Abstract
Tumor-specific Tc9 cells exhibit an excellent antitumor potential in tumor immunotherapy. Identification of factors that contribute to Tc9-cell differentiation may have important clinical significance. In this study, we found that tumor necrosis factor (TNF)-α promotes Tc9 differentiation in vitro, and the TNF-α-induced Tc9 cells display enhanced cell survival and cell proliferation. More importantly, the TNF-α-induced tumor-specific Tc9 cells have increased antitumor capabilities in vivo. TNF-α activates its downstream signaling through 2 cell surface receptors, TNFR1 and TNFR2. In this study, we found that TNF-α promotes Tc9-cell differentiation through TNFR2, but not TNFR1. Furthermore, we found that TNF-α-TNFR2 activates STAT5 and nuclear factor-κB signaling during Tc9-cell differentiation. Blocking STAT5 or nuclear factor-κB by their specific inhibitors partially abrogates TNF-α-induced promotion of Tc9-cell differentiation. Thus, our study demonstrated TNF-α as a potent stimulator of Tc9-cell differentiation and may have important clinical implications.
Key Words: TNF-α, Tc9, TNFR2, STAT5
Adoptive cell therapy (ACT) using tumor-specific T cells has shown significant clinical promises for the treatment of patients with melanoma1; however, complete responses are infrequent, and most other tumors are refractory to ACT.2,3 The antitumor efficacy mediated by ACT is dependent on the cytolytic activity and the in vivo persistence of the transferred tumor-specific effector T cells.1,4 Tumor-specific CD8+ cytotoxic T lymphocytes (CTLs or Tc1 cells) have direct cytolytic activities against tumor cells and are the leading candidate used in ACT of cancers.5 Nevertheless, CTLs display a terminally differentiated phenotype, which leads to a short-term persistence in vivo and limited clinical response in tumor therapy.6,7 Therefore, identification of new classes of effector T cells with enhanced antitumor capability in tumor immunotherapy is urgently needed.
Tumor-specific Tc9 cells are an attractive effector cell candidate for adoptive therapy of cancers. Tc9 cells are a new subset of CD8+ T cells characterized by the secretion of interleukin (IL) 9.8 Similar as Th9 cells, Tc9 cells can be generated by culturing naive CD8+ T cells with the cytokines IL-4 and transforming growth factor (TGF)-β in vitro.8 Tc9 cells display a less exhausted phenotype and less cytolytic capabilities in vitro than Tc1 cells; however, Tc9 cells have a longer in vivo persistence than Tc1 cells.9 In addition, Tc9 cells can differentiate into cytolytic Tc1-like effector cells in vivo.8 Furthermore, tumor-specific Tc9 cells can induce much higher therapeutic efficacy than Tc1 cells in mouse tumor models.8 Therefore, identifying factors that can stimulate Tc9 cell development may have important clinical significance. As the Th9 and Tc9 cells have similar polarizing conditions, factors that can stimulate Th9-cell differentiation may also drive Tc9 differentiation.
Recent investigations showed that cytokines from the tumor necrosis factor (TNF) family, such as OX40L, TL1A, and GITRL, drives Th9-cell differentiation.10–13 We found that TNF-α also promotes Th9-cell differentiation, and the TNF-α-induced tumor-specific Th9 cells display increased antitumor efficacy in mouse tumor models.14 TNF-α is recognized as a pleiotropic cytokine in inflammation, apoptosis, and immune system development.15,16 It also plays a crucial role in the antitumor immunity through promoting the proliferation and differentiation of immune cells.17–20 TNF-α exerts these functions by binding 2 cell surface receptors, TNFR1 and TNFR2, which are both expressed by T cells.21
In this study, we showed that the addition of TNF-α promotes Tc9-cell differentiation and the TNF-α-induced Tc9 cells show increased cell survival and cell proliferation. In addition, the TNF-α-induced tumor-specific Tc9 cells display increased cytotoxic activities against melanoma tumor cells in vivo. Furthermore, our data indicated that TNF-α stimulates Tc9-cell differentiation through TNFR2-dependent signaling. Our results identify TNF-α as a powerful inducer of Tc9 cells and may have important clinical implications.
MATERIALS AND METHODS
Mice and Cell Lines
C57BL/6 (H-2b) mice and OT-I [C57BL/6-Tg (TcraTcrb)1100Mjbn/J] mice were purchased from the Jackson Laboratory and bred in-house at The First Hospital Animal Center of Jilin University. Mice were used in experiments at 6–8 weeks of age. All animal experimental procedures were conducted according to the ethical guidelines of the Animal Ethical Committee of First Hospital of Jilin University.
B16 and B16-OVA melanoma cell lines were purchased from ATCC (Rockville, MD) and were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 100 U/mL penicillin (Invitrogen), and 100 mg/mL streptomycin (Invitrogen). Cells were grown in standard (37°C, 5% CO2) culture incubators.
Reagents and Antibodies
Anti-mouse CD3e and CD28 antibodies [monoclonal antibodies (mAbs)] were purchased from eBiosciences. Recombinant mouse IL-4, TNF-α, and human TGF-β were purchased from R&D Systems. Anti-TNFR1 (Monoclonal Hamster IgG Clone # 55R170) and anti-TNFR2 (Monoclonal Hamster IgG Clone # TR7589.29) blocking antibodies and control immunoglobulin G were purchased from R&D Systems. STAT5 inhibitor was purchased from Santa Cruz. Nuclear factor-κB (NF-κB) inhibitors bortezomib, CAS 213546-53-3, and JSH-23 were purchased from Selleckchem. CFSE (carboxyl-fluorescein diacetate, succinimidyl ester) was purchased from Invitrogen. The OVA (257–264, SII NFE KL) peptide used in the OT-I mouse model was purchased from GL Biochem (Shanghai) Ltd.
