Abstract
Androgen insensitivity syndrome (AIS), manifesting incomplete virilization in 46,XY individuals, is caused mostly by androgen receptor (AR) gene mutations. Therefore, a search for AR mutations is a routine approach in AIS diagnosis. However, some AIS patients lack AR mutations, which complicates the diagnosis. Here, we describe a patient suffering from partial androgen insensitivity syndrome (PAIS) and lacking AR mutations. The whole exome sequencing of the patient and his family members identified a heterozygous FKBP4 gene mutation, c.956T>C (p.Leu319Pro), inherited from the mother. The gene encodes FKBP prolyl isomerase 4, a positive regulator of the AR signaling pathway. This is the first report describing a FKBP4 gene mutation in association with a human disorder of sexual development (DSD). Importantly, the dysfunction of a homologous gene was previously reported in mice, resulting in a phenotype corresponding to PAIS. Moreover, the Leu319Pro amino acid substitution occurred in a highly conserved position of the FKBP4 region, responsible for interaction with other proteins that are crucial for the AR functional heterocomplex formation and therefore the substitution is predicted to cause the disease. We proposed the FKBP4 gene as a candidate AIS gene and suggest screening that gene for the molecular diagnosis of AIS patients lacking AR gene mutations.
Keywords: androgen insensitivity syndrome (AIS), partial androgen insensitivity syndrome (PAIS), disorder of sexual development (DSD), androgen receptor signaling, FKBP4
1. Introduction
Androgens govern the development of reproductive and non-reproductive pathways of the male body during the successive life periods, resulting in human sexual dimorphism. Testosterone and dihydrotestosterone are the most crucial androgens inducing male development. In the male embryo, they are responsible for the development of the Wolffian duct into the internal male sex organs and virilization of external genitalia, respectively. At the puberty period, androgens govern the growth of male internal and external genitalia as well as the growth of skeletal muscle, development of larynx, and general growth spurt. In the adult, androgens regulate spermatogenesis, muscle mass, bone metabolism as well as behavior; for a review, see [1,2,3]. Androgens act via the androgen receptor (AR) signaling pathway. Namely, they bind to the AR in the AR-Hsp90-p23 heterocomplex located within the cytoplasm of androgen-sensitive cells, cause AR activation and translocation into the nucleus, whereby AR functions as a transcription factor of specific genes; for a review, see [3,4]. The expression of these genes, in turn, stimulates cell growth, proliferation, cell cycle progression as well as secretion of specific proteins by AR-expressing cells, resulting in the development of a male phenotype; for review see [3]. The AR-mediated transcription is modulated by FKBP4 protein (also known as FKBP52), which interacts with AR [5] and its chaperon Hsp90 [6]. Thanks to the FKBP4–Hsp90 interaction, the AR receptor gains a high affinity for hormone binding and the AR-mediated transcription becomes upregulated [7,8,9,10].
Loss-of-function mutations in the AR gene (located on the X chromosome) cause the dysregulation of the AR signaling pathway, resulting in androgen insensitivity syndrome (AIS) in 46,XY individuals. This syndrome is characterized by abnormalities of the internal and external genitalia virilization as well as secondary sex characteristics. This occurs despite the presence of hormonally active bilateral testes producing normal levels of testosterone and anti-Müllerian hormone (AMH), for a review, see [11]. The spectrum of AIS phenotypes is broad and classified in three major categories: complete (CAIS), partial (PAIS), and mild androgen insensitivity syndrome (MAIS). CAIS patients display female phenotypic characteristics, like female external genitalia, presence of distal vagina, female breast development, and the absence of prostate and Wolffian structures. However, they lack Müllerian duct derivatives, i.e., upper vagina, Fallopian tubes, and uterus. Those phenotypic females are characterized by primary amenorrhea. The PAIS category is more variable then CAIS. The phallic structure of PAIS patients’ ranges from a penis with varying degree of diminished size and hypospadias, up to a slightly enlarged clitoris. Wolffian structures are fully or partially developed, and the prostate is typically small or impalpable. Müllerian remnants are very rare. Finally, the MAIS category is reported in men representing adolescent gynecomastia and/or suffering from infertility; for a review, see [1]. Although a majority of AIS patients carry hemizygous mutations of the AR gene, in some AIS individuals no AR gene mutations have been found. Namely, around 10% of CAIS and 60–80% of PAIS patients do not carry such mutations; for a review, see [12,13]. Studies of such patients described mutations in some other genes important for sexual development, e.g., in the hypospadias-associated MAMLD1 gene [14] or the NR5A1 gene, encoding steroidogenic factor 1 [15]. Nevertheless, a majority of AIS patients lacking AR mutations are genetically unexplained. It was hypothesized that mutations in the AR co-regulator proteins could account for the AIS phenotype [16]. According to this hypothesis it was shown that a group of AIS patients with no AR gene mutation revealed reduced AR transactivation ability since the mRNA level of an AR target gene was significantly lowered. This data supports the existence of cellular components, besides the AR, affecting androgen signaling during sexual differentiation and suggests their involvement in the etiology of AIS when disrupted. That phenotypic AIS subgroup characterized by a lowered expression of AR target while lacking AR gene mutation is called AIS type II [13]. The hypothesis that the mutations of AR co-activators could be responsible for AIS, has not been confirmed, since no such mutations have been identified so far; for a review, see [17]. Therefore, the elucidation of the molecular background of idiopathic AIS is crucial for achieving a more complete understanding of the etiology of the disease and would be precious for AIS genetic counseling.
