Abstract
Acute colonic diverticulitis (ACD) is an acute episode of severe and prolonged lower abdominal pain due to diverticular inflammation, usually associated with change in bowel movements, fever, and leukocytosis. Worldwide, computed tomography (CT) of the abdomen and pelvis with intravenous contrast is accepted as the best imaging method for evaluating the diverticular inflammation, serving the following functions: confirming the presence of ACD; evaluation of the disease severity and degree; therapy planning guide in presence of complications (such as abscess or intestinal perforation); diagnosis of other diseases that may simulate diverticular inflammation. In the literature, we found values of CT sensitivity for diverticular inflammation from 79% to 99%; CT is useful in differentiating other diseases, which may cause abdominal pain, when diverticular inflammation is not the cause, such as neoplasm, inflammatory bowel disease, appendix inflammations, epiploic appendix inflammation and colon ischemia. The trick to differentiate diverticulitis from other inflammatory diseases that involve the colon is the identification of diverticula in the pathological intestinal loop. In the last years, a radiological classification was created in order to guide the management of ACD in patients treated conservatively or with interventional procedures. The new classification system divides ACD into two groups: complicated and uncomplicated. Uncomplicated ACD is defined if only thickening of the intestinal wall is present, with increase of the perivisceral fat density. Complicated ACD is divided into 4 stages, depending on presence of microperforation without abscess and/or peritoneum involvement (stage 1 A), presence of abscess with diameter ≤4 cm (stage 1 B), presence of abscess with diameter >4 cm (stage 2 A), presence of distant air >5 cm from the pathological loop (stage 2 B), presence of diffuse fluid in at least two distant abdominal quadrants without distant free air (stage 3), presence of diffuse fluid and distant free air (stage 4). In this pictorial essay, we describe CT findings of the ACD and explain classification of the disease and its common and uncommon complications.
A cute colonic diverticulitis (ACD) is an acute episode of severe and prolonged lower abdominal pain due to diverticular inflammation, usually associated with change in bowel movements, fever, and leukocytosis. Worldwide, computed tomography (CT) of the abdomen and pelvis with intravenous (IV) contrast is accepted as the best imaging method for evaluating the diverticular inflammation, serving the following functions: confirming the presence of ACD; evaluation of the disease severity and degree; therapy planning guide in presence of complications (such as abscess or intestinal perforation); diagnosis of other diseases that may simulate diverticular inflammation. In the literature, we found values of CT sensitivity for diverticular inflammation from 79% to 99%; CT is useful in differentiating other diseases, which may cause abdominal pain, when diverticular inflammation is not the cause, such as neoplasm, inflammatory bowel disease, appendix inflammations, epiploic appendix inflammation and colon ischemia (1). The trick to differentiate diverticulitis from other inflammatory diseases that involve the colon is the identification of diverticula in the pathological intestinal loop. In this pictorial essay, we describe CT findings of the ACD and explain classification of the disease and its common and uncommon complications.
Computed tomography
In our hospital, CT exam is performed before and after administration of iodinated contrast agent. Basal scan is not necessary when clinical context is really specific for ACD. The acquisition after 70–80 seconds (portal phase) allows the visualization of the intestinal wall and the extension of the inflammation in the extra-visceral tissues.
Anatomic description or the colon
The entire colon is about 150 cm long, and it is divided into five major segments: cecum, proximal or ascending colon, transverse, descending, and recto-sigmoidal colon. The cecum is the portion of the colon below the ileo-cecal valve, ascending and transverse colon are divided by the hepatic flexure, transverse and descending colon are divided by the splenic flexure, descending and sigmoidal colon are divided by the sigmoidal flexure.
The values of the bowel wall thickness are influenced by the degree of intestinal distension. When the lumen is well distended, the thickness of the bowel wall is less than 3 mm (2); if the loop is not well distended, it can vary from 3 to 5 mm, but a wall thickness of 6–8 mm may still be normal in contracted colonic segments (Fig. 1) (3). The intestinal wall usually shows contrast enhancement after IV iodinated contrast injection. The mucosa can appear as a separate layer because it is the most vascularised layer of the intestinal wall. On the contrary, the submucosa is less vascularised, so it appears as a distinct layer only in case of edema, hemorrhage or infiltration by fat (Fig. 2) (2).
