ABSTRACT
The purpose of this study was to reveal possible consequences of long-bone fracture on cardiac tissue and to analyze the role of systemically elevated danger associated molecular patterns, complement anaphylatoxins and cytokines. Blood samples of mice, pigs, and humans after a fracture were analyzed by ELISAs for complement component 5a (C5a), tumor necrosis factor (TNF), and extracellular histones. In vivo results were completed by in vitro experiments with human cardiomyocytes treated with TNF and extracellular histones. The influence of histones and human plasma after fracture on isolated human polymorphonuclear leukocytes (PMNs) was investigated. An elevation of TNF, C5a, and extracellular histones after long bone fracture was measured. Moreover, the appearance of systemic troponin I levels was observed and structural changes in connexin 43 and desmin were detected. Further, the presence of TNF leads to elevation of reactive oxygen species, troponin I release, and histone appearance in supernatant of human cardiomyocytes. Incubation of human PMNs with histones and plasma of patients after fracture lead to formation of neutrophil extracellular traps. Present results suggest that structural alterations in the heart might be consequences of the complement activation, the release of extracellular histones, and the systemic TNF elevation in the context of a long bone fracture.
Keywords: Complement system, connexin43, damage control orthopedics, early total care, HFABP, polymorphonuclear leukocytes, troponin I
INTRODUCTION
Physical trauma are an increased burden for the society (1) and reflect a major challenge for the future. Despite direct cardiac damage after physical trauma, there is rising evidence for trauma-induced secondary cardiac structural and functional damage (2,3). After multiple trauma in pigs, a functional and structural damage of the heart has been described in previous studies (4); as well as a linkage between systemic cardiac troponin I levels of multiple injured patients and the corresponding survival (5). Further, an association between hip fracture and the appearance of coronary heart disease was made (6,7). Therefore, the question arises whether and how single long bone fractures may affect the heart.
In the present study, different animal models and human samples were analyzed to investigate whether a long bone fracture and the inflammatory consequences are linked to cardiac alterations. In particular, the role of tumor necrosis factor (TNF), complement component 5a (C5a), and extracellular histones after fracture were investigated. TNF is well known to be elevated after fracture (8,9) and to be cardio-depressive (10,11). TNF induces apoptosis in cardiomyocytes and leads to cardiac dysfunction in different models. (10,12–14). Activation of the complement system, especially C5a, caused cardiomyocyte dysfunction during sepsis (15). Likewise, after fracture the complement system has been shown to be activated (16,17). Furthermore, C5a leads to an activation of neutrophil granulocytes, which are able to form neutrophil extracellular traps (NETs) (18,19), containing DNA and extracellular histones. Extracellular histones are described as a mediator of septic cardiomyopathy. Histones caused disequilibrium in the redox status and intracellular [Ca2+]i levels in cardiomyocytes (CMs) and disturbed functional and electrical responses of hearts perfused with histones and induced defects in mitochondrial function (20). Therefore, the NET formation by neutrophils in patients with bone fracture will be investigated in regard of its role in amplifying systemic release of damage-associated molecular patterns (DAMPs) and consequently causing organ damage.
MATERIALS AND METHODS
Mice
All animal experiments were performed according to the international regulations for the care and use of laboratory animals and were approved by the responsible government authority (No. 1096 and 1149, Regierungspräsidium Tübingen, Germany). They were performed according to the guidelines of the Federation of European Laboratory Animal Science Association (FELASA). Mice received either sham treatment or femur osteotomy for 6 and 24 h, respectively. For the respective groups, five to seven animals were used. Briefly, an osteotomy (0.4 mm) was performed at the mid-shaft at the right femur of 12 weeks old, male C57BL/6J-Mice, purchased from Charles River (Sulzfeld, Gemany). General anesthesia was conducted with 2% isoflurane, mice received tramadol for analgesia. The femur fracture was stabilized by using an external fixator (RISystem, Davos, Switzerland). Blood plasma from mice was collected at the end of the experiments, 6 and 24 h after fracture or sham procedure, by puncture of the heart under deep anesthesia. 1 mL whole blood was collected, centrifuged at 4,000 ×g for 10 min and plasma was stored at −80°C. Details of the animal experiment and sample collection were already published in Kroner et al. (21) and Kovtun et al. (22).
Pigs
Animals and anesthesia
All the procedures conformed to the Society of Laboratory Animal Science as well as the National Animal Welfare Law and gained approval from the responsible government authority (“Landesamt für Natur, Umwelt, und Verbraucherschutz”: LANUV-NRW, Germany: AZ TV-No.: 84–02.04.2014.A265). They were performed according to the guidelines of the FELASA. Animal experiments adhere to the Animal Research: Reporting of In Vivo Experiments guidelines for reporting animal research. In the interest of limiting animal numbers, the samples were obtained from a recently conducted prospective randomized experimental study performed by the TREAT Research Group. The model has been previously described in detail by Horst et al. (23). Briefly, eleven 12 to 16-week-old male pigs with a mean body weight of 30 ± 5 kg (Sus scrofa dormestica, Tierzucht GmbH Heinrich, Heinsberg, Germany) were included in the study. Anesthesia was induced and maintained during the study period of 72 h with propofol (1–2 mg/kg body weight). The pigs were orotracheally intubated and ventilation was conducted in biphasic positive airway pressure mode in a lung protective ventilation (6–8 mL/kg body weight). Vital parameters were monitored by electrocardiographic (ECG) recordings and ECG-synchronized pulse oximetry. Fluids were administered by continuous crystalloid infusion (Sterofundin ISO; 2 mL/kg body weight /h).
Fracture surgery
Pigs underwent a femur fracture (n = 8) or sham procedure (n = 3). The Sham procedure included anesthesia but no femur fracture. The fracture group (n = 8) was randomized in two therapy arms (n = 4); external fixation of the femur fracture corresponding to damage control orthopedics (DCO) or femoral nailing appropriate to early total care (ETC) principles.
Sample collection
Whole venous blood samples, containing all physiological blood components, were collected from V femoralis at baseline as well as 1.5, 3.5, 5.5, 24, 48, and 72 h after fracture. At each time point, 6 mL whole venous blood was collected from the pigs. Blood loss was adjusted adequately by continuous crystalloid administration (Sterofundin ISO; 2 mL/kg body weight /h). Samples were kept on ice and serum was obtained by centrifugation (2,000 × g for 15 min at 4°C). After centrifugation, serum was removed and stored at −80°C until analysis. Heart tissue of left ventricles was obtained 72 h after multiple trauma and fixed with 4% formalin followed by embedding in paraffin.
Humans
Human blood sample collection was approved by the Ethical Committee of the University Medical Center Ulm (No. 438/15) as recently published (24) and was in accordance with the Declaration of Helsinki. All the enrolled patients signed the written informed consent form themselves or written informed consent was obtained from the nominated legally authorized representatives on the behalf of participants in accordance with ethical standards. All the methods were performed in accordance with the ethical guidelines of the Ethical Committee of the University Medical Center Ulm (No. 438/15).
In total, 26 patients (age: 32–97 years, mean: 75 years) with metaphyseal/diaphyseal fractures of long bones (femur, tibia, humerus, radius, ulna) treated surgically at the University Medical Center Ulm between January 2016 and January 2018 were included. Exclusion criteria were polytrauma, pregnancy, bone diseases except primary osteoporosis, intake of bisphosphonates or parathyroid hormone, rheumatoid arthritis, open fractures grade 3 or 4 according to Tscherne and Oestern, hepatic or nephritic insufficiency, cancer, intake of steroids, intake of immunosuppressive medication, chemotherapy in the last 3 months, and artificial ventilation after surgery. In further subgroup analyses, patients with femur fracture (AO-31 A1/A2/A3/B2; n = 19) were assigned to two groups at d0: male fracture patients (n = 6, age: 32–97 years, mean: 69 years) and female fracture patients (n = 13, age: 57–87 years, mean: 78 years). Furthermore, 20 healthy volunteers (10 males aged 24–57, mean: 37; 10 females aged 27–87 years, mean: 47 years) donated one blood sample each as controls. The collective of the analyzed serum was published previously by Fischer et al. (24). Peripheral venous blood was obtained from each patient at the day 0 after fracture event. Additionally, blood from seven patients was obtained at day 14 after fracture. Blood samples were centrifuged to get serum, which was stored at −80°C until analysis. Details of sample collection and the patient cohort were previously published by Fischer et al. (24).
ELISAs
Serum and plasma samples were analyzed using ELISA kits according to the manufacturer's instructions. Histones in mice plasma, pig serum, and human plasma were measured by using a cell death detection ELISA kit (Hoffmann-La Roche, Indianapolis, Ind). A histone mixture (containing H2, H2A, H2B, H3, H4) (Sigma, St.-Louis, Mo) was used to establish a standard curve. Cardiac troponin I concentrations in mouse plasma, pig serum samples, human plasma, and supernatant of human cardiomyocytes were measured by using species-specific ultrasensitive troponin-I ELISA kits (Life Diagnostics, West Chester, Pa) and cardiac troponin I human simple Step ELISA Kit (Abcam, Cambridge, UK). C5a was measured in patient and mouse plasma by using a C5a ELISA (DRG Diagnostics, Marburg, Germany; R&D Systems, McKinley, Minn). IL-6 was measured in plasma/serum of mice/humans with fracture by IL-6 ELISA (R&D Systems, McKinley, Minn). TNF concentrations were measured in mice plasma samples by TNF ELISA (R&D Systems, McKinley, Minn). Human TNF was detected in serum of fracture patients measured by TNF ELISA (Abcam, Cambridge, UK).