Here, we report for the first time a potentially causative FKBP4 gene mutation in a patient manifesting PAIS with hypospadias.
2. Results
2.1. PAIS Case Report
The chromosomal analysis of the patient showed a 46,XY karyotype without evidence of mosaicism. At the age of 10, urological examinations revealed the size of penis and testes being smaller, compared to the average at his age: penis length was 25 mm, the right testis 9 mm × 7 mm (0.33 mL), the left one 12 mm × 9 mm (0.565 mL). Pelvic ultrasound investigation did not reveal any structure of Müllerian ducts. The patient was overweight (BMI = 32.2 kg/m2). Later on, at the age of 13, urological examinations revealed the size of penis and testes still smaller compared to the average, namely penis length was 28 mm, the right testis size was 58 mm × 24 mm (7.28 mL), the left one was 60 mm × 21 mm (6.59 mL).
Hormonal investigations at the age of 10 revealed testosterone, anti-Müllerian hormone (AMH), estradiol, luteinizing hormone (LH), follicle-stimulating hormone (FSH), thyroid-stimulating hormone (TSH), prolactin (PRL), dehydroepiandrosterone sulfate (DHEAS), cortisol, and progesterone within the proper range, in accordance with age (Table 1). The human chorionic gonadotropin (hCG) testosterone stimulation test, performed at the age of 10 and 13, revealed a high response of testosterone, i.e., 8-fold and over 7-fold increase in testosterone concentration, respectively (Table 1). This result indicated correct testosterone biosynthesis.
Table 1.
Values of testosterone (baseline and after human chorionic gonadotropin (hCG) stimulation), anti-Müllerian hormone (AMH), estradiol, luteinizing hormone (LH), follicle-stimulating hormone (FSH), thyroid-stimulating hormone (TSH), prolactin (PRL), dehydroepiandrosterone sulfate (DHEAS), cortisol, and progesterone of the partial androgen insensitivity syndrome (PAIS) patient.
| Hormone, Unit | Age 10 Years | Age 10 Years Reference Values [18] | Age 13 Years | Age 13 Years Reference Values [18] | |
|---|---|---|---|---|---|
| Total testosterone, ng/dL | Baseline after hCG |
2.5 | <3–10 | 120 | 18–150 |
| 20.0 | 970 | ||||
| AMH, ng/mL | 167.4 | 7.4–243 | |||
| Estradiol, pg/mL | 0.3 | <1.0 | |||
| LH, mIU/mL | 0.2 | 0.02–0.3 | |||
| FSH, mIU/mL | 0.6 | 0.26–3.0 | |||
| TSH, mIU/L | 3.9 | 0.6–6.3 | |||
| PRL, ng/mL | 12.3 | 3–18 | |||
| DHEAS, µmol/L | 24.8 | 13–115 | |||
| Cortisol, nmol/L | 289.5 | 140–550 | |||
| Progesterone, nmol/L | 2.9 | ≤3.8 |
Taking into consideration (1) the characteristics of the disorder of sexual development (DSD) phenotype of the patient, (2) his male karyotype, (3) the results of the hCG stimulation tests excluding defects in testosterone biosynthesis, (4) the normal AMH level excluding gonadal dysgenesis, and (5) normal LH and FSH levels excluding hypogonadotropic hypogonadism, we concluded that the patient suffered from PAIS.