Figure 1. a, b.
Axial contrast-enhanced CT images show how sigmoid wall thickness can vary (a) when the lumen is well distended (arrow) or (b) when the wall is contracted or the lumen is not distended (arrow).
Figure 2.
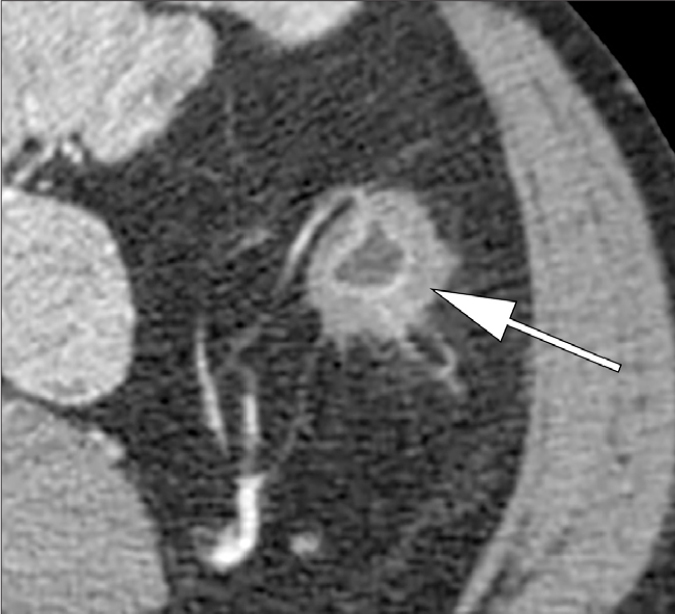
Axial contrast-enhanced CT image shows submucosa as a separate less vascularised layer on CT (arrow) because of the edema of submucosa and hyperemia of the mucosa in a patient with colonic inflammation.
Diverticula and acute colonic diverticulitis
Diverticula are protrusions of the mucosa and submucosa through the muscularis of the intestinal wall (false diverticulum) or herniation of the entire intestinal wall (true diverticulum). Descending and sigmoid colon are the most common affected sites and in these sites diverticula are primarily false. Size of diverticula usually varies from 2–3 mm up to 2 cm. On CT, diverticulosis appears as small, air-filled sacculations of the colonic wall (Fig. 3) (2). ACD is thought to ensue when a diverticulum becomes obstructed by feces, food particles or inflammation, resulting in fecal stasis, mucosal trauma, and ischemia (2).
Figure 3.
Axial contrast-enhanced CT image shows presence of diverticulosis as a small, air-filled sacculation of the colonic wall (arrow).
Management and CT stage of the patients
In 1978, Hinchey et al. (4) formulated a classification in which ACD is classified into four stages; stage I, the abscess is exclusively pericolonic; stage II, it extends to the pelvis; stage III, purulent peritonitis occurs; stage IV, a large perforation of the loop causes peritoneal dissemination of feces. Because this classification could be used correctly only in patients who had undergone surgery, a radiological classification was necessary to create in order to guide the management of ACD (5). Some authors (4, 6–8) have evaluated CT findings in association with the Hinchey classification and have shown how CT findings can successfully direct medical or surgical therapy. In particular, in 2015, the guidelines of ACD were presented and debated during the consensus conference of the World Society of Emergency Surgery (WSES); the final guidelines were approved and published by Sartelli (9). This classification system divides ACD into two groups: uncomplicated and complicated ACD.
