Abstract
Background:
The Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) is a multicenter, randomized, placebo-controlled trial, designed to test whether a statin medication can prevent cardiovascular disease in people with HIV. REPRIEVE recently completed enrollment of 7557 participants at over 100 clinical sites globally. Participant groups of focus were women, and racial and ethnic minorities.
Objective:
To describe recruitment methods and strategies developed by the REPRIEVE Clinical Coordinating Center (CCC) and share best practices learned from the recruitment process.
Methods:
Enrollment targets were agreed upon with the primary funder, the National Heart, Lung, and Blood Institute (NHLBI) and were milestone driven. Milestones included number of sites activated, number of participants enrolled within specific time frames, and proportion of women and minorities enrolled. Strategies to achieve these milestones and included structured interviews with site-designated REPRIEVE Recruitment Champions to develop best practices, development of a multimedia campaign, and site level recruitment support.
Results:
Recruitment initiated March, 2015 and completed March, 2019. The final accrual target was 7500 participants over 48 months. The trial met this target within the time specified. Overall, 10,613 screens were completed, 48% of participants enrolled from sites outside of North America, 32% were female, 44% were black or African American, and 25% were Hispanic or Latino.
Conclusions:
REPRIEVE met its overall projected recruitment goal by using multiple, simultaneous strategies to specifically target a diverse population including minority subgroups. REPRIEVE benefited from the development of recruitment strategies with clear targets and communication of accrual targets to study teams.
Keywords: HIV, REPRIEVE, recruitment, clinical coordinating center, cardiovascular disease, primary prevention
Introduction
Relative to the general population, people with HIV (PWH) have approximately 1.5–2 times the risk of experiencing a cardiovascular disease event (CVD)(1–3). The Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE)(A5332) is the largest long-term, randomized placebo-controlled trial to date to assess statins as a primary CVD prevention strategy in PWH(4). To address this urgent need, REPRIEVE set out to enroll 6500 PWH in 30 months (later increased to 7500 PWH in 48 months). REPRIEVE targeted a population of PWH with low to moderate risk of CVD, based on 2013 ACC/AHA 10-year ASCVD risk score and LDL-cholesterol, to evaluate if a pharmacologic intervention with a statin (pitavastatin calcium 4mg/day) would be beneficial. The trial funding, design, and detailed inclusion and exclusion criteria have been described elsewhere(4). REPRIEVE was designed to fill an important knowledge gap as to the efficacy and safety of a statin strategy in PWH, a population in whom traditional CVD and unique HIV-associated risk factors contribute to overall CVD risk.
Although timely recruitment of the intended study population is vital to the success of all clinical trials, findings suggest less than half achieve their recruitment targets(5). Failure to recruit a full, diverse cohort can lead to biased studies which are underpowered or lack generalizability to the target population(5). It is important for trials to share best practices and lessons learned regarding successful recruitment strategies. Our objective is to describe recruitment methods and strategies developed by the Clinical Coordinating Center (CCC) based at Massachusetts General Hospital (MGH), Boston, MA USA.
Methods
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institute of Allergy and Infectious Diseases; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Recruitment strategy
REPRIEVE leadership developed an organizational structure to carry out recruitment activities and developed comprehensive management and continuous monitoring of recruitment efforts. These efforts were led by the CCC(4). Enrollment targets were agreed upon with the primary funder, the National Heart, Lung, and Blood Institute (NHLBI), and were milestone driven. Milestones included number of sites activated, proportion of women and minorities enrolled (Supplemental Table 1), as well as number of participants enrolled within specific time frames, see Figure 3 in Grinspoon et al., AHJ 2019(4).
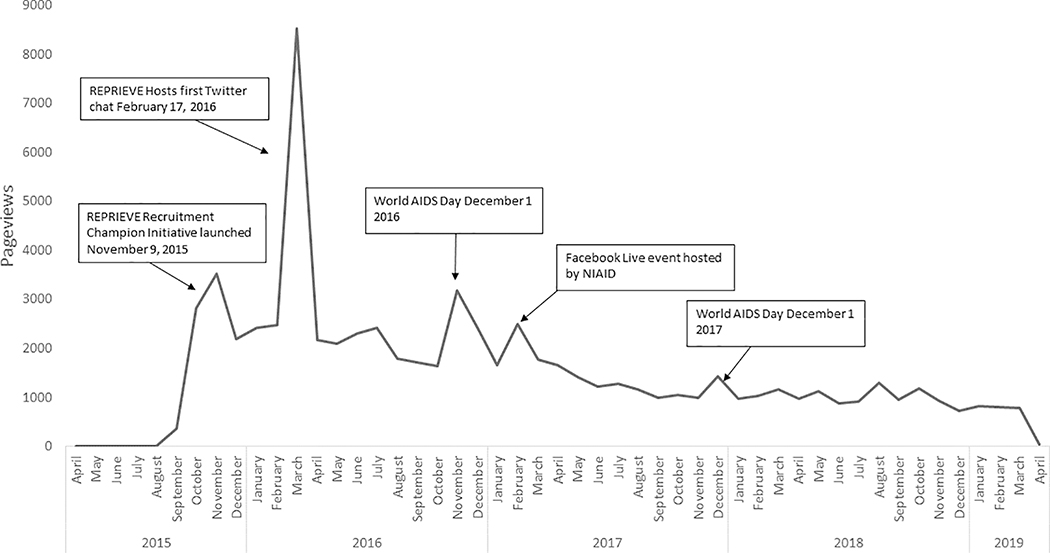
Figure 3. REPRIEVE website traffic, as indicated by pageviews, by month.