2.2. Identification of the FKBP4:c.956T>C (p.Leu319Pro) Mutation in the Patient
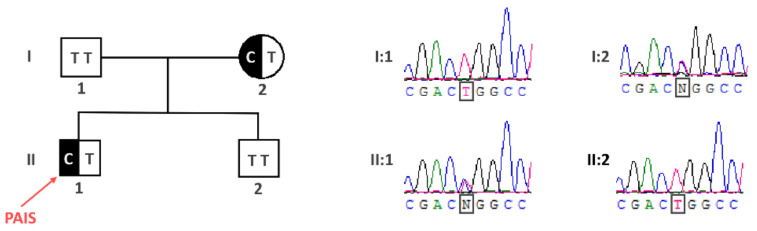
Since AR gene mutations are significantly less frequent in PAIS than in CAIS patients [12], we proceeded straight away with the whole exome sequencing (WES) analysis in search for the genetic background of PAIS in the patient. While the WES analysis did not reveal any AR gene mutation, it uncovered a heterozygous mutation in the FKBP4 autosomal gene encoding an AR interactor [5] being a modulator of AR transcriptional activity [7,8,9,10]. The mutation was a T>C transition NM_002014.4(FKBP4_v001):c.956T>C, causing amino acid substitution NM_002014.4(FKBP4_i001):p.Leu319Pro, hereinafter referred to as FKBP4:c.956T>C (p.Leu319Pro). The patient’s mother was heterozygous for that mutation, while the father carried the wild-type alleles. We confirmed the FKBP4:c.956T>C (p.Leu319Pro) mutation status in all the family members and additionally showed a lack of mutation in the healthy brother by Sanger sequencing (Figure 1).
Figure 1.
The identification of the FKBP4:c.956T>C (p.Leu319Pro) mutation in the partial androgen insensitivity syndrome (PAIS) patient. The pedigree shows the inheritance of the FKBP4 mutation according to an autosomal dominant model, with the phenotype restricted to male individuals.
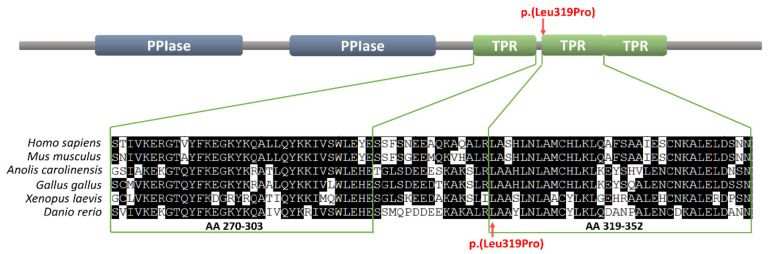
Importantly, the mutation was located within one of three tetratricopeptide repeats (TPR), responsible for interaction with several proteins crucial for AR activity. Moreover, the 319 position in which the Leu319Pro substitution occurred is highly conserved among vertebrate Fkbp4 homologues (Figure 2). The FKBP4:c.956T>C (p.Leu319Pro) variant was not present in any single nucleotide polymorphism (SNP) database (gnomAD, 1000G, ExAC) and the Leu319Pro substitution in human FKBP4 protein has been predicted as disease-causing or disrupting the protein function by in silico algorithms: Meta-SNP, MutationTaster, and PolyPhen-2.
Figure 2.
The Leu319 position within the tetratricopeptide repeat (TPR) domain of the FKBP4 protein and the conservation of that residue among vertebrates from fish to humans. The upper part represents the FKBP4 protein domains organization (PPIase = prolyl isomerase). The lower part represents the alignment of the first two human TPR repeats of human, mouse, lizard, chicken, frog, and zebrafish homologues. The FKBP4:p.Leu319Pro mutation is indicated with arrows.
Besides the FKBP4 gene mutation, six additional heterozygous variants in five genes (AKR1C4, CYP17A1, FREM2, IL17RD, NKD2) involved in sexual development were identified in the patient (among them two were identified in distinct alleles of FREM2 gene). However, three (in FREM2, IL17RD, NKD2 genes) out of the six variants were predicted as neutral using Meta-SNP (Table 2). For that reason, we did not further consider their influence on the patient’s phenotype. The other three variants of AKR1C4, CYP17A1 or FREM2 genes were predicted to be disease-causing (Table 2) but mutations of CYP17A1 and FREM2 genes caused DSD in karyotypic men only when both alleles of those genes were mutated [19,20,21], therefore the heterozygous status of CYP17A1 and FREM2 variants in our patient make their contribution to the DSD phenotype unlikely. However, we did not rule out the modifier effect of those variants. Besides, we cannot exclude the contribution of the AKR1C4 variant to the DSD phenotype, all the more so that heterozygous mutations of this gene have been previously described as potential modifiers of the DSD phenotype [22].
Table 2.