In Vitro Tc9-Cell differentiation
Mouse-naive CD8+ T cells (CD8+CD25−CD62Lhi) were isolated from spleen cells by fluorescence activated cell sorting, as described previously.8,22 Naive CD8+ T cells were cultured at 1×105/well in the presence of plate-bound anti-CD3 (2 μg/mL), anti-CD28 (2 μg/mL), TGF-β (3 ng/mL), and IL-4 (10 ng/mL) with or without the addition of TNF-α (50 ng/mL). Cells from cultures without the addition of TGF-β and IL-4 were used as Tc0 cells. Cells were cultured for 2 days and analyzed by flow cytometry and/or quantitative polymerase chain reaction (qPCR).
To determine the role of TNFR1 and TNFR2 in TNF-α-induced Tc9-cell differentiation, naive CD8+ T cells were cultured under Tc9-cell polarization conditions with or without the addition of TNF-α for 2 days. Anti-TNFR1 (50 μg/mL), anti-TNFR2 (50 μg/mL), or control immunoglobulin G (50 μg/mL) were added in cell cultures. Cells were harvested and analyzed by flow cytometry and/or qPCR.
To explore the signaling pathways involved in TNF-α-induced Tc9-cell differentiation, naive CD8+ T cells were cultured at Tc9 polarization condition with or without the addition of TNF-α. STAT5 inhibitors (10 μg/mL), bortezomib (5 nM), CAS 213546-53-3 (2 μg/mL), JSH-23 (30 μM), or dimethyl sulfoxide as controls were added in the cell cultures. Cells were cultured for 2 days and analyzed by flow cytometry and/or qPCR.
qPCR
qPCR was performed, as described previously.23 Primer sets for Il9, Ifng, Il4, Il5, Il13, Il17a, Sfpi1, Irf4, Tbx21, Gata3, Foxp3, Rorc, Il2, Gzmb, and Prf1 were shown in the previous publications.23,24
Flow Cytometry
Flow cytometry was performed, as described previously.24 Cells were acquired and analyzed by a BD LSRFortessa cytometer. Fluorescence-conjugated mAbs against murine CD8, CD25, CD44, and CD62L were purchased from BD Biosciences. FITC-Annexin V was purchased from BD Biosciences. APC-conjugated mAb against IL-9 was purchased from Biolegend.
Western Blots
Western blot analysis was performed, as described previously.24 Anti-mouse phosphorylated STAT5 (pSTAT5), pSTAT6, pIKKα/β, Bax, Bcl2, Caspase3, cleaved caspase3, Bim, p50, p65, and β-actin were purchased from Cell Signaling Technology (CST).
Adoptive Tumor Immunotherapy
B16-OVA cells (2×105 cells/mouse) were injected subcutaneously into C57BL/6 mice. Tc9 or TNF-α-induced Tc9 cells were generated in the cultures for 2 days. On day 5 after tumor challenge, the B16-OVA tumor-bearing mice were randomly divided into 3 groups (5 mice/group) and treated with Tc9 or TNF-α-induced Tc9 cells by tail vein injection (1×106 cells/mouse). Mice treated by phosphate-buffered saline (PBS) served as controls. Tumor growth was monitored by caliper measurement. Mice were sacrificed when the tumor diameter reached the range between 1.5 and 2 cm. Tumor volume was calculated by the following formula: 3.14×(mean diameter)3/6.
In Vivo CTL Assay
To explore the cytotoxicity of TNF-α-induced Tc9 cells in vivo, naive CD8+ T cells isolated from OT-I mice were cultured under Tc9 polarization conditions in the presence or absence of TNF-α for 2 days. Spleen cells from C57BL/6 mice were pulsed with OT-I OVA peptide in the culture for 2 hours. Cells were labeled with high concentration of CFSE (5 μM) and used as target cells (CFSEhi). Unpulsed spleen cells were labeled with low concentration of CFSE (0.5 μM) and used as nontarget cells (CFSElo). CFSEhi target cells and CFSElo nontarget cells were mixed at 1:1 ratio, and a total of 1×107 cells per mouse were infused into C57BL/6 mice through the tail vein with Tc9 (5×106 cells/mouse) or TNF-α-induced Tc9 (5×106 cells/mouse) cells. Each group contained 5 mice. Six hours after cell injection, mice were sacrificed, and splenocytes were collected and analyzed by flow cytometry.
Statistical Analysis
The Student t test (2 groups) and analysis of variance (≥3 groups) were used to compare various experimental groups. A P-value<0.05 was considered significant.
RESULTS
TNF-α Increases the Induction of Tc9 Cells In Vitro
To explore the role of TNF-α in Tc9-cell differentiation, naive CD8+ T cells were cultured under the Tc9-polarizing conditions with or without the addition of TNF-α for 2 days. TNF-α treatment increased the expression of IL-9 mRNA and protein in Tc9 cells (Figs. 1A, B) and the frequency of Tc9 cells (Fig. 1B). However, TNF-α under Tc0 polarizing conditions could not induce IL-9 expression in T cells and Tc9-cell differentiation (Figs. 1A, B). We also examined the expression of Tc9-cell–related transcription factors. As shown in Figure 1C, the addition of TNF-α had minor effects on the expression of Tc9-related transcription factors Sfpi1 or Irf4 in Tc9 cells, suggesting that TNF-α promotes Tc9-cell differentiation through other Tc9-related transcription factors. In addition, TNF-α treatment had minor effects on the expression of other Tc-related transcription factors and cytokines (Figs. 1C, D), but increased the expression of Il13 and Il2 in Tc9 cells (Fig. 1D). We next examined the expression of Il9 and Il2 in TNF-α-induced Tc9 cells at different time points. We found that the addition of TNF-α could not increase the expression of either Il9 or Il2 in Tc9 cells at hour 6 or hour 12 but began to increase the expression of both Il9 and Il2 at hour 24 (Fig. 1E). Together, these results demonstrated that TNF-α promotes Tc9-cell differentiation in vitro.