Line graph illustrating the number of page views on www.reprievetrial.org by month during the recruitment period (March 2015 – March 2019) and various initiatives that led to increased website traffic.
Role of the Clinical Coordinating Center
A major role of the CCC was to facilitate recruitment by developing efforts to raise awareness about REPRIEVE within the HIV community, focusing on subgroups of interest (women and minority groups), to drive enrollment at clinical research sites (CRSs) and to support CRSs in local recruitment efforts. A major challenge that emerged was engaging a relatively healthy population of PWH with low to moderate CVD risk, but without known CVD, to a long-term, primary prevention trial in which participants were required to add one additional daily medication to often complex medical regimens. In this regard, the CCC was responsible for raising public awareness about CVD risk in HIV, the potential importance and the relative benefits of CVD prevention in PWH, and the role played by the REPRIEVE trial in providing evidence to support either a statin or usual care strategy.
External support to develop public awareness campaign
Public relations agency
The CCC retained a public relations (PR) agency to assist with developing and launching the initial recruitment campaign. Work with this agency took place between August 2015 and July 2016. The objective of this collaboration was to drive enrollment across CRSs. Challenges identified were a lack of awareness of the increased risk of CVD among PWH and infrequent focus by clinicians on CVD health among PWH(6). The CCC team collaborated with the PR agency to create compelling consumer messaging to raise awareness about CVD and HIV and to promote knowledge and enthusiasm about REPRIEVE. The PR agency assisted with key advocacy and media outreach, video development, targeted advertising, and website refinement of the participant pages on the REPRIEVE website.
NIAID Office of Communications and Government Relations
As REPRIEVE is co-funded by the NHLBI and the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH), the NIAID Office of Communications and Government Relations collaborated with the CCC to extend the outreach campaign for this large, unique trial given NIAID’s experience with outreach in the HIV community. This effort focused on placing REPRIEVE messaging on select online HIV channels to reach highly relevant audiences, developing new communication channels to reach those not yet aware of the trial through existing contact strategies, and contacting federal and non-federal partners to convey information about REPRIEVE. Collaboration with NIAID was essential to leverage the strong network of existing NIH media channels and partnerships.
Campaign to raise awareness about REPRIEVE
Media outreach
Outreach to national and regional media was essential to generate public awareness of the increased risk of CVD among PWH and the potential benefits of participation in REPRIEVE. Online and print articles were published in health-focused/HIV-focused publications intended to reach PWH and HIV clinicians (Supplemental Table 2). To bolster enrollment efforts in Africa, media appearances by NIH officials and local CRS representatives were made to discuss the trial on South African radio, television programs and an op-ed was placed in print media (Supplemental Table 2).
Media outlets were chosen based on target audience demographics in regions with multiple CRSs, e.g. Los Angeles, to maximize potential engagement. Messaging in articles included education about the potential risk of CVD in PWH, the benefits for individuals with HIV to continue their legacy of activism by enrolling in clinical trials and highlighting REPRIEVE as an opportunity to contribute to the field of knowledge regarding comorbidities and HIV particularly among women and minorities who are often underrepresented in trials. Outreach to media often took place around HIV/AIDS awareness days such as National HIV/AIDS and Aging Awareness Day and during the American Heart Association’s Heart Health Month with the goal of placing the importance of REPRIEVE into context.
REPRIEVE website
A “participant hub” of the REPRIEVE website was developed in English and Spanish, to share consumer-friendly content written in plain language that included key facts about HIV and CVD and the benefits of participating REPRIEVE. Mechanisms for individuals to reach the CCC or CRS included a toll-free number, a map searchable by city or zip code with contact information for each CRS, and a contact page directing emails to both the CCC and the CRS closest to the candidate’s zip code. Pre-screening questions were also available for interested individuals in Qualtrics Survey Software.
Video promotions
A promotional video was developed in collaboration with the PR agency. The intent of this animated video of approximately 1 minute in duration, was to explain the connection between HIV and CVD using common terms, listing details of the trial, and the ways to learn more about the trial. The video was placed on the home page of the trial website, the REPRIEVE YouTube channel, and was distributed to advocacy groups and all CRSs. CRS staff members were encouraged to share the video with potential participants. Additionally, videos featuring encouraging remarks from NIAID and NHLBI leadership were shared with CRS staff and participants at major enrollment milestones. REPRIEVE also promoted a NIAID Facebook Live video discussion featuring trial participants and CAB members in February 2017 concurrent with the Conference on Retroviruses and Opportunistic Infections in Seattle, WA, USA.
Social media and online promotions
The CCC developed Facebook and Twitter pages, posts and tweets about HIV and CVD were frequently shared, HIV/AIDS Awareness days were recognized, and trial updates provided. Paid advertising on Facebook was also carried out. NIAID assisted the CCC to promote enrollment via NIAID’s social media channels and on the NIH-wide Clinical Trials website. These promotions were shared with the US Department of Health and Human Services HIV.gov program. Additional content was also shared with several Facebook pages catering to long-term survivors of HIV.
