | 350×450 | 1 | NS | 370 |
MRL, magnetic resonance lymphangiography; TR, repetition time; TE, echo time; FOV, field of view; NEX, number of excitations; 3D, three-dimensional; GRE, gradient echo; NS; not specified; FSE, fast spin-echo.
Other sequences can be added to the study, before performing post-contrast sequences, depending on the type of MRI unit used: Signa TwinSpeed HDxt GE, Coronal 3D steady-state free precession (SSFP) balanced (TR/TE, 4/1.9 ms; FOV, 40×40 cm; matrix, 224×192; thickness, 2/1 mm), 3D T2-weighted turbo spin-echo (TR/TE, 2000/680 ms; FOV, 40×40 cm; matrix, 320×224; thickness, 3.5/1 mm) (21); Magnetom Trio Siemens Healthcare, T2-weighted coronal rapid acquisition with spectral fat saturation and sampling perfection with application of optimized contrast using different flip angle evolution (SPACE: TR/TE, 4000/221 ms; flip angle, 120°; section thickness, 1.5 mm; in-plane resolution, 1.0 Å~1.4 mm2; FOV, 350 mm; integrated parallel acquisition technique with acceleration factor of three) (36), Magnetom Avanto Siemens Healthcare, coronal half-Fourier acquisition single-shot turbo spin-echo (HASTE: TR/TE, 2000/696 ms; section thickness, 1.5 mm; FOV, 480×360; matrix, 256×256) (44), Magnetom Trio Siemens Healthcare, coronal HASTE (TR/TE, 2500/400 ms; section thickness, 2 mm; FOV, 380×285; matrix, 256×256) (45).
MRL with both intradermal and intravenous injection of contrast agent
MRL requires intracutaneous injection of an extracellular gadolinium-based contrast agent to allow its uptake by the lymphatic circulation; however, the intravenous injection of the same Gd-based contrast agent used for the lymphangiogram or, more recently, an iron-based blood-pool agent, either before gadolinium injection or any time necessary during the examination, can be used to create a suppression of venous signal (46). However, only a few articles in the literature performed this procedure (46, 48).
Imaging protocol
The initial protocol acquisition is similar to MRL performed with injection of gadolinium-based contrast agent in the interdigital web spaces. Mainly two sequences are used: a heavily T2-weighted 3D turbo spin echo (TSE) with spectral fat suppression and a dynamic fat-suppressed T1-weighted 3D spoiled gradient echo (SPGR) (either a single-echo 3D T1-weighted gradient-echo (GRE) with spectral fat suppression or dual-echo 3D T1-weighted GRE with Dixon reconstruction) before and after the intracutaneous injection of contrast medium. A minimum of 6 dynamic-phase acquisitions at 10-minute intervals (0-10-20-30-40-50 min) are acquired. A fat-suppressed T1-weighted 3D SPGR sequence acquired 180 s after a single dose (0.1 mmol/kg) of the same gadolinium-based contrast agent is added, with the aim to remove venous signal secondary to T2 suppression (46).
Discussion
Nowadays lymphangiographic imaging remains an open issue, although there have been recent advances in the assessment of the lymphatic system, due to the lack of specific diagnostic methods. Lymphoscintigraphy is the most common technique for the study of lymphatic vessels; however, it is limited by the lack of sufficient spatial resolution, essential to highlight lymphatic anatomy, and long acquisition times, making it uncomfortable for the patients, in addition to ionizing radiation exposure (25). The lack of vein representation limits the use of this technique for planning LVA treatment too. MRL represents a valid tool to evaluate lymph nodes and lymphatic channels. MRL has high spatial resolution and the possibility to use 3D sequences without radiation dose exposure to the patient (56), even if it is affected by some limitations such as long acquisition times (less than lymphoscintigraphy, 1 hour vs. 6–8 hours) or the need of manual massage or lymphatic drainage, to facilitate the progression of the administered contrast, in cases of chronic lymphedema. Moreover, MRL offers a good evaluation of deep lymphatic vessels at the expenses of superficial lymphatic vessels and veins (19, 21). The authors should decide the best method to visualize lymphatic vessels through MRL or decide to combine different imaging modalities (ICG-MRI) to overcome limitations of MRL when used as the sole imaging method.