FIGURE 1.
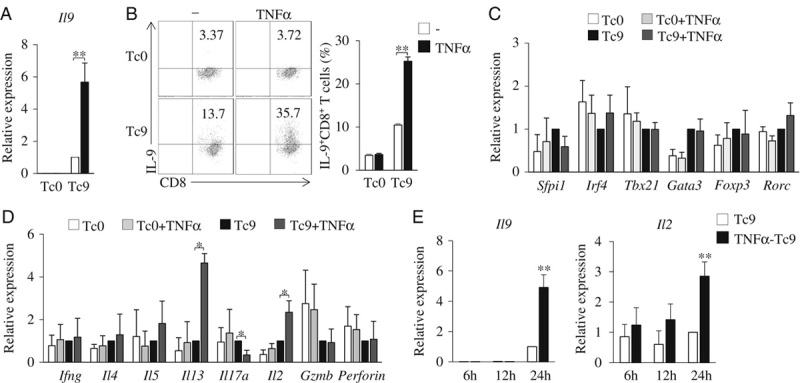
TNF-α promotes the induction of Tc9 cells. A–D, Mouse-naive CD8+ T cells were cultured under Tc9-polarizing conditions with or without the addition of TNF-α for 2 days. Cells cultured without the addition of TGF-β and IL-4 (Tc0) were used as controls. A, Quantitative polymerase chain reaction analysis of Il9 expression in CD8+ T cells. Expression was normalized to Gapdh and set at 1 in cells treated with TGF-β plus IL-4 (Tc9 cells). B, Flow cytometry analysis of IL-9-expressing CD8+ (IL-9+CD8+) T cells. Numbers in the dot plots represent the percentages of IL-9+CD8+ T cells. Right, summarized results of 3 independent experiments obtained as at left. Quantitative polymerase chain reaction analysis of the indicated transcription factors (C), cytokines, and effector molecules (D) in T cells. Expression was normalized to Gapdh and set at 1 in Tc9 cells. E, Mouse-naive CD8+ T cells were cultured under Tc9-polarizing conditions with or without the addition of TNF-α. Cells were collected at the indicated time points, and quantitative polymerase chain reaction analyzed the expression of Il9 and Il2 in Tc cells. Expression was normalized to Gapdh and set at 1 in Tc cells without the addition of TNF-α. Data are representative of 3 (B) independent experiments or presented as mean±SD of 3 (A–E) independent experiments. *P<0.05; **P<0.01. IL indicates interleukin; TGF, transforming growth factor; TNF, tumor necrosis factor.
TNF-α Promotes Tc9-Cell Survival and Proliferation In Vitro
The cell survival and proliferation of tumor-specific T cells is crucial for their persistence in vivo and their antitumor efficacy. We next examined genes related to T-cell survival and proliferation in TNF-α-induced Tc9 cells. We found that both the mRNA and protein expression levels of CD44 were significantly increased in TNF-α-induced Tc9 cells as compared with regular Tc9 cells (Figs. 2A, B). CD44 is a marker of effector and memory T cells that may contribute to T-cell survival and proliferation.25 Indeed, Annexin V staining detected less cell apoptosis of Tc9 cells than of Tc0 cells (Fig. 2C), and TNF-α treatment further reduced the apoptosis of Tc9 cells (Fig. 2C). Ki67 is a marker for cell proliferation.26 We also found that the frequency of Ki67+CD8+ T cells increased in TNF-α-induced Tc9 cells, as compared with regular Tc9 cells (Fig. 2D). We next examined the effects of TNF-α treatment on the cell apoptotic signaling in Tc9 cells. As shown in Figure 2E, TNF-α treatment increased the protein level of Bcl-2 but decreased the protein levels of Bax and cleaved caspase3 in Tc cells cultured under Tc9-polarizing conditions, indicating that TNF-α inhibited the cell apoptotic signaling in Tc9 cells. Together, these results demonstrated that TNF-α increases Tc9-cell survival and proliferation.
FIGURE 2.
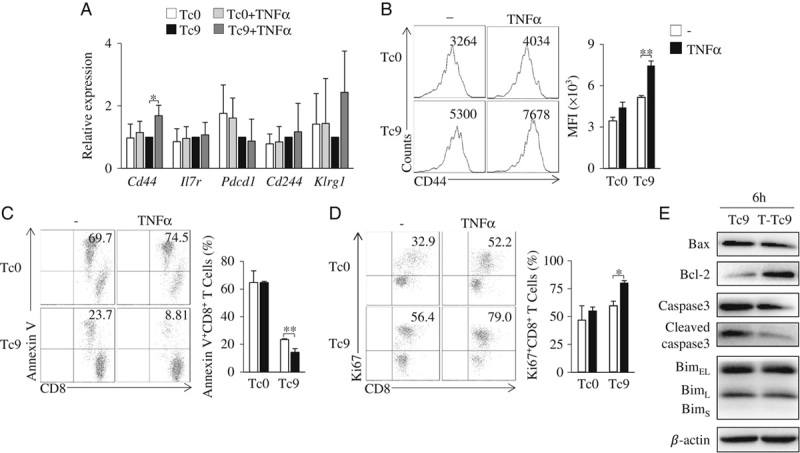
TNF-α increases the survival and proliferation of Tc9 cells in vitro. A–D, Mouse Tc9 cells were generated in the cultures in the presence or absence of TNF-α for 2 days. Tc0 cells were used as controls. A, Quantitative polymerase chain reaction analysis of the indicated molecules in CD8+ T cells. Expression was normalized to Gapdh and set at 1 in Tc9 cells. B, Flow cytometry analysis of CD44 on CD8+ T cells. Numbers in the histograms represent the fluorescence intensity (FI) of CD44. Right, summarized results of 3 independent experiments obtained as at left. Flow cytometry analysis of Annexin V+CD8+ (C) and Ki67+CD8+ (D) T cells. Numbers in the histograms represent the percentages of the double-positive T cells. Right, summarized results of 3 independent experiments obtained as at left. E, Naive CD8+ T cells were cultured under Tc9-polarizing conditions with or without the addition of TNF-α for 6 hours. Western blots examined the protein levels of Bax, Bcl-2, caspase3, cleaved caspase3, and Bim in T cells. β-actin was used as a loading control. Data are representative of 3 (B–E) independent experiments or presented as mean±SD of 3 (A–D) independent experiments. *P<0.05; **P<0.01. IL indicates interleukin; MFI, mean fluorescence intensity; TNF, tumor necrosis factor.
