Supplemental Digital Content is available in the text.
Key Words: cirrhosis, palliative care, supportive care, communication
Background and Aims:
Liver health professionals have difficulty discussing liver cirrhosis and its prognosis with patients and families. Question Prompt Lists (QPLs), which are evidence-based lists of “recommended questions,” may improve communication but need to be designed specifically for the target population. This study aimed to develop and pilot a QPL for patients with cirrhosis.
Methods:
A mixed-methods design in 3 phases. In phase 1 (item generation), potential questions for inclusion in the QPL were identified from 3 sources—a scoping literature review; an online survey; and interviews with patients, family members, and health professionals. In phase 2 (QPL construction), a multidisciplinary expert panel finalized the selection of questions and the format of the QPL. In phase 3 (pilot study), the QPL was assessed for acceptability and feasibility in a hepatology outpatient clinic population.
Results:
From 258 topics initially identified, 30 questions were included in the first draft of the QPL. After review by a multidisciplinary expert panel including patients, the QPL was reduced to 22 questions. In the pilot study, 133/215 eligible patients consented to participate, although only 67/133 used the QPL in their clinic appointment. Among those who used the QPL, all questions were asked at least once. The most commonly asked question related to life expectancy. Most participants expressed support for the content of the QPL.
Conclusions:
A QPL, suitable for use in patients with liver cirrhosis attending hepatology outpatient clinics, has been developed and piloted. The QPL seems to be feasible to use and acceptable to patients and clinicians. Further work is needed to evaluate its effectiveness and to determine optimum delivery in clinical practice.
Liver health professionals (HPs) find it difficult to discuss the liver disease and its prognosis with patients and families,1–3 often not having these discussions with family members until the patient is in the last week of life. Simple approaches are needed to support professionals. Question Prompt Lists (QPLs) are evidence-based lists of “recommended questions” that are given to patients and family members, ahead of an outpatient consultation4 to improve communication.4 They are effective in improving patient participation during the consultation and in stimulating discussions about prognosis.5,6 Both patients and HPs find QPLs useful as a communication tool7 that can help patients to discuss difficult issues without interfering with the flow of the medical consultation.8 They increase the number of questions asked during consultations,5,9 which can lead staff to provide more information to patients.5 QPLs increase the level of patient recall of information and decrease anxiety at follow-up.5 The most effective QPLs have been specifically designed for their target population and are provided to patients before their consultation.9 Implementation works best if there is a clinical champion to support use within the team.4
The majority of studies investigating QPLs have involved people with cancer.9 While a few have been developed in other conditions,10,11 no QPL has yet been developed for people with cirrhosis.
The aim of this study was to develop and pilot a QPL to improve communication in outpatient consultations between patients with liver cirrhosis, their close family members and HPs. The objectives of the study were: (1) to develop a QPL for use in this population and; (2) to explore its acceptability and feasibility among patients, close family members, and HPs.
METHODS
There is no standard methodology for developing QPLs. We used a mixed-methods design based on the approach adopted by previous researchers.7,8,10,11 Development and refinement of the QPL, together with testing acceptability and feasibility was carried out in 3 phases (item generation; development and refinement of QPL; evaluation in a pilot study).
Phase 1—Generation of Potential Question Topics
Potential question topics for inclusion in the QPL were generated from 3 sources.
A scoping literature review of studies concerning the needs of patients with liver cirrhosis.12
An electronic survey of patients with the liver disease using Opinio software.13 The survey was sent to: the British Liver Trust, LiveRNORTH, PSC (Primary Sclerosing Cholangitis) Support, and the Hepatitis C Trust, who circulated an electronic link to their members’ mailing list. The survey asked participants to list those questions that they had asked, or had wanted to ask during medical consultations.