The CCC hosted two Twitter chat events, in February, 2016 to recognize Heart Health Month and in September, 2016 to recognize National HIV/AIDS and Aging Awareness Day. Unique hashtags and logos were developed for each. HIV/AIDS advocacy groups and select HIV clinicians were contacted to promote the event within their communities. Promotional tweets were developed and posted on REPRIEVE’s social media channels. Topics of discussion were developed to guide the discussion geared toward raising awareness about CVD risk in HIV. Thirty-two organizations supported each event.
See Supplemental Table 3 for a list of online resources and platforms.
Sex and gender inclusivity
To ensure enrollment of a sex and gender diverse cohort into REPRIEVE, two efforts were carried out. Sara Looby, Ph.D. and Markella Zanni, M.D. of MGH launched “Follow YOUR Heart”, an evidence-based campaign designed to empower women with HIV to participate in clinical studies including REPRIEVE(7). Funding from NIAID(5R01AI123001–03) supported this campaign which included a website, a video, a song, tailored social media messages and a blog, all developed with guidance from women with or at risk for HIV. The overarching goal of this campaign was to develop innovative strategies to ensure female participation in REPRIEVE to ultimately answer clinically relevant questions about sex-specific outcomes and related mechanisms of CVD among women with HIV. Additionally, the trial team had a goal to be gender inclusive and REPRIEVE was among the first large trials to introduce a case report form to assess self-reported gender identity during trial participation.
Ensuring diversity
It was expected that the REPRIEVE population represent the diversity of PWH and that >50% of participants be from Black or African-American, Native American and Asian race as well as Hispanic or Latino ethnicity. Recruitment strategies to maximize diversity included use of culturally sensitive messages, recognizing via social media messaging days of awareness to reach these communities such as National Latinx AIDS Awareness Day, and placing recruitment advertisements in media outlets recognized in these communities. All recruitment materials including print and online were provided in Spanish. Finally, many CRSs were in communities where representation of these populations is recognized to be high.
Celebrity engagement
Through an altruistic partnership with the Women’s Heart Alliance (WHA), REPRIEVE promoted WHA-branded social media messages and a Health Affairs commentary by NIAID Director Anthony S. Fauci, M.D., and WHA co-founder and entertainer Barbra Streisand highlighting REPRIEVE as an example of sex- and gender-inclusive clinical research design in the HIV and cardiovascular fields. Following the commentary, Ms. Streisand delivered a lecture at the NIH in which she highlighted REPRIEVE’s embedded sex differences analysis to the NIH community. The lecture was also broadcast live on the NIH Facebook page and was featured in a Washington Post article (Supplemental Table 2).
Outreach to educational and advocacy groups
The CCC reached out to over 40 HIV/AIDS advocacy groups. Outreach was performed approximately annually during the recruitment period via phone calls, emails and direct mailing of recruitment materials in English and Spanish for distribution to PWH. Outreach organizations were targeted based on reputation, capacity to engage PWH and geographic proximity to participating CRSs.
Community advisory board
REPRIEVE received support and guidance from ACTG Community Scientific Subcommittee members from its inception. However, as REPRIEVE expanded to CRSs beyond the ACTG Network, a broader representation of community members was needed. A community advisory board (CAB) was developed to share and promote the voices of REPRIEVE participants in trial activities, improve participant engagement, provide a forum for participants to share their input, liaise with trial leaders and interact with other participants. The REPRIEVE CAB met quarterly, activities included discussion of trial activities and review of ongoing and new participant-directed trial materials. CAB members were encouraged to share trial updates and materials within their own communities.
Efforts to support site level recruitment
Recruitment champion initiative
The CCC conducted phone interviews with site identified Recruitment Champions at over 50 REPRIEVE CRSs. Successful recruitment practices identified, included frequent pre-screening of clinic charts, communicating with providers by email, chart alerts/e-flags and CRS staff speaking with potential candidates in-person after introduction by clinic members. Champions shared efforts to maximize incentives and convenience for participants, including remuneration, assistance with transportation, parking and meals, flexible visit hours and coordination of study and clinic visits. Recruitment barriers included participant concerns such as additional pill burden, feeling too healthy to be in a research study, use of a placebo pill, statin safety, time commitment, CRS resources needed to assist with recruitment, and competing studies.
Information gathered from this initiative was shared with all CRSs. To assist CRSs to overcome some barriers, the CCC developed recruitment materials addressing participant concerns, procured additional funds for CRS-level recruitment activities, and established detailed co-enrollment guidelines to enable participants to volunteer for multiple trials when possible. Champions were instrumental to facilitate awareness about REPRIEVE by sharing with potential candidates, posters/flyers, videos, and presentations.
Promotional materials
Utilizing recruitment materials such as flyers, postcards, infographics, brochures and slide presentations is a longstanding recruitment strategy by clinical research staff(8). These materials were developed for REPRIEVE and included plain language about the trial and information to address barriers and shared benefits of participation. To facilitate their distribution, the CCC developed a website portal in which CRS representatives could log in and create customized recruitment materials with the CRS’s contact information. Each CRS was asked to share these materials widely with potential participants, other clinics in their region and community organizations. Template slide presentations were developed and distributed to investigator clinicians to share with non-investigator clinicians (Supplemental Material 2).
Community events
CRS teams were encouraged to promote REPRIEVE at community events such as HIV/AIDS walks and health fairs (Supplemental Material 2).