Uncomplicated ACD
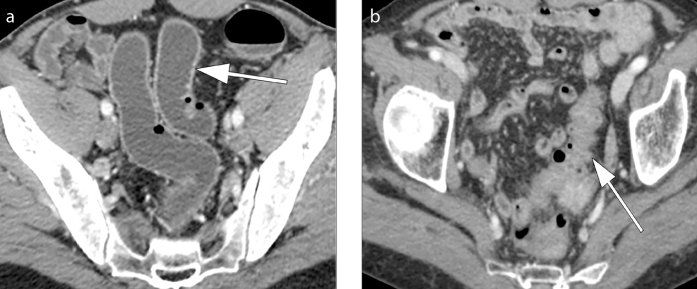
Mural thickening of the colon (Fig. 4) and presence of pericolic fat stranding (Fig. 5) are the typical CT findings in uncomplicated diverticular inflammation. Typically, diverticula are present and often it is possible to identify an inflamed diverticulum in association to these signs (1, 10, 11). Pericolic fat stranding may vary from minimal (so-called dirty fat) to severe inflammation with possible presence of phlegmon (Fig. 5) (1). It is characteristic in ACD that the colonic wall shows a moderate focal thickening with respect to more severe fat stranding (12). The congestion of the mesenteric vessels (so-called centipede sign) and the presence of fluid at the root of the sigmoid mesentery (so-called comma sign) (Figs. 5 and 6) are two other revelatory signs of the inflammatory process (2, 12, 13).
Figure 4. a, b.
Example of wall thickness measure. Coronal (a) and axial (b) contrast-enhanced CT images. The longitudinal centerline of the colon is identified (dotted line). Measurements are made perpendicular to the centerline, excluding abscess and lumen (solid line). Wall thickness is measured with an electronic caliper in the clinical picture archiving and communications system to identify the maximum distance from the serosal-to-mucosal surface of the colon, including the folds and teniae coli (a). In situations where a lumen could not be clearly seen, the entire serosa-to-serosa distance was measured and divided in half (b).
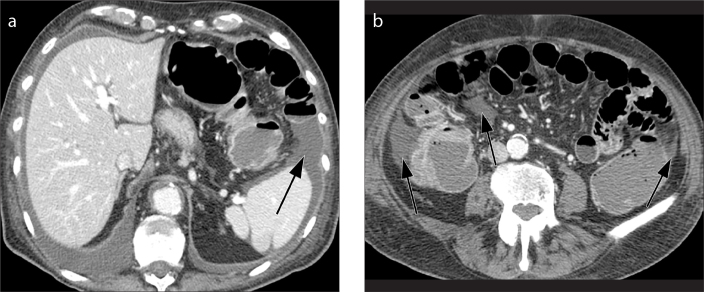
Figure 5. a, b.
Axial contrast-enhanced CT images (a, b) show wall thickening of the left colon (thick arrow) and presence of fat stranding (thin arrow) ranging from minimal “dirty fat” (a) to severe inflammation and phlegmonous changes (b). Thickening and fluid collection of the left latero-conal fascia (so-called comma sign) are also evident in both images (black arrows).
Figure 6. a, b.
Axial contrast-enhanced CT image (a) shows engorgement of the mesenteric vessels, a finding known as the “centipede sign” (arrow). Axial contrast-enhanced CT image (b) shows thickening and fluid collection of the left latero-conal fascia, a finding known as “comma sign” (black arrow).
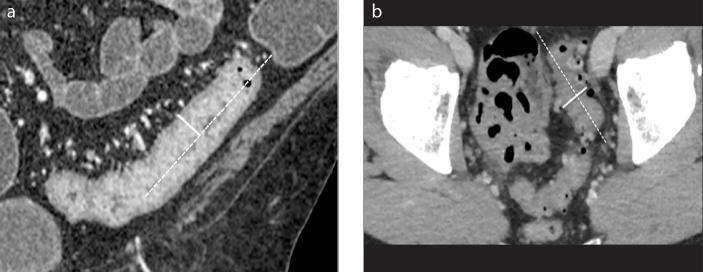
In CT images, we can measure the length of the involved colon and the density of the diverticula. The length of involved colon is expressed in linear measurement (millimetres, mm) and more precisely as percentage with respect to the whole colon (Fig. 7). Diverticula density is defined as the number of diverticula distributed along the colon, obtained by an electronic caliper on axial images (Fig 8). Dickerson et al. (14) adopted a severity scale including minimal (few diverticula, with more than 5 cm of distance in between), mild (diverticula distanced from 1 cm to 5 cm), moderate (each diverticulum within <1 cm of the next one) and severe (several diverticula so close together that they cannot be distinguished from one another). Recent studies have shown that most patients with uncomplicated diverticulitis are discharged after a short hospitalization period and the outpatient treatment has a success rate of approximately 95% (15). The increase of the intestinal wall thickness and the severity grade, and the appearance of complications are significant predictors of the likelihood of diverticulitis recurrence after treatment of the first episode (14).
