Abstract:
Herpes simplex keratitis, caused primarily by human herpes simplex virus type 1 (HSV-1), remains the most common infectious cause of unilateral blindness and vision impairment in the industrialized world. Major advances in the care of HSV keratitis have been driven in large part by the landmark Herpetic Eye Disease Study randomized clinical trials, which were among the first in ophthalmology to reflect emerging trial conventions, including multicenter subject enrollment, double-masking, placebo controls, and a priori sample size determinations. The results of these trials now form much of the evidence basis for the management of this disease. However, management patterns in clinical practice often deviate from evidence-based care. These perceived quality gaps have given rise to the evolving field of implementation science, which is concerned with the methods of promoting the application of evidence-based medicine within routine care. To overcome variations in the quality and consistency of care for HSV keratitis, a range of clinical- and technology-based innovations are proposed. The most pressing needs include the following: a rational and tractable disease classification scheme that provides an immediate link between the anatomical localization of disease (corneal epithelial, stromal, or endothelial) and the appropriate treatment, and the actualization of an electronic medical record system capable of providing evidence-based treatment algorithms at relevant points of care. The latter would also input data to population-wide disease registries to identify implementation-rich targets for quality improvement, education, and research. These innovations may allow us to reduce the human and economic burdens of this highly morbid, and often blinding, disease.
Key Words: herpes simplex virus, keratitis, classification, implementation, diffusion of innovations
“But there were also other fevers… Many had their mouths affected with aphthous ulcerations. There were also many defluxions about the genital parts, ulcerations, and boils (phymata), externally and internally, about the groins. Watery ophthalmies of chronic character, with pains and fungous excrescences of the eyelids, externally and internally, called fig, which destroyed the sight of many persons.”
—Hippocrates, Book II Section III, Of the Epidemics (c. 400 BCE), translated by Francis Adams1
Since antiquity, infections caused by the human herpes simplex virus (HSV) have presented a significant diagnostic and therapeutic challenge for physicians. The first possible descriptions of HSV infections were documented by ancient Greek scholars such as Hippocrates (c. 460 BCE to c. 375 BCE), who used the term “herpes” to describe skin lesions which would “creep” or “crawl” over affected areas.2–4 Medical, cultural, and even political references to such orocutaneous eruptions would appear thereafter, from the Roman emperor Tiberius forbidding the act of kissing during his reign (14–37 AD)5,6 to Shakespeare's description of lesions arising “O'er ladies' lips… with blisters plagues” in his tragedy Romeo and Juliet.7 It was not until the mid to late 19th century that an infectious agent was implicated, with the work of a French dermatologist Vidal8 demonstrating human transmission by the induction of herpetic vesicles in healthy subjects after inoculation with infected fluid. The corneal manifestations of HSV infection also gained recognition around this time,9 with Swiss ophthalmologists Horner and Emmert describing “herpes corneal febrilis”10,11 and “dendritic keratitis”12 respectively, both characterized by a nonspecific prodrome of fever and coryza, followed by vision-reducing corneal disease. HSV was later successfully isolated in experimental rabbit models of herpetic keratitis performed by Löwenstein,13 Gruter,14 and Lipshütz.15 These early investigations at the turn of the 20th century laid the foundation for our current understanding of HSV infections.
HSV keratitis remains a leading infectious cause of blindness worldwide.16 Where ancient Greek physicians may have once treated ocular maledictions with bloodletting and wine to restore the capricious balance of the body's humors,17 great strides have been made in establishing the evidence basis for the care of HSV keratitis. Specifically, the landmark Herpetic Eye Disease Study (HEDS), conducted from the late 1980s to the 1990s, provides guidance as to the role of antiviral therapy and topical corticosteroids in the treatment of, and prophylaxis for, the varied presentations of HSV keratitis. However, patterns of clinical care often radically diverge from what is considered “best practice.”18–20 This disparity has given rise to the field of implementation science, which is principally concerned with methods to systematically translate the evidence basis for any condition into routine care.21,22 Using this framework, we propose a range of clinical innovations to reduce the global burden of HSV keratitis. These include the introduction of a standardized system of disease classification and the resolution of age-old “eminence-based” teachings that may conflict with current best practice. Furthermore, the actualization of a “living,” machine learning–enabled electronic medical record (EMR) would support evidence-based medicine by providing clinicians with point-of-care access to validated diagnostic and management algorithms, in addition to autopopulating disease registries to identify the unmet needs in continuing medical education and research.23 Together, the widespread adoption of these clinical innovations could change the trajectory of overall care for this ancient, highly morbid disease.
CURRENT CARE FOR HSV KERATITIS
The Human Herpes Simplex Viruses
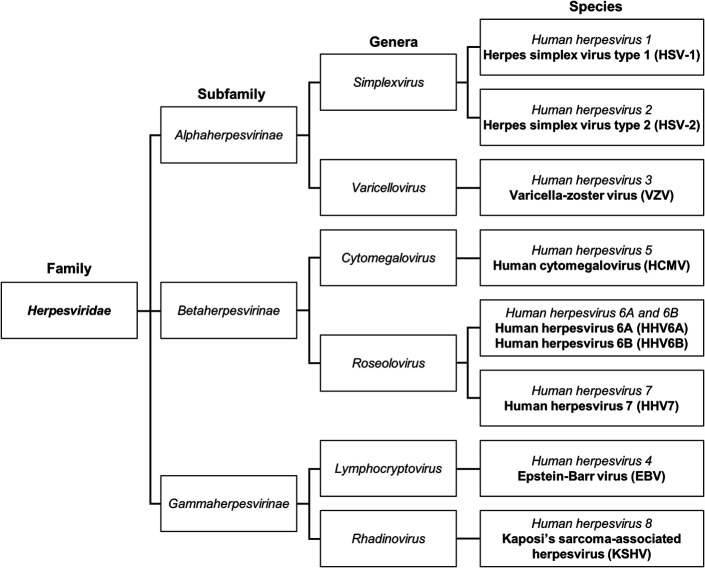
Virus taxonomy is determined by phylogenetic comparisons of validated, whole viral genomes, and is overseen by the International Committee on Taxonomy of Viruses (ICTV).24 In 2009, the ICTV reclassified herpesviruses into the order Herpesvirales,25 comprising hundreds of closely related viruses, all sharing a double-stranded DNA genome, a 20-faceted icosahedral capsid, a surrounding proteinaceous tegument, and an external glycoprotein-laden lipid envelope.24,26 Nine species of these viruses are known to infect humans (Fig. 1).27–29 HSV type 1 (human herpesvirus 1, HSV-1) and HSV type 2 (human herpesvirus 2, HSV-2), of family Herpesviridae, subfamily Alphaherpesvirinae, and genus Simplexvirus, share 40% to 50% nucleotide sequence homology30 and cause a wide array of diseases because of their broad tissue tropisms. These include orofacial and genital mucocutaneous infections, meningoencephalitis, varied presentations of ocular disease, and visceral invasion, the latter occurring in association with severe immune dysfunction.31–33 The ocular manifestations of HSV include adnexal and/or anterior segment disease in the forms of blepharoconjunctivitis, keratitis, trabeculitis, and anterior uveitis, along with posterior segment disease, including acute retinal necrosis and posterior uveitis.34,35 The morbidity and mortality associated with herpetic infections is significant and is compounded by the ability of herpes viruses to establish lifelong viral latency.36–39 HSV-1 and HSV-2 are neurotrophic viruses, which remain dormant in sensory neurons of the dorsal root and trigeminal ganglia.40–46 Viral reactivation can cause disease recrudescence many years after primary infection.
FIGURE 1.
Taxonomy of the family Herpesviridae, as determined by the International Committee on Taxonomy of Viruses and based on phylogenetic analysis of whole viral genomes.24 This truncated chart only includes the 9 species of human herpesviruses. Among these, HSV-1 and HSV-2, representing the subfamily Alphaherpesvirinae and genus Simplexvirus, are indistinguishably associated with keratitis. The third human herpesvirus in this subfamily, varicella-zoster virus, of the genus Varicellovirus, is another cause of corneal infection.
Epidemiology of Ocular HSV
Pooled data suggest that there are 1 to 1.5 million new16,47,48 and 9 million recurrent cases47,48 of ocular HSV each year worldwide, resulting in at least 40,000 cases of new-onset, severe visual disability per year.16 However, diagnosis often relies on nonspecific clinical signs. Furthermore, heterogeneity in the study design and outcome measures, compounded by a paucity of data from low-income countries, add considerable imprecision to current global estimates. The long-held notion that ocular HSV represents the leading infectious cause of blindness in middle-to high-income countries49 stems from epidemiological studies conducted in Denmark,50 Croatia,51 France,52 and the United States,53–55 where the reported incidence rates of total (new and recurrent) cases range from 4.1 to 31.5 per 100,000 persons per year. In the United States, much of the data derive from two retrospective cohort studies from Rochester, MN, conducted over two distinct time periods, 1950 to 1982 and 1976 to 2007.53,54 These studies suggest a rise in the annual incidence of new ocular HSV cases from 8.4 to 11.8 per 100,000 persons per year. Extrapolating the age- and sex-adjusted incidence of 20.7 total cases per 100,000 person years,53 as derived from the 1950 to 1982 cohort, to a US census population of 329,135,084 as of January 2020,56 the number of new and recurrent episodes of ocular HSV now exceeds 68,000 annually. As populations age and grow, we expect the burden associated with decreased visual function, lost productivity, and need for continuing care to also increase.