REPRIEVE outreach toolkit
The REPRIEVE Outreach Toolkit was developed in collaboration with the NIAID Communications and Government Relations office. The toolkit contained information and materials to empower CRSs and facilitate connection with local media, community-based organizations, and their institution’s communications office with the goal of promoting REPRIEVE in their local area. The toolkit also contained key messages, a media engagement template, a press release template, instructions to participate in community events (Supplemental Material 2), and social media messages. The toolkit was made available to every CRS via the REPRIEVE website and its utility was promoted heavily in site newsletters and monthly site calls.
Communications about enrollment status and performance
A variety of communication methods were developed to ensure CRS teams were aware of accrual status; these communications were distributed frequently. Weekly emails were sent using a protocol-wide email distribution list that included site investigators, study coordinators, among others. Emails contained updates including number of participants enrolled overall and in the past week. A figure illustrating predefined NIH target enrollment zones with accrual progress of the trial was also included(4). CRSs enrolling at least one participant in the previous week were recognized and CRSs exceeding accrual targets were highlighted by including their photo and recruitment best practices utilized at the CRS. To further bolster enrollment, enrollment challenges were identified and communicated in newsletters. Monthly site calls were held and included similar content.
Site Selection and Performance Committee
The Site Selection and Performance Committee (SSPC), a committee inclusive of REPRIEVE leadership and CRS staff, had overall responsibility for monitoring enrollment at the site level. Expectations regarding enrollment performance was included in the Site Performance Plan (SPP) made available to each CRS. During the recruitment period, the SSPC had monthly meetings to discuss accrual status of each CRS and identified CRSs not meeting targets. When a CRS had difficulty activating, screening and/or enrolling per the metrics outlined in the SPP, a staged process of increased contact was implemented between the SSPC and the CRS team. This process included email communications and phone calls led by leadership and the CRS team to review progress, identify enrollment barriers, and engage in problem solving. The goal of communication from the SSPC was not to penalize sites for not meeting enrollment targets, but rather to relay support and encouragement during recruitment. The SSPC ensured communications to sites were kept in a positive and encouraging tone. Additionally, the SSPC communicated accrual status via monthly site scorecards distributed via the protocol-wide email list and biannual evaluation letters emailed to each site PI and study coordinator. Each of these communications reminded CRSs of enrollment metrics and expectations while the biannual evaluation letters required CRSs not meeting expectations to submit a corrective action plan addressing plans to meet expectations for the next evaluation period.
Results
Screening and Enrollment
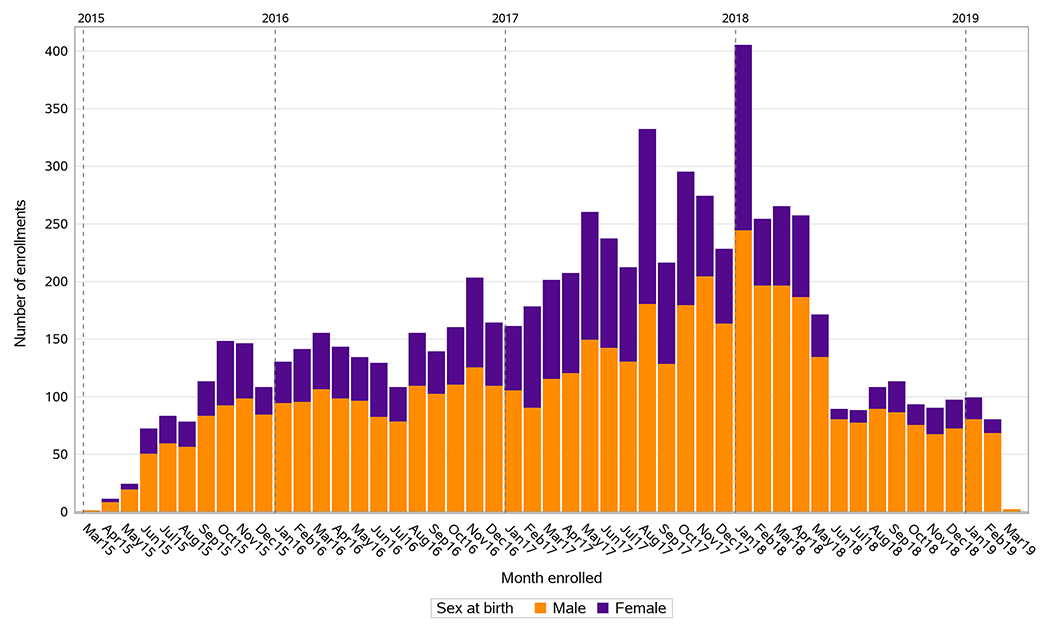
7557 participants were enrolled over 48 months (March 2015 to March 2019), surpassing the target accrual of 7500. One-hundred-thirty CRSs were initially activated and 125 enrolled participants. Figure 1 illustrates enrollment by month and sex during the recruitment period. The highest enrollment yield was between May 2017 and April 2018. Enrollment slowed somewhat but continued at a steady pace after April 2018, when future enrollments were limited to participants with ASCVD risk score ≥5.0%.
Figure 1. Enrollment during REPRIEVE (A5332) recruitment period, by month.
Bar graph illustrating monthly enrollment numbers during the recruitment period between March 2015 and March 2019. Enrollment by sex at birth illustrated by stacked bar graphs.