TNF-α-induced Tc9 Cells Exhibit Increased Antitumor Efficacy In Vivo
To examine the antitumor efficacy of TNF-α-induced Tc9 cells, we used Tc9 cells generated from OT-I mice. We first examined the OT-I Tc9 cells. Similarly, TNF-α-induced OT-I Tc9 cells expressed higher levels of IL-9 mRNA and protein than regular OT-I Tc9 cells (Figs. 3A, B). We next used OT-I Tc9 cells and TNF-α-induced OT-I Tc9 cells to treat B16-OVA-bearing C57BL/6 mice. Tc9 cell treatment exhibited greater inhibition on melanoma tumor growth than PBS controls (Fig. 3C), and TNF-α-induced Tc9 cells further inhibited melanoma tumor growth, as compared with regular Tc9 cells (Fig. 3C). These results demonstrated that TNF-α-induced Tc9 cells have increased antitumor efficacy in vivo.
FIGURE 3.
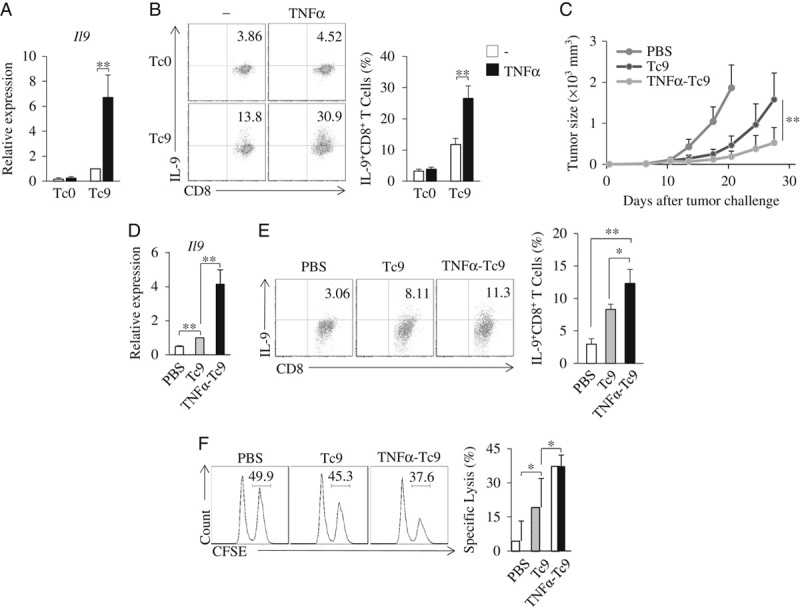
TNF-α increases Tc9 cell antitumor efficacy in vivo. A and B, Naive CD8+ T cells from OT-I mice were cultured under Tc9-polarizing conditions in the presence or absence of TNF-α for 2 days. A, Quantitative polymerase chain reaction analysis of Il9 in T cells. B, Flow cytometry analysis of IL-9+CD8+ T cells. Numbers in the dot plots represent the percentages of IL-9+CD8+ T cells. C, C57BL/6 mice were injected subcutaneously with 2×105 B16-OVA cells. On day 5 after tumor challenge, the B16-OVA tumor-bearing mice were randomly divided into 3 groups with 5 mice per group. Naive CD8+ T cells from OT-I mice were cultured under Tc9-polarizing conditions in the presence or absence of TNF-α for 2 days. Tc9 or TNFα-treated Tc9 cells were injected intravenously into the B16-OVA tumor-bearing mice (1×106 cells/mouse). Mice treated by PBS served as controls. Experiments were repeated twice. Shown are the tumor growth curves. Results are presented as mean±SD of the combined data from 2 independent experiments (n=10/group). D and E, B16-OVA cells (2×106/mouse) were injected intravenously into C57BL/6 mice. Naive CD8+ T cells from OT-I mice were cultured under Tc9-polarizing conditions in the presence or absence of TNF-α for 2 days. On day 5 after tumor injection, mice received Tc9 cells or TNFα-treated Tc9 cells via tail vein injection (1×106 cells/mouse). Mice that received PBS were used as controls. On day 2 after T-cell transfusion, cells were isolated from the lung tumor tissues. D, Quantitative polymerase chain reaction analysis of Il9 in T cells. E, Flow cytometry analysis of IL-9+CD8+ T cells. Numbers in the dot plots represent the percentages of IL-9+CD8+ T cells. F, In vivo CTL assay. CFSEhi target cells and CFSElo nontarget cells were mixed at 1:1 ratio and injected into the tail veins of C57BL/6 mice with Tc9 or TNFα-Tc9 cells generated from OT-I mice. Mice treated with labeled cells and PBS served as controls. After 6 hours, splenocytes were collected and flow cytometry analyzed the CFSE-labeling cells. Numbers in the histograms represent percentages of CFSEhi target cells. Right, summarized specific lysis results of 3 independent experiments obtained as at left. Data are representative of 3 (B, E, F) independent experiments or presented as mean±SD of 3 (A, B, D–F) independent experiments. *P<0.05; **P<0.01. CFSE indicates carboxyl-fluorescein diacetate, succinimidyl ester; IL, interleukin; PBS, phosphate-buffered saline; TNF, tumor necrosis factor.