Clinical Manifestations of HSV Keratitis: The Role of Classification
A simple and unambiguous disease classification system is critical to the implementation of evidence-based therapy based on stages or manifestations of the disease. HSV keratitis includes at least four distinct entities, each with its own pathophysiology and varied responses to therapeutic interventions. Historical characterizations of disease patterns observed in HSV keratitis include terms such as “dendritic,”57–60 “geographic,”57–60 “amoeboid ulceration,”61–63 “interstitial,”59,64,65 “immune stromal,”57,58,64,65 “necrotizing,”57,58,66–69 and “disciform,”57–60,66,68,70,71 that are routinely used as primary classifiers of disease. Morphological descriptions can indeed be useful in generating a weighted differential diagnosis. For example, corneal epithelial ulceration with a dendritic shape and terminal bulbs at the edges most commonly represents HSV epithelial keratitis, although not always.72–75 However, other terms are misleading and prone to inconsistent application. “Geographic” keratitis, a term applied to large areas of denuded corneal stroma resulting from HSV epithelial infection, is vague and nondescript and can lead to misdiagnosis, for example, in patients with persistent corneal epithelial defects. “Immune stromal” keratitis, used to describe corneal inflammation due to HSV in which viral replication is not a prominent feature, suggests the false narrative that other variations of HSV stromal keratitis do not involve immune activation. The characterizations of keratitis as “interstitial” or “necrotizing” are more appropriately applied to tissue biopsies examined by histopathology. In Japan, “disciform” keratitis is considered a type of stromal keratitis.76 In the United States, the term typically refers to endothelial keratitis but has also been applied to stromal keratitis presenting with round or oval infiltrates.66,68 In addition, “disciform” does not capture other patterns of endothelial disease (eg, diffuse and linear) that have the same pathogenesis and treatment. Unfortunately, much of the HSV keratitis lexicon has been formally codified in the International Classification of Diseases. Confusion in diagnostic terminology may contribute to misapplications of therapy, lost opportunities to treat the disease in its earliest stages, and even frank medical errors.
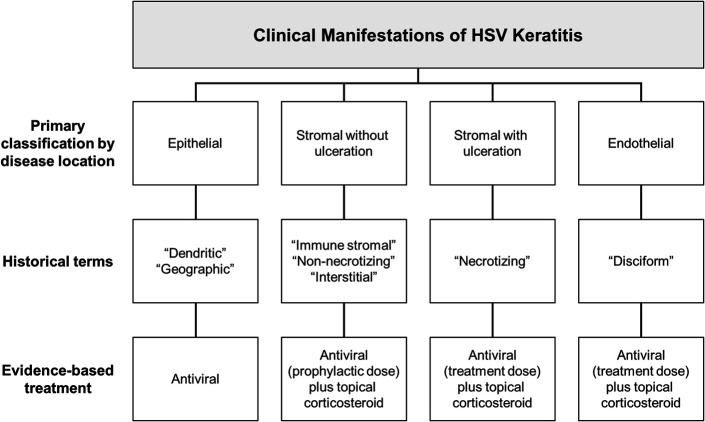
A comprehensive classification scheme based on the anatomical layer of the cornea most prominently involved—epithelium, stroma, or endothelium—is an easily implemented clinical innovation (Fig. 2) that enables anyone experienced in slit-lamp examination to easily synthesize the clinical signs and pathognomonic features associated with HSV keratitis. Implicit to this classification is that identification of the corneal layer most clearly involved correlates well with the unique pathogenic mechanisms that in turn mandate specific therapeutic measures. With standardized nomenclature, this system also provides a logical approach to understanding the epidemiology and natural history of the disease. As such, it has broad applications, from aiding the conduct of systematic reviews and meta-analyses to allowing clinicians to properly counsel patients regarding prognosis. HSV epithelial keratitis, typically a self-limiting process, results from the cytopathic effects of corneal epithelial infection and cell death. The condition is characterized by the presence of an epithelial ulcer with raised grey edges, typically with a leafy, branching appearance (dendrite), although larger (geographic) ulcers may bare the epithelial basement membrane (Fig. 3). Stromal keratitis occurs either with or without epithelial ulceration. Stromal keratitis without ulceration, the more common form, is often described as “nonnecrotizing,” “immune-stromal,” and “interstitial.” Focal, multifocal, or diffuse HSV-related stromal inflammation with intact epithelium is believed to represent immunopathology in the relative absence of viral replication.71,77–82 Stromal keratitis with overlying epithelial ulceration, most likely the result of stromal HSV reactivation,83–85 is characterized by severe, “necrotizing” inflammation and a proclivity for scar formation and progressive neovascularization. Finally, endothelial “disciform” keratitis is believed to result from infection of the corneal endothelium.86–88 This form of keratitis is typically associated with a distinct area of corneal edema with underlying keratic precipitates, although diffuse and linear patterns can also occur. The remaining, uninvolved cornea is characteristically clear. This clinical picture is not to be confused with anterior uveitis associated with secondary keratic precipitates, which, unlike endothelial keratitis, is distinguished by a pronounced anterior chamber reaction. Although all forms of HSV keratitis are commonly recurrent, the risk is greatest in stromal keratitis, which is the most likely to result in corneal scarring, thinning, and neovascularization.89,90
FIGURE 2.
Overview of clinical manifestations and management of HSV keratitis, classified by anatomical localization. For full treatment recommendations, see Herpes Simplex Keratitis: A Treatment Guideline (2014) by White and Chodosh47 as published by the American Academy of Ophthalmology.
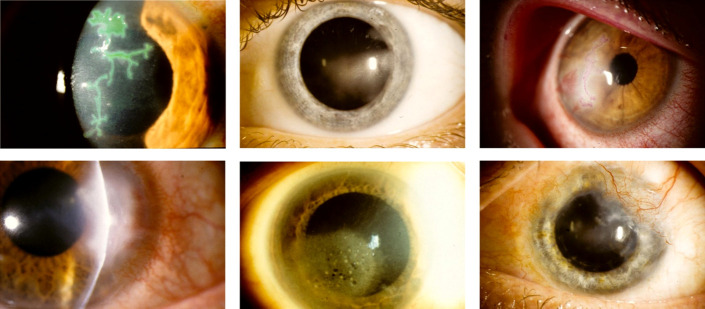
FIGURE 3.
Clinical photography of varying manifestations of HSV keratitis. Top row: epithelial keratitis with pathognomonic dendritic ulcer, visualized with lissamine green staining (left); stromal keratitis without ulceration (middle); mixed epithelial and stromal keratitis, visualized with rose bengal dye staining (right). Bottom row: stromal keratitis with ulceration (left); endothelial keratitis (middle); chronic, scarring stromal keratitis with limbal neovascularization (right).
Treatment of HSV Keratitis
Before the discovery of specific antiviral agents, ophthalmologists treated HSV keratitis with a wide range of approaches, including manual debridement, chemical cautery, photoreactive dyes, surgery, antiseptics, and antibiotics.91 More extreme treatments included intramuscular injections of placental extract,92 subconjunctival injections of autologous blood,93 snake venom,94 and radiotherapy.95 In 1962, Kaufman et al demonstrated the first evidence of clinical benefit for the newly synthesized antiviral, idoxuridine, first in rabbits96 and then in humans.97 Small, uncontrolled studies followed investigating other antiviral agents such as trifluridine, vidarabine, and acyclovir, mostly in the care of HSV epithelial keratitis.35,98 Treatments for HSV keratitis evolved concurrently with advances in the conduct of randomized clinical trials (RCTs) during the 1980s. Emerging trial conventions, emphasizing the importance of methodologic rigor, included the designation of control and/or placebo groups, explicit specification of recruitment criteria, masking, and pretrial determinations of sample size requirements. The National Institutes of Health-funded, placebo-controlled, double-masked, multicenter HEDS RCTs,99 completed in the 1990s, were the first in HSV keratitis to include a priori power-based calculations for enrollment targets.100 The so-called HEDS-I101 included the Herpes Stromal Keratitis Not on Steroid (HEDS-SKN),102 Herpes Stromal Keratitis on Steroid Treatment (HEDS-SKS),103 and the HSV Iridocyclitis Receiving Topical Steroids (HEDS-IRT)104 trials. HEDS-II105 consisted of the HSV Epithelial Keratitis (HEDS-EKT)106 and Acyclovir Prevention (HEDS-APT) trials.107 A third study, the Ocular HSV Recurrence Factor Study (HEDS-RFS), was a questionnaire-based study that investigated precipitating factors for recurrence.108 The HEDS trials formed the evidence basis for the publication of a treatment guideline by White and Chodosh47 in 2014 (https://www.aao.org/clinical-statement/herpes-simplex-virus-keratitis-treatment-guideline).
Treatment of HSV Epithelial Keratitis
The use of antiviral therapy for HSV epithelial keratitis is supported by decades of clinical trial data. In the United States, Food and Drug Administration–approved topical antiviral formulations include trifluridine solution (1%), ganciclovir gel (0.15%), and a newly available acyclovir ointment (3%), whereas systemic formulations include acyclovir, valacyclovir, and famciclovir. Treatments that have been superseded include antivirals that are no longer manufactured, such as idoxuridine and vidarabine, or those that are effective but associated with unacceptable systemic toxicity, including oral valganciclovir, foscarnet, and cidofovir.47 The effectiveness of antiviral therapy for HSV epithelial keratitis was confirmed in an updated Cochrane systematic review and meta-analysis conducted by Wilhelmus, which included 137 randomized studies involving 8333 eyes.98 This study confirmed that the earliest antivirals, idoxuridine and vidarabine, were superior to controls, and that vidarabine, trifluridine, acyclovir, and brivudine were superior to idoxuridine in 2-week healing rates.98 Comparisons of topical ganciclovir to topical acyclovir in 28 studies involving 2062 eyes showed a modest advantage of ganciclovir in healing rates (relative risk: 1.34, 95% confidence interval [CI]: 1.20–1.51), although study heterogeneity and publication bias may have confounded this finding. Taken as a whole, the literature suggests that topical trifluridine, acyclovir, and ganciclovir are, at a minimum, not inferior to each other in the treatment of HSV epithelial keratitis.