Approximately 10,000 screens were completed. The overall recruitment yield (R-factor), which is calculated by dividing the total number enrolled by the total number of screens, was 71%, however the R-factor varied slightly across participant groups of focus (women and racial/ethnic minorities). Table 1a provides a comparison of enrollment, screen failures, and recruitment yield for each group. By racial category, participants who identified as ‘White’ had a relatively higher recruitment yield of 76%. Participants screened who were Hispanic or Latino ethnicity had a higher recruitment yield than participants who were not. Similarly, males who screened for the trial were more likely to enroll than females.
Table 1a.
REPRIEVE(A5332) recruitment yield by sex at birth, age, race, and ethnicity.
| Total Screened (N=10,613) | Screen Fail (N=3056) | Enrolled (N=7557) | R-Factor (%) | |
|---|---|---|---|---|
| Sex | ||||
| Male | 7020 (66) | 1862 (27) | 5158 (73) | 73 |
| Female | 3593 (34) | 1194 (33) | 2399 (67) | 67 |
| Age | ||||
| <50 years | 4866 (46) | 1177 (24) | 3689 (76) | 76 |
| ≥50 years | 5713 (54) | 1845 (32) | 3868 (68) | 68 |
| Race | ||||
| Black or African American | 4963 (47) | 1639 (33) | 3324 (67) | 67 |
| White | 3229 (30) | 785 (24) | 2444 (76) | 76 |
| Asian | 1510 (14) | 371 (25) | 1139 (75) | 75 |
| More than one race | 290 (3) | 182 (63) | 108 (37) | 37 |
| Other | 313 (3) | 0 (0) | 313 (100) | 100 |
| Ethnicity | ||||
| Hispanic or Latino | 2439 (23) | 543 (22) | 1896 (78) | 78 |
| Not Hispanic or Latino | 7737 (73) | 2394 (31) | 5343 (69) | 69 |
| Unknown | 437 (4) | 119 (27) | 318 (73) | 73 |
Note: the column totals do not equal the total number screened as there are other subgroups screened that are not included.
All results are reported as N (%)
Despite higher recruitment yields among Whites and males, REPRIEVE successfully enrolled a diverse cohort of participants and surpassed all subgroup targets put forth at the beginning of the trial. Table 1b highlights initial enrollment targets for participant groups, compared to actual enrollment. REPRIEVE enrolled 10% more women than initially planned and achieved great diversity across racial groups, with the majority of the study population identifying as non-White. International CRSs contributed greatly to the enrollment of women and racial minorities. Participant populations were majority female in Thailand, South Africa, and Botswana. Enrollment by country and sex is illustrated in Table 2.
Table 1b.
Comparison of planned enrollment and actual enrollment by race, sex at birth, and ethnicity.
| Planned Enrollment | Actual Enrollment | |
|---|---|---|
| Sex | ||
| Male | 5850 (78) | 5158 (68) |
| Female | 1650 (22) | 2399 (32) |
| Race | ||
| Black or African American | 3225 (43) | 3324 (44) |
| White | 4050 (54) | 2444 (32) |
| Asian | 188 (2.5) | 1139 (15) |
| Ethnicity | ||
| Hispanic or Latino | 1275 (17) | 1896 (25) |
| Not Hispanic or Latino | 6225 (83) | 5343 (71) |
All results are reported as N (%)
Table 2.
REPRIEVE (A5332) enrollment by sex at birth, by country
| Total (N=7557) |
Africa Botswana (N=281) |
Africa South Africa (N=570) |
Africa Uganda (N=181) |
Africa Zimbabwe (N=125) |
Asia India (N=504) |
Asia Thailand (N=590) |
N.America Haiti (N=140) |
N.America US/Canada (N=3919) |
S.America Brazil (N=1099) |
S.America Peru (N=148) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex at birth | |||||||||||
| Male | 5,158 (68) | 104 (37) | 195 (34) | 88 (49) | 95 (76) | 375 (74) | 257 (44) | 81 (58) | 3,050 (78) | 777 (71) | 136 (92) |
| Female | 2,399 (32) | 177 (63) | 375 (66) | 93 (51) | 30 (24) | 129 (26) | 333 (56) | 59 (42) | 869 (22) | 322 (29) | 12 (8) |
| Race | |||||||||||
| White | 2444 (32) | 0 (0) | 2 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (0) | 0 (0) | 1967 (50) | 464 (42) | 9 (6) |
| Black or African American | 3324 (44) | 280 (100) | 567 (99) | 181 (100) | 125 (100) | 0 (0) | 0 (0) | 140 (100) | 1680 (43) | 349 (32) | 2 (1) |
| Asian | 1139 (15) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 504 (100) | 588 (100) | 0 (0) | 45 (1) | 2 (0) | 0 (0) |
| Other | 650 (9) | 1 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 227 (6) | 284 (26) | 137 (93) |
All results are reported as N (%)
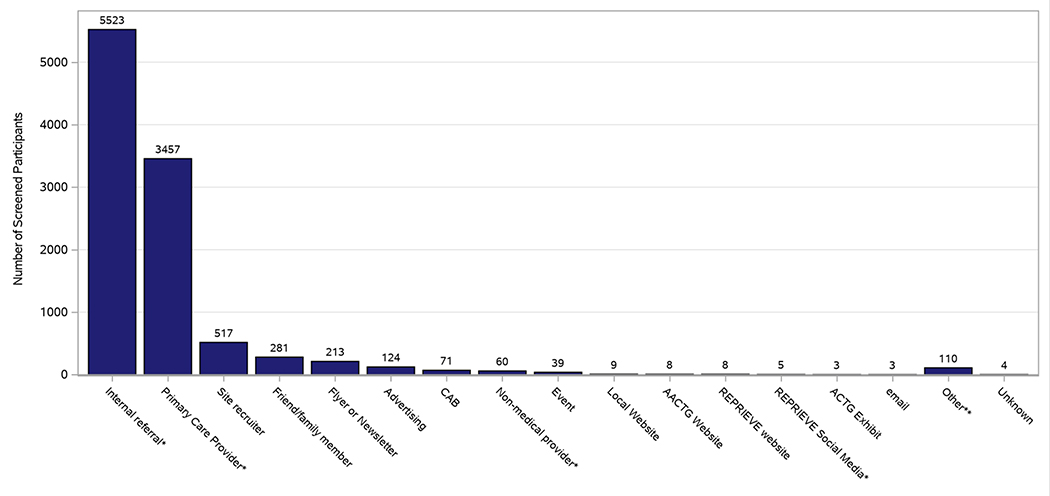
Figure 2 illustrates how individuals screened heard about REPRIEVE. Most commonly this resulted from an internal referral within the participant’s clinic. In addition, 33% heard about the study through their primary care provider (PCP). Despite efforts to generate an online presence through advertisements and social media platforms, only 146 individuals reported hearing about REPRIEVE through one of these mechanisms.
