Supplemental Digital Content is available in the text.
Keywords: endocarditis, multidisciplinary, pathway
Abstract
Clinical pathways can be useful when disparate clinical-pathologic groups converge on a common diagnostic and therapeutic trajectory. The progressive increase in the incidence of endocarditis in the US has included higher-risk subjects whose candidacy for aggressive cardiac surgical intervention may be highly resource-intensive, prohibitively high risk, or delayed and possibly deferred by comorbidities. We sought to define the sequence, application, and resolution of multidisciplinary endocarditis team decision-making in 4 distinct clinical groups.
Infectious endocarditis (IE) in the US and worldwide has increased in incidence and complexity1–3 with a persistent overall mortality rate of 30%. Contemporary estimates of the global burden of IE4 indicate that the incidence of IE has increased by about 30% from 1990 to 2017, with an unadjusted increase in IE mortality by 45%. Contrary to earlier estimates from literature reviews (3 to 7 cases of IE/100,000 population in the US), more robust data analysis has found a recent (2017) incidence of IE of 19.0/100,000 in US, and 21.3/100,000 in Western Europe.4 This has made IE the fourth most common systemic infection in the US.
Although community-acquired IE still predominates in this disease population, there has been an increase in nosocomial IE, due, in part, to a rise in invasive central venous and arterial testing and therapies. Both community-acquired and nosocomial infections seem to be facilitated by a rise in incidence of age-related native and prosthetic valve disease, survivors of complex congenital heart disease, implanted cardiovascular implantable electrophysiology devices, and maintenance hemodialysis in chronic renal failure. The rise in bacteremia due to Staphylococcus species has enhanced the number of cases of acute IE with a more rapid and destructive course.5
Certainly, the IE patient group most challenging to manage acutely and chronically has been those persons with substance use disorder (SUD). In the last 15 years, drug-associated IE hospitalizations have increased about 12-fold in some areas of US, and the incidence of IE cases associated with SUD has increased from 6% to 8% to over 30% of cases in large urban referral centers.6,7 Although this population is younger, and acute survival is general better than in the average IE population, cardiac surgery is required more often, length of stay is more prolonged, and long-term survival is often worse, especially when addiction has not been addressed or managed.8–14
Because IE may present in nonspecific ways to a variety of specialists and primary caregivers, there is the potential for delay in diagnosis and treatment in a system with an unorganized approach to management of the disease. Management of IE often suffers from a lack of coordination and ownership.
The European experience has accumulated convincing evidence that early recognition and prompt medical treatment of affected IE patients, as well as identification of individuals who may be early and late candidates for surgical intervention (up to 50%–60% of cases of IE), can optimize outcomes in endocarditis. Formalizing multidisciplinary endocarditis management teams with protocols endorsed by the American College of Cardiology and the European Society of Cardiology15–17 has resulted in achieving more uniform antibiotic treatment strategies, fostered earlier diagnosis of both the primary disease and its complications, and accelerated the timing of urgent cardiac surgery, with improved in-hospital survival and reduced comorbidities.18–20 Despite these strong recommendations, explicit clinical pathways for endocarditis care have yet to be published in the US.
The development and implementation of our multidisciplinary endocarditis management team and pathway is the focus of this article.
METHODS
The University of Washington medical system (UW Medicine) is a Washington State-based not-for-profit healthcare system, which includes 3 academic medical centers [University of Washington Medical Center (UWMC), Harborview Medical Center (HMC), VA Puget Sound Medical Center], Valley Medical Center, 18 neighborhood clinics, Airlift Northwest (an acute care air transport service for 5 northwest states), and numerous regional hospital alliances.
The nature, patient demographics, and different specialty care profiles of our 2 main campuses (HMC and UWMC) prompted a more in-depth analysis of the clinical flow of our endocarditis care. Harborview Medical Center is a King County safety net hospital committed to care of the indigent and underserved but is also a tertiary referral center as a level 1 trauma center, stroke center, heart center, and burn center; referrals for cardiac surgery are made to UWMC. UWMC is a regional tertiary and quaternary referral center for cardiothoracic surgery, electrophysiology, advanced heart failure and transplantation, and cancer care, among other specialties. Thus, our endocarditis care pathway needed to account for both site-specific and interinstitutional management. Our hope was that this pathway would not only serve both University endocarditis management but also facilitate communication and referral guidelines from more distant hospitals.
Initial collaboration around IE care began in early 2017, with core multidisciplinary champions from UWMC and HMC represented by Cardiology, Cardiothoracic Surgery, and Infectious Disease; additional stakeholders from Laboratory Medicine, Addiction Medicine, Hospital Medicine, Information Systems, Medical Ethics, Palliative Care, and Social Services were included for consultation and implementation.
Key Decision Points in Development and Implementation
The committee identified as its first principal that the members of the clinical management team have a direct relationship with both diagnostic and therapeutic activities. In order to standardize communication among the members of the management team, we also undertook to revise and enhance protocols for the diagnosis of endocarditis by transthoracic and transesophageal echocardiography, and to establish protocols for recommending transesophageal echocardiography based on clinical and transthoracic echocardiographic findings21–25 (Fig. 1). It had been a long-standing practice of the infectious disease consulting service to review daily all cases of documented bacteremia. This activity was then linked to findings from the echocardiography lab.