NIMRL
NIMRL represents a relatively new imaging protocol to study lymphatic vessels without the injection of contrast media; it is a great option, especially for allergic and pediatric patients, overcoming all the problems related to contrast media complications after intracutaneous injection (57). Patients undergoing MRL with contrast media can experience pain and swelling at the sites of intracutaneous contrast injections (6). Moreover NIMRL, through heavily T2-weighted and fat-suppressed sequences, is able to evaluate the subcutaneous soft tissues to delineate the presence, severity, and extent of lymphedema, as well as associated soft-tissue changes such as adipose deposition and fibrosis avoiding the need of contrast media injection (58, 42). NIMRL seems to be superior for the analysis of proximal lymphatic ducts, such as inguinal and iliac, compared to contrast-enhanced MRL, and it is suitable for detecting the malformation of the deep lymphatic system, especially lymphatic dilatation deformity (41). The major limitation of NIMRL is represented by only few diagnostic experiences with this technique present in the literature, so larger patient case series are needed to validate this procedure; moreover the scanty number of articles comparing this imaging modality with intraoperative findings, makes it difficult to determine if NIMRL is sufficient by itself to provide a correct pre-procedural and diagnostic tool. Furthermore, NIMRL cannot show normal or hypoplastic lymphatic vessels (59) and distal lymphatic vessels because of its lesser spatial resolution compared with contrast-enhanced MRL. Moreover, in the absence of contrast medium, no information is gathered about vessels’ functionality (both lymphatics and veins), which is essential to identify lymphatics suitable for performing LVA treatment; in this sense, contrast-enhanced MRL has a great advantage since the vessels visualized in this way have at least a residual undamaged pump to drain the contrast agent (16, 42).
Using NIMRL, blood and lymphatic vessel features can appear similar, both in MIP reconstructions and in acquisition sequences, since these vessels are closer; thus, distinguishing between the lymphatics and blood vessels can be difficult (38). However some articles in literature (21–23) have described the possibility to differentiate venus from lymphatic vessels at the same time with NIMRL: caliber, morphology and beaded appearance of vessels have been explored with good histology-proven results (21). Furthermore to improve this possibility, Crescenzi et al. (39) tried to implement some procedures to insure blood-water signal suppression: the authors utilized a long echo time sequence (MRI unit 3.0 T Philips Healthcare, TEeffective=600 ms; TEequivalent=491 ms). Since 3.0 T lymphatic T2 is 610 ms (5), 3.0 T venous and arterial blood–water T2 are less than 50 ms and 150 ms, respectively, including microvessels with a hematocrit <0.30 (60), and a long TSE pulse train (TSEfactor=90; shot duration=1187 ms), which serves to inefficiently refocus fast-flowing spins, as is the case for blood–water.
MRL with gadolinium-based contrast agent
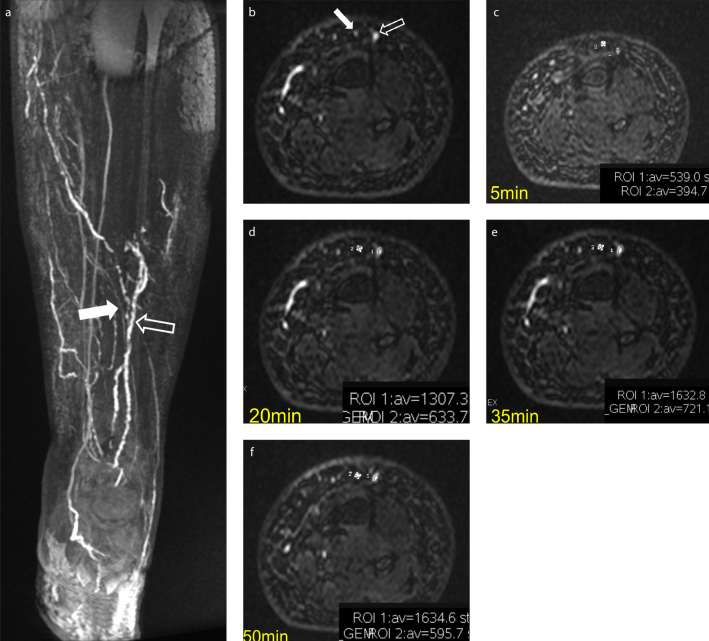
MRL with intradermal injections of gadolinium-based contrast agent was introduced with the aim to improve lymphatic vessels disease interpretation. Contrast-enhanced MRL imaging is used to map the lymphatic vessels throughout high-resolution 3D sequences, with the advantage of dynamic monitoring of the lymphatic transit with a minimally invasive technique. It provides good morphologic and functional information in a single examination, with high spatial and temporal resolution, depicting the drainage pattern, lymph node position, lymphatics, and venous structures, as well as the severity of lymphedema (Fig. 1) (23, 36, 38). Moreover contrast-enhanced