The off-label use of oral antivirals—which are at least as effective as their topical counterparts35,98,109,110—is preferred by many corneal specialists.19 Oral acyclovir, the most commonly used antiviral for ocular HSV, has good ocular penetration111 and is well tolerated and safe,112,113 although dose adjustment is required in moderate-to-severe renal impairment and in the elderly.114 By contrast, topical antivirals are limited by poor intraocular bioavailability115 and side effects such as ocular surface toxicity,116–118 allergic reactions, and punctal and nasolacrimal duct stenosis.119 One report suggested an association between trifluridine and corneal epithelial dysplasia.120 Although topical and oral antivirals are independently effective in HSV keratitis, there is insufficient and conflicting evidence for combining modalities to accelerate healing.106,121 Finally, nonspecific therapies are only minimally effective at best. For example, manual debridement alone has been shown to be inadequate.122–124 Topical administration of the experimental biologic agent interferon has only a modest benefit over placebo, and its addition to specific antiviral therapy does not alter recovery time.98 Corticosteroids are contraindicated in HSV epithelial keratitis, and when used can cause prolonged infection and conversion of a dendritic epithelial ulcer to that of a larger “geographic” morphology.
Treatment of HSV Stromal Keratitis
The preferred treatment for HSV stromal keratitis is an oral antiviral agent combined with a topical corticosteroid, the latter tapered over a period greater than 10 weeks.47 Stromal keratitis with intact epithelium can be treated with prophylactic doses of antiviral medication, whereas ulcerating disease often requires therapeutic doses from its early stages. Although the requirement for antiviral therapy in HSV stromal keratitis has never been disputed, historically the role of topical corticosteroids was controversial. The introduction of corticosteroids to ophthalmology in the 1950s led to two opposing schools of thought regarding their use in herpetic eye disease, now known as the decades-long “Steroid Wars.”125,126 The “West-Coast” school, led by Thygeson and Hogan at the Proctor Foundation and the University of California, San Francisco, expressed severe reservations regarding the use of corticosteroids for any indication involving HSV keratitis.127–129 The “East-Coast” school, led by Kaufman, Laibson, and Pavan-Langston, among others, advocated for their use in select circumstances, including HSV stromal and endothelial disease.130,131 The lack of clear clinical guidance led to the initiation of HEDS-SKN, which commenced in 1989. This trial randomized 106 patients with active HSV stromal keratitis, all treated with topical trifluridine at baseline, to the addition of either 1% prednisolone sodium phosphate or placebo, tapered over a 10-week period.102 Over 90% of participants had stromal keratitis without epithelial ulceration. The main outcome measure was time to treatment failure, defined as the worsening of stromal inflammation or development of uveitis at any visit, no change in stromal inflammation within 2 weeks of treatment commencement, or the occurrence of an adverse event. By the end of treatment, 26% of the corticosteroid group and 73% of the placebo group had failed treatment. The median time to treatment failure was far longer in the corticosteroid group (98 days, 95% CI: 81 to >120 days) than that in the placebo group (17 days, 95% CI: 14–27 days). Those treated with prednisolone had a shorter median time to clinical resolution (26 days, 95% CI: 14–49 vs. 72 days, 95% CI: 44–123). These unequivocal results, obtained during an interim analysis commissioned by the study's Data and Safety Monitoring Board, led to the early termination of enrollment well short of the initial recruitment target of 178 participants. Of note, the proportion of participants who later reached a study endpoint, as determined at 16 weeks after initiation of the study drug, that is, 6 weeks after tapering off prednisolone or placebo, increased to 49% in the corticosteroid group and 76% in the placebo group. This suggested that a 10-week tapered course of topical corticosteroid was insufficient, leaving patients susceptible to early disease recurrence immediately after drug cessation.
Treatment of HSV Endothelial Keratitis
HSV endothelial keratitis is relatively uncommon and was not directly addressed by the HEDS trials. The current evidence basis for its management is limited to a few, relatively small studies conducted in the 1980s and 1990s.113,118,132–134 Three RCTs showed that in patients treated with 3% topical acyclovir at baseline, the addition of topical betamethasone at concentrations ranging from 0.01% to 0.1% resulted in more rapid resolution compared with the addition of placebo.118,132,133 An open-label RCT later demonstrated that either oral or topical acyclovir, when added to 0.05% topical prednisolone, resulted in similar mean healing times (25.9 days vs. 25.3 days).134 However, oral treatment was associated with faster resolution of symptoms than topical treatment, with a greater degree of visual recovery.134 Therefore, existing evidence supports a combination of an oral antiviral and a topical corticosteroid for the management of HSV endothelial keratitis. Oral antiviral agents penetrate the aqueous humor at therapeutic levels,111 and because HSV endothelial keratitis likely involves viral replication in the posterior cornea,86–88 agents with adequate anterior chamber penetration are preferred. Among the available topical antivirals in the United States, only acyclovir reliably achieves therapeutic levels within the aqueous humor.115,135,136
Prophylaxis for HSV Keratitis
Two arms of the HEDS trials addressed the issue of prophylaxis against recurrence of HSV keratitis. In the HEDS-EKT study, 287 patients with acute HSV epithelial keratitis were treated with topical trifluridine at baseline and randomized to either a 3-week course of concomitant oral acyclovir (400 mg 5 times a day) or placebo.106 At the end of a 12-month follow-up period, no difference was observed between treatment groups in the proportion of patients who later, after the resolution of epithelial keratitis, developed stromal keratitis or iritis (11% vs. 10%). This study demonstrated that oral acyclovir given briefly during an episode of HSV epithelial keratitis does not prevent later stromal keratitis or iritis. The subsequent HEDS-APT study randomly assigned 703 patients with a history of ocular HSV in the preceding year to a 12-month course of prophylactic oral acyclovir (400 mg) or placebo twice daily. All participants were followed up for 6 months after the cessation of treatment, resulting in a total trial duration of 18 months.41 Study participants on oral acyclovir had a significantly reduced cumulative probability of ocular HSV recurrence compared with placebo (19% vs. 32%, P < 0.001). The benefit of oral acyclovir was specific to stromal keratitis, as was shown in a subgroup analysis of the 337 participants who had a history of at least one previous episode of stromal keratitis. Importantly, the prophylactic benefit of oral acyclovir was in effect only when taking the drug; the risk of recurrent stromal keratitis returned to baseline immediately after drug cessation. HEDS-APT did not determine whether prophylaxis beyond 12 months would have successfully reduced recurrences after treatment cessation. Existing data suggest that oral prophylaxis should be considered on an indefinite or long-term basis for high-risk patients, including those with a history of recurrences, atopy,137–139 a corneal allograft in the setting of previous ocular HSV,140–143 or immune compromise.144–146 It is also reasonable to prescribe oral antiviral prophylaxis to patients before scheduled ocular surgery including photorefractive procedures147,148 whenever there is a known history of HSV keratitis, and to maintain prophylaxis until topical corticosteroids are tapered successfully.
IMPLEMENTATION GAPS IN EVIDENCE-BASED CARE
Dissemination and implementation science is the study of methods that promote the systematic translation of evidence-informed practices within a field of interest.149–152 In medicine, the extent to which clinical care is informed by evidence-based medicine—that is, the conscious and discerning use of clinical trial data, systematic reviews, meta-analyses, and clinical practice guidelines—has become an area of intense scholarship.153–156 “Quality gaps”157 and implementation stasis in medicine are not new phenomena, with even the most transformative of medical innovations (eg, penicillin, smallpox vaccination, and hand hygiene) not entering routine patient care until many years after their discovery.158 Empirically, it has been estimated that a “17-year odyssey”159 elapses between the publication of original clinical research and the incorporation of their findings into standard care.160 In part, this is because of the growing recognition that RCTs, the gold standard of medical evidence, are conducted under carefully controlled “experimental” conditions that do not entirely replicate real-life settings. The exclusion of various groups of study participants, often for purely practical reasons, can limit the generalizability of trial results161 as can recruitment criteria for entities such as HSV keratitis, which are diagnosed according to the clinical criteria alone.102 Other factors that contribute to implementation inertia include the time required to “deimplement”162,163 nonevidence-based practices and often the need to establish the appropriate health infrastructure to deliver such care. Overall, clinicians are slow to adopt evidence-based recommendations.164,165
The roots of medical implementation research are found in social and behavioral science, including the literature surrounding the diffusion of innovations. Proponents of diffusion theory study the factors that determine the rate at which innovations are adopted within a specified community,166,167 which classically approximates a sigmoid curve.166,168,169 In its initial phases, the innovation typically fails to gain traction apart from a few early adopters. Further implementation is only achieved with time, attrition, and sustained behavioral and systemic changes.170 In ophthalmology, practice patterns in the care of HSV keratitis are highly variable. This was demonstrated by a 2010 survey of 595 US-based eye care providers, including optometrists, comprehensive ophthalmologists, and cornea specialists, who were asked to identify their preferred treatment choices for the following three uncomplicated cases of HSV keratitis: epithelial, stromal, and a repeated stromal recurrence.19 Although more than 95% of respondents correctly selected a topical or oral antiviral agent for the treatment of epithelial keratitis, only 82% of cornea-trained ophthalmologists elected to treat stromal keratitis correctly with a combination of antiviral and topical corticosteroid therapy. For the prevention of stromal recurrences, 12 years after publication of HEDS-APT, only 62% of corneal specialists correctly elected to prescribe a prophylactic oral antiviral agent. Such disparities in the care of HSV keratitis are not unique to the United States,52,171–173 suggesting an overall lack of adoption of HEDS-derived recommendations.