Figure 7. a, b.
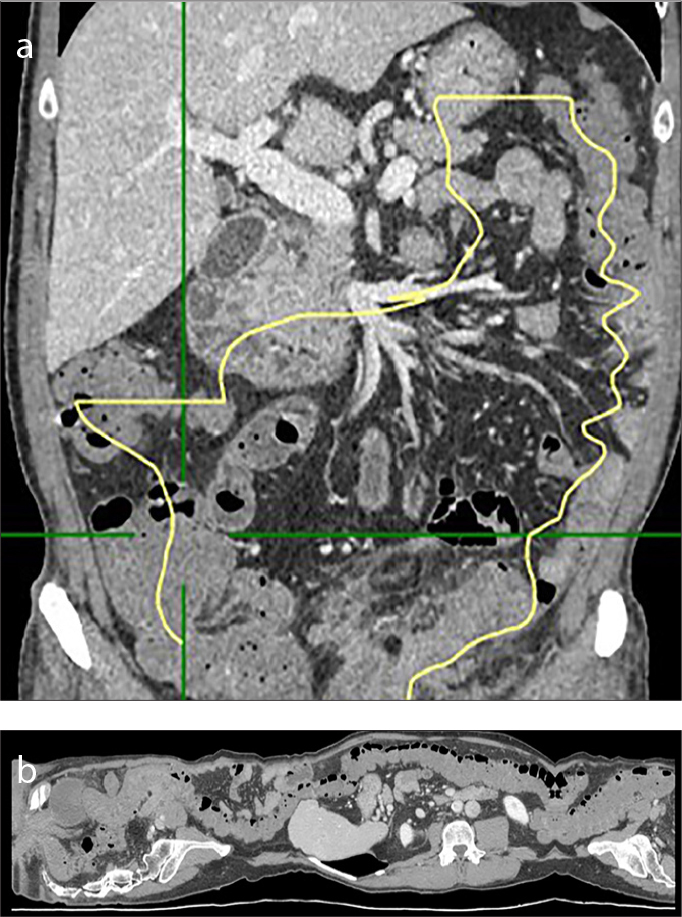
Example of length measure. Length of involved colon, expressed as linear measure (centimeters, cm) or, more precisely, as percentage with respect to the whole colon. In our hospital, we use a specific vascular post-processing 2D and 3D software (Vue PACS, Carestream), usually employed for vasal segmentation to identify the tubular structure of the colon (a) and measure the different tracts manually. The software allows the creation of a virtual image (b) and the measurement of the length of the colon despite the fact that it is a tubular viscera with a flexuous course, often curved and with some convoluted traits.
Figure 8.
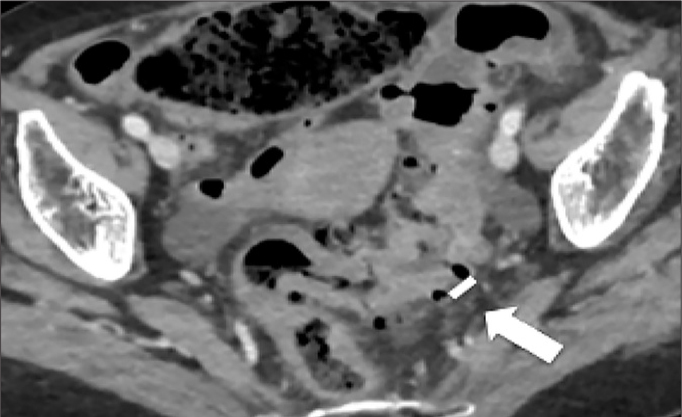
Example of diverticula density. Axial contrast-enhanced CT image shows the minimum distance between two adjacent diverticula (arrow). This case is a moderate grade (each diverticulum is next to another for less than 1 cm).