Semistructured interviews (individual or group) were conducted with patients with cirrhosis, close family members and HPs, identified from hepatology outpatient clinics of a liver transplant unit based in Southern England (June to December 2017). During each interview (conducted by S.D. or J.T.S.L.), participants were asked to list questions that they considered were important to ask during medical consultations, and about their perspectives about HP communication (Appendix I—topic guide, Supplemental Digital Content 1, http://links.lww.com/JCG/A555). All interviews were recorded using a digital voice recorder. Two researchers (J.T.S.L./S.D.) independently extracted a list of potential topics for inclusion in the QPL from each interview. All participants were given information about the study and gave their informed consent to take part.
Phase 2—Construction of the QPL
The lead researcher (J.T.S.L.) grouped the question topics identified in phase 1 into themes. Question topics covering the same or similar issues were merged. The number of potential question topics was reduced using a methodology similar to that used for the development and refinement of the quality of life measures.14,15 A multidisciplinary expert panel consisting of 4 liver specialists, 3 palliative care HPs, 3 academic palliative care researchers and 2 patient representatives further reduced the initial list of question topics to a more manageable number. Interviews were then conducted with 8 people with cirrhosis, 3 family members, and 3 liver clinicians. A focus group with 6 liver clinicians was also conducted. Participants were shown the draft QPL and were asked to comment on: its comprehensiveness; clarity; relevance; practical issues regarding its implementation; potential barriers to use; and additional resources that might be required to facilitate its introduction. The draft QPL was also sent to leads of voluntary organizations involved in phase 1 of the study to obtain their opinions about its content and format. The expert panel was then reconvened, the number of items was reduced and the QPL was revised (Appendix II, Supplemental Digital Content 1, http://links.lww.com/JCG/A555) ready for piloting.
Phase 3: Pilot Study
Eligible patients for the pilot study were adults, with a diagnosis of cirrhosis, attending selected clinics at a tertiary liver unit in Southern England and identified by their liver clinicians. Patients provided written informed consent and were given a copy of the QPL before their scheduled consultation. Participants were asked to identify up to 3 questions from the QPL that they wanted to ask during their appointment. After the consultation, patients were asked whether the QPL was used, and if so, which questions were asked. Those who did not use the QPL were asked why this was the case. Data were collected about participants’ age, gender, ethnicity, and cause of liver disease.
Semistructured interviews were conducted with a convenience sample of 10 study participants (8 of whom had used the QPL during their consultation and 2 of whom had not), 1 family member (non-QPL user), and 6 HPs (4 consultants and 2 clinical nurse specialists) working in clinics where the QPL was piloted. Patient and family member participants were approached at least 24 hours after initial consent was provided and were interviewed by telephone using a topic guide (Appendix III, Supplemental Digital Content 1, http://links.lww.com/JCG/A555) once further informed consent was obtained. Interviews were written-up by researchers contemporaneously.
Analytic Methods
A mixture of qualitative and quantitative analytic methods were used for analysis. Descriptive statistics were used to describe demographic data for participants in the pilot QPL study and in the qualitative studies. Interviews and focus groups were subjected to thematic content analysis, and a frequency count was conducted on themes identified.
Ethics
Approval for different aspects of the study were obtained from UCL REC (3552/003) and Yorkshire & The Humber-Sheffield Research Ethics Committee (17/YH/0042).
RESULTS
Phase 1—Generation of Potential Question Topics
Detailed results from the systematic literature review have been published elsewhere.12 In summary, 19 research studies were identified, involving 1413 patients, 31 family carers, and 733 HPs. From these papers, 18 potential question topics were identified (Appendix iv, Supplemental Digital Content 1, http://links.lww.com/JCG/A555).
The online survey was completed by 278 respondents. However, only those responses (n=78) from patients identified with liver cirrhosis or liver failure were used to generate potential questions for the QPL. Respondents were predominately white UK females, aged 45 to 64 years, diagnosed with autoimmune conditions (Table 1). From these responses, 165 potential question topics were identified (Appendix iv, Supplemental Digital Content 1, http://links.lww.com/JCG/A555).