Historically, medical practitioners have drawn on “eminence-based”174–176 or “tradition-based”177,178 teachings to guide patient care, leaning on the authority, experience, and wisdom of senior physicians and leading experts. However, the development of the modern RCT, comparative effectiveness research,179 and evidence-based medicine has shifted the emphasis to data-driven clinical decision-making. Despite this change in medical culture, eminence- and evidence-based medicine can be complementary.180,181 Clinical medicine and its evidence basis are replete with knowledge gaps, and there are perfectly sound clinical, logistical, and/or ethical reasons why an RCT cannot or should not be pursued for a particular clinical question. It is the physicians' training and clinical acumen that enable them to meaningfully engage with medical evidence so that treatment plans can be tailored according to patient demographics, comorbidities, financial means, health priorities, and individual preferences.155,182 However, the failure to distinguish those nonevidence-based elements of clinical practice, passed down to students by eminent teachers and repeated without interrogation, prevents us from asking the right questions and slows progress toward more effective treatments.
ADOPTION OF INNOVATION IN HSV KERATITIS
Myths in the Care of HSV Keratitis
Without acknowledgment, eminence-based teachings can prolong clinical myths, which can compromise the quality of patient care. As discussed earlier, historical classification systems for HSV keratitis obfuscate clinical trial outcomes, render treatment guidelines more difficult to interpret, and therefore do not offer a tractable link between clinical diagnosis and the appropriate evidence-based management. Another common pitfall is the incomplete reading of published evidence. For example, in the HEDS-SKN trial,102 topical prednisolone and placebo were tapered over 10 weeks, after which a high proportion of participants experienced worsening disease. However, it would be a misapplication of the trial results to conclude that topical corticosteroids were ineffective. Rather, the trial indicated that there are subsets of patients with active stromal keratitis who may need a prolonged or indefinite taper of topical corticosteroids.183 Similarly, it would be a misinterpretation of the HEDS-APT41 study to surmise that 12 months of treatment, the designated length of oral acyclovir or placebo, were sufficient for longer-term prophylaxis. In the 6 months after treatment cessation, study subjects experienced recurrences at similar rates regardless of their previously assigned treatment, suggesting that there are select patients with a history of HSV stromal keratitis who may require indefinite prophylaxis. Competent and nuanced medical practice should not be “ritualistic”177; the delivery of personalized care requires systematic engagement with the literature, hierarchical appraisal of studies that vary in strength,184 and thoughtful application of clinical practice guidelines.164,185
Clinical Equipoise
The recognition of eminence-based teachings in medicine may also highlight examples of clinical equipoise within physician practice. This principle refers to the circumstances where there may be genuine uncertainty regarding the efficacy of treatment modalities for a given condition.186–188 Equipoise is the primary philosophical, ethical, and clinical reasoning on which appropriately designed RCTs are conducted. Although the HEDS trials directly addressed many of the clinical controversies once present in the eye care community, significant questions remain. For instance, the optimal treatments for HSV stromal keratitis with ulceration and HSV endothelial keratitis, both uncommon entities, were not effectively addressed by the HEDS trials. Any approach to their treatment is therefore eminence-based at best. In addition, the finer details of antiviral prophylaxis have not been resolved. Although the HEDS-APT trial showed that 400 mg of acyclovir administered twice daily is effective in reducing the rate of recurrence of HSV stromal keratitis, it is not known whether an increased dose would be more effective, whether other oral antivirals such as valacyclovir and famciclovir are superior to acyclovir, or whether a long-term, low-frequency topical corticosteroid has any additional role in prophylaxis.19
The Promise of a Diversified Electronic Medical Record
Mechanisms to better implement evidence-based medical innovations range from improving access to digitalized forms of continuing medical education,189 to offering financial incentives for following predetermined algorithms of care,190–192 and to restrictions on the scope of practice by training and professional degree.193 However, one technological innovation with the potential to dramatically improve health care is a diversified, “smart” EMR. Originally touted as the panacea to the limitations of the study record-keeping,194 the principal benefits of current EMR systems include improved efficiency in billing and better cross-institutional access to legible medical records within increasingly large healthcare systems. However, despite billions of dollars in investment,195 poor intersystem compatibility continues to hamper longitudinal health record-keeping and the centralized coordination of multidisciplinary care across disparate healthcare networks.196 With counterintuitive workflows, overabundance of clinically irrelevant tools, and excessive alert systems, current EMR applications often distract physicians from patient care and contribute to physician burnout and dissatisfaction.197,198 In a 2018 survey of over 500 primary care physicians conducted by Stanford Medical School, only 8% of respondents reported that they found clinical value in their EMR.199
A reenvisioned EMR would incorporate artificial intelligence and machine learning to provide enhanced physical and laboratory examination cues to the physician, generate data-driven and ranked differential diagnosis lists, and offer point-of-care access to validated clinical algorithms.200 Automated input of patient data to Health Insurance Portability and Accountability Act-compliant registries, such as the eye disease registry, Intelligent Research in Sight (IRIS),201 would permit data mining heuristics.202,203 This could guide research in previously inconceivable ways, well beyond binary decision-making to questions of disease epidemiology, quality improvement, and utilization management. This “smart” EMR would facilitate comparative effectiveness research and provide postmarketing surveillance of evidence-based interventions, enabling continuous fine-tuning of best care. When confronted with diagnostic or therapeutic dilemmas for which evidence is scarce or unavailable, this EMR would enable physicians to guide treatment with real-time analyses of pooled patient data stratified by relevant clinical variables.204 A diversified EMR would also allow researchers to answer questions of clinical equipoise with data from sample sizes far larger than RCTs could ever enroll, providing a powerful complement to traditional study designs.
CONCLUSION
A common misconception in medicine is that the mere existence of an evidence basis inevitably translates into improvements in clinical practice. However, it has been shown repeatedly that a passive approach to the adoption of new evidence is rarely sufficient. For HSV keratitis, current gaps in care are addressed only in part by the uptake of a classification of HSV keratitis based on anatomical localization. A closer review of eminence-based patterns of treatment can assist in dispelling the persistent myths that currently impact patient care, and enable us to identify examples of clinical equipoise for future clinical trials. Furthermore, an EMR that functions beyond its record-keeping role to provide immediate access to validated management algorithms in real time, while also serving as a source of data for population-wide disease studies, is needed to improve the implementation of evidence-based care into routine practice. The more rapid adoption of clinical innovations in HSV keratitis will enable the delivery of the highest quality of care, and reduce the complications and morbidity associated with this age-old blinding disorder.
Footnotes
Supported in part by an unrestricted grant to the Department of Ophthalmology, Harvard Medical School, from Research to Prevent Blindness, NY, NY; and by the Dozoretz Family Private Foundation.
Presented in part at the Kyoto Cornea Club 25th Annual Meeting; November 30, 2019; Kyoto, Japan, with travel supported by Santen Pharmaceutical Co., Ltd.