The infiltration of effector T cells into tumor sites is associated with their antitumor effects. We next examined the tumor-infiltrating capability of TNF-α-induced Tc9 cells. OT-I Tc9 cells and TNF-α-induced OT-I Tc9 cells were transfused to B16-OVA-bearing C57BL/6 mice. CD8+ T cells from the lung tumor tissues were collected and analyzed. Cells from mice receiving TNF-α-induced Tc9 cells expressed higher levels of Il9 (Fig. 3D) and contained higher percentages of IL-9-expressing Tc cells than cells from mice transfused with Tc9 cells or PBS controls (Fig. 3E), indicating the increased tumor-infiltrating capability of TNF-α-induced Tc9 cells. In addition, TNF-α-induced OT-I Tc9 cells exhibited higher CTL activity than regular OT-I Tc9 cell in vivo (Fig. 3F). Together, these results demonstrated that TNF-α-induced Tc9 cells have increased antitumor capacity in vivo.
TNF-α Enhances Tc9-Cell Differentiation Through TNFR2
TNF-α has 2 cell surface receptors, TNFR1 and TNFR2.15 CD8+ T cells express both TNFR1 and TNFR2.27,28 We next explored the role of TNFR1 and TNFR2 in TNF-α-induced Tc9-cell differentiation. Naive CD8+ T cells were cultured under Tc9-polarizing conditions in the presence of anti-TNFR1 (αR1) or anti-TNFR2 (αR2)-blocking antibodies with or without the addition of TNF-α. The addition of αR2 largely abrogated TNF-α-induced upregulation of IL-9 expression in Tc9 cells (Figs. 4A, B), while the addition of αR1 exhibited minor effects on TNF-α-induced Tc9-cell differentiation (Figs. 4A, B), indicating that TNF-α drives Tc9-cell differentiation via TNFR2 but not TNFR1. In addition, the addition of αR2 but not αR1 inhibited TNF-α-induced Tc9 survival (Fig. 4C) and proliferation (Fig. 4D). Together, these results indicated that TNF-α stimulates Tc9-cell differentiation through TNFR2-mediated signaling.
FIGURE 4.
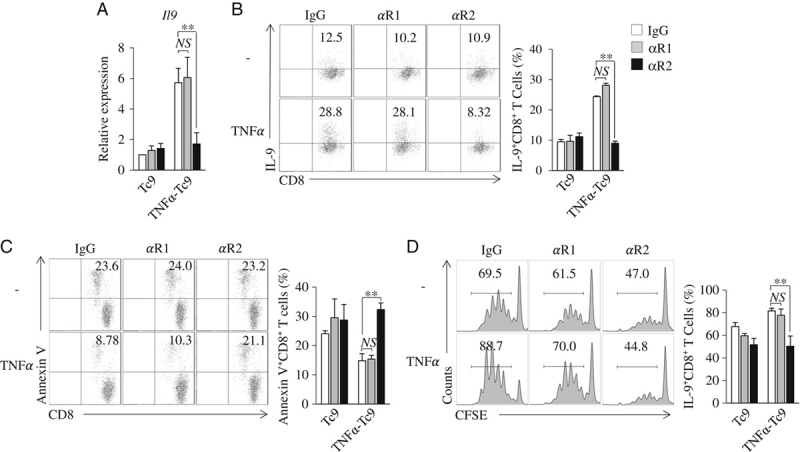
TNF-α drives Tc9-cell differentiation through TNFR2. A–C, CD8+ naive T cells were cultured under Tc9-polarizing conditions in the presence of TNFR1 (αR1) or TNFR2 (αR2)-blocking antibodies or an isotype control IgG with or without (−) the addition of TNF-α for 2 days. A, Quantitative polymerase chain reaction analysis of Il9 expression in CD8+ T cells. Expression was normalized to Gapdh and set at 1 in Tc9 cells. B, Flow cytometry analysis of IL-9+CD8+ T cells. Numbers in the dot plots represent the percentages of IL-9+CD8+ T cells. Right, summarized results of 3 independent experiments obtained as at left. C, Flow cytometry analyzed Annexin V+CD8+ T cells. Numbers in the histograms represent the percentages of Annexin V+CD8+ T cells. Right, summarized results of 3 independent experiments obtained as the left. D, Naive CD8+ T cells were labeled with CFSE and cultured under Tc9-polarizing conditions in the presence of TNFR1 (αR1) or TNFR2 (αR2)-blocking antibodies or an isotype control IgG with or without (−) addition of TNF-α for 2 days. Flow cytometry analyzed CFSE-stained T cells. Numbers in the histograms represent the percentage of CFSE-stained T cells. Right, summarized results of 3 independent experiments obtained as the left. Data are representative of 3 (B–D) independent experiments or presented as mean±SD of 3 (A–D) independent experiments. **P<0.01. CFSE indicates carboxyl-fluorescein diacetate, succinimidyl ester; Ig, immunoglobulin; IL, interleukin; NS, nonsignificant; TNF, tumor necrosis factor.