TABLE 1.
Participant Demographic Details—Online Survey, Semistructured Interviews (Phase 1)
| Online Survey | n (%) |
|---|---|
| Patient | N=78 |
| Age range (y) | |
| 18-34 | 4 (5) |
| 35-44 | 8 (10) |
| 45-54 | 19 (25) |
| 55-64 | 28 (36) |
| 65-74 | 15 (19) |
| >75 | 4 (5) |
| Ethnicity | |
| White—UK | 71 (91) |
| White—other | 6 (7.5) |
| Mixed—white UK/African | 1 (1.5) |
| Gender | |
| Male | 27 (35) |
| Female | 51 (65) |
| Cause of cirrhosis | |
| Autoimmune (PBC, AIH, PSC) | 42 (54) |
| Nonalcohol fatty liver disease | 14 (18) |
| Alcohol-related liver disease | 10 (13) |
| Viral (HBV/HCV) | 3 (4) |
| Other (hemochromatosis, Budd-Chiari, SCS, cryptogenic) | 5 (6) |
| Unknown | 4 (5) |
| Semistructured Interviews | n (%) |
| Patient | N=12 |
| Age | |
| Mean (SD) | 51 (11) |
| Minimum-maximum | 31-69 |
| Gender | |
| Male | 8 (67) |
| Female | 4 (33) |
| Ethnicity | |
| White—UK | 7 (58) |
| White—other | 3 (25) |
| South Asian | 1 (7.5) |
| Other | 1 (7.5) |
| Cause of cirrhosis | |
| Alcohol-related liver disease | 6 (50) |
| NASH/NAFLD | 2 (50) |
| Hepatitis C | 1 (3) |
| Unknown | 3 |
| Close family member | N=6 |
| Relationship status | |
| Parent | 3 |
| Son | 2 |
| Spouse | 1 |
| Gender | |
| Male | 3 (50) |
| Female | 3 (50) |
| Ethnicity | |
| White—UK | 6 (100) |
| Cause of patient cirrhosis | |
| Alcohol-related liver disease | 3 (50) |
| NASH | 1 (17) |
| Unknown | 2 (33) |
| Health professional | |
| Profession | |
| Consultant hepatologist | 5 |
| Registrar/clinical fellow | 2 |
| Transplant nurse coordinators | 4 |
| Clinical nurse specialist | 1 |
| Alcohol liaison nurse | 1 |
| Dietician | 1 |
| Gender | |
| Male | 6 (43) |
| Female | 8 (57) |
| Ethnicity | |
| White—UK | 12 (86) |
| Black—UK | 1 (12) |
| South Asian | 1 (12) |
AIH indicates autoimmune hepatitis; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; SCS, secondary bilary cirrhosis.
Semistructured interviews were conducted with 12 patients with cirrhosis [mainly male, median age: 51 y with a diagnosis of alcohol-related liver disease (ARLD)], 6 close family members (mainly fathers or sons) and 14 HPs (7 doctors, 6 nurses, and 1 allied health professionals). These generated a further 78 question topics (Appendix iv, Supplemental Digital Content 1, http://links.lww.com/JCG/A555).
Thus, 258 potential question topics were identified. After merging similar issues together and removing duplicates, a reduced list of 82 question topics was constructed. Topics with similar themes were grouped into the following categories: symptom issues; understanding my liver disease; management of my liver disease test/scan results; medication; the impact of liver disease on my life; progression of liver disease, and life expectancy; transplant issues.