MRL can identify the “dermal backflow” (an area of progressive interstitial dispersion of the contrast medium in soft tissue due to proximal obstruction of lymph drainage) (24), which is related to high intralymphatic pressure and excess of lymphatic fluid. This feature is indicative of proximal lymphatic obstruction with alternate pathways of transport and is generally seen after a mean time of 15–20 min from the injection of the contrast media, with increasing intensity over time (24). The immediate visualization of contrast media extravasation, and the correct identification of the point of obstruction, allows faster and more accurate planning for LVA treatment.
Figure 1. a–f.
Clinical example of the kinetic of enhancement (ROI 1 = lymphatic; empty arrow; ROI 2= vein, white arrow). Coronal and axial 3D T1 gradient-echo sequences with SPECtral inversion at Lipid (FSPGR) show different morphology and diameter of lymphatic vessels (empty arrow) and veins (white arrow) (a, b). Taking into account the kinetic of enhancement at 5, 20, 35, 50 minutes after subcutaneous injection of contrast media, there is a clear increase in the region of interest (ROI) values in the affected lymphatic vessel, while in the vein, an uptrend followed by a down trend is observed (c–f).
Limitation of contrast-enhanced MRL are higher costs related to the presence of contrast medium and the longer acquisition time compared with NIMRL. Contrast-enhanced MRL examination takes approximately 1 h and 15 min (21), while NIMRL takes half an hour including patient positioning and image acquisition (35), so longer acquisition time means further costs. Even if also NIMRL could allow the differentiation between lymphatic vessels and veins, contrast-enhanced MRL allows for better differentiation between these two anatomical structures, even though sometimes, there can be venous contamination, because gadolinium chelates-based contrast is water soluble and diffusible (22). Some authors (45) highlight the possibility to reduce venous contamination by using intradermal injection rather than subcutaneous injection. Mazzei et al. (21) used a precaution to reduce contamination by withdrawing the syringe plunger before the injection in order to avoid cannulation of a small vein. Moreover, Mazzei at al. (21), performed a steady-state free precession (SSFP) balanced electrocardiography-triggered sequence (FIESTA, GE), with spectral fat saturation, before the injection of the contrast medium, which allowed correct visualization of the venous system, lymphedema and lymph nodes, without adding too much time to the full exam (Fig. 2). Mitsumori et al. (46) performed an intravenous systemic and subdermal injection of Gd-based contrast with the aim to overcome contrast-MRL limitation and to correctly distinguish lymphatic from venous vessels. They supported the idea to identify all venous vessels with contrast intravenous injection and technologically remove these vessels from those evidenced in subdermal contrast sequences, obtaining only lymphatic vessels. However, they provided no histological confirmation. Some studies introduce USPIO (<50 nm) as alternative to gadolinium-based contrast agent alone to study lymphatic vessels (26, 61). USPIO contrast decreases relaxation times in lymph nodes due to its specificity for the reticuloendothelial system present in normal nodes, but is less represented in neoplastic nodes. However, this contrast is not very widespread; it is used for evaluating metastatic nodes, but less commonly used for mapping lymphatic vessels (62). Moreover, due to the negative-contrast nature of the detection, small lesions can be missed (26). Clearly, more extensive studies are needed to confirm the advantages of intravenous contrast media injection associated with standardized contrast-enhanced MRL.
Figure 2. a–c.