Complicated diverticulitis
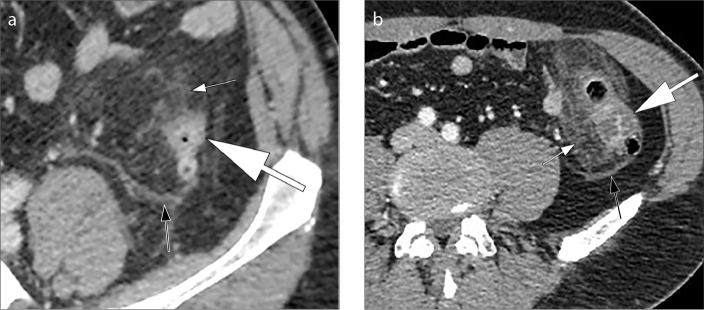
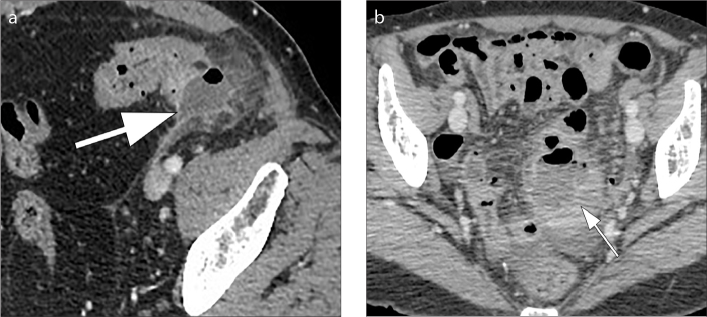
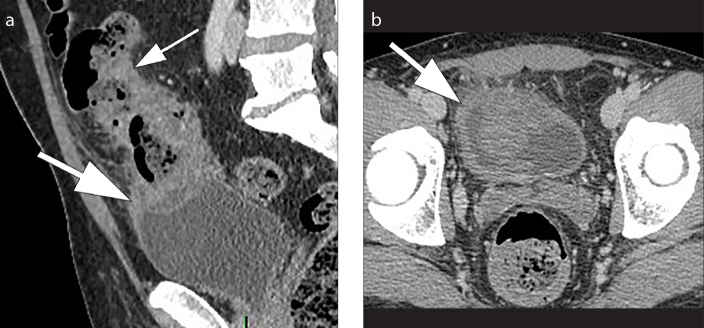
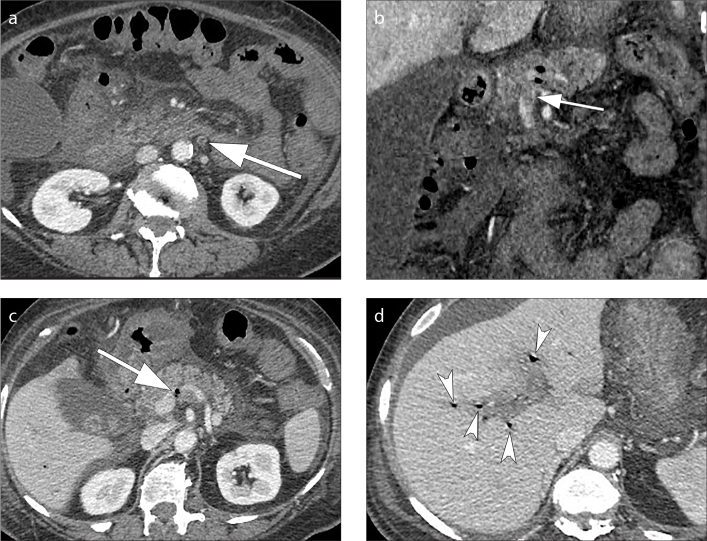
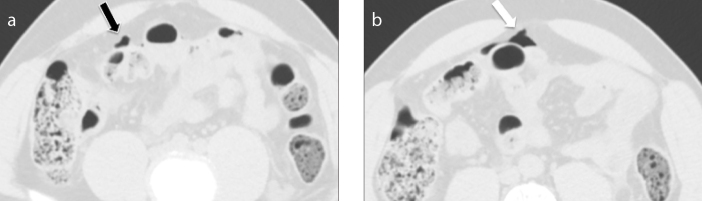
A diverticulitis is complicated when CT findings indicate complications such as abscess, intestinal occlusion, hepatic abscesses, fistulas and ascending septic thrombophlebitis of the inferior mesenteric vein (1). An abscess is a fluid collection delimited by a hyperdense wall, with or without air inside (Fig. 9). Fistulas are communications of the intestinal loop with an abscess, another intestinal loop, bladder or skin. The colovesical fistulas are the most common and they are suspected when air is seen inside the bladder in association with a thickening of the bladder wall adjacent to the involved segment of the colon, usually the sigmoid (Fig. 10). Mesenteric vein thrombosis consists in filling defect of this vessel and its branches, associated with mesenteric congestion and stranding (Fig 11). Microperforation can also be a complication of diverticulitis and appears as small extra-luminal pockets of air (Fig. 12). Pneumoperitoneum is not a common finding in patients with diverticulitis (Fig. 13).