Phase 2—Construction of the QPL
At the initial expert panel meeting, 82 potential question topics were converted into 30 questions for inclusion in a first draft of the QPL. This number of questions is in line with the size of previously developed QPLs.11 These questions initially included 6 that were specifically relevant to transplant patients. Patients, family members, and users/health professionals associated with voluntary liver organizations considered the content of the QPL to be reasonably comprehensive but suggested further questions, in particular concerning diet. Clinicians were supportive of the draft QPL, but thought it contained many generic questions, which were impractical to answer in the time allocated for an outpatient appointment. Clinicians advised removing questions that they felt could more easily be addressed using other resources (eg, factsheets) rather than requiring a personal response (eg, what is cirrhosis?). Clinicians also advised removal of transplant questions, as they were not considered to be relevant to most patients and may cause upset for those patients in whom a transplant was not indicated.
The expert panel was then reconvened and produced a final version of the QPL, containing 22 questions and links/advice about sources of information and points of contact for support (Appendix I—finHPal version QPL, Supplemental Digital Content 1, http://links.lww.com/JCG/A555).
Phase 3—Pilot Study
Between December 2018 and March 2019, 808 patients at 64 liver outpatient clinics were screened for inclusion in the study. A total of 215 patients were considered eligible (on screening of notes) of whom 133 were approached by researchers and gave consent to participate. Eighty-two patients from the list either did not attend or were not interested in participating. Participants were predominantly white UK males with a mean age of 62 years and mainly had a diagnosis of ARLD or nonalcoholic steatohepatitis (Table 2—demographic details). Patients who used or did not use the QPL had similar features in terms of gender, ethnicity, and age, but those who used the QPL were less likely to have a diagnosis of ARLD.
TABLE 2.
Participant Demographic Detail—Pilot Study (Phase 3)
| n (%) | |
|---|---|
| Total sample | N=133 |
| Age | |
| Mean (SD) | 62 (12) |
| Minimum-maximum | 27-85 |
| Gender | |
| Male | 77 (62) |
| Female | 47 (38) |
| Ethnicity | |
| White—UK | 71 (57) |
| White—other | 19 (15) |
| South Asian | 17 (14) |
| Other | 8 (6) |
| Missing | |
| Cause of cirrhosis where recorded | N=125 |
| ARLD | 63 (47) |
| NASH/NAFLD | 21 (16) |
| Autoimmune (PSC/PBC/AIH) | 18 (14) |
| Viral (HBV/HCV) | 12 (9) |
| HCC | 1 (1) |
| Cryptogenic | 2 (2) |
| Portal hyper/HV+SV thrombosis | 3 (2) |
| Mixed | 5 (4) |
| Missing | 8 (6) |
| QPL users | N=67 |
| Age | |
| Mean (SD) | 63 (10) |
| Minimum-maximum | 35-85 |
| Gender | |
| Male | 43 (64) |
| Female | 24 (36) |
| Ethnicity | |
| White—UK | 37 (55) |
| White—European | 11 (17) |
| South Asian | 12 (18) |
| Other | 3 (4) |
| Missing | 4 (6) |
| Cause of cirrhosis | |
| ARLD | 25 (37) |
| NASH/NAFLD | 13 (19) |
| Viral (HBV/HCV) | 9 (13) |
| Autoimmune (PSC/PBC/AIH) | 11 (16) |
| HCC | 1 (1) |
| Heme/ARLD | 1 (1) |
| NASH/ARLD | 2 (3) |
| HV thrombosis | 1 (1) |
| Missing | 4 (6) |
AIH indicates autoimmune hepatitis; ARLD, alcohol-related liver disease; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HV, hepatic vein; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; QPL, Question Prompt List; SV, splenic vein.
Of 133 participants, 67 both read and used the QPL during their medical consultation, 54 did not use it and 12 participants left the hospital after their outpatient consultation before researchers were able to interview them (Fig. 1). Among those who used the QPL, all questions were asked at least once (Table 1). The most frequently asked question was, “What is my life expectancy?” Requests were made by at least 10 participants to be referred to a local charity, which offers help with practical and social issues (Table 3).
FIGURE 1.