Immunohistochemistry
For immunohistochemistry porcine, formalin-fixed left ventricles were used. For complement component 5a receptor (C5aR1) staining polyclonal Rabbit anti CD88/C5aR1 (Acris Antibodies, Herford, Germany) was used as primary antibody. For Connexin (Cx43) staining rabbit anti-pig Cx43 (Cell Signaling Technology, Danvers, Mass) was used. Nitrotyrosine staining was performed using antinitrotyrosine (Merckmillipore, Darmstadt, Germany). Dako REAL Detection System (Dako, Glostrup, Denmark) was used as a secondary system. Signal density was measured in nine randomly chosen, distinct fields of vision from each slide using an Axio ImagerM.1 microscope and the Zeiss AxioVision software 4.9 (Zeiss, Jena, Germany). Results are presented as mean density of each group (arbitrary units).
Confocal imaging
For the confocal imaging, the left ventricles of pigs 72 h after fracture (DCO and ETC) (n = 8) or after sham treatment (n = 3) were analyzed. Therefore, formalin fixed and paraffin embedded heart tissue from left ventricles were used from the pigs.
For α-actinin-2 staining rabbit anti-α-actinin (clone N1N3) (GeneTex, Ivine, Calif) was used as primary and goat antirabbit (AF-488) as secondary antibody (Jackson Immuno research Laboratories, West Grove, Pa). For desmin staining mouse antidesmin (GeneTex, Irvine, Calif) was deployed as primary and goat antimouse (AF-647) as secondary antibody (Jackson Immuno Research Laboratories, West Grove, Pa). Following staining sections were mounted with ProLong Gold Antifade Reagent (Invitrogen, Carlsbad, Calif). Confocal imaging was performed using Leica SP8 (Leica microsystems, Wetzlar, Germany). Evaluation of fluorescence-intensity was conducted by the Software Image J x1 (25). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed by using CF 488 TUNEL Apoptosis Detection Kit (Biotium, Fremont, Calif). A counterstain with Höchst 33342 (Sigma, Darmstadt, Germany) was done. Results of TUNEL positive nuclei were presented in percentage of whole nuclei number.
Determination of RNA expression of C5aR1 in pig left ventricle homogenates by real-time quantitative PCR analysis
Total RNA was isolated from pig heart homogenates using TRIZOL (Life Technologies, Carlsbad, Calif) according to manufacturer's instructions. cDNA was then obtained by using SuperScript IV VILO Master Mix with ezDNAse enzyme (Thermo Fisher, Waltham, Mass) and was then amplified by using PowerUp SYBR Green Master Mix (Life Technologies, Carlsbad, Calif). Amplification was performed by using Quant Studio 3 (Applied Biosystems, Waltham, Mass). mRNA expression of the respective genes was normalized to GAPDH expression. Calculation of the relative quantitative mRNA expression was done with the cycle threshold methodΔΔCt algorithm. C5aR1-Primer: Forward: 5‘-AGAATATGGACCCCATGGTTG-3‘, Reverse: 5‘-GTTCTGGAAGAGGCATCCAC-3‘
In vitro incubation of cardiomyocytes (CMs) with inflammation-cocktail (IF-C) and TNF
Human cardiomyocytes (iPS) (Cellular Dynamics, Madison, Wis) were cultured for 10 days at 37°C and 7% CO2 in iCell medium (Cellular Dynamics, Madison, Wis). After 10 days an incubation with either inflammation-cocktail (IF-C), including 500 ng/mL C3a, 10 ng/mL C5a, 250 pg/mL IL-1β, 500 pg/mL IL-6, 150 pg/mL IL-8, and 10 ng/mL TNF, or with only 250 pg/mL TNF (at 37°C and 7% CO2) for 6 h followed. After 6 h, supernatant and cell lysates were frozen at −20°C. Supernatant was used to measure extracellular histones detected by cell death detection ELISA kit (Hoffmann-La Roche, Indianapolis, Ind) and to investigate troponin I concentration by using human troponin I simple step ELISA kit (Abcam, Cambridge, UK). To assess caspase-3 in treated cardiomyocytes, Caspase-Glow 3/7 Assay (Promega, Madison, Wis) was used. Cytosolic reactive oxygen species (ROS) were measured by immunofluorescence by using CellROX Deep Red Reagent (Life Technologies, Carlsbad, Calif). Cell lysates were used to isolate mRNA for qPCR measurements. cDNA was then obtained by using SuperScript IV VILO Master Mix with ezDNAse enzyme (Thermo Fisher, Waltham, Mass) and was then amplified by using PowerUp SYBR Green Master Mix (Life Technologies, Carlsbad, Calif). Amplification was performed by using Quant Studio 3 (Applied Biosystems, Waltham, Mass). mRNA expression of the respective genes was normalized to GAPDH expression. Calculation of the relative quantitative mRNA expression was done with the cycle threshold method ΔΔCT algorithm. The following primers were utilized: for mRNA expression of IL-1ß forward 5’-GCAAGGGCTTCAGGCAGGCCGCG-3’ and reverse 5’-GGTCATTCTCCTGGAAGGTCTGTGGGC-3’; for NLRP3 mRNA expression forward 5’-CTTCTCTGATGAGGCCCAAG-3’ and reverse 5’-GCAGCAAACTGGAAAGGAAG-3’; mRNA of C5aR1 forwards 5’-GGAGACCAGAACATGAACTCCTT-3’ and reverse 5’-ATCCACAGGGGTGTTGAGGT -3’; for C3aR mRNA expression forwards 5’-AGACAGGACTCGTGGAGACA-3’ and reverse 5’-AGACGCCATTGCTAAACTTCAAA-3’.
Mitochondrial respiration with seahorse XFe96 analyzer
Human cardiomyocytes (iPS) (Cellular Dynamics, Madison, Wis) were seeded in special Seahorse XFe96 cell culture plates (Agilent Technologies, Santa Clara, Calif) and were cultured for 10 days in iCell maintenance medium (Cellular Dynamics, Madison, Wis) at 37°C and 7% CO2. After the cultivation, cells were treated either with 250 pg/mL TNF or with the above mentioned IF-C for 6 h at 37°C and 7% CO2. After exposure of cells to TNF or IF-C, mitochondrial respiration was measured. Mitochondrial respiration was measured by using the Seahorse XFe96 Analyzer (Agilent Technologies, Santa Clara, Calif) and the Seahorse XF Cell Mito Stress Test Kit (Agilent Technologies, Santa Clara, Calif). Immediately after the experiment, cells were fixed with 4% formalin at 4°C overnight. Then cells were stained with 0.2% Janus-Green solution, washed and resolved with 0.5 M hydrochloric acid. OD was measured at 630 nm. The oxygen consumption rate (OCR) values were normalized to the OD 630 nm values, respectively. Results were evaluated using Seahorse Wave 2.4 software (Agilent Technologies, Santa Clara, Calif).
In vitro incubation with histones
Human cardiomyocytes (iPS) (Cellular Dynamics, Madison, Wis) were cultured for 10 days at 37°C and 7% CO2 in iCell medium (Cellular Dynamics, Madison, Wis). After 10 days an incubation with 100 μg/mL histones (Sigma, St Louis, Mo) for 6, 12, and 24 h followed. Control cells were incubated with PBS in iCell medium. Troponin I was measured by human cardiac troponin simple step ELISA kit (Abcam, Cambridge, UK) in supernatant of treated cardiomyocytes.
Isolation and treatment of human polymorphonuclear leucocytes (PMNs)
Human blood sample collection was approved by the Ethical Committee of the University Medical Center Ulm (local Approval no 94/14). Human blood plasma was used to isolate human PMNs from healthy control patients.
For PMN isolation 30 mL human whole blood was collected with S-Monovette, coated with 3.2% citrate (Sarstedt, Nümbrecht, Germany). Whole blood was mixed with 0.9% NaCl solution (Fresenius Kabi, Bad Homburg, Germany). Afterward, whole blood was layered at Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden) and was centrifuged for 30 min with 340 × g at room temperature. Cells from whole blood, containing also leukocytes, were separated by density gradient during centrifugation. After centrifugation, the supernatant was discarded and the pellet, containing the leukocytes, was collected. The pellet was incubated for 30 min with Dextran (from Leuconostoc spp, Sigma, St. Louis, Mo). After incubation, the pellet was discarded and the supernatant was filled up with 0.9% NaCl (Fresenius Kabi, Bad Homburg, Germany) and was once again centrifuged for 5 min with 340 × g. After this centrifugation, the supernatant was discarded, the pellet was lysed by adding double-distilled water and was refilled afterward with 2.7% NaCl solution (Sigma Aldrich, St. Louis, Mo). Afterward, another centrifugation step followed for 5 min with 340 × g. Then, the supernatant was discarded and the pellet, containing the neutrophils, was resuspended in Hanks’ balanced salt solution (HBSS, Sigma Aldrich, St. Louis, Mo) Isolated neutrophils were cultured with DMEM (Gibco, Paisley, Scotland, UK), supplemented with 10% fetal bovine serum, 1% L-Glutamin, and 1% penicillin/streptomycin for 1.5 h. Isolated neutrophils were incubated with a mixture of histones (Sigma, St.-Louis, Mo). For Figure 6B histone concentrations of 0.05, 0.5, 1, 10, and 20 μg/mL and LPS concentrations of 5, 10, 20, and 50 μg/mL were used and incubated for 2 h.
Fig. 6.
In vitro influence of TNF (250 pg/mL) presence to human cardiomyocytes.