TNF-α Enhances Tc9-Cell Differentiation Through STAT5 and NF-κB Pathways
We previously found that TNF-α/TNFR2 induces Th9-cell differentiation through STAT5 and NF-κB signaling pathways.14 We hypothesized that TNF-α also stimulates Tc9-cell differentiation through STAT5 and NF-κB pathways. To address these issues, we first examined the role of STAT5 in TNF-α-induced Tc9-cell differentiation. Naive CD8+ T cells were cultured under Tc9-polarizing conditions with or without the addition of TNF-α, phosphorylated STAT5 (pSTAT5) in T cells was examined. TNF-α increased the protein levels of pSTAT5 in Tc cells, as compared with untreated controls (Fig. 5A), indicating that TNF-α increases the activation of STAT5 signaling during Tc9-cell differentiation. IL-4 activates STAT6 in T cells.29,30 To examine the role of STAT6 signaling in TNF-α-induced Tc9-cell differentiation, we also examined pSTAT6 in TNF-α-treated Tc cells. The addition of TNF-α exerted minor effects on the protein levels of pSTAT6 in Tc cells under Tc9-polarizing conditions (Fig. 5A), indicating that TNF-α cannot further increase the activation of STAT6 during Tc9-cell differentiation. To examine the role of STAT5 in TNF-α-induced Tc9-cell differentiation, a STAT5-specific inhibitor (STAT5i) was used during Tc9-cell differentiation. STAT5i exerted minor effects on the differentiation of regular Tc9 cells, as demonstrated by the similar expression levels of IL-9 mRNA and protein in Tc9 cells with or without the addition of STAT5i (Figs. 5C, D). However, the addition of STAT5i partially abrogated TNF-α-induced promotion of Tc9-cell differentiation, as demonstrated by the lower expression of IL-9 mRNA and protein in TNF-α-induced Tc9 cells with the addition of STAT5i compared with the dimethyl sulfoxide controls (Figs. 5C, D). These results demonstrated that TNF-α induces Tc9-cell differentiation through STAT5 signaling pathway.
FIGURE 5.
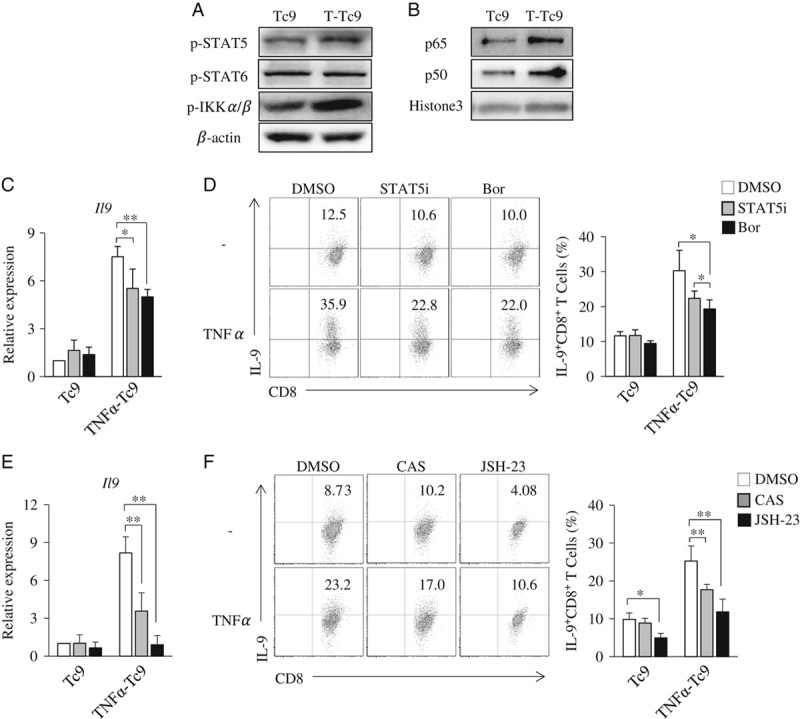
TNF-α enhances Tc9-cell differentiation through STAT5 and NF-κB. A, Naive CD8+ T cells were cultured under Tc9-polarizing conditions with or without the addition of TNF-α for 3 hours. Western blots examined the protein levels of the phosphorylated STAT5 (pSTAT5), IKKα/β (p-IKKα/β), and phosphorylated STAT6 (pSTAT6) in T cells. β-actin was used as a loading control. B, Naive CD8+ T cells were cultured under Tc9-polarizing conditions with or without the addition of TNF-α for 6 hours. Western blots examined the protein levels of the p65 and p50 in the nucleus of T cells. Histone3 was used as a loading control. C–F, Naive CD8+ T cells were cultured under Tc9-polarizing conditions in the presence or absence of TNF-α with or without (DMSO) the addition of STAT5 inhibitor (STAT5i) or NF-κB inhibitors [bortezomib (Bor), CAS 213546-53-3 (CAS), and JSH-23] for 2 days. C and E, Quantitative polymerase chain reaction analysis of Il9 expression in T cells. D and F, Flow cytometry analysis of IL-9-expressing CD8+ (IL-9+CD8+) T cells. Numbers in the dot plots represent the percentages of IL-9+CD8+ T cells. Right, summarized results of 3 independent experiments obtained as at left. Data are representative of 3 (A, B, D, F) independent experiments or presented as mean±SD of 3 (C–F) independent experiments. *P<0.05; **P<0.01. DMSO indicates dimethyl sulfoxide; IL, interleukin; NF-κB, nuclear factor-κB; TNF, tumor necrosis factor.
We next examined the role of NF-κB signaling in TNF-α-induced Tc9-cell differentiation. Western blots were used to examine the protein levels of NF-κB signaling molecules. TNF-α treatment increased the protein levels of pIKKα/β (Fig. 5A) and promoted p65 and p50 nuclear translocation (Fig. 5B) in Tc cells under Tc9-polarizing conditions, indicating that TNF-α upregulates NF-κB signaling during Tc9-cell differentiation.