REFERENCES
- 1.Hippocrates. Of the Epidemics, Book II. 2009. F. Adams, trans. [The Massachusetts Institute of Technology Internet Classics Archive Website]. Available at: http://classics.mit.edu/Hippocrates/epidemics.mb.txt. Accessed October 25, 2019. [Google Scholar]
- 2.Thygeson P. Historical observations on herpetic keratitis. Surv Ophthalmol. 1976;21:82–90. [DOI] [PubMed] [Google Scholar]
- 3.Wildy P. Herpes viruses: a background. Br Med Bull. 1985;41:339–344. [DOI] [PubMed] [Google Scholar]
- 4.Roizman B, Whitley RJ. The nine ages of herpes simplex virus. Herpes. 2001;8:23–27. [PubMed] [Google Scholar]
- 5.Ighani A. Herpes—a not so simple(x) history. JAMA Dermatol. 2017;153:910. [DOI] [PubMed] [Google Scholar]
- 6.Taylor MW. Herpesvirus. In: Taylor MW, ed. Viruses and Man: A History of Interactions. Cham, Switzerland: Springer International Publishing; 2014:249–265. [Google Scholar]
- 7.Wildy P. Herpes: history and classification. In: Kaplan A, ed. The Herpesviruses. New York, NY: Academic Press; 1973:1–22. [Google Scholar]
- 8.Vidal E. Inoculabilité des pustules d'ecthyma. Ann Dermatol Syphiligr. 1873;2:350–358. [Google Scholar]
- 9.Fox LW. Diseases of the Eye. New York, NY: Appleton; 1904. [Google Scholar]
- 10.Horner F. Die Krankheiten des Auges im Kindesalter. DMW-Deutsche Medizinische Wochenschrift. 1883;9:501–502. [Google Scholar]
- 11.Horner F. On corneal herpes [Ueber Herpes cornealis]. Klinische Monatsblätter für Augenheilkunde. 1871;9:321–337. [Google Scholar]
- 12.Emmert E. Keratitis dendritica exulcdrans mycotica: an undescribed form of ulcerative keratitis [Keratitis dendritica exulcerans mycotica: Eine noch nicht beschriebene Form ulcerirender Hornhautentzündung]. Zentralblatt für praktische Augenheilkunde. 1885;9:302–311. [Google Scholar]
- 13.Löwenstein A. Aetiologische Untersuchungen über den fieberhaften Herpes. Munch Med Wochenschr. 1919;66:769–770. [Google Scholar]
- 14.Grüter W. Experimentelle und klinische Untersuchungen über den sogenannten Herpes corneae. Klin Monatsbl f Augenh. 1920;65:398. [Google Scholar]
- 15.Lipschütz B. Untersuchungen über die Ätiologie der Krankheiten der Herpesgruppe (Herpes zoster, Herpes genitalis, Herpes febrilis). Archiv für Dermatologie und Syphilis. 1921;136:428–482. [Google Scholar]
- 16.Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;57:448–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirschberg J. The History of Ophthalmology. Vol 1: Antiquity. Bonn, Germany: Verlag J.P. Wayenborgh; 1982. [Google Scholar]
- 18.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–2645. [DOI] [PubMed] [Google Scholar]
- 19.Guess SM, Butt AL, Neely SB, et al. Dissemination of knowledge from randomized clinical trials for herpes simplex virus keratitis. Arch Ophthalmol. 2010;128:1624–1625. [DOI] [PubMed] [Google Scholar]
- 20.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362:1225–1230. [DOI] [PubMed] [Google Scholar]
- 21.Eccles MP, Mittman BS. Welcome to implementation science. Implement Sci. 2006;1:1. [Google Scholar]
- 22.Burnham JP, Geng E, Venkatram C, et al. Putting the dissemination and implementation in infectious diseases. Clin Infect Dis. 2020;71:218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liebman DL, Chiang MF, Chodosh J. Realizing the promise of electronic health records: moving beyond “paper on a screen.” Ophthalmology. 2019;126:331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davison AJ. Herpesvirus systematics. Vet Microbiol. 2010;143:52–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davison AJ, Eberle R, Ehlers B, et al. The order Herpesvirales. Arch Virol. 2009;154:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roizman B, Furlong D. The replication of herpesviruses. In: Fraenkel-Conrat H, Wagner RR, eds. Reproduction: DNA Animal Viruses. Boston, MA: Springer US; 1974:229–403. [Google Scholar]
- 27.Roizman B. Herpesviridae. In: Fields BN, Knipe DM, Howley PM, eds. Fields Virology. Vol II Philadelphia, PA: Lippincott-Raven Publishers; 1996:2221–2230. [Google Scholar]
- 28.Whitley RJ. Herpesviruses. In: Baron S, ed. Medical Microbiology. 4th ed Galveston, TX: University of Texas Medical Branch at Galveston; 1996. [PubMed] [Google Scholar]
- 29.Davison AJ. Evolution of sexually transmitted and sexually transmissible human herpesviruses. Ann N Y Acad Sci. 2011;1230:E37–E49. [DOI] [PubMed] [Google Scholar]
- 30.Kieff E, Hoyer B, Bachenheimer S, et al. Genetic relatedness of type 1 and type 2 herpes simplex viruses. J Virol. 1972;9:738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiffer JT, Corey L. Herpes simplex virus. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. Vol II Philadelphia, PA: Churchill Livingstone; 2015:1713–1730. [Google Scholar]
- 32.Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57:737–763. [DOI] [PubMed] [Google Scholar]
- 33.Steiner I, Kennedy PG, Pachner AR. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 2007;6:1015–1028. [DOI] [PubMed] [Google Scholar]
- 34.Liesegang TJ. Ocular herpes simplex infection: pathogenesis and current therapy. Mayo Clin Proc. 1988;63:1092–1105. [DOI] [PubMed] [Google Scholar]
- 35.Guess S, Stone DU, Chodosh J. Evidence-based treatment of herpes simplex virus keratitis: a systematic review. Ocul Surf. 2007;5:240–250. [DOI] [PubMed] [Google Scholar]
- 36.Johnson DC, Baines JD. Herpesviruses remodel host membranes for virus egress. Nat Rev Microbiol. 2011;9:382–394. [DOI] [PubMed] [Google Scholar]
- 37.Stevens JG. Human herpesviruses: a consideration of the latent state. Microbiol Rev. 1989;53:318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pepose JS, Keadle TL, Morrison LA. Ocular herpes simplex: changing epidemiology, emerging disease patterns, and the potential of vaccine prevention and therapy. Am J Ophthalmol. 2006;141:547–557. [DOI] [PubMed] [Google Scholar]
- 39.Burnet FM, Williams SW. Herpes simplex: a new point of view. Med J Aust. 1939;1:637–642. [Google Scholar]
- 40.Margolis TP, Dawson CR, LaVail JH. Herpes simplex viral infection of the mouse trigeminal ganglion. Immunohistochemical analysis of cell populations. Invest Ophthalmol Vis Sci. 1992;33:259–267. [PubMed] [Google Scholar]
- 41.Herpetic Eye Disease Study Group. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N Engl J Med. 1998;339:300–306. [DOI] [PubMed] [Google Scholar]
- 42.Wilson AC, Mohr I. A cultured affair: HSV latency and reactivation in neurons. Trends Microbiol. 2012;20:604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roizman B, Whitley RJ. An inquiry into the molecular basis of HSV latency and reactivation. Annu Rev Microbiol. 2013;67:355–374. [DOI] [PubMed] [Google Scholar]
- 44.Perng GC, Dunkel EC, Geary PA, et al. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J Virol. 1994;68:8045–8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tullo AB, Shimeld C, Blyth WA, et al. Spread of virus and distribution of latent infection following ocular herpes simplex in the non-immune and immune mouse. J Gen Virol. 1982;63:95–101. [DOI] [PubMed] [Google Scholar]
- 46.Asbell PA, Centifanto-Fitzgerald YM, Chandler JW, et al. Analysis of viral DNA in isolates from patients with recurrent herpetic keratitis. Invest Ophthalmol Vis Sci. 1984;25:951–954. [PubMed] [Google Scholar]
- 47.White ML, Chodosh J. Herpes Simplex Virus Keratitis: A Treatment Guideline. Hoskins Center for Quality Eye Care and American Academy of Ophthalmology Website; 2014. Available at: https://www.aao.org/clinical-statement/herpes-simplex-virus-keratitis-treatment-guideline. Accessed July 9, 2019. [Google Scholar]
- 48.Wilhelmus KR. Epidemiology of ocular infections. In: Tasman W, Jaeger EA, eds. Duane's Foundations of Clinical Ophthalmology. Vol 2 Philadelphia, PA: Lippincott Williams & Wilkins; 2005: chapter 43. [Google Scholar]
- 49.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. [DOI] [PubMed] [Google Scholar]
- 50.Norn MS. Dendritic (herpetic) keratitis. I. Incidence—seasonal variations—recurrence rate—visual impairment—therapy. Acta Ophthalmol (Copenh). 1970;48:91–107. [DOI] [PubMed] [Google Scholar]
- 51.Ribaric V. The incidence of herpetic keratitis among population. Ophthalmologica. 1976;173:19–22. [DOI] [PubMed] [Google Scholar]
- 52.Labetoulle M, Auquier P, Conrad H, et al. Incidence of herpes simplex virus keratitis in France. Ophthalmology. 2005;112:888–895. [DOI] [PubMed] [Google Scholar]
- 53.Liesegang TJ, Melton LJ, III, Daly PJ, et al. Epidemiology of ocular herpes simplex. Incidence in Rochester, MN, 1950 through 1982. Arch Ophthalmol. 1989;107:1155–1159. [DOI] [PubMed] [Google Scholar]
- 54.Young RC, Hodge DO, Liesegang TJ, et al. Incidence, recurrence, and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976–2007: the effect of oral antiviral prophylaxis. Arch Ophthalmol. 2010;128:1178–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stanzel TP, Diaz JD, Mather R, et al. The epidemiology of herpes simplex virus eye disease in Northern California. Ophthalmic Epidemiol. 2014;21:370–377. [DOI] [PubMed] [Google Scholar]