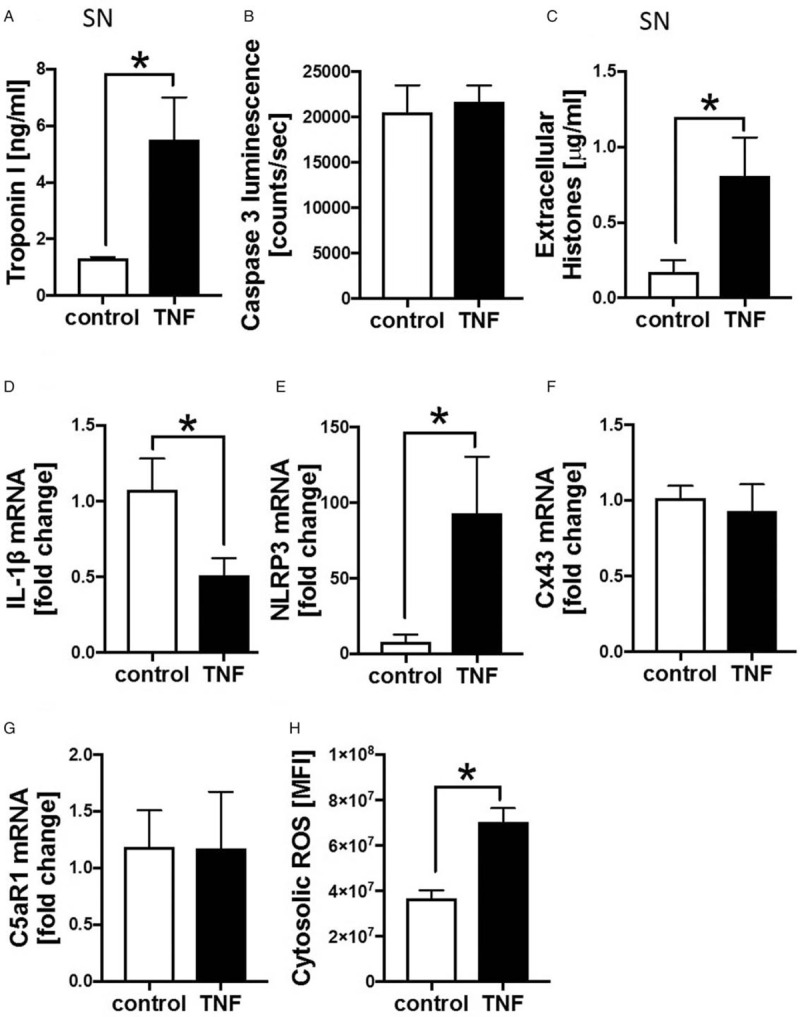
A, Troponin I concentration in ng/mL in supernatant (SN) of human cardiomyocytes treated with 250 pg/mL TNF for 6 h compared with control cardiomyocytes measured by ELISA, n = 6. B, Caspase 3 luminescence in counts/seconds measurement of human cardiomyocytes after incubation in the absence (control) and in the presence of 250 pg/mL TNF for 6 h (TNF), n = 6. C, Appearance of extracellular histones in μg/mL in supernatant of human cardiomyocytes incubated with 250 pg/mL TNF for 6 h compared with control cardiomyocytes, n = 6 measured by using ELISA. mRNA expression of IL-1ß (D), NLRP3 (E), Cx43 (F), and C5aR1 (G) of cardiomyocytes incubated in the absence (control) and in the presence of TNF for 6 h, n = 6. H, Reactive oxygen species (ROS) in human cardiomyocytes treated with TNF (TNF) for 6 h and controls (control) assessed by Cell ROX Red Reagent, n = 6, ∗differences to control were significant, P < 0.05.
Isolated PMNs were also incubated with serum from patients with a fracture or from healthy controls for 2 h. The used serum samples from fracture patients as well as from healthy controls were randomly selected from the present serum collective of the study. Additional propidium iodide (Sigma, St. Louis, Mo) and Hoechst was added to the wells for staining extracellular DNA/NETs. After incubation PMNS were washed twice with PBS, were fixed with 4% Formalin, and were mounted with ProLong Gold Antifade Reagent (Invitrogen, Carlsbad, Calif). The whole experiment was repeated three times.
Statistical procedures
All values were expressed as means ± SEM. Data were analyzed by one-way ANOVA followed by Dunnett, Tukey, or Holm-Sidak multiple comparison test. P≤0.05 was considered statistically significant. GraphPad Prism 7.0 software was used for statistical analysis (GraphPad Software Inc, San Diego, Calif).
RESULTS
Systemic inflammation and release of extracellular histones after fracture in mice, pigs, and humans
To determine systemic inflammation after long bone fracture, the systemic release of the inflammatory and cardio-depressive mediators C5a, C3a, TNF and of extracellular histones were measured in mouse, pig, and human blood samples (Fig. 1). Mice subjected to femur fracture demonstrated increased systemic complement activation by enhanced release of C5a (Fig. 1A) and of C3a (Fig. 1B) compared with sham-treated animals 6 h after fracture. Systemic IL-6 concentrations in blood samples of mice 6 h after fracture were elevated significantly, whereas systemic TNF was enhanced slightly but not significantly (Fig. 1, C and D). Extracellular histone concentration in plasma was elevated 24 h after fracture in mice (Fig. 1E). In pigs subjected to femur fracture, extracellular histones in serum samples were significantly increased 1.5 and 3 h after trauma compared with baseline (Fig. 1F). In humans, extracellular histones (Fig. 1G) were increased systemically compared with healthy controls 0 and 14 days after meta/diaphyseal fracture of long bones. Further, after fracture C5a (Fig. 1I) and TNF (Fig. 1H) were slightly elevated systemically at day 0 and 14 days after fracture compared with healthy controls.
Fig. 1.
Systemic inflammation and release of extracellular histones after fracture in mouse, pig and human.
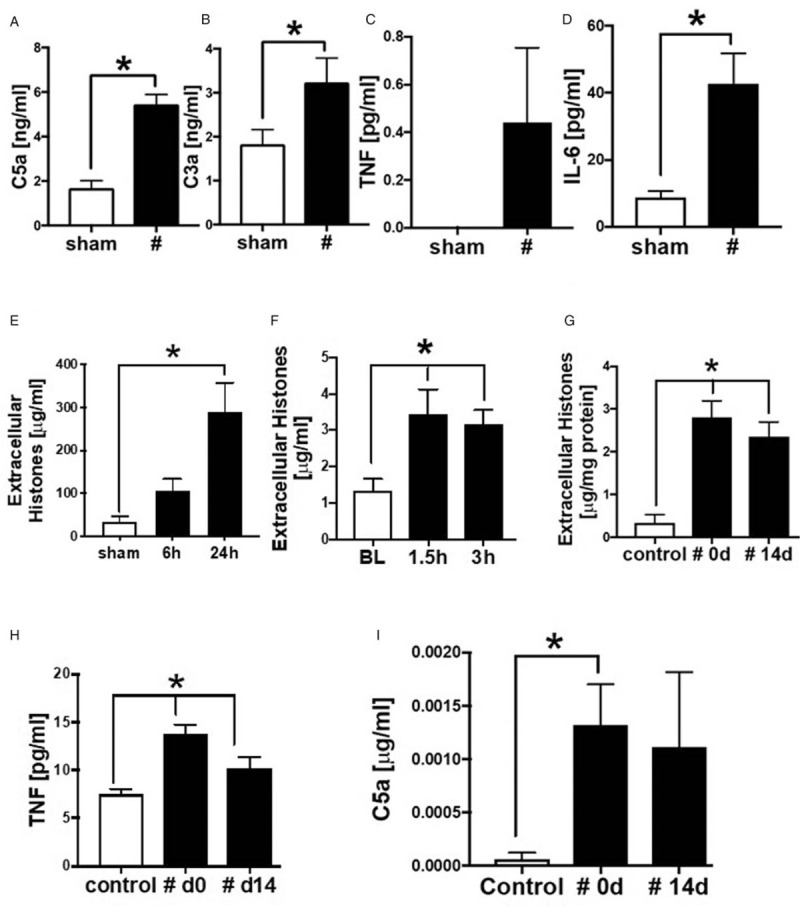
A, C5a concentration in plasma of mice with a fracture (#), compared with sham treated animals measured 6 h after fracture, n = 5–7. B, C3a concentration in μg/mL in plasma of mice with a fracture (#), compared with sham-treated animals measured 6 h after fracture, n = 5–7. (C) TNF levels in μg/mL and (D) Il-6 levels in pg/mL in plasma of mice with fracture, compared with sham-treated animals measured 6 h after fracture, n = 5–7. E, Extracellular histone appearance in plasma from mice (C57BL/6) 6 and 24 h after fracture or after sham treatment. F, Time course for extracellular histone appearance in serum from pigs after fracture (black bars, n = 48) compared with sham (white bars, n = 3). G, Time course for extracellular histone appearance in plasma from patients with fracture (#), at day of fracture (0 d, n = 25) and 14 days after fracture (14 d, n = 6) (both black bars). H, TNF concentration in pg/mL in plasma from patients with fracture (#), at day of fracture (0 d, n = 26) and 14 days after fracture (14 d) (both black bars, n = 7), compared with healthy controls (white bar) n = 20. I, C5a concentration in plasma from patients with fracture (#), at day of fracture (0 d, n = 26) and 14 days after fracture (14 d, n = 7) (both black bars), compared with healthy controls (white bar), n = 20. ∗differences to sham/baseline (BL)/ control procedure were significant, P < 0.05.
Hemodynamic alterations in pigs after fracture and systemic Troponin I concentrations in pigs and humans after fracture
Since mice, pigs, and humans showed increased levels of inflammatory mediators after long bone fracture, which were all previously shown to be cardio-depressive, cardiac injury was analyzed in the respective species (Table 1, Fig. 2). To estimate cardiac injury after fracture in pigs and humans, troponin I as a specific marker for cardiac cell damage was assessed at different time points after trauma (Fig. 2). Cardiac troponin I was elevated systemically in pooled serum from pigs of both treatment groups (DCO and ETC) 1.5 and 3 h after trauma compared with baseline (Fig. 2A). The troponin I release could not be explained by apoptosis of cardiac cells: no differences in apoptotic positive cardiomyocytes were measured between sham and fracture animals (Fig. 2B). Furthermore, cardiac troponin I in human plasma was detected after fracture. Here, an elevation at the day of fracture (day 0) was measured in plasma of patient with fracture compared with healthy controls (Fig. 2C). The systemic troponin I release from humans after fracture was independent of age and there was no statistic correlation between systemic troponin I release and age (R2 = 0.003) (Fig. 2D).