List of heterozygous variants in autosomal genes involved in sexual development identified in the partial androgen insensitivity syndrome (PAIS) patient.
| Gene/Protein | Associated Recessive Diseases | Related Pathway | SNP ID | Frequency (gnomAD) | DNA Change | Protein Change |
Mother | Father | Meta-SNP |
|---|---|---|---|---|---|---|---|---|---|
|
FKBP4/ Peptidyl-prolyl cis-trans isomerase FKBP4 |
----- | AR signaling | - | - | c.956T>C | p.Leu319Pro | Het | - | Disease |
|
AKR1C4/ aldo-keto reductase family 1 member C4 |
AKR1C4 may act as a modifier of 46,XY disorder of sex development due to testicular 17,20-lyase deficiency [22] |
Alternative pathways of testicular androgen biosynthesis | rs533399756 | 0.00007946 | c.704T>C | p.Leu235Pro | - | Het | Disease |
|
CYP17A1/ Steroid 17-alpha-hydroxylase/17,20 lyase |
46,XY disorder of sex development due to isolated 17,20-lyase deficiency [19,20] | Steroid hormone biosynthesis | rs373661758 | 0.00001443 | c.910G>A | p.Val304Met | - | Het | Disease |
|
FREM2/ FRAS1-related extracellular matrix protein 2 |
Fraser Syndrome 2 (characterized by ambiguous genitalia in 46,XY individuals) [21] |
Extracellular matrix-receptor interaction | rs200316547 | 0.00022000 | c.996G>T | p.Gln332His | Het | - | Disease |
| rs142821775 | 0.00002850 | c.6445A>G | p.Met2149Val | - | Het | Neutral | |||
|
IL17RD/ Interleukin-17 receptor D |
Hypogonadotropic hypogonadism 18 [23] | RET signaling | - | - | c.1164G>C | p.Glu388Asp | Het | Neutral | |
|
NKD2/ Protein naked cuticle homolog 2 |
----- | Inhibitor of WNT signaling | - | - | c.809G>A | p.Arg270Lys | - | Het | Neutral |
The data of whole-exome sequencing of the described patient, his mother and his father are available from the BioProject database (ID PRJNA669263), biosample accessions: SAMN16449689, SAMN16449690, SAMN16449691.
3. Discussion
The number of AIS patients with no AR gene mutation is significant, which makes their genetic diagnosis difficult. Here, we report a patient manifesting a PAIS phenotype, lacking any AR gene mutation and instead carrying a novel FKBP4 gene variant. The gene seems to be a strong candidate for PAIS, since the knockout of its homologue in the mouse caused a phenotype consistent with PAIS, including hypospadias [24]. Moreover, the function of that gene is linked to an AR signaling pathway, i.e., the FKBP4 protein interacts with AR [5] and its chaperone Hsp90 [6], leading to enhanced androgen binding and the upregulation of AR-mediated transcription [7,8,9,10]. Previously, a search for a FKBP4 gene mutation in a group of patients with hypospadias was described but no variant associated with that condition was found [25]. The FKBP4 variant identified in our PAIS patient carries a missense p.Leu319Pro mutation. This mutation is considered causative for several reasons. Firstly, it is located within the TPR domain, which is crucial for FKBP4-Hsp90 interaction within the AR heterocomplex, as well as for FKBP4 interaction with S100A1 and S100A2 proteins, which regulate FKBP4-Hsp90 complex formation [26]. Taking into consideration that the presence of FKBP4 in the AR heterocomplex enhances AR ligand binding, a mutation in the domain vital for FKBP4–Hsp90 interaction could impair testosterone binding by its receptor and finally causing AIS. Secondly, the Leu319Pro substitution is situated within a position, which is conserved across vertebrates (Figure 2), indicating its critical significance for FKBP4 protein function. Thirdly, the p.Leu319Pro substitution in the human FKBP4 protein has been predicted as disease-causing by several predictors of functional effects of human SNPs, and this variant is not present in any SNP database. Fourthly, the mutation in the PAIS patient described here has been inherited from its mother and was not present in the healthy brother (Figure 1). Such a pedigree may indicate an autosomal dominant model of inheritance, with the DSD phenotype restricted to karyotypic men. However, this dominant mode of inheritance is not in line with the mouse model, in which heterozygous Fkbp4 mutant males did not display hypospadias and were fertile, in contrast to homozygous Fkbp4 mutant animals displaying a phenotype corresponding to PAIS [24]. Still, it is possible that the gene is imprinted in humans and only the maternal allele is expressed. Alternatively, discrepancy between the human and the mouse model for dosage requirement could be taken into account, similarly to some other autosomal genes involved in sexual development. For instance, a heterozygous mutation of the SOX8 gene, encoding a transcriptional factor closely related to SRY (the other allele being the wild-type), caused a 46,XY DSD phenotype [27]. Meanwhile, the homozygous Sox8 knockout did not affect the sex determination process. Instead, it caused Sertoli cell function impairment in the adult, leading to infertility or decreased fertility in males [28]. Therefore, we assume that discrepancy between the phenotype of the patient carrying the FKBP4 heterozygous variant and a lack of the abnormal phenotype in heterozygous knockout in mice does not rule out a causative effect of the FKBP4 variant in the patient.