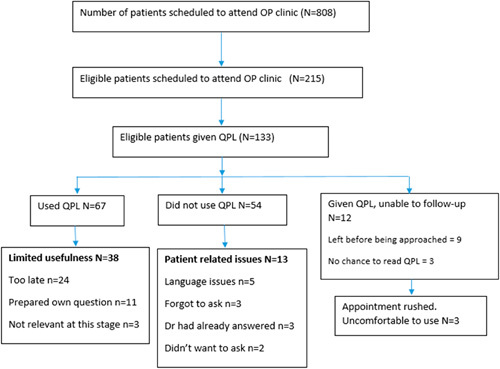
Patient recruitment and use of QPL. OP indicates outpatient; QPL, Question Prompt List.
TABLE 3.
Frequency of Questions Asked From the Question Prompt List (n=66)
| Questions Asked | Total |
|---|---|
| What is my life expectancy | 21 |
| What symptoms should I look out for? | 19 |
| What is my treatment plan (short term/long term)? | 19 |
| Is there any special diet that I shall follow? | 12 |
| How can I improve my liver function? | 12 |
| Can I travel abroad? | 12 |
| What is the side effect of my medication | 10 |
| What can I expect from the future? | 10 |
| Support hub | 10 |
| What do my test results mean? | 9 |
| Can I have occasional alcohol/low alcohol? | 8 |
| What should I do if symptoms occur? | 7 |
| What is cirrhosis | 6 |
| Can I drive? | 6 |
| What is liver disease | 5 |
| I am feeling worried/low. Where can I get help? | 5 |
| Will liver disease affect my ability to work? | 4 |
| What is my liver medication trying to achieve | 4 |
| What is my liver medication trying to achieve? | 4 |
| I am concerned about my sex life | 4 |
| Can you review my liver medication? | 4 |
| Why do I have liver disease | 3 |
| Wills | 1 |
| Will liver cancer spread to other parts of the body? | 1 |
| What is the cause of liver disease | 1 |
| What are the success of the operation | 1 |
| Is she going to develop HE again? | 1 |
| How quick would her deterioration be at the EOL? | 1 |
| Help at home | 1 |
| Effects on breathing difficulties | 1 |
| Carvedhiol supply | 1 |
| Can she get some bereavement support or counseling? | 1 |
| Effects of other medication on liver? | 1 |
EOL indicates end of life; HE, hepatic encephalopathy.
Patient Experience With the QPL
A thematic content analysis of the open comments made in feedback by participants suggested that the QPL’s main benefit was to act as a general prompt/reminder (n=20). In some cases, the QPL acted as a specific prompt for issues such as life expectancy (n=4), travel (n=2), or work (n=1). Some participants felt that it helped in organizing questions (n=4) or in generating new questions (n=3). A few stated that it had helped legitimize asking questions to their doctor.
Detailed interviews with a subsample of participants showed that they were positive about the QPL. They found it easy to use, well-structured and with questions clearly presented. One participant felt that they had not had adequate time to consider the QPL before their consultation. All participants felt that they would have no problems in asking many of the questions on the QPL, except for the question on “life expectancy,” about which 3 participants expressed some reluctance. Two participants indicated that they would be fearful of asking this question, although 1 had said that he would have asked this question sooner if he had been given the QPL earlier. One felt that, since he had asked his allotted 3 questions, and because the clinic was busy, his additional questions could wait for another occasion.
Study participants who did not use the QPL during their consultation felt that it only had limited usefulness for them (38/55). Most (n=24) felt that the QPL had come too late for themselves, having lived with the disease for several years. During this time, attendance at repeated clinic appointments had provided plenty of opportunities to ask doctors the questions that they wanted an answer, although they reflected that they may have benefitted from the QPL if it had been given at an earlier point in their illness. Some participants who did not use the QPL (n=11) felt they were able to prepare their own questions without needing a communication prompt. Three felt that QPL items were not relevant to them or their specific liver disease. Five participants had limited English language skills so were unable to use the QPL, but wanted to take it home to be translated by family members. Three had expressed a desire to use the QPL in their appointment and had identified questions to ask, but had forgotten to ask these questions. Three had said that the HP had answered the questions they had wanted to ask and therefore they did not need to use the QPL. Two participants had decided that they did not want to use the QPL. Finally, 3 participants said that although they were planning to use the QPL, their appointment was running late and they felt rushed, which in turn made them uncomfortable asking their clinician any additional questions.