Figure 2. How participants screened heard about REPRIEVE.
Bar graph illustrating how participants screened heard about the REPRIEVE Trial.
Impact of digital and social media outreach
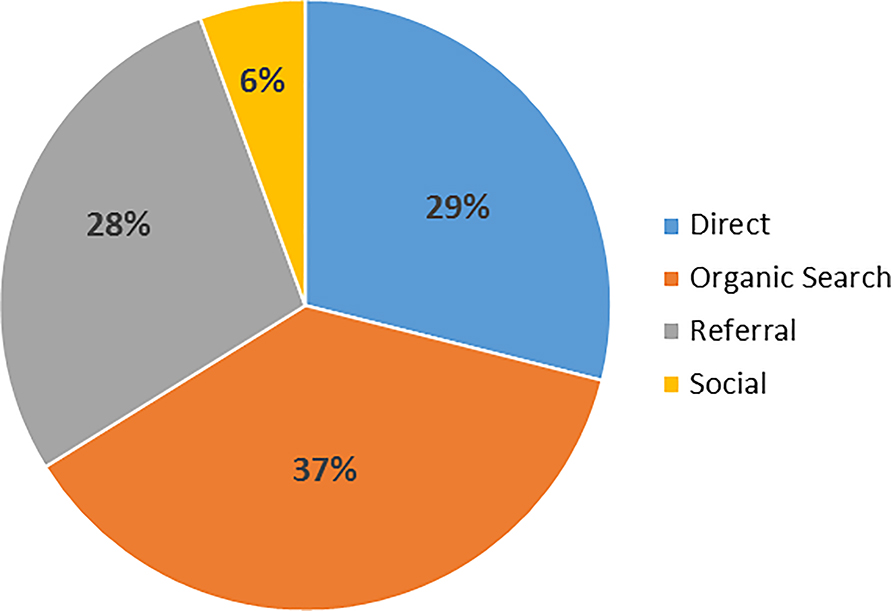
While few individuals cited the REPRIEVE website as a reference source for the trial, results from Google Analytics indicate high volumes of traffic to the site. Google Analytics was used throughout the recruitment process to monitor website traffic. The REPRIEVE website was viewed approximately 73,810 times during the recruitment period. Most sessions originated from servers within the United States. Figure 3 illustrates the number of page views by month and various initiatives that led to increased website traffic. The highest traffic occurred in March 2016 with a total of 8,521 pageviews, directly following the first REPRIEVE Twitter chat. Increases in traffic to the site also occurred around World AIDS Day (December 1). Results from Google Analytics indicate how individuals navigated to the website (Figure 4). Most visitors discovered the site through an “organic search,” (visit originated from unpaid search results), others navigated directly to the URL, or from a referral (clicking a link from another site). Less website traffic than anticipated originated from social media platforms such as Facebook and Twitter.
Figure 4. Results from Google Analytics indicating how individuals navigated to the website www.reprievetrial.org.
Pie chart illustrating results from Google Analytics showing the most common ways individuals navigated to the REPRIEVE website (www.reprievetrial.org).
Analysis of the impact of the #HIVheartchat revealed over 1000 associated tweets, 250 unique relevant tweets, and 129 participants from target groups. Twenty-seven percent were from public health and advocacy organizations, 22% identified as health care professionals, 12% identified as activists and journalists and 5% as PWH. Tweets about REPRIEVE reached approximately 139,000 unique Twitter accounts.
REPRIEVE was featured in over 40 articles in influential media outlets (Supplemental Table 2). Results from Google Analytics indicate that the Boston Herald article was the 12th largest referral source for reprievetrial.org at the time of publication (March 2016).
REPRIEVE website users were prompted to complete a pre-screening survey in Qualtrics Survey Software if interested. Approximately 954 pre-screening surveys were initiated utilizing this pre-screening tool. Of the surveys initiated, 571 individuals qualified for further screening into the study.