The six additional heterozygous variants identified in our patient in genes AKR1C4, CYP17A1, FREM2, IL17RD, NKD2, involved in sexual development, do not seem to be causative for the patient’s phenotype. Namely, three among these variants were predicted to be neutral (Table 2) and for that reason we did not further consider their influence on the patient’s phenotype. The other three variants of AKR1C4, CYP17A1 or FREM2 genes were predicted to be disease causing (Table 2). We consider the contribution of the AKR1C4 variant to the DSD phenotype, as heterozygous mutations of this gene have been described as potential modifier of the DSD phenotype [22]. The mutations of CYP17A1 and FREM2 genes instead caused DSD in karyotypic men only when both alleles of those genes were mutated [19,20,21], therefore the heterozygous status of CYP17A1 and FREM2 variants in our patient make their contribution to the DSD phenotype less likely. However, we cannot rule out the modifier effect of those variants. Thus, although the described patient is a compound heterozygote of the FREM2 gene, the variant inherited from the father was ruled out as causative due to the predicted neutral character. We assumed that the single FREM2 variant inherited from the mother, however, predicted as disease causing is not sufficient to cause the DSD phenotype as ambiguous genitalia in karyotypic men are present in case both FREM2 alleles are mutated [21]. Still there is possibility that the FREM2 variant might contribute to the patient’s phenotype.
Altogether, although we do not provide direct evidence for FKBP4 mutation causality, our findings justify placing the FKBP4 gene within a DSD target gene panel, specifically as an AIS candidate gene. The identification of more PAIS cases carrying a FKBP4 mutation will provide stronger confirmation for its implication in an androgen insensitivity phenotype and could contribute to the diagnosis of AIS patients lacking AR mutations.
4. Materials and Methods
4.1. Patient Description
A male patient, of Armenian origin, was born after full-term pregnancy (38 weeks of gestation) via vaginal delivery without complications. At birth, the patient manifested a scrotal form of hypospadias, small phallus (18 mm) with ventral chordae, bilateral cryptorchidism (testes inside the internal inguinal ring). The fourth degree of virilization was assessed according to the Prader scale. At the age of 6, the patient underwent surgery due to hypospadias, accompanied by orchiopexy. At the age of 10, a comprehensive examination, such as karyotyping, urological examinations (including testicle and pelvic ultrasound investigation), and hormonal analysis (including testosterone synthesis stimulation test) were performed. At the age of 13, urological examinations and the testosterone synthesis stimulation test were repeated. The patient expressed male psycho-sexual identity. His parents consented to endocrine and genetic studies.
4.2. Hormonal Analysis
At the age of 10, the patient was subjected to a complex hormonal examination. The levels of AMH, LH, FSH, TSH, PRL, DHEAS, cortisol, and progesterone were determined using the cobas e 411 instrument (Roche Diagnostics, Risch-Rotkreuz, Switzerland) and applying commercial kits (catalogue numbers: 6331076190, 11732234122, 11775863122, 11731459122, 3203093190, 3000087122, 6687733190, 7092539190 respectively (Roche Diagnostics, Risch-Rotkreuz, Switzerland). The efficiency of testosterone synthesis was examined upon 3-day testosterone stimulation with 1500 IU hCG (Pregnyl, Organon, India Ltd.) [29]. The hCG test was repeated at the age of 13.
4.3. DNA Isolation and Whole Exome Sequencing (WES)
Genomic DNA from the blood samples of the proband, his parents, and healthy brother was isolated by using the DNA mini kit (Qiagen, Hilden, Germany). Exome capture was performed on the proband and parents’ DNA samples by using the SureSelect Human All Exon v3 kit (Agilent Inc.®). Sequencing was performed on Illumina HiSeq 2000 device, and Fastq files were obtained using the Illumina CASAVA v1.8.1 software. The raw data were analyzed using our bioinformatic pipeline hosted on the Vital-IT Center of the Swiss Institute of Bioinformatics (SIB; [30]), as previously described [30]. WES data were analyzed using the VariantMaster software [31] in order to identify de novo variants, as well as variants with different Mendelian inheritance models (dominant with reduced penetrance, recessive or X-linked).
4.4. Sanger Sequencing
The mutation identified in the FKBP4 gene was validated by Sanger sequencing in the patient, as compared to his parents and the healthy brother. Exon 8 of FKBP4 gene, in which the mutation was identified, was amplified together with flanking intronic regions on DNA of the proband, his mother, father and brother, using FKBP4_E8_F:AACCTCTTGTGGCCATGTGT and FKBP4_E8_R:GTCACCAAGGGGAAGTTTCA primers. The obtained 386 bp amplicons were sequenced in both directions using the above primers, in separate reactions.
4.5. Bioinformatics
The data of the Genome Aggregation Database (gnomAD) [32], the 1000 genomes project (1000G) [33], and the Exome Aggregation Consortium (ExAC) Browser [34] were used to check for the variant allele frequency identified in genes involved in sexual development. The Meta-SNP integrating four algorithms (PANTHER, PhD-SNP, sorting intolerant from tolerant—SIFT and screening for non-acceptable polymorphisms—SNAP) [35], MutationTaster [36], and PolyPhen-2 [37] predictors of functional effect of SNP were used to predict the disease-causing variants identified in the genes involved in sexual development.