Noninvasive MRL performed through heavily T2-weighted sequences without subcutaneous contrast media injection allow lymphatics visualization; however, in case of severe lymphedema, the hyperintensity of epifascial areas could prevent the visualization of the underlying lymphatics (a). T2 sequences, if used alone, are not useful to give functional information about the run-time of enhancement of lymphatics. A balanced sequence ECG-triggered (3D steady-state free precession, SSFP) can show both the epifascial distribution of lymphedema and a map of veins (b, arrow), whereas a post-contrast T1-weighted 3D with fat saturation sequence can visualize both lymphatics (arrowhead) and veins (arrows), allowing functional information as well (c).
Indocyanine green-combined MRI and ICGL
The recent introduction of ICG-combined MRI and ICGL aims to overcome the limitations of using MRL alone. Combined ICG-MRI offers more sensitive imaging and provides the surgeon with an improved understanding of lymphatic function and anatomy (31). The information obtained by the combination of these two techniques, allows the selection of safe, efficient and most effective locations for LVA, because ICGL alone is able to detect only superficial lymphatics vessels (31), and the addition of MRL to preoperative planning provides an accurate individual evaluation of the entire lymphatic system, from superficial to deep (31, 63–65).
Conclusion
Lymphedema is an important medical issue worldwide. MRL imaging of lymphatics has introduced a change in the assessment and management of lymphatic pathologies, despite lymphoscintigraphy being the most common technique today. This relatively new approach and minimally invasive technique continues to develop according to the presence of higher Tesla MRI units and implements sequences focused on lymphatic imaging (9, 66). However, there is no agreement yet on the best technique to perform high-resolution MRL (67).
Main points.
MRL is a valid method to diagnose lymphedema and to map lymphatic vessels, which is fundamental to follow the disease development and to plan the appropriate surgical treatment.
NIMRL, through heavily T2-weighted and fat-suppressed sequences, is able to evaluate the presence, severity, and extent of lymphedema, as well as associated soft-tissue changes, avoiding the need of contrast media injection.
Contrast-enhanced MRL can map the lymphatic vessels throughout high-resolution 3D sequences with the advantage of dynamic monitoring of the lymphatic transit with high spatial and temporal resolution, and a minimally invasive technique.
Indocyanine green-combined MRI aims to overcome the limitations of imaging by MRL alone, with more sensitive imaging.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Stout NL, Brantus P, Moffatt C. Lymphoedema management: an international intersect between developed and developing countries. Similarities, differences and challenges. Glob Public Health. 2012;7:107–123. doi: 10.1080/17441692.2010.549140. [DOI] [PubMed] [Google Scholar]
- 2.Rockson SG, Rivera KK. Estimating the population burden of lymphedema. Ann N Y Acad Sci. 2008;1131:147–154. doi: 10.1196/annals.1413.014. [DOI] [PubMed] [Google Scholar]
- 3.Zuther JE, Norton S. Lymphedema management: the comprehensive guide for practitioners. 3rd ed. Vol. 3. New York: Thieme Medical Publishers; 2005. [Google Scholar]
- 4.Kim EY, Hwang HS, Lee HY, et al. Anatomic and functional evaluation of central lymphatics with noninvasive magnetic resonance lymphangiography. Medicine (Baltimore) 2016;95:e3109. doi: 10.1097/MD.0000000000003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rane S, Donahue PM, Towse T, et al. Clinical feasibility of noninvasive visualization of lymphatic flow with principles of spin labeling MR imaging: implications for lymphedema assessment. Radiology. 2013;269:893–902. doi: 10.1148/radiol.13120145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitsumori LM, McDonald ES, Wilson GJ, et al. MR lymphangiography: How I do it. J Magn Reson Imaging. 2015;42:1465–1477. doi: 10.1002/jmri.24887. [DOI] [PubMed] [Google Scholar]