Clinician Experience With the QPL
The 6 clinicians interviewed (2 clinical nurse specialists, 4 consultant hepatologists) identified no problems with using the QPL in their clinics, although 1 consultant suggested that it would have more use in a nurse-led clinic as they have more time in their appointments to answer questions. Clinicians highlighted several potential benefits of the QPL, such as empowering patients to ask questions and making them more aware of existing support services and sources of information. Some clinicians felt that newly referred patients or those recently given a diagnosis of cirrhosis were the ideal patient group in whom to use the QPL, whereas another thought it should be given to all patients with liver disease. Some clinicians felt that the QPL would be less beneficial to patients on the transplant waiting list or those who had been regularly attending the clinic for a long period.
Suggestions for Improving the Delivery of the QPL in Clinical Practice
Findings from QPL users, QPL nonusers and HPs highlighted that careful consideration would be required about how best to deliver the QPL were it to be implemented more widely. Most participants appreciated receiving the QPL while waiting for their appointment as this provided adequate time to decide which questions to ask. Some patients suggested that it might be preferable to receive the QPL by post, before their appointment, as it would have provided them with an opportunity to discuss questions with significant others and to have more time to consider their question selection. One clinician felt that it was important to have access to information leaflets so that they could provide patients with written material because there may not be adequate time to answer the questions verbally during the appointment.
DISCUSSION
This is the first study to develop and pilot a specific QPL for patients with cirrhosis to address their informational needs. As with previous studies, our findings demonstrate the feasibility and acceptability of using this type of communication aid in secondary care.10,11 Our results show the potential of the QPL to improve communication between patients and liver HPs and to increase patient participation in clinical consultations. Patients felt that the QPL empowered them to ask questions and clinicians recognized the need to respond to patients’ queries. As with previous studies,4 we found that patients not using the QPL, said that they may have done so if it had been given at an earlier stage in their illness. Our study highlighted specific areas in the care of cirrhosis patients where information was lacking particularly concerning the progression of liver disease and life expectancy.
Although our study showed the acceptability and utility of the QPL in increasing patients’ capacity to ask relevant questions, our findings did not specifically explore how the QPL should be implemented in clinical practice. In keeping with previous research,9 we found that most patients and clinicians felt that providing the QPL to patients on their arrival to the clinic provided sufficient time for it to be considered before the consultation. Nonetheless, some patients forgot to use the QPL. Significant memory problems are a particular problem for patients with cirrhosis, particularly those with hepatic encephalopathy16 so clinicians may need to prompt patients to use the QPL during their appointments.
Clinicians need to be aware that differences in ethnicity, gender, or other cultural factors between themselves and their patients may present barriers to asking difficult questions. Clinicians also need to be aware that patients’ willingness to use the QPL may be influenced by the cause or severity of their liver disease. This is illustrated by our finding that patients with ARLD were less likely to use the QPL. The use of the QPL may also be affected by patients’ fluency in understanding written English.
Current evidence suggest that the provision of information increases if patients use a QPL during their consultations. However, there is less evidence about whether QPLs improve patient recall, reduce anxiety or improve satisfaction.9 Further research will be required to evaluate the impact of the QPL on other relevant patient outcomes such as patient empowerment17 or satisfaction with the quality of health communication.18
Future research would also need to consider the health-economic impact of the QPL. Increased consultation times have a financial cost in both publically and privately funded health care systems. In the latter, it may be necessary to develop time-dependent billing codes to take into account any resulting additional consultation time.