Figure 9. a, b.
Abscess. Axial contrast-enhanced CT image (a) shows a heterogeneous collection (diameter <4 cm) with air bubble inside and contrast-enhancing walls (arrow) in the left colon; pericolic haziness is present. CT image (b) of another patient shows an abscess (arrow) with diameter >4 cm near the sigmoid colon.
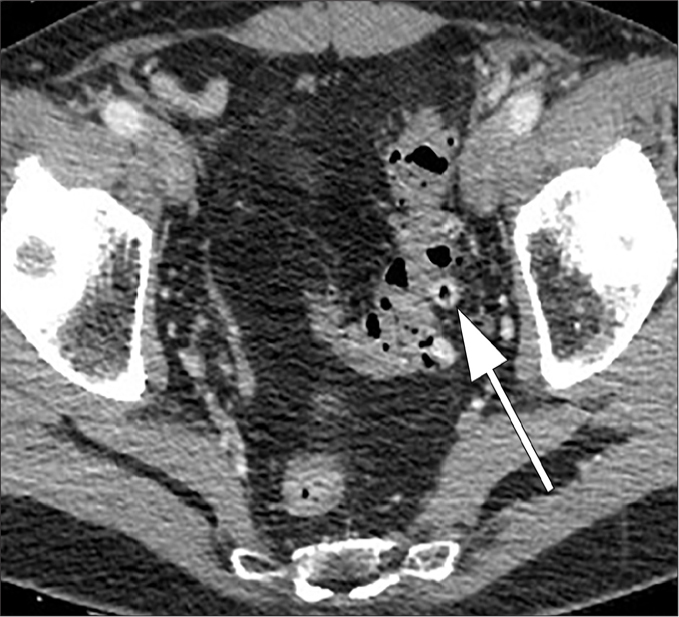
Figure 10. a, b.
Colovesical fistula. Sagittal contrast-enhanced CT image (a) and axial contrast-enhanced CT image (b) show marked thickening of the bladder wall (thick arrow) adjacent to the pathological sigmoid tract (thin arrow), suggestive for colovesical fistula.
Figure 11. a–d.
Pylephlebitis or septic thrombophlebitis. Axial (a) and coronal (b) contrast-enhanced CT images show thrombus formation in the inferior mesenteric vein (a, b, arrows); note the presence of intravascular air in the main portal vein (c, arrow) and in the intrahepatic branches of the portal vein (d, multiple arrowheads).
Figure 12.
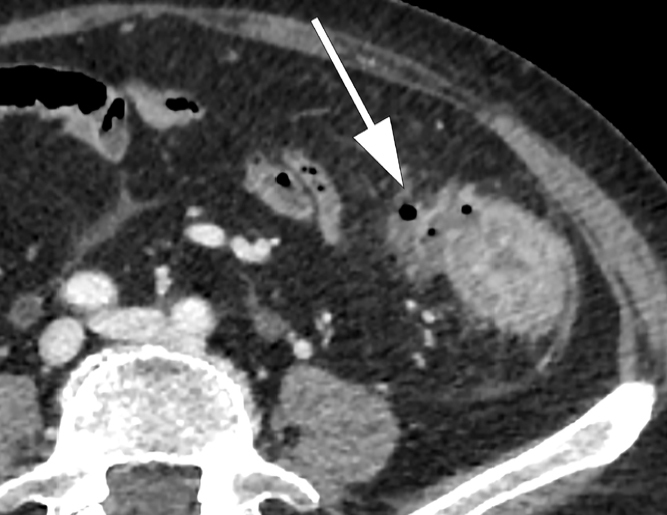
Microperforation. Axial contrast-enhanced CT image shows the presence of small extra-luminal pockets of air (arrow) and increased density of the pericolic fat within 5 cm of the inflamed bowel wall.