To explore the role of NF-κB signaling in TNF-α-induced Tc9-cell differentiation, bortezomib, an inhibitor of NF-κB pathway, was used during Tc9-cell differentiation. Bortezomib exerted minor effects on the differentiation of regular Tc9 cells, as demonstrated by the similar expression levels of IL-9 mRNA and protein in Tc9 cells, with or without the addition of bortezomib (Figs. 5C, D). However, the addition of bortezomib partially abrogated the upregulation of the expression levels of IL-9 mRNA and protein in TNF-α-induced Tc9 cells (Figs. 5C, D). To further confirm the role of NF-κB in TNF-α-induced Tc9-cell differentiation, other 2 NF-κB inhibitors (CAS 213546-53-3 and JSH-23) were used. Both CAS 213546-53-3 and JSH-23 were found to abolish the upregulation of IL-9 expression in TNF-α-induced Tc9 cells (Figs. 5E, F). These results demonstrated that TNF-α stimulates Tc9-cell differentiation through NF-κB pathway.
Together, these results demonstrated that TNF-α stimulates Tc9-cell differentiation through STAT5 and NF-κB signaling pathways.
DISCUSSION
We recently identified tumor-specific Tc9 cells as a potent antitumor effector Tc-cell subset in tumor immunotherapy, better than Tc1 cells.8,9 Therefore, strategies of more efficient induction of Tc9 cells may have important clinical significance. In this study, we found that TNF-α treatment promotes Tc9-cell differentiation, and Tc-cell proliferation and survival. More importantly, TNF-α-induced Tc9 cells induce more potent antitumor efficacy than regular Tc9 cells in adoptive immunotherapy in mouse tumor models. Multiple mechanisms may be involved in the increased antitumor efficacy of TNF-α-induced Tc9 cells. Generally, the antitumor efficacy of Tc cells depends on their in vivo persistence and tumor-specific cytotoxicity. TNF-α-induced Tc9 cells exhibit higher tumor-specific cytotoxicity than regular Tc9 cells in vivo. TNF-α increases the expression of IL-9 and IL-2 in Tc9 cells. Both IL-9 and IL-2 promote T-cell proliferation and survival,8,31,32 which may contribute to the in vivo persistence and antitumor efficacy of TNF-α-induced Tc9 cells. CD44 is a marker of effector T cells.25 Thus, our data demonstrated that TNF-α is a potent inducer of Tc9 cells.
TNF-α has 2 receptors, TNFR1 and TNFR2.16 In this study, we found that TNF-α drives Tc9-cell differentiation through TNFR2 signaling. It is interesting to note that TNFR2 is the main TNF receptor on CD8+ T cells and maintains CD8+ T cells at memory and effector stages.33,34 We found that TNF-α/TNFR2 signaling further activates STAT5 and NF-κB pathways, which are required for TNF-α-induced Tc9-cell differentiation. STAT5 signaling is critical for CD8+ T-cell development and function,35,36 and the activation of NF-κB pathway can inhibit CD8+ T cells from exhaustive Tc-cell differentiation.37 Therefore, our data demonstrated that TNF-α promotes Tc9-cell differentiation through TNFR2-induced STAT5 and NF-κB signal pathways.
TNF-α stimulates the expression of IL-2 in Tc9 cells. IL-2 was shown to signal through STAT5 and promote T-cell proliferation and survival, suggesting the potential of indirect induction of Tc9 cells by TNF-α. However, TNF-α activates STAT5 at the early stage (at hour 3) of Tc9-cell differentiation. In addition, TNF-α begins to stimulate the expression of IL-2 and IL-9 in Tc9 cells at hour 24, and the expression of IL-2 and IL-9 follows the same temporal patterns in TNF-α-induced Tc9 cells. These observations indicate that TNF-α can promote Tc9-cell differentiation by directly activating STAT5 signaling.
In this study, we found that TNF-α-induced Tc9 cells express increased IL-13, which is a cytokine expressed in Tc2 cells, suggesting that the addition of TNF-α during Tc9 differentiation may result in the induction of Tc2-like cells. However, TNF-α-induced Tc9 cells did not have increased expression of Gata3, the major transcription factor of Tc2 cells, and the activation of STAT5 and NF-κB can also stimulate the expression of IL-13 in T cells.38,39 On the basis of these observations, we believe that TNF-α stimulates the expression of IL-13 in Tc9 cells.
In conclusion, our study demonstrates that TNF-α potently promotes Tc9-cell differentiation. Moreover, TNF-α-induced Tc9 cells induce potent antitumor efficacy in adoptive immunotherapy in mouse tumor models. Moreover, TNF-α promotes Tc9-cell differentiation through TNFR2-mediated STAT5 and NF-κB pathways during Tc9 differentiation. Thus, our study demonstrated TNF-α as a potent stimulator of Tc9-cell differentiation and may have important clinical implications.
Acknowledgments
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
Supported by funds from National Natural Science Foundation of China (81372536 to S.W. and 81802839 to J.C.). All authors have declared that there are no financial conflicts of interest with regard to this work.
Footnotes
S.W. and Y.Y.: initiated the study. S.W.: designed the experiments and wrote the paper. S.W., S.Y., H.Z., J.C., Y.J., and T.Q.: performed the experiments and statistical analyses. S.G.: provided critical suggestions to this study.