4.6. Accession Numbers
Accession numbers of Homo sapiens mRNAs used are: FKBP4 NM_002014.3, AKR1C4 NM_001818, CYP17A1 NM_000102, FREM2 NM_207361, IL17RD NM_017563, NKD2 NM_001271082. Accession numbers of vertebrate FKBP4 proteins used are: Homo sapiens NP_002005.1, Mus musculus NP_034349, Anolis carolinensis XP_008109533, Gallus gallus NP_001006250, Xenopus laevis NP_001084593, Danio rerio XP_005173673.
Acknowledgments
We would like to thank the late Anna Spik for contribution to investigation and project administration. We dedicate this work to her.
Abbreviations
| AR | androgen receptor |
| AIS | androgen insensitivity syndrome |
| PAIS | partial androgen insensitivity syndrome |
| CAIS | complete androgen insensitivity syndrome |
| DSD | disorder of sexual development |
| BMI | body mass index |
| AMH | anti-Müllerian hormone |
| LH | luteinizing hormone |
| FSH | follicle-stimulating hormone |
| TSH | thyroid-stimulating hormone |
| PRL | prolactin |
| DHEAS | dehydroepiandrosterone sulfate |
| hCG | human chorionic gonadotropin |
| WES | whole exome sequencing |
| TPR | tetratricopeptide repeat |
| PPIase | prolyl isomerase |
| SNP | single nucleotide polymorphism |
| FKBP4 | peptidyl-prolyl cis-trans isomerase FKBP4 |
| AKR1C4 | aldo-keto reductase family 1 member C4 |
| CYP17A1 | Steroid 17-alpha-hydroxylase/17,20 lyase |
| FREM2 | FRAS1-related extracellular matrix protein 2 |
| IL17RD | interleukin-17 receptor D |
| NKD2 | rotein naked cuticle homolog 2 |
| gnomAD | The Genome Aggregation Database |
| 1000G | The 1000 Genomes Project |
| ExAC | The Exome Aggregation Consortium |
| SIFT | sorting intolerant from tolerant |
| SNAP | screening for non-acceptable polymorphisms |
Author Contributions
Conceptualization, K.K.-Z., J.J., S.N.; data curation, R.M., P.S., B.J.S., M.P.S.; K.K.-Z.; formal analysis, S.N., J.J., K.K.-Z.; funding acquisition, J.J., S.N.; investigation, E.I., R.M., P.S., B.J.S., M.S., M.P.S., H.H., T.S., L.L., S.N., J.J., K.K.-Z.; methodology, E.I., R.M., P.S., B.J.S., M.S.; project administration, K.K.-Z.; resources, T.S., S.N., J.J.; software, P.S., B.J.S.; supervision, K.K.-Z., validation, E.I.; visualization, K.K.-Z., E.I., L.L.; writing—original draft, K.K.-Z.; writing—review and editing, E.I., R.M., P.S., B.J.S., M.S., M.P.S., H.H., L.L., S.N., J.J., K.K.-Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Swiss National Science Foundation, grant number SCOPES IZ73Z0_152347/1.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hughes I.A., Davies J.D., Bunch T.I., Pasterski V., Mastroyannopoulou K., MacDougall J. Androgen insensitivity syndrome. Lancet. 2012;380:1419–1428. doi: 10.1016/S0140-6736(12)60071-3. [DOI] [PubMed] [Google Scholar]
- 2.Murashima A., Kishigami S., Thomson A., Yamada G. Androgens and mammalian male reproductive tract development. Biochim. Biophys. Acta. 2015;1849:163–170. doi: 10.1016/j.bbagrm.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Shukla G.C., Plaga A.R., Shankar E., Gupta S. Androgen receptor-related diseases: What do we know? Andrology. 2016;4:366–381. doi: 10.1111/andr.12167. [DOI] [PubMed] [Google Scholar]
- 4.Davies T.H., Sanchez E.R. Fkbp52. Int. J. Biochem. Cell Biol. 2005;37:42–47. doi: 10.1016/j.biocel.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Cheung-Flynn J., Prapapanich V., Cox M.B., Riggs D.L., Suarez-Quian C., Smith D.F. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol. Endocrinol. 2005;19:1654–1666. doi: 10.1210/me.2005-0071. [DOI] [PubMed] [Google Scholar]
- 6.Nair S.C., Toran E.J., Rimerman R.A., Hjermstad S., Smithgall T.E., Smith D.F. A pathway of multi-chaperone interactions common to diverse regulatory proteins: Estrogen receptor, Fes tyrosine kinase, heat shock transcription factor Hsf1, and the aryl hydrocarbon receptor. Cell Stress Chaperones. 1996;1:237–250. doi: 10.1379/1466-1268(1996)001<0237:APOMCI>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Leon J.T., Iwai A., Feau C., Garcia Y., Balsiger H.A., Storer C.L., Suro R.M., Garza K.M., Lee S., Kim Y.S., et al. Targeting the regulation of androgen receptor signaling by the heat shock protein 90 cochaperone FKBP52 in prostate cancer cells. Proc. Natl. Acad. Sci. USA. 2011;108:11878–11883. doi: 10.1073/pnas.1105160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guy N.C., Garcia Y.A., Cox M.B. Therapeutic Targeting of the FKBP52 Co-Chaperone in Steroid Hormone Receptor-Regulated Physiology and Disease. Curr. Mol. Pharm. 2015;9:109–125. doi: 10.2174/1874467208666150519114115. [DOI] [PubMed] [Google Scholar]