Figure 13. a, b.
Axial contrast-enhanced CT images (a, b) show free air bubbles arranged anteriorly to the bowel wall (a, black arrow) and between the intestinal loops on the right side (b, white arrow); this finding may help to determine the site of perforation. Using lung window is useful to identify air bubbles between the intestinal loops.
Complicated ACD is divided into the following stages and the stage influences the therapeutic choice (9):
Stage 1A: presence of diverticular inflammation with microperforation, without abscess and/or peritoneum involvement. CT shows presence of pericolic air or a little pericolic fluid (Fig. 12). Pericolic air is defined when air bubbles are within 5 cm of the involved intestinal loop; no distant air is evident on the CT. A broad-spectrum antibiotic is the choice therapy.
Stage 1B: presence of an abscess with diameter <4 cm (Fig. 9a). Antibiotics or percutaneous drainage are the choice therapy.
Stage 2A: presence of an abscess >4 cm (Fig. 9b). In this stage a percutaneous drainage with intravenous antibiotic is the choice therapy.
Stage 2B: presence of distant air (> 5 cm from the pathological loop) in absence of diffuse peritoneal fluid (Fig 13). Conservative therapy is possible in selected cases. The commonly practiced surgical option is intestinal resection.
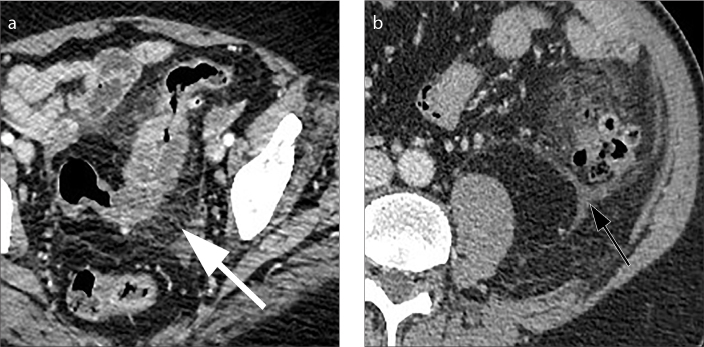
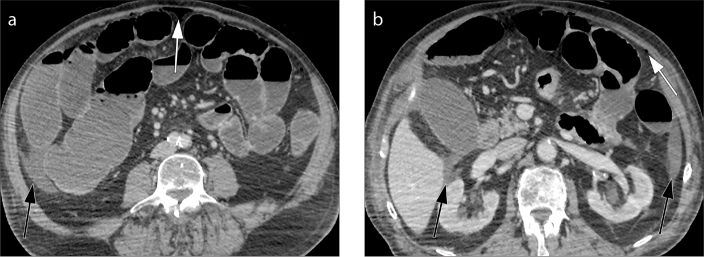
Stage 3: presence of diffuse fluid in at least two distant abdominal quadrants without distant free air (Fig. 14). The therapy is peritoneal lavage, or colonic resection.
Stage 4: presence of diffuse fluid and distant free air secondary to constant hole in the colic wall (Fig. 15). Hartmann resection is the operative choice.
Figure 14. a, b.
Axial contrast-enhanced CT images (a, b) show presence of diffuse fluid in at least two distant abdominal quadrants (black arrows) without distant free air.
Figure 15. a, b.
Axial contrast-enhanced CT images (a, b) show presence of diffuse fluid in at least two distant abdominal quadrants (black arrows) with distant free air (white arrows).
Conclusion
Although the management of ACD depends on different elements such as the presence of peritoneum involvement, clinical status and physiological reserve of the patient, this CT classification may help decide whether surgical therapy is needed or not. In addition, CT can be useful in order to guide interventional procedures.
Main points.
CT of the abdomen and pelvis is accepted as the best imaging method for evaluating the diverticular inflammation, serving the following functions: confirming the presence of ACD; evaluation of the disease severity and degree; therapy planning guide in presence of complications (such as abscess or intestinal perforation); diagnosis of other diseases that may simulate diverticular inflammation.