REFERENCES
- 1. Gattinoni L, Powell DJ, Jr, Rosenberg SA, et al. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lauss M, Donia M, Harbst K, et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat Commun. 2017;8:1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parmiani G, Castelli C, Rivoltini L, et al. Immunotherapy of melanoma. Semin Cancer Biol. 2003;13:391–400. [DOI] [PubMed] [Google Scholar]
- 4. Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ye Z, Tang C, Xu S, et al. Type 1 CD8+ T cells are superior to type 2 CD8+ T cells in tumor immunotherapy due to their efficient cytotoxicity, prolonged survival and type 1 immune modulation. Cell Mol Immunol. 2007;4:277–285. [PubMed] [Google Scholar]
- 6. Yu Y, Cho HI, Wang D, et al. Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J Immunol. 2013;190:1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joshi NS, Cui W, Chandele A, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu Y, Hong B, Li H, et al. Tumor-specific IL-9-producing CD8+ Tc9 cells are superior effector than type-I cytotoxic Tc1 cells for adoptive immunotherapy of cancers. Proc Natl Acad Sci USA. 2014;111:2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu Y, Wang Q, Yi Q. Anticancer Tc9 cells: long-lived tumor-killing T cells for adoptive therapy. Oncoimmunology. 2014;3:e28542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meylan F, Siegel RM. TNF superfamily cytokines in the promotion of Th9 differentiation and immunopathology. Semin Immunopathol. 2017;39:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiao X, Fan Y, Li J, et al. Guidance of super-enhancers in regulation of IL-9 induction and airway inflammation. J Exp Med. 2018;215:559–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang D, Li H, Duan YY, et al. TL1A modulates the severity of colitis by promoting Th9 differentiation and IL-9 secretion. Life Sci. 2019;231:116536. [DOI] [PubMed] [Google Scholar]
- 13. Kim IK, Kim BS, Koh CH, et al. Glucocorticoid-induced tumor necrosis factor receptor-related protein co-stimulation facilitates tumor regression by inducing IL-9-producing helper T cells. Nat Med. 2015;21:1010–1017. [DOI] [PubMed] [Google Scholar]
- 14. Jiang Y, Chen J, Bi E, et al. TNF-alpha enhances Th9 cell differentiation and antitumor immunity via TNFR2-dependent pathways. J Immunother Cancer. 2019;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell. 2004;116:491–497. [DOI] [PubMed] [Google Scholar]
- 16. Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. [DOI] [PubMed] [Google Scholar]
- 17. Croft M. The TNF family in T cell differentiation and function—unanswered questions and future directions. Semin Immunol. 2014;26:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. [DOI] [PubMed] [Google Scholar]
- 19. Valencia X, Stephens G, Goldbach-Mansky R, et al. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salomon BL, Leclerc M, Tosello J, et al. Tumor necrosis factor alpha and regulatory t cells in oncoimmunology. Front Immunol. 2018;9:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang S, Wang J, Brand DD, et al. Role of TNF-TNF receptor 2 signal in regulatory T cells and its therapeutic implications. Front Immunol. 2018;9:784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu Y, Hong S, Li H, et al. Th9 cells promote antitumor immune responses in vivo. J Clin Invest. 2012;122:4160–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu N, Jiang Y, Chen J, et al. IL-33 drives the antitumor effects of dendritic cells via the induction of Tc9 cells. Cell Mol Immunol. 2019;16:644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao Y, Chu X, Chen J, et al. Dectin-1-activated dendritic cells trigger potent antitumour immunity through the induction of Th9 cells. Nat Commun. 2016;7:12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baaten BJ, Li CR, Deiro MF, et al. CD44 regulates survival and memory development in Th1 cells. Immunity. 2010;32:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan PH, Bay BH, Yip G, et al. Immunohistochemical detection of Ki67 in breast cancer correlates with transcriptional regulation of genes related to apoptosis and cell death. Mod Pathol. 2005;18:374–381. [DOI] [PubMed] [Google Scholar]
- 27. Dostert C, Grusdat M, Letellier E, et al. The TNF family of ligands and receptors: communication modules in the immune system and beyond. Physiol Rev. 2019;99:115–160. [DOI] [PubMed] [Google Scholar]
- 28. Wortzman ME, Clouthier DL, McPherson AJ, et al. The contextual role of TNFR family members in CD8(+) T-cell control of viral infections. Immunol Rev. 2013;255:125–148. [DOI] [PubMed] [Google Scholar]
- 29. Koch S, Sopel N, Finotto S. Th9 and other IL-9-producing cells in allergic asthma. Semin Immunopathol. 2017;39:55–68. [DOI] [PubMed] [Google Scholar]
- 30. Song GY, Chung CS, Chaudry IH, et al. IL-4-induced activation of the Stat6 pathway contributes to the suppression of cell-mediated immunity and death in sepsis. Surgery. 2000;128:133–138. [DOI] [PubMed] [Google Scholar]
- 31. Van Snick J, Goethals A, Renauld JC, et al. Cloning and characterization of a cDNA for a new mouse T cell growth factor (P40). J Exp Med. 1989;169:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. [DOI] [PubMed] [Google Scholar]
- 33. Calzascia T, Pellegrini M, Hall H, et al. TNF-alpha is critical for antitumor but not antiviral T cell immunity in mice. J Clin Invest. 2007;117:3833–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ye LL, Wei XS, Zhang M, et al. The significance of tumor necrosis factor receptor type II in CD8(+) regulatory T Cells and CD8(+) effector T cells. Front Immunol. 2018;9:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yao Z, Cui Y, Watford WT, et al. Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci USA. 2006;103:1000–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kelly J, Spolski R, Imada K, et al. A role for Stat5 in CD8+ T cell homeostasis. J Immunol. 2003;170:210–217. [DOI] [PubMed] [Google Scholar]
- 37. Xu Y, Wang Y, Yang Q, et al. A versatile supramolecular nanoadjuvant that activates NF-kappaB for cancer immunotherapy. Theranostics. 2019;9:3388–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Visekruna A, Ritter J, Scholz T, et al. Tc9 cells, a new subset of CD8(+) T cells, support Th2-mediated airway inflammation. Eur J Immunol. 2013;43:606–618. [DOI] [PubMed] [Google Scholar]
- 39. Guo L, Wei G, Zhu J, et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci USA. 2009;106:13463–13468. [DOI] [PMC free article] [PubMed] [Google Scholar]