- 9.Riggs D.L., Cox M.B., Tardif H.L., Hessling M., Buchner J., Smith D.F. Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling. Mol. Cell. Biol. 2007;27:8658–8669. doi: 10.1128/MCB.00985-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riggs D.L., Roberts P.J., Chirillo S.C., Cheung-Flynn J., Prapapanich V., Ratajczak T., Gaber R., Picard D., Smith D.F. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003;22:1158–1167. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottlieb B., Beitel L.K., Nadarajah A., Paliouras M., Trifiro M. The androgen receptor gene mutations database: 2012 update. Hum. Mutat. 2012;33:887–894. doi: 10.1002/humu.22046. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed S.F., Bashamboo A., Lucas-Herald A., McElreavey K. Understanding the genetic aetiology in patients with XY DSD. Br. Med. Bull. 2013;106:67–89. doi: 10.1093/bmb/ldt008. [DOI] [PubMed] [Google Scholar]
- 13.Hornig N.C., Ukat M., Schweikert H.U., Hiort O., Werner R., Drop S.L., Cools M., Hughes I.A., Audi L., Ahmed S.F., et al. Identification of an AR Mutation-Negative Class of Androgen Insensitivity by Determining Endogenous AR Activity. J. Clin. Endocrinol. Metab. 2016;101:4468–4477. doi: 10.1210/jc.2016-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogata T., Sano S., Nagata E., Kato F., Fukami M. MAMLD1 and 46,XY disorders of sex development. Semin. Reprod. Med. 2012;30:410–416. doi: 10.1055/s-0032-1324725. [DOI] [PubMed] [Google Scholar]
- 15.Tuhan H., Anik A., Catli G., Onay H., Aykut A., Abaci A., Bober E. A novel mutation in steroidogenic factor (SF1/NR5A1) gene in a patient with 46 XY DSD without adrenal insufficiency. Andrologia. 2017;49 doi: 10.1111/and.12589. [DOI] [PubMed] [Google Scholar]
- 16.Adachi M., Takayanagi R., Tomura A., Imasaki K., Kato S., Goto K., Yanase T., Ikuyama S., Nawata H. Androgen-insensitivity syndrome as a possible coactivator disease. N. Engl. J. Med. 2000;343:856–862. doi: 10.1056/NEJM200009213431205. [DOI] [PubMed] [Google Scholar]
- 17.Gulia C., Baldassarra S., Zangari A., Briganti V., Gigli S., Gaffi M., Signore F., Vallone C., Nucciotti R., Costantini F.M., et al. Androgen insensitivity syndrome. Eur. Rev. Med. Pharm. Sci. 2018;22:3873–3887. doi: 10.26355/eurrev_201806_15272. [DOI] [PubMed] [Google Scholar]
- 18.Babler E.K. Clinical Handbook of Pediatric Endocrinology. 2nd ed. Taylor and Francis India; New Delhi, India: 2014. [Google Scholar]
- 19.Biason-Lauber A., Leiberman E., Zachmann M. A single amino acid substitution in the putative redox partner-binding site of P450c17 as cause of isolated 17,20-lyase deficiency. J. Clin. Endocrinol. Metab. 1997;82:3807–3812. doi: 10.1210/jcem.82.11.4380. [DOI] [PubMed] [Google Scholar]
- 20.Geller D.H., Auchus R.J., Mendonca B.B., Miller W.L. The genetic and functional basis of isolated 17,20-lyase deficiency. Nat. Genet. 1997;17:201–205. doi: 10.1038/ng1097-201. [DOI] [PubMed] [Google Scholar]
- 21.Van Haelst M.M., Maiburg M., Baujat G., Jadeja S., Monti E., Bland E., Pearce K., Fraser Syndrome Collaboration G., Hennekam R.C., Scambler P.J. Molecular study of 33 families with Fraser syndrome new data and mutation review. Am. J. Med. Genet. A. 2008;146:2252–2257. doi: 10.1002/ajmg.a.32440. [DOI] [PubMed] [Google Scholar]