Table 1.
Mean values (± SD) of hemodynamic parameters of pigs after long bone fracture receiving fracture provision either according to damage control orthopedics (DCO) or to early total care principles (ETC)
| n | BL | 3.5 h | 5.5 h | 24 h | 48 h | 72 h | P value | |
| HR | 12 | 94.6 | 78.1 | 83.8 | 67.7 | 67.8 | 76.8 | 0.001 |
| MAP | 12 | 73.1 | 73.8 | 77.6 | 68.5 | 81.2 | 85.5 | <0.001 |
| RR | 12 | 99.1 | 105.3 | 105.7 | 104.6 | 121.1 | 116.9 | <0.001 |
P values were determined for changes over time per group. n = 12 animals per group. Heart rate (HR) in beats/min, mean arterial pressure (MAP) in mm Hg and systolic RR-interval in ms.
Fig. 2.
Troponin I concentration in pig and human after fracture.
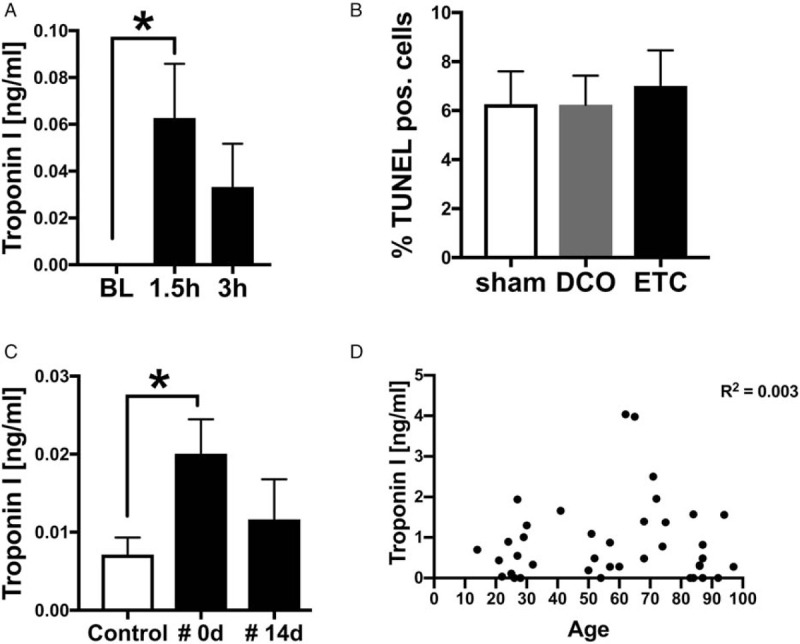
A, Time course of cardiac specific troponin I in ng/mL in serum of pigs after fracture (n = 8) compared with sham procedure (n = 3). B, Apoptotic cells in percentage detected by TUNEL staining of left ventricle in sham (n = 3) and after fracture and damage control treatment (DCO, n = 4) or early total care (ETC, n = 4) treatment. C, Concentrations of cardiac troponin I in ng/mL in plasma of patients with fracture (#) at day of fracture (0 d, n = 26) and at day 14 after fracture (14 d, n = 7) compared with healthy controls (white bars, n = 20). Correlation analysis if systemic troponin I values (D). Systemic troponin I values (ng/mL) were correlated with the age of the patients. R2 = 0.003. ∗ differences to control procedure were significant, P < 0.05.
Pigs showed increased systemic inflammation as well as the presence of the myocardial injury marker troponin I; therefore, the hemodynamic parameters of the animals were analyzed after long bone fracture. To determine the hemodynamic alterations in pigs after long bone fracture, the heart rate (HR), the mean arterial pressure (MAP), as well as the systolic RR-interval were measured by ECG recordings (Table 1). The data from the pigs of both treatment groups (DCO and ETC) were combined for the analysis (n = 12). The HR decreased significantly during the observation period. Moreover, the MAP as well as the systolic RR interval increased significantly over the observation time.
Structural alterations in the porcine heart after long bone fracture: α-actinin, desmin, and translocation of Cx43
Since pigs showed hemodynamic alterations, the left ventricular expression of the cardiac structure proteins α-actinin and desmin were analyzed (Fig. 3). Both structure proteins are associated with impaired cardiac function after trauma. The expression of α-actinin in CMs was increased 72 h after fracture, followed by DCO treatment compared with sham procedure (Fig. 3A), which was determined by fluorescence intensity measurement. Comparable results showed the measurement of fluorescence intensity of desmin staining of the left ventricles. The expression of desmin was increased in the ETC compared with sham treated animals (Fig. 3B).
Fig. 3.
Structural alterations in the porcine heart after fracture: α-actinin, desmin, and translocation of Cx43.
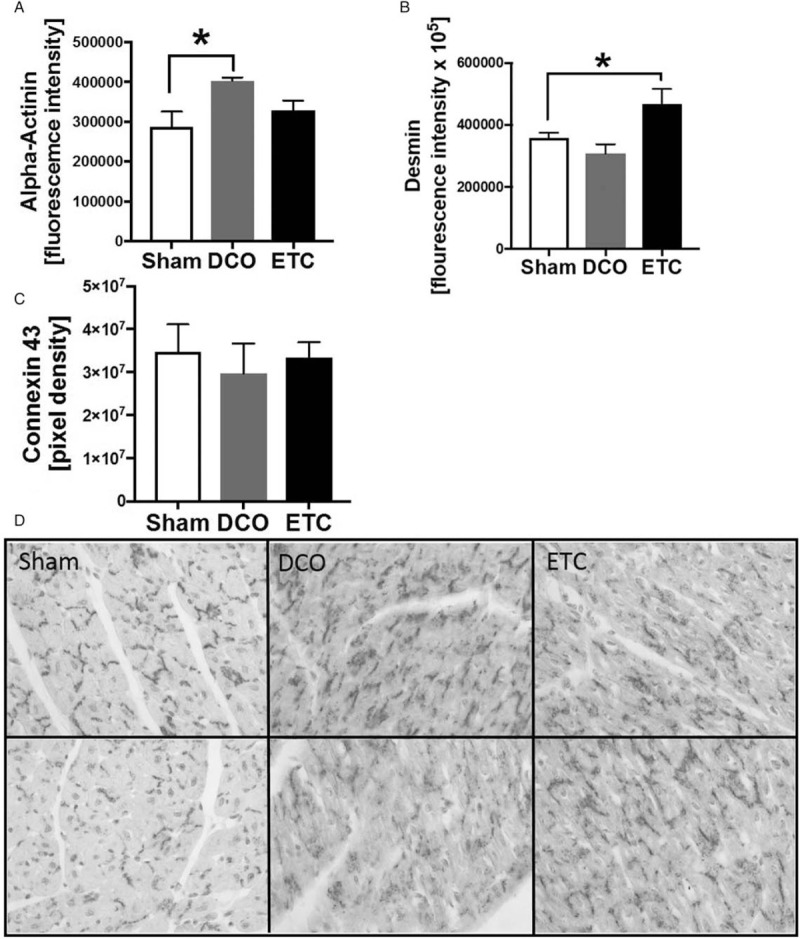
A, Fluorescence intensity of α2-actinin staining and confocal imaging in animals treated with fracture and Damage Control Orthopedics (DCO, n = 4), or fracture and Early Total Care (ETC, n = 4) compared with sham (n = 3). B, Fluorescence intensity of α2-desmin staining and confocal imaging in animals treated with fracture and Damage Control Orthopedics (DCO, n = 4), or fracture and Early Total Care (ETC, n = 4) compared with sham (n = 3). C, Results of density measurement of Connexin 43 (Cx43) staining in left ventricular tissue after fracture and DCO (n = 4) or ETC (n = 4) treatment and in sham-treated animals (white bar, n = 3). D, Representative distribution of Cx43 staining of the left ventricle of sham-treated animals and pigs after fracture and damage control (DCO, middle) treatment and early total care (ETC, right), ∗ differences to sham were significant, P < 0.05.
Left ventricular alterations of the gap junction protein Cx43 are associated with severe arrhythmia. Therefore, alterations of the left ventricular expression of Cx43 after long bone fracture were analyzed in pigs. To determine whether gap junction proteins in the heart were altered after fracture immunohistochemistry staining of Cx43 gap junction protein in left ventricular tissue sections was performed. In sham-treated animals Cx43 was located in intercalated discs whereas Cx43 was translocated and scattered into the cytosol (Fig. D) after fracture followed by DCO or ETC. No differences in total Cx43 protein expression in left ventricles after femur fracture were observed in pigs (Fig. 3C).
Local inflammation in porcine heart after long bone fracture
After trauma, the activation of the complement system is associated with impaired cardiac function (Fig. 4). Since mice and humans showed increased systemic levels of the complement activation products C3a and C5a after long bone fracture, the left ventricular expression of the C5a receptor 1 (C5aR1) was determined in pigs after isolated long bone fracture. There was a reduction of the complement receptor C5aR1 72 h after fracture in both treatment groups (DCO and ETC) compared with the sham group (Fig. 4A), whereas mRNA expression of C5aR1 was not significantly influenced by fracture in pigs, after 72 h (Fig. 4B).
Fig. 4.
Local inflammation.
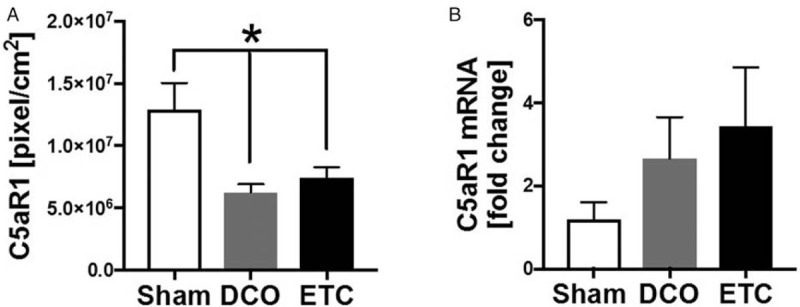
A, Changes in density of C5aR1 staining in left ventricular tissue of pigs 72 h after fracture, followed by DCO (n = 4) or ETC (n = 4) treatment as well as in sham-treated pigs (n = 3). B, mRNA expression of C5aR in left ventricles of pigs 72 h after fracture and DCO (n = 4) or ETC treatment (n = 4) or sham procedure (n = 3) ∗ differences to sham were significant, P < 0.05.