- 7.Shah C, Vicini FA. Breast cancer-related arm lymphedema: incidence rates, diagnostic techniques, optimal management and risk reduction strategies. Int J Radiat Oncol Biol Phys. 2011;81:907–914. doi: 10.1016/j.ijrobp.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 8.Tiwari P, Coriddi M, Salani R, et al. Breast and gynecologic cancer-related extremity lymphedema: a review of diagnostic modalities and management options. World J Surg Oncol. 2013;11:237. doi: 10.1186/1477-7819-11-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cellina M, Gibelli D, Floridi C, Oliva G. Volumetric analysis of Non-contrast Magnetic Resonance Lymphangiography in patients affected by lower extremities primary lymphedema. Radiol Med. 2020;125:432–435. doi: 10.1007/s11547-019-01122-9. [DOI] [PubMed] [Google Scholar]
- 10.Beesley V, Janda M, Eakin E, et al. Lymphedema after gynecological cancer treatment: prevalence, correlates, and supportive care needs. Cancer. 2007;109:2607–2614. doi: 10.1002/cncr.22684. [DOI] [PubMed] [Google Scholar]
- 11.Hardy D, Williams A. Best practice guidelines for the management of lipoedema. Br J Community Nurs. 2017;(Suppl 10):S44–S48. doi: 10.12968/bjcn.2017.22.Sup10.S44. [DOI] [PubMed] [Google Scholar]
- 12.Gebruers N, Verbelen H, De Vrieze T, et al. Current and future perspectives on the evaluation, prevention and conservative management of breast cancer related lymphoedema: A best practice guideline. Eur J Obstet Gynecol Reprod Biol. 2017;216:245–253. doi: 10.1016/j.ejogrb.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 13.Mihara M, Hara H, Furniss D, et al. Lymphaticovenular anastomosis to prevent cellulitis associated with lymphoedema. Br J Surg. 2014;101:1391–1396. doi: 10.1002/bjs.9588. [DOI] [PubMed] [Google Scholar]
- 14.Cormier JN, Rourke L, Crosby M, et al. The surgical treatment of lymphedema: a systematic review of the contemporary literature (2004–2010) Ann Surg Oncol. 2012;19:642–651. doi: 10.1245/s10434-011-2017-4. [DOI] [PubMed] [Google Scholar]
- 15.Schaverien MV, Badash I, Patel KM, et al. Vascularized lymph node transfer for lymphedema. Semin Plast Surg. 2018;32:28–35. doi: 10.1055/s-0038-1632401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campisi C, Boccardo F, Zilli A, et al. Long-term results after lymphatic-venous anastomoses for the treatment of obstructive lymphedema. Microsurgery. 2001;21:135–139. doi: 10.1002/micr.1025. [DOI] [PubMed] [Google Scholar]
- 17.Phillips GSA, Gore S, Ramsden A, et al. Lymphaticovenular anastomosis in the treatment of secondary lymphoedema of the legs after cancer treatment. J Plast Reconstr Aesthet Surg. 2019;72:1184–1192. doi: 10.1016/j.bjps.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Lucarelli RT, Ogawa M, Kosaka N, et al. New approaches to lymphatic imaging. Lymphat Res Biol. 2009;7:205–214. doi: 10.1089/lrb.2009.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzei MA, Gentili F, Mazzei FG, et al. High-resolution MR lymphangiography for planning lymphaticovenous anastomosis treatment: a single-centre experience. Radiol Med. 2017;122:918–927. doi: 10.1007/s11547-017-0795-x. [DOI] [PubMed] [Google Scholar]
- 20.Goss JA, Maclellan RA, Greene AK. Lymphoscintigraphic evaluation of systemic tracer uptake in patients with primary lymphedema. Ann Plast Surg. 2019;82(4S Suppl 3):S212–S214. doi: 10.1097/SAP.0000000000001839. [DOI] [PubMed] [Google Scholar]