The two most common CT findings in uncomplicated diverticulitis are mural thickening of the colon and presence of pericolic fat stranding.
Diverticulitis is complicated when abscess, bowel obstruction, hepatic abscess, fistula, and vein thrombosis are present.
CT findings may be used to guide clinical management.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.DeStigter KK, Keating DP. Imaging update: acute colonic diverticulitis. Clin Colon Rectal Surg. 2009;22:147–155. doi: 10.1055/s-0029-1236158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandes T, Oliveira MI, Castro R, Araújo B, Viamonte B, Cunha R. Bowel wall thickening at CT: simplifying the diagnosis. Insights Imaging. 2014;5:195–208. doi: 10.1007/s13244-013-0308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiesner W, Mortelé KJ, Ji H, Ros PR. Normal colonic wall thickness at CT and its relation to colonic distension. J Comput Assist Tomogr. 2002;26:102–106. doi: 10.1097/00004728-200201000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg. 1978;12:85–109. [PubMed] [Google Scholar]
- 5.Klarenbeek BR, de Korte N, van der Peet DL, Cuesta MA. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207–214. doi: 10.1007/s00384-011-1314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker ME. Imaging and interventional techniques in acute left-sided diverticulitis. J Gastrointest Surg. 2008;12:1314–1317. doi: 10.1007/s11605-008-0490-2. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser AM, Jiang JK, Lake JP, et al. The management of complicated diverticulitis and the role of computed tomography. Am J Gastroenterol. 2005;100:910–917. doi: 10.1111/j.1572-0241.2005.41154.x. [DOI] [PubMed] [Google Scholar]
- 8.Ambrosetti P. Acute diverticulitis of the left colon: value of the initial CT and timing of elective colectomy. J Gastrointest Surg. 2008;12:1318–1320. doi: 10.1007/s11605-008-0489-8. [DOI] [PubMed] [Google Scholar]
- 9.Sartelli M, Catena F, Ansaloni L. WSES Guideline for the management of acute left sided colonic diverticulitis in the emergency setting. World J Emerg Surg. 2016;11:37. doi: 10.1186/s13017-016-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kircher MF, Rhea JT, Kihiczak D, Novelline RA. Frequency, sensitivity and specificity of individual signs of diverticulitis on thin-section helical CT with colonic contrast material: experience with 312 cases. AJR Am J Roentgenol. 2002;178:1313–1318. doi: 10.2214/ajr.178.6.1781313. [DOI] [PubMed] [Google Scholar]
- 11.Werner A, Diehl SJ, Farag-Soliman M, Düber C. Multi-slice spiral CT in routine diagnosis of suspected acute left-sided colonic diverticulitis: a prospective study of 120 patients. Eur Radiol. 2003;13:2596–2603. doi: 10.1007/s00330-003-1887-7. [DOI] [PubMed] [Google Scholar]
- 12.Pereira JM, Sirlin CB, Pinto PS, Jeffrey RB, Stella DL, Casola G. Disproportionate fat stranding: a helpful CT sign in patients with acute abdominal pain. Radiographics. 2004;24:703–715. doi: 10.1148/rg.243035084. [DOI] [PubMed] [Google Scholar]
- 13.Padidar AM, Jeffrey RB, Jr, Mindelzun RE, Dolph JF. Differentiating sigmoid diverticulitis from carcinoma on CT scans: mesenteric inflammation suggests diverticulitis. AJR Am J Roentgenol. 1994;163:81–83. doi: 10.2214/ajr.163.1.8010253. [DOI] [PubMed] [Google Scholar]
- 14.Dickerson EC, Chong ST, Ellis JH. Recurrence of colonic diverticulitis: identifying predictive CT findings—retrospective cohort study. Radiology. 2017;285:850–858. doi: 10.1148/radiol.2017161374. [DOI] [PubMed] [Google Scholar]
- 15.van Dijk ST, Bos K, de Boer MGJ, et al. A systematic review and meta-analysis of outpatient treatment for acute diverticulitis. Int J Colorectal Dis. 2018;33:505–512. doi: 10.1007/s00384-018-3015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]