Study Strengths
The QPL was developed using the perspectives of all relevant stakeholders, including patients, their family members, and HPs. Our expert panel consisted of a multidisciplinary team of academic and clinical palliative care and hepatology researchers and 2 “experts by experience,” who ensured that the development of the QPL took into account patients’ perspectives. Development of the QPL used robust mixed methodology, incorporating an iterative process with several cycles of the patient, carer, and clinician feedback.
Study Limitations
This study took place in a large, tertiary referral, liver unit and so its findings may not be transferable to other settings. Another limitation of our study was that, because of resource and practical constraints, we were only able to produce the QPL in an English language version. Since the QPL was evaluated as part of a research study, participants were actively screened for suitability for inclusion and were supported by the research team to use the QPL before their consultation. This additional support is unlikely to be available if the QPL were to be adopted for use in clinical practice unless additional resources were to be made available.
Implications for Practice and Future Research
Subject to local modification and evaluation we believe that the QPL would be appropriate for use with patients with liver cirrhosis caused by any etiology in other liver outpatient clinics. Our results suggest that the QPL may be more useful for patients recently diagnosed with cirrhosis and during their first few visits to the clinic. It would be important for future studies to include a health-economic analysis and validated patient-reported outcomes to assess the overall costs and benefits of introducing the QPL into routine practice. The QPL was developed in a predominately white UK population, so further studies are needed to validate the tool in populations from different ethnicities and countries, to ensure that the QPL is culturally appropriate.
CONCLUSIONS
We have developed a brief QPL, which is acceptable and feasible to use in patients with liver cirrhosis attending hepatology outpatient clinics in secondary care. Further work is needed to define the best way of delivering and evaluating this communication aid.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jcge.com.
ACKNOWLEDGMENTS
The authors acknowledge the input of Dr Federico Riccardio in constructing both the online survey and the associated database. They also acknowledge the following organizations for their involvement in sending out invitations to their members for the online survey: the British Liver Trust, PSC Support, LiveRNORTH, and the Hepatitis C Trust. The authors acknowledge the valuable contribution of our Patient Public Involvement representative, Janet Walsh, and Perry Gibbs. The also acknowledge the University of Adger (Norway) in allowing Professor Gudrun Rohde to do her sabbatical as an Affiliate Academic with the Marie Curie Palliative Care Research Department, University College London, during the period January-June 2017. Professor Rohde was instrumental in identifying the articles used to select items for the development of the QPL. The authors acknowledge the input of Dr Bella Vivat and Elena Marcus with their methodological advice with developing the QPL. They also acknowledge the support of the UCLH BRC (Biomedical Research Centre). Most importantly, the authors acknowledge all the participants (service users, their family members, and HPs) who took part in various parts of this research study.
Footnotes
J.T.S.L., S.D., L.G., R.C., J.W., A.M., D.T., and P.S.: responsible for the study concept and design. J.T.S.L., S.D., L.G., J.-L.C., and C.C.: responsible to the acquisition of the data. J.T.S.L., S.D., L.G., C.C., R.C., J.W., J.-L.C., A.M., D.T., and P.S.: responsible for analysis or interpretation of the data. J.T.S.L.: drafted the initial manuscript. S.D., P.S., L.G., C.C., R.C., J.W., J.-L.C,. A.M., and D.T.: revised the manuscript critically for important intellectual content.
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors, but Research Department responsible for conducting this study is provided core funding by Marie Curie (Grant reference: MCCC-FPO-16-U). P.S., J.T.S.L. and S.D.’s post is supported by the Marie Curie core and programme grant funding (grants MCCC-FCO-16-U and MCCC-FPO-16-U). The funder played no role in the collection, analysis, and interpretation of data, in the writing of the report; and in the decision to submit the article for publication.
The authors declare that they have nothing to disclose.