In vitro influence of inflammatory mediators and TNF on human cardiomyocytes
Mice, pigs, and humans showed increased systemic levels of inflammatory mediators after isolated long bone fracture; therefore, the effects of the TNF as well as of a defined inflammation-cocktail were analyzed on human cardiomyocytes in vitro (Figs. 5 and 6). In the presence of inflammation-cocktail and in the presence of TNF, the mitochondrial respiration was impaired compared with control cells (Fig. 5A). The mitochondrial basal respiration (Fig. 5B), the mitochondrial spare respiratory capacity (Fig. 5C), as well as the mitochondrial maximal respiration (Fig. 5D) were significantly decreased in the cells when exposed to inflammation-cocktail and to TNF. Furthermore, the amount of cytosolic ROS significantly enhanced in the presence of inflammation-cocktail (Fig. 5E), compared with the control cells. The mRNA expression of IL-1β significantly decreased in the presence of inflammation-cocktail (Fig. 5F), whereas mRNA expression of NLRP3 inflammasome significantly enhanced in the cells (Fig. 5G), compared with the control cells.
Fig. 5.
In vitro influence of inflammation-cocktail (IF-C) or TNF (250 pg/mL) on human cardiomyocytes.
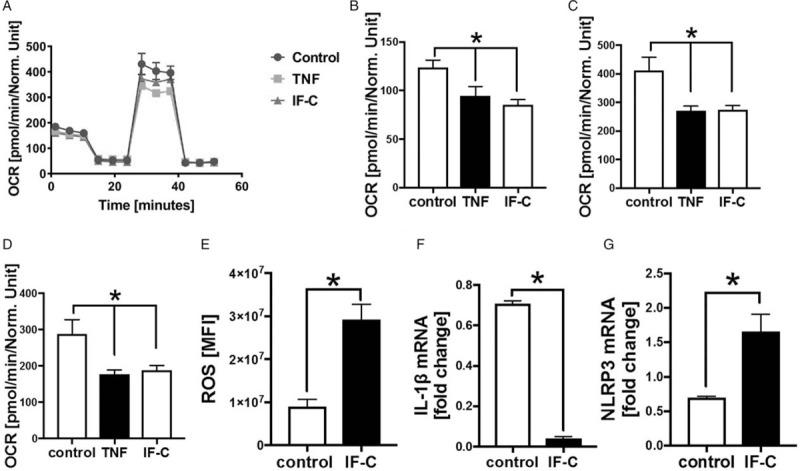
A, Mitochondrial respiration of human cardiomyocytes during MitoStress Assay, measured with Seahorse XFe96 Analyzer. Oxygen consumption rate (OCR) in pmol/min/Norm. Unit. Cells were treated for 6 h either with inflammation-cocktail (IF-C) or with 250 pg/mL TNF. Control group was only treated with cell culture medium. n = 6. B, Mitochondrial basal respiration of human cardiomyocytes. Oxygen consumption rate (OCR) in pmol/min/Norm. Unit. Cells were treated with inflammation cocktail (IF-C) or with 250 pg/mL TNF for 6 h. Control group was only treated with cell culture medium. Mitochondrial respiration was measured with MitroStress Assay using the Seahorse XFe96 Analyzer. n = 6. C, Mitochondrial spare respiratory capacity of human cardiomyocytes. OCR) in pmol/min/Norm. Unit. Cells were treated with inflammation cocktail (IF-C) or with 250 pg/mL TNF for 6 h. Control group was only treated with cell culture medium. Mitochondrial respiration was measured with MitroStress Assay using the Seahorse XFe96 Analyzer. n = 6. D, Mitochondrial maximal respiration of human cardiomyocytes. Oxygen consumption rate (OCR) in pmol/min/Norm. Unit. Cells were treated with inflammation cocktail (IF-C) or with 250 pg/mL TNF for 6 h. Control group was only treated with cell culture medium. Mitochondrial respiration was measured with MitroStress Assay using the Seahorse XFe96 Analyzer. n = 6. E, Reactive oxygen species (ROS) in human cardiomyocytes treated with inflammation-cocktail (IF-C) for 6 h and controls (control) assessed by Cell ROX Red Reagent, n = 6. mRNA expression of IL-1β (F) and NLRP3 inflammasome (G) of human cardiomyocytes treated with inflammation-cocktail (IF-C). Control cells were only treated with cell culture medium. mRNA expression in fold change. ∗ differences to control were significant, P < 0.05.
Human CMs were incubated with recombinant human TNF for 6 h. Here, troponin I concentrations in supernatants were significantly increased in the presence of TNF (Fig. 6A). Activity of caspase 3 indicating CM apoptosis was not elevated in the presence of TNF (Fig. 6B). In contrast, extracellular histone concentration in supernatant was significantly elevated in the presence of TNF (Fig. 6C). Further, TNF was associated with reduced mRNA expression of IL-1ß in CMs compared with controls after 6 h (Fig. 6D), whereas mRNA expression of NLRP3 in the presence of TNF was increased (Fig. 6E). Neither the expression of Cx43 (Fig. 6F) nor C5aR1 (Fig. 6G) was affected by the presence of TNF of human CMs. Though, cytosolic ROS was elevated in CMs in the presence of TNF compared with CM in the absence of TNF (Fig. 6H).
Effects of extracellular histones
Pigs and humans showed enhanced systemic levels of extracellular histones; consequently, we analyzed the ability of human PMNs to make NET formation in the presence of extracellular histones, amplifying the inflammatory response after isolated long bone fracture (Fig. 7). The incubation of human PMNs with histones led to NET-formation in a dose-dependent manner, measured by immunofluorescence of extracellular DNA (Fig. 7A), whereas the positive control was accomplished by LPS-incubation. Additionally, incubation of PMNs with plasma of patients with fracture resulted in a significant increase in extracellular DNA compared with PMNs treated with plasma from healthy controls (Fig. 7B). Incubation of human CMs with histone mixture for 6, 12, and 24 h was associated with increased troponin I concentrations in supernatant compared with controls (Fig. 7C).
Fig. 7.
Effects of extracellular histones.
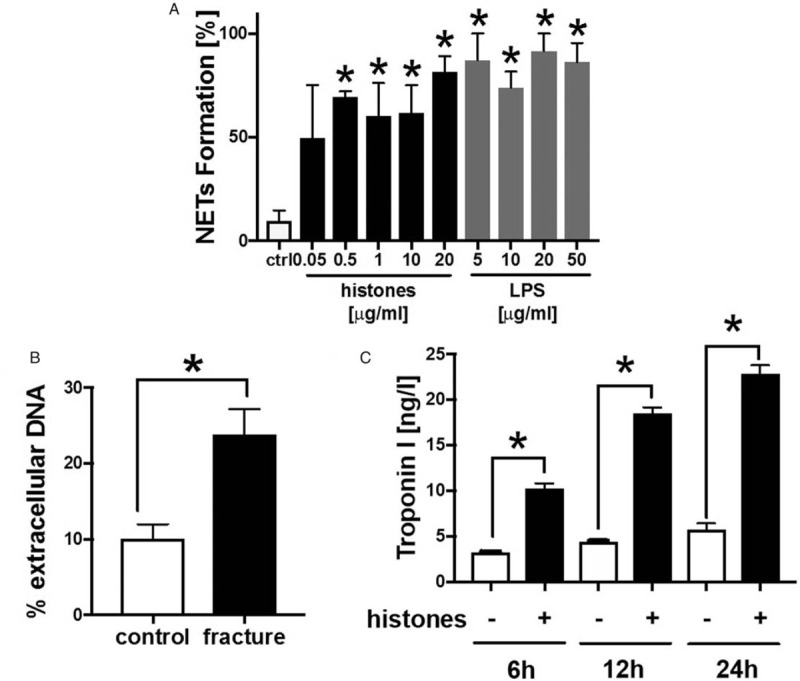
A, NET formation in human PMNs in percentage after treatment with different concentrations of histones (mix of histones) and LPS for 6 h compared with control-PMNs, n = 6. B, Extracellular DNA in percentage measured in PMNs treated with plasma of healthy controls (n = 4) and of patients with fracture (n = 6) for 2 h. C, Troponin I concentration in supernatant of human cardiomyocytes treated with histones (6, 12, 24 h, +) compared with untreated cardiomyocytes (−), n = 6. ∗differences to controls were significant P < 0.05.
DISCUSSION
In this study, we showed a systemic elevation of different inflammatory biomarkers and DAMPs, such as C5a, TNF, and extracellular histones after long bone fracture in the species human, pig, and mouse. Moreover, we observed hemodynamic and structural cardiac alterations in pigs, which might indicate for a secondary cardiac damage after isolated femur fracture.