- 21.Mazzei FG, Gentili F, Guerrini S, et al. MR lymphangiography: a practical guide to perform it and a brief review of the literature from a technical point of view. Biomed Res Int. 2017;2017 doi: 10.1155/2017/2598358. 2598358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cellina M, Oliva G, Menozzi A, et al. Non-contrast magnetic resonance lymphangiography: an emerging technique for the study of lymphedema. Clin Imaging. 2019;53:126–133. doi: 10.1016/j.clinimag.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Gennaro P, Borghini A, Chisci G, et al. Could MRI visualize the invisible? An Italian single center study comparing magnetic resonance lymphography (MRL), super microsurgery and histology in the identification of lymphatic vessels. Eur Rev Med Pharmacol Sci. 2017;21:687–694. [PubMed] [Google Scholar]
- 24.Gennaro P, Chisci G, Mazzei F, et al. Magnetic resonance lymphangiography: How to prove it? J Magn Reson Imaging. 2016;44:509–510. doi: 10.1002/jmri.25147. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida RY, Kariya S, Ha-Kawa S, Tanigawa N. Lymphoscintigraphy for imaging of the lymphatic flow disorders. Tech Vasc Interv Radiol. 2016;19:273–276. doi: 10.1053/j.tvir.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Munn LL, Padera TP. Imaging the lymphatic system. Microvasc Res. 2014;96:55–63. doi: 10.1016/j.mvr.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinmonth JB. Lymphangiography in man; a method of outlining lymphatic trunks at operation. Clin Sci. 1952;11:13–20. [PubMed] [Google Scholar]
- 28.Mangialardi ML, Lorenzano V, Pagliara D, et al. Indocyanine green lymphography, lymphoscintigraphy, and genetic analysis in nonsyndromic primary lymphedema: the distal dermal backflow grading system and the print sign. J Reconstr Microsurg. 2020;36:157–164. doi: 10.1055/s-0039-1698748. [DOI] [PubMed] [Google Scholar]
- 29.Pieper CC, Hur S, Sommer CM, et al. Back to the future: lipiodol in lymphography-from diagnostics to theranostics. Invest Radiol. 2019;54:600–615. doi: 10.1097/RLI.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 30.Clément O, Luciani A. Imaging the lymphatic system: possibilities and clinical applications. Eur Radiol. 2004;14:1498–1507. doi: 10.1007/s00330-004-2265-9. [DOI] [PubMed] [Google Scholar]
- 31.Pons G, Clavero JA, Alomar X, et al. Preoperative planning of lymphaticovenous anastomosis: The use of magnetic resonance lymphangiography as a complement to indocyanine green lymphography. J Plast Reconstr Aesthet Surg. 2019;72:884–891. doi: 10.1016/j.bjps.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 32.Szuba A, Shin WS, Strauss HW, et al. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med. 2003;44:43–57. [PubMed] [Google Scholar]
- 33.Partsch H. Assessment of abnormal lymph drainage for the diagnosis of lymphedema by isotopic lymphangiography and by indirect lymphography. Clin Dermatol. 1995;13:445–450. doi: 10.1016/0738-081X(95)00085-T. [DOI] [PubMed] [Google Scholar]
- 34.Bluemel C, Herrmann K, Giammarile F, et al. EANM practice guidelines for lymphoscintigraphy and sentinel lymph node biopsy in melanoma. Eur J Nucl Med Mol Imaging. 2015;42:1750–1766. doi: 10.1007/s00259-015-3135-1. [DOI] [PubMed] [Google Scholar]
- 35.Bae JS, Yoo RE, Choi SH, et al. Evaluation of lymphedema in upper extremities by MR lymphangiography: Comparison with lymphoscintigraphy. Magn Reson Imaging. 2018;49:63–70. doi: 10.1016/j.mri.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 36.Liu NF, Lu Q, Liu PA, et al. Comparison of radionuclide lymphoscintigraphy and dynamic magnetic resonance lymphangiography for investigating extremity lymphoedema. Br J Surg. 2010;97:359–365. doi: 10.1002/bjs.6893. [DOI] [PubMed] [Google Scholar]