- 56.United States Census Bureau. Monthly Population Estimates for the United States: April 1, 2010 to December 1, 2020 (NA-EST2019-01). US Federal Statistical System Website; 2019. Available at: https://www2.census.gov/programs-surveys/popest/tables/2010-2019/national/totals/na-est2019-01.xlsx?#. Accessed January 4, 2020. [Google Scholar]
- 57.Suresh PS, Tullo AB. Herpes simplex keratitis. Indian J Ophthalmol. 1999;47:155–165. [PubMed] [Google Scholar]
- 58.Holland EJ, Schwartz GS. Classification of herpes simplex virus keratitis. Cornea. 1999;18:144–154. [DOI] [PubMed] [Google Scholar]
- 59.Serna-Ojeda JC, Ramirez-Miranda A, Navas A, et al. Herpes simplex virus disease of the anterior segment in children. Cornea. 2015;34(suppl 10):S68–S71. [DOI] [PubMed] [Google Scholar]
- 60.Hinzpeter EN, Naumann GOH. Cornea and sclera. In: Naumann GOH, Apple DJ, eds. Pathology of the Eye. New York, NY: Springer; 1986:317–412. [Google Scholar]
- 61.Coster DJ, Jones BR, McGill JI. Treatment of amoeboid herpetic ulcers with adenine arabinoside or trifluorothymidine. Br J Ophthalmol. 1979;63:418–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coster DJ, McKinnon JR, McGill JI, et al. Clinical evaluation of adenine arabinoside and trifluorothymidine in the treatment of corneal ulcers caused by herpes simplex virus. J Infect Dis. 1976;133(suppl):A173–A177. [DOI] [PubMed] [Google Scholar]
- 63.Collum LM, Logan P, McAuliffe-Curtin D, et al. Randomised double-blind trial of acyclovir (Zovirax) and adenine arabinoside in herpes simplex amoeboid corneal ulceration. Br J Ophthalmol. 1985;69:847–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwartz GS, Harrison AR, Holland EJ. Etiology of immune stromal (interstitial) keratitis. Cornea. 1998;17:278–281. [PubMed] [Google Scholar]
- 65.Sharma S. Keratitis. Biosci Rep. 2001;21:419–444. [DOI] [PubMed] [Google Scholar]
- 66.Streilein JW, Dana MR, Ksander BR. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol Today. 1997;18:443–449. [DOI] [PubMed] [Google Scholar]
- 67.Shi W, Chen M, Xie L. Amniotic membrane transplantation combined with antiviral and steroid therapy for herpes necrotizing stromal keratitis. Ophthalmology. 2007;114:1476–1481. [DOI] [PubMed] [Google Scholar]
- 68.Wilhelmus KR. Diagnosis and management of herpes simplex stromal keratitis. Cornea. 1987;6:286–291. [DOI] [PubMed] [Google Scholar]
- 69.Heiligenhaus A, Li H, Hernandez Galindo EE, et al. Management of acute ulcerative and necrotising herpes simplex and zoster keratitis with amniotic membrane transplantation. Br J Ophthalmol. 2003;87:1215–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meyers-Elliott RH, Pettit TH, Maxwell WA. Viral antigens in the immune ring of Herpes simplex stromal keratitis. Arch Ophthalmol. 1980;98:897–904. [DOI] [PubMed] [Google Scholar]
- 71.Pepose JS. Herpes simplex keratitis: role of viral infection versus immune response. Surv Ophthalmol. 1991;35:345–352. [DOI] [PubMed] [Google Scholar]
- 72.Chodosh J, Miller D, Stroop WG, et al. Adenovirus epithelial keratitis. Cornea. 1995;14:167–174. [PubMed] [Google Scholar]
- 73.Chodosh J, Gan YJ, Sixbey JW. Detection of Epstein-Barr virus genome in ocular tissues. Ophthalmology. 1996;103:687–690. [DOI] [PubMed] [Google Scholar]
- 74.Wilhelmus KR. Ocular involvement in infectious mononucleosis. Am J Ophthalmol. 1981;91:117–118. [DOI] [PubMed] [Google Scholar]
- 75.Pflugfelder SC, Huang A, Crouse C. Epstein-Barr virus keratitis after a chemical facial peel. Am J Ophthalmol. 1990;110:571–573. [DOI] [PubMed] [Google Scholar]
- 76.Inoue Y. Review of clinical and basic approaches to corneal endotheliitis. Cornea. 2014;33(suppl 11):S3–S8. [DOI] [PubMed] [Google Scholar]
- 77.Mercadal CM, Bouley DM, DeStephano D, et al. Herpetic stromal keratitis in the reconstituted scid mouse model. J Virol. 1993;67:3404–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Newell CK, Martin S, Sendele D, et al. Herpes simplex virus-induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J Virol. 1989;63:769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Metcalf JF, Kaufman HE. Herpetic stromal keratitis-evidence for cell-mediated immunopathogenesis. Am J Ophthalmol. 1976;82:827–834. [DOI] [PubMed] [Google Scholar]
- 80.Foster CS. Herpes simplex virus—induced destructive corneal disease. Eye (Lond). 1989;3:194–203. [DOI] [PubMed] [Google Scholar]
- 81.Heiligenhaus A, Bauer D, Meller D, et al. Improvement of HSV-1 necrotizing keratitis with amniotic membrane transplantation. Invest Ophthalmol Vis Sci. 2001;42:1969–1974. [PubMed] [Google Scholar]
- 82.Thomas J, Gangappa S, Kanangat S, et al. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- 83.Holbach LM, Font RL, Baehr W, et al. HSV antigens and HSV DNA in avascular and vascularized lesions of human herpes simplex keratitis. Curr Eye Res. 1991;10(suppl):63–68. [DOI] [PubMed] [Google Scholar]
- 84.Liesegang TJ. Biology and molecular aspects of herpes simplex and varicella-zoster virus infections. Ophthalmology. 1992;99:781–799. [DOI] [PubMed] [Google Scholar]
- 85.Lass JH, Krishnan N, Velasco ME, et al. Human herpes simplex stromal keratitis: an immunoperoxidase and electron microscopic study. Cornea. 1983;2:147–158. [Google Scholar]
- 86.Sundmacher R, Neumann-Haefelin D. Herpes simplex virus isolations from the aqueous humor of patients suffering from focal iritis, endotheliitis, and prolonged disciform keratitis with glaucoma (author's transl) [in German]. Klin Monbl Augenheilkd. 1979;175:488–501. [PubMed] [Google Scholar]
- 87.Robin JB, Steigner JB, Kaufman HE. Progressive herpetic corneal endotheliitis. Am J Ophthalmol. 1985;100:336–337. [DOI] [PubMed] [Google Scholar]
- 88.Holbach LM, Asano N, Naumann GO. Infection of the corneal endothelium in herpes simplex keratitis. Am J Ophthalmol. 1998;126:592–594. [DOI] [PubMed] [Google Scholar]
- 89.Kaye S, Choudhary A. Herpes simplex keratitis. Prog Retin Eye Res. 2006;25:355–380. [DOI] [PubMed] [Google Scholar]
- 90.Lobo AM, Agelidis AM, Shukla D. Pathogenesis of herpes simplex keratitis: the host cell response and ocular surface sequelae to infection and inflammation. Ocul Surf. 2019;17:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilhelmus KR. The treatment of herpes simplex virus epithelial keratitis. Trans Am Ophthalmol Soc. 2000;98:505–532. [PMC free article] [PubMed] [Google Scholar]
- 92.Kronenberg B. The use of placental extract in the treatment of herpetic keratitis and superficial punctate keratitis. Am J Ophthalmol. 1948;31:1101–1106. [DOI] [PubMed] [Google Scholar]
- 93.Sokolova NS. Treatment of herpetic and dystrophic keratitis with subconjunctival administration of autogenous blood [in Russian]. Vestn Oftalmol. 1963;76:32–35. [PubMed] [Google Scholar]
- 94.Clark WB, Baldone JA, Thomas CI. The use of Sanders neurotoxoid I (modified snake venom) in the treatment of recurrent herpes simplex of the cornea: progress report. South Med J. 1962;55:947–951. [DOI] [PubMed] [Google Scholar]
- 95.Lederman M. Herpetic infections of the outer eye. Trans Ophthalmol Soc U K. 1956;76:193–207. [PubMed] [Google Scholar]
- 96.Kaufman H, Martola EL, Dohlman C. Use of 5-iodo-2'-deoxyuridine (IDU) in treatment of herpes simplex keratitis. Arch Ophthalmol. 1962;68:235–239. [DOI] [PubMed] [Google Scholar]
- 97.Kaufman HE. Clinical cure of herpes simplex keratitis by 5-iodo-2-deoxyuridine. Proc Soc Exp Biol Med. 1962;109:251–252. [DOI] [PubMed] [Google Scholar]
- 98.Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2015;1:CD002898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dawson CR. The herpetic eye disease study. Arch Ophthalmol. 1990;108:191–192. [DOI] [PubMed] [Google Scholar]
- 100.Dawson CR, Jones DB, Kaufman HE, et al. Design and organization of the herpetic eye disease study (HEDS). Curr Eye Res. 1991;10(suppl):105–110. [DOI] [PubMed] [Google Scholar]
- 101.Herpetic Eye Disease Study Group. Herpetic Eye Disease Study I (HEDS-I) National Institutes of Health and U.S. National Library of Medicine Website; 1999. Available at: https://clinicaltrials.gov/ct2/show/NCT00000138. Accessed October 30, 2019. [Google Scholar]
- 102.Wilhelmus KR, Gee L, Hauck WW, et al. Herpetic Eye Disease Study. A controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology. 1994;101:1883–1895; discussion 1895–1886. [DOI] [PubMed] [Google Scholar]
- 103.Barron BA, Gee L, Hauck WW, et al. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology. 1994;101:1871–1882. [DOI] [PubMed] [Google Scholar]
- 104.Herpetic Eye Disease Study Group. A controlled trial of oral acyclovir for iridocyclitis caused by herpes simplex virus. Arch Ophthalmol. 1996;114:1065–1072. [DOI] [PubMed] [Google Scholar]
- 105.Herpetic Eye Disease Study Group. Herpetic Eye Disease Study II (HEDS II). National Institutes of Health and U.S. National Library of Medicine Website; 1999. Available at: https://clinicaltrials.gov/ct2/show/NCT00000139. Accessed October 30, 2019. [Google Scholar]