Structural alterations of cardiac tissue after fracture were indicated by an increased α-actinin expression in pig hearts after femur fracture, with DCO treatment. We previously showed alterations in cardiac α-actinin expression in pigs after experimental polytrauma, which was associated with impaired cardiac function. α-actinin is linked with L-type Ca2+-channels and reacts as responder to mechanical stretch by hemodynamic adaption (26). Interestingly, Z-disc-associated proteins like α-actinin are not only altered due to direct mechanical trauma on the heart as shown after multiple trauma in pigs, but also to fracture. Alterations in α-actinin expression might be to systemic inflammation after fracture. Furthermore, the expression of desmin increased in the present study in porcine heart tissue, which was previously shown after experimental polytrauma in pigs (4). Cardiac desminopathies lead to cardiomyopathy, conduction defects and to arrhythmias (27,28). Desminopathies were associated with impaired mechanical properties of CMs and alterations of the calcium amplitude (29). Increased desmin concentrations were observed in guinea pigs with heart failure (30) and in mice with diastolic dysfunction (31). Additionally, the distribution and function of the ryanodine receptor is affected by modification of desmin (32). Alterations in cardiac desmin expression might be also to systemic inflammation. TNF was previously described to induce the cleavage of desmin by caspase-6, leading to a loss of localization of desmin at the intercalated discs of CMs (33). Moreover, remodeling of gap junction proteins and relocalization of Cx43 were described in a mouse model of desmin-related cardiomyopathy (34). In the present study, pigs showed likewise a translocation of Cx43 from the intercalated discs into the cytosol of the CMs after isolated long bone fracture. Endocytosis of Cx43 was associated with changes in spread of electrical activation and was further associated with arrhythmias and cardiac dysfunction (35,36). To our knowledge this is the first time that alterations in cardiac z-disc and gap junction proteins were described after an isolated long bone fracture. Summarized, in the present study, pigs showed alterations in the left ventricular expression of cardiac structure proteins as well as in the localization of the gap junction protein Cx43 after isolated long bone fracture. These structural cardiac alterations after long bone fracture might predispose to cardiac dysfunction and to cardiac damage.
Interestingly, there was evidence for cardiac injury after fracture due to increased levels of circulating troponin I in humans and pigs. Currently, troponin I is considered an important diagnostic marker for cardiac damage in the clinical use, as well as after trauma (37). Troponin I has been reported to be elevated after heart contusion in 15% to 45% of the cases and was described as a reliable tool for the detection of post-traumatic heart complications, especially in combination with echocardiography (38,39). Enhanced systemic troponin values were associated with increased catecholamine requirement as well as with an increased mortality rate of the patients after trauma (5). In previous studies we observed systemic troponin I elevations after experimental polytrauma in mice and pigs (4,40) and after experimental blunt chest trauma in rats (41). There, enhanced troponin values were associated with impaired cardiac function and cardiac damage. However, here we describe a systemic, but mechanically-independent elevation of troponin I, which might indicate for cardiac injury after long bone fracture in humans and in pigs. The systemic troponin I elevation in this study was not dependent on the age of the patients. The findings of this study are in contrast to those of a previous study in which no cardiac troponin I but skeletal troponin I was systemically detectable after soft tissue- and orthopedic injuries in humans (42). Nevertheless, we do not think that the release of skeletal troponin I distorted the present data due to highly specificity and sensitivity of the used immunoassays. The release of cardiac troponin I in pigs was not due to cardiomyocyte apoptosis, but might be induced by inflammatory cytokines. Notably, the systemic cardiac troponin I by itself is not a sufficient diagnostic tool for myocardial injury and patients with systemic elevated troponin I values must have at least one of five further diagnostic criteria for the definition of myocardial injury (43,44). However, no echocardiographic parameters were assessed from the patients in the current study but this issue has to be addressed in future studies to show myocardial injury after long bone fracture. Moreover, the systemic release of cardiac troponin I was described in various diseases and also in the context of emotional and psychosocial stress (44–46). Certainly, we could not exclude stress-induced systemic release of cardiac troponin I in humans, but we think that the release of cardiac troponin I in this study might be due to the enhanced inflammation after long bone fracture. This inflammatory condition of long bone fracture might predispose to cardiac injury.
Based on the findings of cardiac structural alterations and of enhanced systemic cardiac troponin I levels in pigs after long bone fracture, we analyzed hemodynamic parameters. The HR of the animals decreased significantly during the observation period, whereas the MAP as well as the systolic RR-interval significantly increased over the observation time. These hemodynamic findings might indicate for cardiac functional alterations after fracture and might be further linked to cardiac structural changes and to cardiac injury. However, this assumption of cardiac dysfunction after long bone fracture has to be examined in more detail in future studies by echocardiographic analysis. Summarized, the occurrence of systemic troponin I as well as the alterations in hemodynamic parameters might indicate for cardiac injury after isolated long bone fracture.
In this context the activation of the complement system may play an important role. Especially, the complement activation product C5a was elevated after long bone fracture in mice and humans in the present study. C5a and its receptors (C5aR1/2) were associated with cardiac dysfunction during experimental sepsis in mice (47). During ischemia/reperfusion, the complement system plays a critical role in endothelial transmigration, developing cardiac diseases. In the absence of the C5aR, the expression of matrix metalloprotease and junction adhesion molecule -A was reduced, leading to reduced infarct size and to leucocyte recruitment (48). Considering other cardiac pathologies, C5a was described as powerfully cardio-depressive via C5aR1 interactions on the membrane of cardiomyocytes. This C5a-C5aR1 interaction reduced cardiomyocyte contractility and the ability of relaxation, leading to reduced cardiac output, to amongst multi-organ failure, to cardiac shock and to sudden cardiac death (20,49,50). Moreover, C5a triggers a massive increase of cellular ROS and [Ca2+]i in isolated rat CMs (15). Disturbed calcium homeostasis leads to impaired electrophysiological function of CMs and consecutively aggravated contractility (15). Increased ROS further reduced left ventricular function (51). Furthermore, C5a induces the release of cytokines such as IL-6 and TNF in CMs in vitro (52). In addition, C5a is able to induce release of extracellular histones, which is ameliorated in the absence of C5aR1 (20,53). In vitro activation of neutrophils by C5a leads to enhanced histone concentrations, caused by neutrophil extracellular trap (NET) formation (18). Summarized, the increased systemic C5a levels might contribute to the present cardiac alterations after isolated long bone fracture.
Furthermore, in the present study, extracellular histones were elevated systemically after long bone fracture in mice, pigs, and humans, which was also described previously after experimental multiple trauma (4), blunt chest trauma (41), during sepsis (20,54) and in humans after trauma (55). Histones were released from damaged cells after trauma (56,57), mediated by neutrophils and their NETs (58). Increased levels of circulating histones were associated with acute lung injury and with septic complications after burn injury (59). Perfusion of mouse hearts with extracellular histones leads to reduction of left ventricular pressure and to impaired contractility (20). Besides, incubation of human CMs with histones induced an increase in cytosolic ROS in a dose-dependent manner (20). The present data showed similarly an elevation of ROS in human CMs in the presence of TNF. ROS induce a cytosolic calcium increase by modulating and blocking calcium handling proteins. Further, histones are able to interact with the phospholipid-membrane of cells, which lead to higher permeability and consequently to enhanced cellular calcium influx (55,60–62). Interestingly, in this study the in vitro exposure of human CMs to TNF induced extracellular histone release into supernatant fluids, which might additionally enhance the cardio-depressive effects. This additional release of extracellular histones from CMs might further recruit other immune cells such as neutrophils, intensifying the systemic immune response. Moreover, the CMs themselves are affected by the release of extracellular histones as described above, leading to an enhanced cardio-depressive effect, which might predispose to cardiac dysfunction. Summarized, increased systemic levels of extracellular histones after long bone fracture may affect the heart in the present study, by inducing the systemic release of troponin I.
In the present report we demonstrated to the best of our knowledge for the first time that histones themselves are able to activate human neutrophils by inducing NET-formation. Neutrophils are present in fracture hematoma after bone injury (17). Consequently, this may result in an amplification of local as well as of systemic histone concentrations, which might contribute to compromised organ function after long bone fracture. Moreover, histone application in mice induces the systemic release of further cytokines such as TNF, IL-1ß, IL-6, and IL-10 (63), which is mediated via histone-TLRs interactions (64–68).
Besides C5a and extracellular histones, the release of TNF after long bone fracture is well described and correlated with the results in the present study (8,9). The systemic release of TNF in the present study might also contribute to the cardiac alterations after long bone fracture. Blockade of TNF in experimental burn injury has been shown to improve cardiac function (69). Also, TNF disturbed the maximum extent of cardiac myocyte shortening in cultured rat CMs, which was reversible after the removal of TNF (14). However, the systemic conditions after single long bone fracture might quite differ from them after severe polytrauma (with blunt chest trauma) or during sepsis. After polytrauma, the development of cardiac dysfunction is obvious due to the extent of systemic inflammation by the release of inflammatory cytokines and damages molecules, induced by severe tissue trauma. During sepsis the inflammation has a much larger extent compared with fracture, since additional involvement of exogenous factors induces cardiac dysfunction and multiple organ failure. Moreover, patients who died from sepsis often exhibit suppressed immune response, which makes translation to systemic condition of single long bone fracture difficult (70). Nevertheless, we think that the molecular mechanisms which affect the heart after single long bone fracture might be the same as after polytrauma or during sepsis. The absence of C5aR1/2 improved cardiac function in mice (15) during sepsis. Moreover, usage of an antibody with neutralizing activity to histone H2A and H4 ameliorated cardiac function in septic mice (20). Furthermore, inhibition of TNF production in endotoxemic rats preserved myocardial contractile function (71). Consequently, these cardio-depressive mediators are involved in the development of cardiac dysfunction after trauma and during sepsis and might also predispose to cardiac damage after long bone fracture. For sure, many other cytokines are systemically released after fracture such as IL-1β, IL-6, IL-8, TGFβ, and G-CSF, which further may contribute to cardiac alterations after fracture and to the development of cardiac dysfunction (72,73).