- 37.Notohamiprodjo M, Weiss M, Baumeister RG, et al. MR lymphangiography at 3.0 T: correlation with lymphoscintigraphy. Radiology. 2012;264:78–87. doi: 10.1148/radiol.12110229. [DOI] [PubMed] [Google Scholar]
- 38.Lohrmann C, Foeldi E, Speck O, et al. High-resolution MR lymphangiography in patients with primary and secondary lymphedema. AJR Am J Roentgenol. 2006;187:556–561. doi: 10.2214/AJR.05.1750. [DOI] [PubMed] [Google Scholar]
- 39.Crescenzi R, Donahue PMC, Hartley KG, et al. Lymphedema evaluation using noninvasive 3T MR lymphangiography. J Magn Reson Imaging. 2017;46:1349–1360. doi: 10.1002/jmri.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arrivé L, Derhy S, Dlimi C, et al. Noncontrast magnetic resonance lymphography for evaluation of lymph node transfer for secondary upper limb lymphedema. Plast Reconstr Surg. 2017;140:806e–811e. doi: 10.1097/PRS.0000000000003862. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi H, Kuboyama S, Abe H, et al. Clinical feasibility of noncontrast-enhanced magnetic resonance lymphography of the thoracic duct. Chest. 2003;124:2136–2142. doi: 10.1378/chest.124.6.2136. [DOI] [PubMed] [Google Scholar]
- 42.Arrivé L, Derhy S, Dahan B, et al. Primary lower limb lymphoedema: classification with non-contrast MR lymphography. Eur Radiol. 2018;28:291–300. doi: 10.1007/s00330-017-4948-z. [DOI] [PubMed] [Google Scholar]
- 43.Arrivé L, Derhy S, El Mouhadi S, et al. Noncontrast magnetic resonance lymphography. J Reconstr Microsurg. 2016;32:80–86. doi: 10.1055/s-0035-1549133. [DOI] [PubMed] [Google Scholar]
- 44.Liu NF, Yan ZX, Wu XF, et al. Magnetic resonance lymphography demonstrates spontaneous lymphatic disruption and regeneration in obstructive lymphedema. Lymphology. 2013;46:56–63. [PubMed] [Google Scholar]
- 45.White RD, Weir-McCall JR, Budak MJ, et al. Contrast-enhanced magnetic resonance lymphography in the assessment of lower limb lymphoedema. Clin Radiol. 2014;69:e435–444. doi: 10.1016/j.crad.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Mitsumori LM, McDonald ES, Neligan PC, et al. Peripheral magnetic resonance lymphangiography: techniques and applications. Tech Vasc Interv Radiol. 2016;19:262–272. doi: 10.1053/j.tvir.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Neligan PC, Kung TA, Maki JH. MR lymphangiography in the treatment of lymphedema. J Surg Oncol. 2017;115:18–22. doi: 10.1002/jso.24337. [DOI] [PubMed] [Google Scholar]
- 48.Bellin MF, Beigelman C, Precetti-Morel S. Iron oxide-enhanced MR lymphography: initial experience. Eur J Radiol. 2000;34:257–264. doi: 10.1016/S0720-048X(00)00204-7. [DOI] [PubMed] [Google Scholar]
- 49.Maki JH, Neligan PC, Briller N, et al. Dark blood magnetic resonance lymphangiography using dual-agent relaxivity contrast (DARC-MRL): a novel method combining gadolinium and iron contrast agents. Curr Probl Diagn Radiol. 2016;45:174–179. doi: 10.1067/j.cpradiol.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Bashir MR, Bhatti L, Marin D, et al. Emerging applications for ferumoxytol as a contrast agent in MRI. J Magn Reson Imaging. 2015;41:884–898. doi: 10.1002/jmri.24691. [DOI] [PubMed] [Google Scholar]
- 51.Müller A, Fries P, Jelvani B, et al. Magnetic resonance lymphography at 9.4 T using a gadolinium-based nanoparticle in rats: investigations in healthy animals and in a hindlimb lymphedema model. Invest Radiol. 2017;52:725–733. doi: 10.1097/RLI.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 52.Arrivé L, Azizi L, Lewin M, et al. MR lymphography of abdominal and retroperitoneal lymphatic vessels. AJR Am J Roentgenol. 2007;189:1051–1058. doi: 10.2214/AJR.07.2047. [DOI] [PubMed] [Google Scholar]
- 53.Arrivé L, Monnier-Cholley L, Cazzagon N, et al. Non-contrast MR lymphography of the lymphatic system of the liver. Eur Radiol. 2019;29:5879–5888. doi: 10.1007/s00330-019-06151-6. [DOI] [PubMed] [Google Scholar]