- 22.Fluck C.E., Meyer-Boni M., Pandey A.V., Kempna P., Miller W.L., Schoenle E.J., Biason-Lauber A. Why boys will be boys: Two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am. J. Hum. Genet. 2011;89:201–218. doi: 10.1016/j.ajhg.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miraoui H., Dwyer A.A., Sykiotis G.P., Plummer L., Chung W., Feng B., Beenken A., Clarke J., Pers T.H., Dworzynski P., et al. Mutations in FGF17, IL17RD, DUSP6, SPRY4, and FLRT3 are identified in individuals with congenital hypogonadotropic hypogonadism. Am. J. Hum. Genet. 2013;92:725–743. doi: 10.1016/j.ajhg.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yong W., Yang Z., Periyasamy S., Chen H., Yucel S., Li W., Lin L.Y., Wolf I.M., Cohn M.J., Baskin L.S., et al. Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J. Biol. Chem. 2007;282:5026–5036. doi: 10.1074/jbc.M609360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beleza-Meireles A., Barbaro M., Wedell A., Tohonen V., Nordenskjold A. Studies of a co-chaperone of the androgen receptor, FKBP52, as candidate for hypospadias. Reprod. Biol. Endocrinol. 2007;5:8. doi: 10.1186/1477-7827-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimamoto S., Kubota Y., Tokumitsu H., Kobayashi R. S100 proteins regulate the interaction of Hsp90 with Cyclophilin 40 and FKBP52 through their tetratricopeptide repeats. FEBS Lett. 2010;584:1119–1125. doi: 10.1016/j.febslet.2010.02.055. [DOI] [PubMed] [Google Scholar]
- 27.Portnoi M.F., Dumargne M.C., Rojo S., Witchel S.F., Duncan A.J., Eozenou C., Bignon-Topalovic J., Yatsenko S.A., Rajkovic A., Reyes-Mugica M., et al. Mutations involving the SRY-related gene SOX8 are associated with a spectrum of human reproductive anomalies. Hum. Mol. Genet. 2018;27:1228–1240. doi: 10.1093/hmg/ddy037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Bryan M.K., Takada S., Kennedy C.L., Scott G., Harada S., Ray M.K., Dai Q., Wilhelm D., De Kretser D.M., Eddy E.M., et al. Sox8 is a critical regulator of adult Sertoli cell function and male fertility. Dev. Biol. 2008;316:359–370. doi: 10.1016/j.ydbio.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes I.A., Deeb A. Androgen resistance. Best Pr. Res. Clin. Endocrinol. Metab. 2006;20:577–598. doi: 10.1016/j.beem.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Callier P., Calvel P., Matevossian A., Makrythanasis P., Bernard P., Kurosaka H., Vannier A., Thauvin-Robinet C., Borel C., Mazaud-Guittot S., et al. Loss of function mutation in the palmitoyl-transferase HHAT leads to syndromic 46,XY disorder of sex development by impeding Hedgehog protein palmitoylation and signaling. PLoS Genet. 2014;10:e1004340. doi: 10.1371/journal.pgen.1004340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santoni F.A., Makrythanasis P., Nikolaev S., Guipponi M., Robyr D., Bottani A., Antonarakis S.E. Simultaneous identification and prioritization of variants in familial, de novo, and somatic genetic disorders with VariantMaster. Genom. Res. 2014;24:349–355. doi: 10.1101/gr.163832.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alfoldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P., et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genomes Project C., Abecasis G.R., Auton A., Brooks L.D., DePristo M.A., Durbin R.M., Handsaker R.E., Kang H.M., Marth G.T., McVean G.A. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karczewski K.J., Weisburd B., Thomas B., Solomonson M., Ruderfer D.M., Kavanagh D., Hamamsy T., Lek M., Samocha K.E., Cummings B.B., et al. The ExAC browser: Displaying reference data information from over 60 000 exomes. Nucleic Acids Res. 2017;45:D840–D845. doi: 10.1093/nar/gkw971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capriotti E., Calabrese R., Fariselli P., Martelli P.L., Altman R.B., Casadio R. WS-SNPs&GO: A web server for predicting the deleterious effect of human protein variants using functional annotation. BMC Genom. 2013;14:S6. doi: 10.1186/1471-2164-14-S3-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarz J.M., Cooper D.N., Schuelke M., Seelow D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat. Methods. 2014;11:361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 37.Adzhubei I.A., Schmidt S., Peshkin L., Ramensky V.E., Gerasimova A., Bork P., Kondrashov A.S., Sunyaev S.R. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]