Discussion
Recruitment of REPRIEVE was challenging due to the ambitious target of enrolling 7500 participants in 48 months. While introducing sites in 11 countries was pivotal to the successful enrollment of a diverse cohort, it added challenges due to cultural differences, language barriers and differing regulatory guidelines. The various challenges during the recruitment period now serve as lessons learned for future intervention trials of similar magnitude. Despite these challenges, recruitment was tremendously successful. An important takeaway from the recruitment process is the importance of developing a recruitment plan, with pre-determined targets, real-time tracking methods and utilization of a broad range of strategies to support local recruitment.
One recruitment challenge was awareness of the problem. REPRIEVE is a primary prevention trial of CVD in HIV; CVD is not always recognized among PWH as a potentially serious condition. Moreover, engaging relatively healthy PWH and adding one additional daily medication to an often-complex medication regimen was a significant recruitment barrier. Targeted messaging was necessary to raise awareness of the increased risk for CVD among PWH. Since CVD and HIV were not commonly mentioned together in the mainstream media, the CCC developed specific messaging in recruitment materials and conducted outreach to national and regional media outlets to spread messaging and generate public awareness about CVD risk and HIV. Messaging was targeted to explain the critical need for a randomized trial, equipoise as to the potential importance of statins for CVD prevention in HIV, and lack of current data in this regard.
Print and online media outlets had the greatest yield in terms of driving traffic to the REPRIEVE website compared to social media. Results indicated that articles published increased traffic to the website, demonstrating value in targeted media outreach and influencer engagement. Results from Google Analytics further support these findings, most visitors discovered the REPRIEVE website through unpaid search results and referrals from another site.
Most recruitment efforts were successfully executed at the CRS level with support from the CCC. Given ambitious recruitment goals, the CCC team felt that reliance on local referrals from clinicians might not be sufficient to meet the accrual targets within the required timeframe. However, our findings indicate that 85% of individuals who screened heard about the study from a referral within their local clinic or PCP. A significant number of individuals heard about REPRIEVE from a site recruiter, flier or newsletter, while fewer heard about the study through advertising and social media. These results lead to the conclusion that traditional recruitment strategies, such as a conversation with a familiar provider, informational brochures or fliers, may be more successful in recruiting participants compared to more contemporary techniques such as social media(9). An alternative explanation for this finding is that while potential candidates may become aware of a trial via online mechanisms, it is the face-to-face contact and time that a provider takes to explain a trial that is ultimately the mechanism driving individuals to enroll. We strongly believe the promotional materials and messages about the relationship between CVD and HIV were essential in promoting discussion about REPRIEVE and encouraged providers to discuss the trial with their patients.
As is true for most coordinating centers, the REPRIEVE CCC was limited in its capacity to make direct contact and encourage enrollment of potential candidates and participants, due to privacy regulations and the trial design. These activities occurred at the site level after informed consent was obtained. Additionally, the CCC did not collect comprehensive data on pre-screening activities that were carried out at the site level. Many CRSs utilized existing databases prior to screening to identify potential candidates, and the results of such pre-screening were not entered in the electronic data capture system. Capture of such data may have provided more granularity in real time as to why individuals did not qualify. Moreover, we did not track uptake of fliers and promotional materials at the site level. Together, these limitations made it difficult to understand with certainty how participants came to be recruited into the trial. Prospective, systematic efforts to collect information on pre-screening activities should be developed to facilitate recruitment and efforts in clinical trials as well as to be able to replicate pre-screening activities by other clinical trials.
Based on our experience and results presented, we believe the strongest recruitment tool is a trusted clinic or clinician. The most successful CRSs had study teams who were invested in the success of the trial, and who could effectively and impartially communicate knowledge of the trial to potential candidates, addressing knowledge gaps as to the relevance of CVD in the HIV population, importance of CVD prevention, and the potential risks and benefits trial participation. Provision of materials and detailed, clear information to study teams enabled this process. While online and print media, social media, trial websites and other outreach mechanisms may serve as useful tools to inform potential candidates about a clinical trial, based on our findings, these efforts were not the primary method that REPRIEVE participants became aware of the study. While these tools serve as platforms to promote messaging about a trial, efforts of central coordination to enable and cultivate motivated study teams is more effective to achieve enrollment goals in such large trials. Therefore, providing CRSs, community clinics, and local providers with the information and tools necessary to identify potential candidates and promote a trial within their community and patient population is essential for successful recruitment.
Finally, although the REPRIEVE trial is a large multi-center trial conducted globally among PWH, recruitment practices including developing a campaign to raise awareness, gleaning insight from study staff about recruitment practices, and productive performance evaluation implemented by the CCC can be adapted to other clinical trials of any scale and focus of research to ensure successful enrollment of the trial.
Supplementary Material
Acknowledgments
Grants and Funding Support
This project was supported by NIH Research Grant Number U01 HL123336 (to SKG and PD); U01 HL123339 (to UH and HJR) funded by the Office of AIDS Research, Office of the Director, NIH and the National Heart, Lung and Blood Institute, NIH with additional support from the National Institute of Allergy and Infectious Diseases, NIH under UM1 AI068634, UM1 AI068636, UM1 AI106701; Investigator initiated grant, study drug and blinded matching placebo from Kowa Pharmaceuticals America, Inc. (to SKG); Investigator initiated grant from Gilead Sciences, Inc. (to SKG and MVZ).