- 54.Shaikh R, Biko DM, Lee EY. MR imaging evaluation of pediatric lymphatics: overview of techniques and imaging findings. Magn Reson Imaging Clin N Am. 2019;27:373–385. doi: 10.1016/j.mric.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 55.Jeon JY, Lee SH, Shin MJ, et al. Three-dimensional isotropic fast spin-echo MR lymphangiography of T1-weighted and intermediate-weighted pulse sequences in patients with lymphoedema. Clin Radiol. 2016;71:e56–63. doi: 10.1016/j.crad.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 56.Liu N, Zhang Y. Magnetic resonance lymphangiography for the study of lymphatic system in lymphedema. J Reconstr Microsurg. 2016;32:66–71. doi: 10.1055/s-0034-1384213. [DOI] [PubMed] [Google Scholar]
- 57.Pintaske J, Martirosian P, Graf H, et al. Relaxivity of Gadopentetate Dimeglumine (Magnevist), Gadobutrol (Gadovist), and Gadobenate Dimeglumine (MultiHance) in human blood plasma at 0.2, 1.5, and 3 Tesla. Invest Radiol. 2006;41:213–221. doi: 10.1097/01.rli.0000197668.44926.f7. Erratum in: Invest Radiol 2006;41:859. [DOI] [PubMed] [Google Scholar]
- 58.Jara H, Barish MA, Yucel EK, et al. MR hydrography: theory and practice of static fluid imaging. AJR Am J Roentgenol. 1998;170:873–882. doi: 10.2214/ajr.170.4.9530026. [DOI] [PubMed] [Google Scholar]
- 59.Liu N, Wang C, Sun M. Noncontrast three-dimensional magnetic resonance imaging vs lymphoscintigraphy in the evaluation of lymph circulation disorders: a comparative study. J Vasc Surg. 2005;41:69–75. doi: 10.1016/j.jvs.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 60.Zhao JM, Clingman CS, Narvainen MJ, et al. Oxygenation and hematocrit dependence of transverse relaxation rates of blood at 3T. Magn Reson Med. 2007;58:592–597. doi: 10.1002/mrm.21342. [DOI] [PubMed] [Google Scholar]
- 61.Firouznia K, Amirmohseni S, Guiti M, et al. MR relaxivity measurement of iron oxide nano-particles for MR lymphography applications. Pak J Biol Sci. 2008;11:607–612. doi: 10.3923/pjbs.2008.607.612. [DOI] [PubMed] [Google Scholar]
- 62.Harisinghani MG, Saksena M, Ross RW, et al. A pilot study of lymphotrophic nanoparticle-enhanced magnetic resonance imaging technique in early stage testicular cancer: a new method for noninvasive lymph node evaluation. Urology. 2005;66:1066–1071. doi: 10.1016/j.urology.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 63.Mazzei FG, Volterrani L, Guerrini S, et al. Reduced time CT perfusion acquisitions are sufficient to measure the permeability surface area product with a deconvolution method. Biomed Res Int. 2014;2014 doi: 10.1155/2014/573268. 573268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mazzei MA, Squitieri NC, Sani E, et al. Differences in perfusion CT parameter values with commercial software upgrades: a preliminary report about algorithm consistency and stability. Acta Radiol. 2013;54:805–811. doi: 10.1177/0284185113484643. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto T, Yoshimatsu H, Narushima M, et al. Indocyanine green lymphography findings in primary leg lymphedema. Eur J Vasc Endovasc Surg. 2015;49:95–102. doi: 10.1016/j.ejvs.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 66.Abdelfattah U, Jaimez PM, Clavero JA, Bellantonio V, Pons G, Masia J. Correlation between superficial and deep lymphatic systems using magnetic resonance lymphangiography in breast cancer-related lymphedema: clinical implications. J Plast Reconstr Aesthet Surg. 2020;73:1018–1024. doi: 10.1016/j.bjps.2019.11.053. [DOI] [PubMed] [Google Scholar]
- 67.Gentili F, Guerrini S, Mazzei FG, Volterrani L, Mazzei MA. MRL as one-shot examination for patients suffering from lymphedema. Radiol Med. 2020;125:798–799. doi: 10.1007/s11547-020-01162-6. [DOI] [PubMed] [Google Scholar]