- 106.Herpetic Eye Disease Study Group. A controlled trial of oral acyclovir for the prevention of stromal keratitis or iritis in patients with herpes simplex virus epithelial keratitis. Arch Ophthalmol. 1997;115:703–712. [DOI] [PubMed] [Google Scholar]
- 107.Wilhelmus KR, Beck RW, Moke PS, et al. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. Herpetic Eye Disease Study Group. N Engl J Med. 1998;339:300–306. [DOI] [PubMed] [Google Scholar]
- 108.Herpetic Eye Disease Study Group. Predictors of recurrent herpes simplex virus keratitis. Cornea. 2001;20:123–128. [DOI] [PubMed] [Google Scholar]
- 109.Collum LM, McGettrick P, Akhtar J, et al. Oral acyclovir (Zovirax) in herpes simplex dendritic corneal ulceration. Br J Ophthalmol. 1986;70:435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Collum LM, Akhtar J, McGettrick P. Oral acyclovir in herpetic keratitis. Trans Ophthalmol Soc U K. 1985;104:629–632. [PubMed] [Google Scholar]
- 111.Hung SO, Patterson A, Rees PJ. Pharmacokinetics of oral acyclovir (Zovirax) in the eye. Br J Ophthalmol. 1984;68:192–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gnann JW, Jr, Barton NH, Whitley RJ. Acyclovir: mechanism of action, pharmacokinetics, safety and clinical applications. Pharmacotherapy. 1983;3:275–283. [DOI] [PubMed] [Google Scholar]
- 113.Collum LM, Grant DM. A double-blind comparative trial of acyclovir and adenine arabinoside in combination with dilute betamethasone in the management of herpetic disciform keratitis. Curr Eye Res. 1987;6:221–224. [DOI] [PubMed] [Google Scholar]
- 114.Blum MR, Liao SH, de Miranda P. Overview of acyclovir pharmacokinetic disposition in adults and children. Am J Med. 1982;73:186–192. [DOI] [PubMed] [Google Scholar]
- 115.Poirier RH, Kingham JD, de Miranda P, et al. Intraocular antiviral penetration. Arch Ophthalmol. 1982;100:1964–1967. [DOI] [PubMed] [Google Scholar]
- 116.Lass JH, Langston RH, Foster CS, et al. Antiviral medications and corneal wound healing. Antivir Res. 1984;4:143–157. [DOI] [PubMed] [Google Scholar]
- 117.Wilson FM., II Adverse external ocular effects of topical ophthalmic medications. Surv Ophthalmol. 1979;24:57–88. [DOI] [PubMed] [Google Scholar]
- 118.Collum LM, Power WJ, Collum A. The current management of herpetic eye disease. Doc Ophthalmol. 1992;80:201–205. [DOI] [PubMed] [Google Scholar]
- 119.Pakdel F, Bahmani Kashkouli M. Lacrimal drainage obstruction associated with topical and systemic medications. J Ophthalmic Vis Res. 2009;4:270–271. [PMC free article] [PubMed] [Google Scholar]
- 120.Maudgal PC, Van Damme B, Missotten L. Corneal epithelial dysplasia after trifluridine use. Graefes Arch Clin Exp Ophthalmol. 1983;220:6–12. [DOI] [PubMed] [Google Scholar]
- 121.Colin J, Chastel C, Kaufman HE, et al. Combination therapy for dendritic keratitis with acyclovir and vidarabine. J Ocul Pharmacol. 1987;3:39–42. [DOI] [PubMed] [Google Scholar]
- 122.Parlato CJ, Cohen EJ, Sakauye CM, et al. Role of debridement and trifluridine (trifluorothymidine) in herpes simplex dendritic keratitis. Arch Ophthalmol. 1985;103:673–675. [DOI] [PubMed] [Google Scholar]
- 123.Jones BR, Coster DJ, Fison PN, et al. Efficacy of acycloguanosine (Wellcome 248U) against herpes-simplex corneal ulcers. Lancet. 1979;1:243–244. [DOI] [PubMed] [Google Scholar]
- 124.Hung SO, Patterson A, Clark DI, et al. Oral acyclovir in the management of dendritic herpetic corneal ulceration. Br J Ophthalmol. 1984;68:398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gordon YJ. The evolution of antiviral therapy for external ocular viral infections over twenty-five years. Cornea. 2000;19:673–680. [DOI] [PubMed] [Google Scholar]
- 126.McAllum PJ, McGhee CN. Prescribing trends in infectious keratitis: a survey of New Zealand ophthalmologists. Clin Exp Ophthalmol. 2003;31:496–504. [DOI] [PubMed] [Google Scholar]
- 127.Thygeson P, Hogan MJ, Kimura SJ. The unfavorable effect of topical steroid therapy on herpetic keratitis. Trans Am Ophthalmol Soc. 1960;58:245–262. [PMC free article] [PubMed] [Google Scholar]
- 128.Dawson CR, Togni B. Herpes simplex eye infections: clinical manifestations, pathogenesis and management. Surv Ophthalmol. 1976;21:121–135. [DOI] [PubMed] [Google Scholar]
- 129.Thygeson P, Kimura SJ. Deep forms of herpetic keratitis. Am J Ophthalmol. 1957;43:109–113. [DOI] [PubMed] [Google Scholar]
- 130.Laibson PR. Current therapy of herpes simplex virus infection of the cornea. Int Ophthalmol Clin. 1973;13:39–52. [PubMed] [Google Scholar]
- 131.Pavan-Langston D. Diagnosis and management of herpes simplex ocular infection. Int Ophthalmol Clin. 1975;15:19–35. [DOI] [PubMed] [Google Scholar]
- 132.Collum LM, Logan P, Ravenscroft T. Acyclovir (Zovirax) in herpetic disciform keratitis. Br J Ophthalmol. 1983;67:115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Power WJ, Hillery MP, Benedict-Smith A, et al. Acyclovir ointment plus topical betamethasone or placebo in first episode disciform keratitis. Br J Ophthalmol. 1992;76:711–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Porter SM, Patterson A, Kho P. A comparison of local and systemic acyclovir in the management of herpetic disciform keratitis. Br J Ophthalmol. 1990;74:283–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Collum LM, Logan P, Hillary IB, et al. Acyclovir in herpes keratitis. Am J Med. 1982;73:290–293. [DOI] [PubMed] [Google Scholar]
- 136.Pavan-Langston D, Nelson DJ. Intraocular penetration of trifluridine. Am J Ophthalmol. 1979;87:814–818. [DOI] [PubMed] [Google Scholar]
- 137.Prabriputaloong T, Margolis TP, Lietman TM, et al. Atopic disease and herpes simplex eye disease: a population-based case-control study. Am J Ophthalmol. 2006;142:745–749. [DOI] [PubMed] [Google Scholar]
- 138.Margolis TP, Ostler HB. Treatment of ocular disease in eczema herpeticum. Am J Ophthalmol. 1990;110:274–279. [DOI] [PubMed] [Google Scholar]
- 139.Rezende RA, Hammersmith K, Bisol T, et al. Comparative study of ocular herpes simplex virus in patients with and without self-reported atopy. Am J Ophthalmol. 2006;141:1120–1125. [DOI] [PubMed] [Google Scholar]
- 140.van Rooij J, Rijneveld WJ, Remeijer L, et al. Effect of oral acyclovir after penetrating keratoplasty for herpetic keratitis: a placebo-controlled multicenter trial. Ophthalmology. 2003;110:1916–1919; discussion 1919. [DOI] [PubMed] [Google Scholar]
- 141.Ghosh S, Jhanji V, Lamoureux E, et al. Acyclovir therapy in prevention of recurrent herpetic keratitis following penetrating keratoplasty. Am J Ophthalmol. 2008;145:198–202. [DOI] [PubMed] [Google Scholar]
- 142.Garcia DD, Farjo Q, Musch DC, et al. Effect of prophylactic oral acyclovir after penetrating keratoplasty for herpes simplex keratitis. Cornea. 2007;26:930–934. [DOI] [PubMed] [Google Scholar]
- 143.Barney NP, Foster CS. A prospective randomized trial of oral acyclovir after penetrating keratoplasty for herpes simplex keratitis. Cornea. 1994;13:232–236. [DOI] [PubMed] [Google Scholar]
- 144.Hodge WG, Margolis TP. Herpes simplex virus keratitis among patients who are positive or negative for human immunodeficiency virus: an epidemiologic study. Ophthalmology. 1997;104:120–124. [DOI] [PubMed] [Google Scholar]
- 145.Fillet AM. Prophylaxis of herpesvirus infections in immunocompetent and immunocompromised older patients. Drugs Aging. 2002;19:343–354. [DOI] [PubMed] [Google Scholar]
- 146.Young TL, Robin JB, Holland GN, et al. Herpes simplex keratitis in patients with acquired immune deficiency syndrome. Ophthalmology. 1989;96:1476–1479. [DOI] [PubMed] [Google Scholar]
- 147.de Rojas Silva V, Rodriguez-Conde R, Cobo-Soriano R, et al. Laser in situ keratomileusis in patients with a history of ocular herpes. J Cataract Refract Surg. 2007;33:1855–1859. [DOI] [PubMed] [Google Scholar]
- 148.Levy J, Lapid-Gortzak R, Klemperer I, et al. Herpes simplex virus keratitis after laser in situ keratomileusis. J Refract Surg. 2005;21:400–402. [DOI] [PubMed] [Google Scholar]
- 149.Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press (US); 2001. [PubMed] [Google Scholar]
- 150.Proctor EK, Landsverk J, Aarons G, et al. Implementation research in mental health services: an emerging science with conceptual, methodological, and training challenges. Adm Pol Ment Health. 2009;36:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chambers DA, Feero WG, Khoury MJ. Convergence of implementation science, precision medicine, and the learning health care system: a new model for biomedical research. JAMA. 2016;315:1941–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rabin BA, Purcell P, Naveed S, et al. Advancing the application, quality and harmonization of implementation science measures. Implement Sci. 2012;7:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Glasgow RE, Vinson C, Chambers D, et al. National Institutes of Health approaches to dissemination and implementation science: current and future directions. Am J Public Health. 2012;102:1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shojania KG, Grimshaw JM. Evidence-based quality improvement: the state of the science. Health Aff (Millwood). 2005;24:138–150. [DOI] [PubMed] [Google Scholar]