In the present study, we treated human CMs with an inflammation cocktail, including the inflammatory cytokines IL-1β, IL-6, IL-8, and TNF, as well as the anaphylatoxins C3a and C5a, which all have been shown to be elevated systemically after fracture. In the present study, the presence of the inflammation cocktail impaired mitochondrial respiration of the human cardiomyocytes and increased the amount of cellular ROS, which might be mediated via NLRP3 inflammasome activation and IL-1β release. Since the observed effects of inflammation-cocktail on human cardiomyocytes were multifactorial and could be mediated through synergistic interactions, we selected the inflammatory TNF for the further experiments to specify the cardio-depressive effects of the single fracture-specific inflammatory cytokines. In the present study, presence of TNF increased the release of troponin I, extracellular histones as well as the amount of cytosolic ROS in human CMs in vitro. The caspase-3 activity as well as the number of apoptotic cells in left ventricles of pigs were not altered in the present study, indicating an apoptosis-independent release of troponin I after long bone fracture. This is in contrast to previous studies, which showed a correlation between myocardial TNF concentrations, cardiac dysfunction, and CM apoptosis during sepsis as well as in chronic heart failure (12,13). Furthermore, incubation of CMs with plasma of mice receiving mechanical trauma increased apoptosis in cells, which was ameliorated in the presence of an anti-TNF-antibody (74). Also, elevation of caspases 3, 8, 9, and the pro-apoptotic factor BCL-2 associated X protein were described in isolated rat CMs in the presence of IL-1ß and TNF (10). Therefore, the release of the cardio-depressive inflammatory cytokine TNF after isolated long bone fracture might contribute to the present cardiac structural alterations.
One major limitation of this study is the age discrepancy between the pigs and the humans. Whilst the pigs are juvenile, the patients in this study are adult. These age differences may bias the data and should be always considered when investigating and interpreting cardiac function, cardiac morphology but also systemic inflammation after fracture. Consequently, further studies have to be performed with younger patients to determine cardiac alterations after fracture. Despite the age differences of the used species we were able to show indications for cardiac alterations and cardiac injury in both species. Moreover, besides the difficulties in interpreting the data, the authors want to underline that one advantage of the present study is the comparison between the different species and models with regard to cardiac alterations after single long bone fracture.
Summarized, in the present study we demonstrated systemic as well as local cardiac alterations after isolated long bone fracture. These alterations were linked to the systemic release of inflammatory mediators and DAMPs. Finally, the observed systemic as well as local cardiac alterations after isolated long bone fracture might predispose for secondary cardiac injury. However, this has to be addressed in more detail in future studies.
Footnotes
BW and IL contributed equally to this work.
This work was conducted in the framework of the CRC 1149 funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project number 251293561. Furthermore, funding for the porcine experiments was granted by the AO Project no. S-14-14P.
The authors report no conflicts of interest.
REFERENCES
- 1.Shakur H, Roberts I, Piot P, Horton R, Krug E, Mersch J. A promise to save 100 000 trauma patients. Lancet 2012; 380 (9859):2062–2063. [DOI] [PubMed] [Google Scholar]
- 2.Hanschen M, Kanz K-G, Kirchhoff C, Khalil PN, Wierer M, van Griensven M, Laugwitz K-L, Biberthaler P, Lefering R, Huber-Wagner S. Blunt cardiac injury in the severely injured—a retrospective multicentre study. PLoS One 2015; 10 (7):e0131362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crown LA, Hawkins W. Commotio cordis. Am Fam Physician 1997; 55 (7):2467–2470. [PubMed] [Google Scholar]
- 4.Kalbitz M, Schwarz S, Weber B, Bosch B, Pressmar J, Hoenes FM, Braun CK, Horst K, Simon TP, Pfeifer R, et al. Cardiac depression in pigs after multiple trauma—characterization of posttraumatic structural and functional alterations. Sci Rep 2017; 7 (1):17861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalbitz M, Pressmar J, Stecher J, Weber B, Weiss M, Schwarz S, Miltner E, Gebhard F, Huber-Lang M. The role of troponin in blunt cardiac injury after multiple trauma in humans. World J Surg 2017; 41 (1):162–169. [DOI] [PubMed] [Google Scholar]
- 6.Tsai CH, Lin CL, Hsu HC, Chung WS. Increased risk of coronary heart disease in patients with hip fracture. Osteoporos Int 2015; 26 (6):1849–1855. [DOI] [PubMed] [Google Scholar]
- 7.Chiang CH, Liu CJ, Chen PJ, Huang CC, Hsu CY, Chen ZY, Chan WL, Huang PH, Chen TJ, Chung CM, et al. Hip fracture and risk of acute myocardial infarction. J Bone Miner Res 2013; 28 (2):404–411. [DOI] [PubMed] [Google Scholar]
- 8.Giganti MG, Liuni F, Celi M, Gasbarra E, Zenobi R, Tresoldi I, Modesti A, Bei R, Tarantino U. Changes in serum levels of TNF-alpha, IL-6, OPG, RANKL and their correlation with radiographic and clinical assessment in fragility fractures and high energy fractures. J Biol Regul Homeost Agents 2012; 26 (4):671–680. [PubMed] [Google Scholar]
- 9.Yu MD, Su BH, Zhang XX. Morphologic and molecular alteration during tibia fracture healing in rat. Eur Rev Med Pharmacol Sci 2018; 22 (5):1233–1240. [DOI] [PubMed] [Google Scholar]
- 10.Wu H, Wang G, Li S, Zhang M, Li H, Wang K. TNF-alpha- mediated-p38-dependent signaling pathway contributes to myocyte apoptosis in rats subjected to surgical trauma. Cell Physiol Biochem 2015; 35 (4):1454–1466. [DOI] [PubMed] [Google Scholar]
- 11.Natanson C, Eichenholz PW, Danner RL, Eichacker PQ, Hoffman WD, Kuo GC, Banks SM, MacVittie TJ, Parrillo JE. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med 1989; 169 (3):823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, Thompson M, Giroir B. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation 1998; 97 (14):1375–1381. [DOI] [PubMed] [Google Scholar]
- 13.Lee AS, Chen WP, Kuo YL, Ho YJ, Lee SS, Su MJ. Thaliporphine preserves cardiac function of endotoxemic rabbits by both directly and indirectly attenuating NFkappaB signaling pathway. PLoS One 2012; 7 (6):e39174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med 1996; 183 (3):949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalbitz M, Fattahi F, Herron TJ, Grailer JJ, Jajou L, Lu H, Huber-Lang M, Zetoune FS, Sarma JV, Day SM, et al. Complement destabilizes cardiomyocyte function in vivo after polymicrobial sepsis and in vitro. J Immunol 2016; 197 (6):2353–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrnthaller C, Huber-Lang M, Kovtun A, Rapp AE, Kemmler J, Gebhard F, Ignatius A. C5aR inhibition in the early inflammatory phase does not affect bone regeneration in a model of uneventful fracture healing. Eur J Med Res 2016; 21 (1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergdolt S, Kovtun A, Hagele Y, Liedert A, Schinke T, Amling M, Huber-Lang M, Ignatius A. Osteoblast-specific overexpression of complement receptor C5aR1 impairs fracture healing. PLoS One 2017; 12 (6):e0179512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grailer JJ, Canning BA, Kalbitz M, Haggadone MD, Dhond RM, Andjelkovic AV, Zetoune FS, Ward PA. Critical role for the NLRP3 inflammasome during acute lung injury. J Immunol 2014; 192 (12):5974–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood 2009; 114 (21):4613–4623. [DOI] [PubMed] [Google Scholar]
- 20.Kalbitz M, Grailer JJ, Fattahi F, Jajou L, Herron TJ, Campbell KF, Zetoune FS, Bosmann M, Sarma JV, Huber-Lang M, et al. Role of extracellular histones in the cardiomyopathy of sepsis. FASEB J 2015; 29 (5):2185–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroner J, Kovtun A, Kemmler J, Messmann JJ, Strauss G, Seitz S, Schinke T, Amling M, Kotrba J, Froebel J, et al. Mast cells are critical regulators of bone fracture-induced inflammation and osteoclast formation and activity. J Bone Miner Res 2017; 32 (12):2431–2444. [DOI] [PubMed] [Google Scholar]
- 22.Kovtun A, Bergdolt S, Wiegner R, Radermacher P, Huber-Lang M, Ignatius A. The crucial role of neutrophil granulocytes in bone fracture healing. Eur Cell Mater 2016; 32:152–162. [DOI] [PubMed] [Google Scholar]
- 23.Horst K, Simon TP, Pfeifer R, Teuben M, Almahmoud K, Zhi Q, Santos SA, Wembers CC, Leonhardt S, Heussen N, et al. Characterization of blunt chest trauma in a long-term porcine model of severe multiple trauma. Sci Rep 2016; 6:39659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer V, Kalbitz M, Muller-Graf F, Gebhard F, Ignatius A, Liedert A, Haffner-Luntzer M. Influence of menopause on inflammatory cytokines during murine and human bone fracture healing. Int J Mol Sci 2018; 19 (7): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ. Nat Methods 2012; 9 (7):671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pyle WG, Solaro RJ. At the crossroads of myocardial signaling: the role of Z-discs in intracellular signaling and cardiac function. Circ Res 2004; 94 (3):296–305. [DOI] [PubMed] [Google Scholar]
- 27.Klauke B, Kossmann S, Gaertner A, Brand K, Stork I, Brodehl A, Dieding M, Walhorn V, Anselmetti D, Gerdes D, et al. De novo desmin-mutation N116S is associated with arrhythmogenic right ventricular cardiomyopathy. Hum Mol Genet 2010; 19 (23):4595–4607. [DOI] [PubMed] [Google Scholar]