REFERENCES
- 1.Low J, Davis S, Vickerstaff V, et al. Advanced chronic liver disease in the last year of life: a mixed methods study to understand how care in a specialist liver unit could be improved. BMJ Open. 2017;7:e016887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimbell B, Boyd K, Kendall M, et al. Managing uncertainty in advanced liver disease: a qualitative, multiperspective, serial interview study. BMJ Open. 2015;5:e009241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hudson B, Hunt V, Waylen A, et al. The incompatibility of healthcare services and end-of-life needs in advanced liver disease: a qualitative interview study of patients and bereaved carers. Palliat Med. 2018;32:908–918. [DOI] [PubMed] [Google Scholar]
- 4.Dimoska A, Butow PN, Lynch J, et al. Implementing patient question-prompt lists into routine cancer care. Patient Educ Couns. 2012;86:252–258. [DOI] [PubMed] [Google Scholar]
- 5.Brandes K, Linn AJ, Butow PN, et al. The characteristics and effectiveness of Question Prompt List interventions in oncology: a systematic review of the literature. Psychooncology. 2015;24:245–252. [DOI] [PubMed] [Google Scholar]
- 6.Brandes K, Butow PN, Tattersall MHN, et al. Advanced cancer patients’ and caregivers’ use of a Question Prompt List. Patient Educ Couns. 2014;97:30–37. [DOI] [PubMed] [Google Scholar]
- 7.Eggly S, Tkatch R, Penner LA, et al. Development of a question prompt list as a communication intervention to reduce racial disparities in cancer treatment. J Cancer Educ. 2013;28:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayton J, Butow P, Tattersall M, et al. Asking questions can help: development and preliminary evaluation of a question prompt list for palliative care patients. Br J Cancer. 2003;89:2069–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sansoni JE, Grootemaat P, Duncan C. Question Prompt Lists in health consultations: a review. Patient Educ Couns. 2015;98:1454–1464. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed R, Raynor DK, McCaffery KJ, et al. The design and user-testing of a question prompt list for attention-deficit/hyperactivity disorder. BMJ Open. 2014;4:e006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lederer S, Fischer MJ, Gordon HS, et al. A question prompt sheet for adult patients with chronic kidney disease. BMC Nephrol. 2016;17:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Low JT, Rohde G, Pittordou K, et al. Supportive and palliative care in people with cirrhosis: International systematic review of the perspective of patients, family members and health professionals. 2018;69:1260–1273. [DOI] [PubMed] [Google Scholar]
- 13.Objectmanager. Opinio v7.11; 2018. Available at: www.objectplanet.com/opinio/. Accessed April 26, 2018.
- 14.Kavadas V, Blazeby J, Conroy T, et al. Development of an EORTC disease-specific quality of life questionnaire for use in patients with liver metastases from colorectal cancer. Eur J Cancer. 2003;39:1259–1263. [DOI] [PubMed] [Google Scholar]
- 15.Heffernan N, Cella D, Webster K, et al. Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy–hepatobiliary questionnaire. J Clin Oncol. 2002;20:2229–2239. [DOI] [PubMed] [Google Scholar]
- 16.Weissenborn K, Giewekemeyer K, Heidenreich S, et al. Attention, memory, and cognitive function in hepatic encephalopathy. Metab Brain Dis. 2005;20:359–367. [DOI] [PubMed] [Google Scholar]
- 17.Werbrouck A, Swinnen E, Kerckhofs E, et al. How to empower patients? A systematic review and meta-analysis. Transl Behav Med. 2018;8:660–674. [DOI] [PubMed] [Google Scholar]
- 18.Arraras JI, Wintner LM, Sztankay M, et al. EORTC QLQ-COMU26: a questionnaire for the assessment of communication between patients and professionals. Phase III of the module development in ten countries. Support Care Cancer. 2017;25:1485–1494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jcge.com.