- 155.Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn't. BMJ. 1996;312:71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Evidence-Based Medicine Working Group. Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA. 1992;268:2420–2425. [DOI] [PubMed] [Google Scholar]
- 157.Shojania KG, McDonald KM, Wachter RM, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol 1: Series Overview and Methodology). Rockville, MD: Agency for Healthcare Research and Quality (US); 2004. [PubMed] [Google Scholar]
- 158.Colditz GA. The promise and challenges of dissemination and implementation research. In: Brownson RC, Colditz GA, Proctor EK, eds. Dissemination and Implementation Research in Health. 2nd ed New York, NY: Oxford University Press; 2017:1–18. [Google Scholar]
- 159.Green LW, Ottoson JM, Garcia C, et al. Diffusion theory and knowledge dissemination, utilization, and integration in public health. Annu Rev Public Health. 2009;30:151–174. [DOI] [PubMed] [Google Scholar]
- 160.Balas EA, Boren SA. Managing clinical knowledge for health care improvement. Yearb Med Inform. 2000:65–70. [PubMed] [Google Scholar]
- 161.Sox HC, Greenfield S. Comparative effectiveness research: a report from the Institute of Medicine. Ann Intern Med. 2009;151:203–205. [DOI] [PubMed] [Google Scholar]
- 162.van Bodegom-Vos L, Davidoff F, Marang-van de Mheen PJ. Implementation and de-implementation: two sides of the same coin? BMJ Qual Saf. 2017;26:495–501. [DOI] [PubMed] [Google Scholar]
- 163.Prasad V, Ioannidis JP. Evidence-based de-implementation for contradicted, unproven, and aspiring healthcare practices. Implement Sci. 2014;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. [DOI] [PubMed] [Google Scholar]
- 165.Davis DA, Taylor-Vaisey A. Translating guidelines into practice. A systematic review of theoretic concepts, practical experience and research evidence in the adoption of clinical practice guidelines. Can Med Assoc J. 1997;157:408–416. [PMC free article] [PubMed] [Google Scholar]
- 166.Rogers EM. Diffusion of Innovations. New York, NY: Simon and Schuster; 2010. [Google Scholar]
- 167.Dearing JW. Evolution of diffusion and dissemination theory. J Public Health Manag Pract. 2008;14:99–108. [DOI] [PubMed] [Google Scholar]
- 168.Brancheau JC, Wetherbe JC. The adoption of spreadsheet software: testing innovation diffusion theory in the context of end-user computing. Inf Syst Res. 1990;1:115–143. [Google Scholar]
- 169.Gatignon H, Robertson TS. A propositional inventory for new diffusion research. J Consum Res. 1985;11:849–867. [Google Scholar]
- 170.Glasgow RE, Chambers D. Developing robust, sustainable, implementation systems using rigorous, rapid and relevant science. Clin Transl Sci. 2012;5:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ziahosseini K, Ikram K. Current clinical practice of consultant ophthalmologists in treating herpetic eye disease in the United Kingdom. Eye (Lond). 2009;23:993–994. [DOI] [PubMed] [Google Scholar]
- 172.Alkhayyal MA, Stone DU. Practice patterns for herpes simplex keratitis: a survey of ophthalmologists in Gulf Coast countries. Saudi J Ophthalmol. 2017;31:61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cabrera-Aguas M, Robaei D, McCluskey P, et al. Clinical translation of recommendations from randomized trials for management of herpes simplex virus keratitis. Clin Exp Ophthalmol. 2018;46:1008–1016. [DOI] [PubMed] [Google Scholar]
- 174.Bassand JP, Priori S, Tendera M. Evidence-based vs. 'impressionist' medicine: how best to implement guidelines. Eur Heart J. 2005;26:1155–1158. [DOI] [PubMed] [Google Scholar]
- 175.Yee J. Eminence-based medicine: the king is dead. Adv Chronic Kidney Dis. 2012;19:1–2. [DOI] [PubMed] [Google Scholar]
- 176.Isaacs D, Fitzgerald D. Seven alternatives to evidence based medicine. BMJ. 1999;319:1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bourgault AM, Upvall MJ. De-implementation of tradition-based practices in critical care: a qualitative study. Int J Nurs Pract. 2019;25:e12723. [DOI] [PubMed] [Google Scholar]
- 178.Gerber A, Lauterbach K. Evidence-based medicine: why do opponents and proponents use the same arguments? Health Care Anal. 2005;13:59–71. [DOI] [PubMed] [Google Scholar]
- 179.Iglehart JK. Prioritizing comparative-effectiveness research—IOM recommendations. N Engl J Med. 2009;361:325–328. [DOI] [PubMed] [Google Scholar]
- 180.Dickersin K, Straus SE, Bero LA. Evidence based medicine: increasing, not dictating, choice. BMJ. 2007;334(suppl 1):s10. [DOI] [PubMed] [Google Scholar]
- 181.Tonelli MR. Integrating evidence into clinical practice: an alternative to evidence-based approaches. J Eval Clin Pract. 2006;12:248–256. [DOI] [PubMed] [Google Scholar]
- 182.Greenhalgh T. Is my practice evidence-based? BMJ. 1996;313:957–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Weiner G. Demystifying the Ocular Herpes Simplex Virus. American Academy of Ophthalmology Website; 2013. Available at: https://www.aao.org/eyenet/article/demystifying-ocular-herpes-simplex-virus. Accessed October 29, 2019. [Google Scholar]
- 184.Guyatt GH, Haynes RB, Jaeschke RZ, et al. Users' guides to the medical literature: XXV. Evidence-based medicine: principles for applying the users' guides to patient care. Evidence-Based Medicine Working Group. JAMA. 2000;284:1290–1296. [DOI] [PubMed] [Google Scholar]
- 185.Kelly SP; Royal College of Ophthalmologists. Guidance on patient safety in ophthalmology from the Royal College of Ophthalmologists. Eye (Lond). 2009;23:2143–2151. [DOI] [PubMed] [Google Scholar]
- 186.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317:141–145. [DOI] [PubMed] [Google Scholar]
- 187.Weijer C, Shapiro SH, Cranley Glass K. For and against: clinical equipoise and not the uncertainty principle is the moral underpinning of the randomised controlled trial. Br Med J. 2000;321:756–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Miller PB, Weijer C. Equipoise and the duty of care in clinical research: a philosophical response to our critics. J Med Philos. 2007;32:117–133. [DOI] [PubMed] [Google Scholar]
- 189.Chen C, Svec DM, Chen JH. Evolving Medical Education for a Digital Future. Medscape Website; 2018. Available at: https://www.medscape.com/viewarticle/906569. Accessed January 5, 2020. [Google Scholar]
- 190.Shortell SM, Zazzali JL, Burns LR, et al. Implementing evidence-based medicine: the role of market pressures, compensation incentives, and culture in physician organizations. Med Care. 2001;39:I62–I78. [PubMed] [Google Scholar]
- 191.Christianson JB, Knutson DJ, Mazze RS. Physician pay-for-performance. Implementation and research issues. J Gen Intern Med. 2006;21(suppl 2):S9–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Clough JD, McClellan M. Implementing MACRA: implications for physicians and for physician leadership. JAMA. 2016;315:2397–2398. [DOI] [PubMed] [Google Scholar]
- 193.Schleiter KE. Ophthalmologists, optometrists, and scope of practice concerns. Virtual Mentor. 2010;12:941–945. [DOI] [PubMed] [Google Scholar]
- 194.Atherton J. Development of the electronic health record. Virtual Mentor. 2011;13:186–189. [DOI] [PubMed] [Google Scholar]
- 195.Reis ZSN, Maia TA, Marcolino MS, et al. Is there evidence of cost benefits of electronic medical records, standards, or interoperability in hospital information systems? Overview of systematic reviews. JMIR Med Inform. 2017;5:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tang PC, Ash JS, Bates DW, et al. Personal health records: definitions, benefits, and strategies for overcoming barriers to adoption. J Am Med Inform Assoc. 2006;13:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836–848. [DOI] [PubMed] [Google Scholar]
- 198.Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO Study. J Am Med Inform Assoc. 2014;21:e100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Stanford Medical School. White paper: the future of electronic health records. 2018. Available at: http://med.stanford.edu/content/dam/sm/ehr/documents/EHR-White-Paper.pdfv. Accessed October 26, 2019.
- 200.Demner-Fushman D, Seckman C, Fisher C, et al. A prototype system to support evidence-based practice. AMIA Annu Symp Proc. 2008:151–155. [PMC free article] [PubMed] [Google Scholar]
- 201.Chiang MF, Sommer A, Rich WL, et al. The 2016 American Academy of Ophthalmology IRIS Registry (Intelligent Research in Sight) Database: characteristics and methods. Ophthalmology. 2018;125:1143–1148. [DOI] [PubMed] [Google Scholar]
- 202.Hripcsak G, Albers DJ. Next-generation phenotyping of electronic health records. J Am Med Inform Assoc. 2013;20:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kushida CA, Nichols DA, Jadrnicek R, et al. Strategies for de-identification and anonymization of electronic health record data for use in multicenter research studies. Med Care. 2012;50(suppl):S82–S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Frankovich J, Longhurst CA, Sutherland SM. Evidence-based medicine in the EMR era. N Engl J Med. 2011;365:1758–1759. [DOI] [PubMed] [Google Scholar]