- 28.Taylor MR, Slavov D, Ku L, Di Lenarda A, Sinagra G, Carniel E, Haubold K, Boucek MM, Ferguson D, Graw SL, et al. Prevalence of desmin mutations in dilated cardiomyopathy. Circulation 2007; 115 (10):1244–1251. [DOI] [PubMed] [Google Scholar]
- 29.Hnia K, Ramspacher C, Vermot J, Laporte J. Desmin in muscle and associated diseases. Cell Tissue Res 2015; 360 (3):591–608. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Li F, Campbell SE, Gerdes AM. Chronic pressure overload cardiac hypertrophy and failure in guinea pigs: II. Cytoskeletal remodeling. J Mol Cell Cardiol 1999; 31 (2):319–331. [DOI] [PubMed] [Google Scholar]
- 31.Sheng J-J, Feng H-Z, Pinto JR, Wei H, Jin J-P. Increases of desmin and alpha-actinin in mouse cardiac myofibrils as a response to diastolic dysfunction. J Mol Cell Cardiol 2016; 99:218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramspacher C, Steed E, Boselli F, Ferreira R, Faggianelli N, Roth S, Spiegelhalter C, Messaddeq N, Trinh L, Liebling M, et al. Developmental alterations in heart biomechanics and skeletal muscle function in desmin mutants suggest an early pathological root for desminopathies. Cell Rep 2015; 11 (10):1564–1576. [DOI] [PubMed] [Google Scholar]
- 33.Panagopoulou P, Davos CH, Milner DJ, Varela E, Cameron J, Mann DL, Capetanaki Y. Desmin mediates TNF-alpha-induced aggregate formation and intercalated disk reorganization in heart failure. J Cell Biol 2008; 181 (5):761–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gard JJ, Yamada K, Green KG, Eloff BC, Rosenbaum DS, Wang X, Robbins J, Schuessler RB, Yamada KA, Saffitz JE. Remodeling of gap junctions and slow conduction in a mouse model of desmin-related cardiomyopathy. Cardiovasc Res 2005; 67 (3):539–547. [DOI] [PubMed] [Google Scholar]
- 35.Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res 2001; 88 (3):333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agullo-Pascual E, Cerrone M, Delmar M. Arrhythmogenic cardiomyopathy and Brugada syndrome: diseases of the connexome. FEBS Lett 2014; 588 (8):1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mair J. Tissue release of cardiac markers. Clin Chem Lab Med 1999; 37 (11–12):1084. [DOI] [PubMed] [Google Scholar]
- 38.Korff S, Katus HA, Giannitsis E. Differential diagnosis of elevated troponins. Heart 2006; 92 (7):987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bock JS, Benitez RM. Blunt cardiac injury. Cardiol Clin 2012; 30 (4):545–555. [DOI] [PubMed] [Google Scholar]
- 40.Braun CK, Kalbitz M, Halbgebauer R, Eisele P, Messerer DAC, Weckbach S, Schultze A, Braumuller S, Gebhard F, Huber-Lang MS. Early structural changes of the heart after experimental polytrauma and hemorrhagic shock. PLoS One 2017; 12 (10):e0187327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalbitz M, Amann EM, Bosch B, Palmer A, Schultze A, Pressmar J, Weber B, Wepler M, Gebhard F, Schrezenmeier H, et al. Experimental blunt chest trauma-induced myocardial inflammation and alteration of gap-junction protein connexin 43. PLoS One 2017; 12 (11):e0187270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onuoha GN, Alpar EK, Dean B, Tidman J, Rama D, Laprade M, Pau B. Skeletal troponin-I release in orthopedic and soft tissue injuries. J Orthop Sci 2001; 6 (1):11–15. [DOI] [PubMed] [Google Scholar]
- 43.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, et al. Third universal definition of myocardial infarction. Circulation 2012; 126 (16):2020–2035. [DOI] [PubMed] [Google Scholar]
- 44.Arshed S, Luo HX, Zafar S, Regeti K, Malik N, Alam M, Yousif A. Elevated troponin I in the absence of coronary artery disease. J Clin Med Res 2015; 7 (10):820–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramaraj R. Stress cardiomyopathy. Postgrad Med J 2007; 83 (982):543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziegelstein RC. Depression and tako-tsubo cardiomyopathy. Am J Cardiol 2010; 105 (2):281–282. [DOI] [PubMed] [Google Scholar]
- 47.Kalbitz M, Fattahi F, Grailer JJ, Jajou L, Malan EA, Zetoune FS, Huber-Lang M, Russell MW, Ward PA. Complement-induced activation of the cardiac NLRP3 inflammasome in sepsis. FASEB J 2016; 30 (12):3997–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller M, Herzog C, Larmann J, Schmitz M, Hilfiker-Kleiner D, Gessner JE, Theilmeier G. The receptor for activated complement factor 5 (C5aR) conveys myocardial ischemic damage by mediating neutrophil transmigration. Immunobiology 2013; 218 (9):1131–1138. [DOI] [PubMed] [Google Scholar]
- 49.Hoesel LM, Niederbichler AD, Ward PA. Complement-related molecular events in sepsis leading to heart failure. Mol Immunol 2007; 44 (1–3):95–102. [DOI] [PubMed] [Google Scholar]
- 50.Niederbichler AD, Hoesel LM, Westfall MV, Gao H, Ipaktchi KR, Sun L, Zetoune FS, Su GL, Arbabi S, Sarma JV, et al. An essential role for complement C5a in the pathogenesis of septic cardiac dysfunction. J Exp Med 2006; 203 (1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 2006; 71 (2):310–321. [DOI] [PubMed] [Google Scholar]
- 52.Atefi G, Zetoune FS, Herron TJ, Jalife J, Bosmann M, Al-Aref R, Sarma JV, Ward PA. Complement dependency of cardiomyocyte release of mediators during sepsis. FASEB J 2011; 25 (7):2500–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, Zetoune FS, Gerard NP, Cianflone K, Kohl J, et al. Functional roles for C5a receptors in sepsis. Nat Med 2008; 14 (5):551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alhamdi Y, Abrams ST, Cheng Z, Jing S, Su D, Liu Z, Lane S, Welters I, Wang G, Toh C-H. Circulating histones are major mediators of cardiac injury in patients with sepsis. Crit Care Med 2015; 43 (10):2094–2103. [DOI] [PubMed] [Google Scholar]
- 55.Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, Wang SS, Brohi K, Kipar A, Yu W, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med 2013; 187 (2):160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holdenrieder S, Stieber P, Bodenmuller H, Busch M, Pawel, Schalhorn A, Nagel D, Seidel D. Circulating nucleosomes in serum Ann N Y. Acad Sci 2001; 945:93–102. [DOI] [PubMed] [Google Scholar]
- 57.van der Vaart M, Pretorius PJ. The origin of circulating free DNA. Clin Chem 2007; 53:2215. [DOI] [PubMed] [Google Scholar]
- 58.Remijsen Q, Vanden Berghe T, Wirawan E, Asselbergh B, Parthoens E, Rycke Rde, Noppen S, Delforge M, Willems J, Vandenabeele P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res 2011; 21 (2):290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hampson P, Dinsdale RJ, Wearn CM, Bamford AL, Bishop JRB, Hazeldine J, Moiemen NS, Harrison P, Lord JM. Neutrophil dysfunction, immature granulocytes, and cell-free DNA are early biomarkers of sepsis in burn-injured patients. Ann Surg 2017; 265 (6):1241–1249. [DOI] [PubMed] [Google Scholar]
- 60.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med 2009; 15 (11):1318–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kleine TJ, Lewis PN, Lewis SA. Histone-induced damage of a mammalian epithelium. Am J Physiol 1997; 273 (6 pt 1):C1925–C1936. [DOI] [PubMed] [Google Scholar]
- 62.Kleine TJ, Gladfelter A, Lewis PN, Lewis SA. Histone-induced damage of a mammalian epithelium. Am J Physiol 1995; 268 (5 pt 1):C1114–C1125. [DOI] [PubMed] [Google Scholar]
- 63.Kawai C, Kotani H, Miyao M, Ishida T, Jemail L, Abiru H, Tamaki K. Circulating extracellular histones are clinically relevant mediators of multiple organ injury. Am J Pathol 2016; 186 (4):829–843. [DOI] [PubMed] [Google Scholar]
- 64.Huang H, Evankovich J, Yan W, Nace G, Zhang L, Ross M, Liao X, Billiar T, Xu J, Esmon CT, et al. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology 2011; 54 (3):999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang X, Li L, Liu J, Lv B, Chen F. Extracellular histones induce tissue factor expression in vascular endothelial cells via TLR and activation of NF-kappaB and AP-1. Thromb Res 2016; 137:211–218. [DOI] [PubMed] [Google Scholar]
- 66.Silk E, Zhao H, Weng H, Ma D. The role of extracellular histone in organ injury. Cell Death Dis 2017; 8 (5):e2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allam R, Scherbaum CR, Darisipudi MN, Mulay SR, Hagele H, Lichtnekert J, Hagemann JH, Rupanagudi KV, Ryu M, Schwarzenberger C, et al. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol 2012; 23 (8):1375–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol 2011; 187 (5):2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giroir BP, Horton JW, White DJ, McIntyre KL, Lin CQ. Inhibition of tumor necrosis factor prevents myocardial dysfunction during burn shock. Am J Physiol 1994; 267 (1 pt 2):H118–H124. [DOI] [PubMed] [Google Scholar]
- 70.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD2, Kreisel D, Krupnick AS, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011; 306 (23):2594–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meng X, Ao L, Meldrum DR, Cain BS, Shames BD, Selzman CH, Banerjee A, Harken AH. TNF-alpha and myocardial depression in endotoxemic rats. Am J Physiol 1998; 275 (2):R502–R508. [DOI] [PubMed] [Google Scholar]
- 72.Li H, Liu J, Yao J, Zhong J, Guo L, Sun T. Fracture initiates systemic inflammatory response syndrome through recruiting polymorphonuclear leucocytes. Immunol Res 2016; 64 (4):1053–1059. [DOI] [PubMed] [Google Scholar]
- 73.Volpin G, Cohen M, Assaf M, Meir T, Katz R, Pollack S. Cytokine levels (IL-4, IL-6, IL-8 and TGFbeta) as potential biomarkers of systemic inflammatory response in trauma patients. Int Orthop 2014; 38 (6):1303–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li S, Tao L, Jiao X, Liu H, Cao Y, Lopez B, Luan R-H, Christopher T, Ma XL. TNFalpha-initiated oxidative/nitrative stress mediates cardiomyocyte apoptosis in traumatic animals. Apoptosis 2007; 12 (10):1795–1802. [DOI] [PubMed] [Google Scholar]