The study investigators would like to thank the study participants, site staff and study-associated personnel, REPRIEVE Community Advisory Board members including Angel Luis Hernandez, Alicia Diggs, Janice Jarrells, Robert Ettinger, Shirley Selvage, ACTG Community Scientific Subcommittee member Kate Starr and Clinical Trials Specialists (Barbara Bastow, Laura Moran, and Jhoanna Roa) for their efforts to make this trial possible. See Supplemental Material 1 for supplemental author list.
Disclosures
Kathleen V. Fitch, M.S.N. has received support from an educational grant from Gilead Sciences, Inc. and Merck & Co., Inc., unrelated to this project
Steven K. Grinspoon, M.D. has received grant support through his institution from Kowa
Pharmaceuticals America, Inc. and Gilead Sciences, Inc. for the conduct of the study; from Theratechnologies, and Navidea unrelated to this project and received consulting fees from Theratechnologies, Navidea, Gilead, Merck, and Bristol Myers Squibb, all unrelated to this Project.
Sara E. Looby, is a non-paid board member of the non-profit organization Healing Our Community Collaborative, Inc. Boston, MA, unrelated to this project. Received grant support from the Claflin Distinguished Scholar Award from the MGH Executive Committee on Research, and the William F. Connell Family.
Edgar T. Overton, M.D. has received grant support through his institution from Gilead Sciences and ViiV unrelated to this project and received consulting fees from ViiV and Merck.
Markella V. Zanni, M.D. has received grant support through her institution from Gilead Sciences for the conduct of the study.
Carl J. Fichtenbaum, M.D., has received grant support through his institution from Gilead Sciences, ViiV Healthcare, Janssen, Pfizer, Merck, Amgen and Cytodyn unrelated to this project. He also receives consulting fees for advisory board from Janssen.
Judith A. Aberg, M.D. has received research grants from Bristol-Myers Squibb, Gilead Sciences and Viiv Healthcare, and received scientific advisory board personal fees from Gilead, Janssen, Merck, and ViiV Healthcare, all unrelated to the present study
Carlos Malvestutto, M.D. received consulting fees from ViiV Healthcare unrelated to this project.
Beverly Alston-Smith, M.D. is an employee of the National Institutes of Health.
Katharine Cooper-Arnold, M.D. is an employee of the National Institutes of Health.
Laura R. Sanchez, B.A. has no disclosures to report.
Anne Rancourt, M.P.S. is an employee of the National Institutes of Health.
Sharlaa Badal-Faesen, M.D. has not disclosures to report.
Sandra Wagner Cardoso, M.D. has no disclosures to report.
Anchalee Avihingsanon, M.D., Ph.D., has received research grants though her institution from Gilead Science, ViiV Healthcare, and received scientific advisory fee from ViiV Healthcare.
Sandesh Patil, M.D. M.P.H. is an employee of Johns Hopkins University, Baltimore, U.S.A. at Byramjee Jeejeebhoy Medical College Clinical Research Site, Pune, India.
Judith Lavelle, M.S. is an employee of the National Institutes of Health.
Karin L. Klingman, M.D. is an employee of the National Institutes of Health.
Patrice Desvigne-Nickens, M.D. is an employee of the National Institutes of Health.
Udo Hoffmann M.D., M.P.H., has received grant support through his institution from Kowa. Pharmaceuticals America, Inc. for the conduct of the study and has received grant support from Siemens Healthcare, the American College of Radiology Imaging Network and HeartFlow Inc. unrelated to this project.
Heather J. Ribaudo, Ph.D. has no disclosures to report.
Pamela S. Douglas M.D. has no disclosures to report.
Kathleen Melbourne, PharmD., is an employee of Gilead Sciences and owns stock in Gilead Sciences.
Craig Sponseller, M.D. is an employee of Kowa Pharmaceuticals America, Inc.
Footnotes
Disclaimer
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institute of Allergy and Infectious Diseases; the National Institutes of Health; or the U.S. Department of Health and Human Services.
ClinicalTrials.gov Identifier: NCT02344290
References
- 1.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007. July;92(7):2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013. April 22;173(8):614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV. Circulation. 2018. September 11;138(11):1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grinspoon SK, Fitch KV, Overton ET, Fichtenbaum CJ, Zanni MV, Aberg JA, et al. Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). Am Heart J 2019. June;212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treweek S, Pitkethly M, Cook J, Kjeldstrom M, Taskila T, Johansen M, et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev 2010. April 14(4):MR000013. [DOI] [PubMed] [Google Scholar]
- 6.Ladapo JA, Richards AK, DeWitt CM, Harawa NT, Shoptaw S, Cunningham WE, et al. Disparities in the Quality of Cardiovascular Care Between HIV-Infected Versus HIV-Uninfected Adults in the United States: A Cross-Sectional Study. J Am Heart Assoc 2017. November 14;6(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanni MV, Fitch K, Rivard C, Sanchez L, Douglas PS, Grinspoon S, et al. Follow YOUR Heart: development of an evidence-based campaign empowering older women with HIV to participate in a large-scale cardiovascular disease prevention trial. HIV Clin Trials. 2017. March;18(2):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsey TM, Snyder JK, Lovato LC, Roumie CL, Glasser SP, Cosgrove NM, et al. Recruitment strategies and challenges in a large intervention trial: Systolic Blood Pressure Intervention Trial. Clin Trials. 2016. June;13(3):319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volkmann ER, Claiborne D, Currier JS. Determinants of participation in HIV clinical trials: the importance of patients’ trust in their provider. HIV Clin Trials. 2009. Mar-Apr;10(2):104–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.