FIGURE 1.
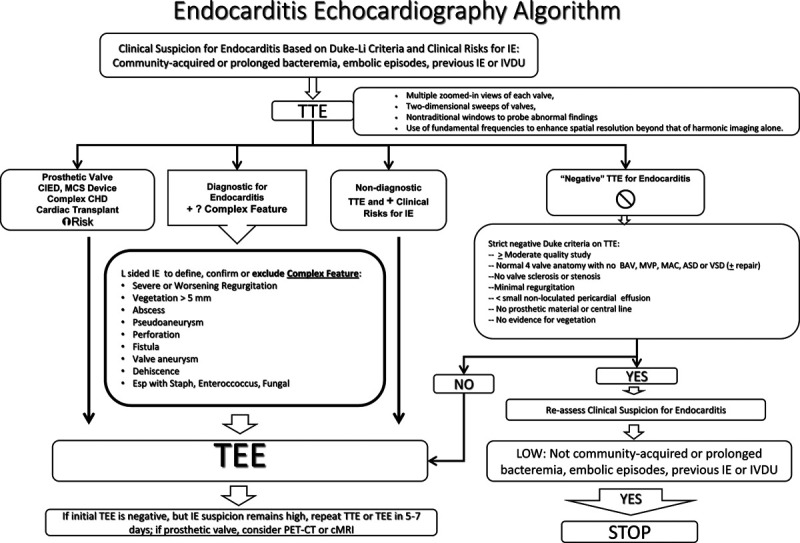
Integrated protocol algorithm for transthoracic echocardiography, with indications for transesophageal echocardiography. CHD indicates congenital heart disease; CIED, cardiac implanted electrophysiology device; IVDU, intravenous drug use; MCS, mechanical circulatory support; TEE, transesophageal echocardiogram. Adapted with permission from Clin Infect Dis. 2000;30:633–638 and Clin Infect Dis. 2012;54:1230–1239.21,22
Thus, the echocardiography reader and the infectious disease specialist are charged with the responsibility of providing an initial diagnosis of endocarditis based on the modified Duke Li criteria, then notifying the primary physician (inpatient or outpatient) of the findings. If a definite or probable diagnosis of endocarditis were made, the severity of the findings would be discussed with the cardiology consult team, who was then charged with the responsibility of assuming comprehensive cardiology care and recommendations and contacting cardiothoracic surgery for consultation based on the urgency of the findings in accordance with guidelines published by the American College of Cardiology.13
Just as in other large urban academic medical centers, we faced the need to put in perspective and commit to a management approach in those patients who, while they may meet criteria for urgent or emergency surgery (based on the development of heart failure or complex endocarditis complications such as severe regurgitation, abscess, persistent bacteremia or recurrent embolization), other comorbidities or social constraints made it difficult or prohibitive to proceed with cardiac surgery on a short-term basis. The most prominent groups facing these limitations were persons with SUD, and those who had had a major intracranial event, either bland or hemorrhagic stroke. In early 2018, a larger multidisciplinary group met in two 90-minute forums with members of the University Medical Ethics committee, to discuss and develop approaches to the complex decision-making processes in endocarditis medical and cardiothoracic surgical care. As a result of these discussions, we were successful in receiving departmental support for expanded consultation resources in addiction medicine. We were also able to outline a more explicit approach to defining the circumstances under which a complex patient may proceed to cardiothoracic surgery or have such surgery deferred to a more conservative medical approach or to palliative care. In addition, we enlisted Information Technology Services to construct an Electronic Medical Record-embedded IE checklist to collate information essential to management and referral for potential cardiac surgery and outpatient handoffs (Supplement 1, available at http://links.lww.com/HPC/A217).
Development of an Endocarditis Management Pathway
To understand our endocarditis population more fully, we (E.F.G.) undertook a detailed direct chart review of 18 months (July 2016 to December 2017) of endocarditis hospitalizations at HMC and UWMC. These charts were identified by both billing and discharge coding data, with endocarditis confirmed or refuted according to the modified Duke Li criteria, after review of clinical, bacteriologic, and imaging data. The results are summarized in Table 1. Key findings include:
TABLE 1.
Results of a Retrospective Chart Review of 213 Unique Patients With Discharge Diagnosis of IE Over 18 Months, 166 of Whom Had True IE by Modified Duke Li Criteria
| HMC | N | UWMC | N | ||
|---|---|---|---|---|---|
| Total true IE | 66* | Total true IE | 106 | ||
| Not IE as coded (excluded) | 20 | Not IE as coded (excluded) | 21 | ||
| Median age | 47.0 | Median age | 47.5 | ||
| % Male | 62.3% | % Male | 84.9% | ||
| SUD | 46 | SUD | 33 | ||
| % SUD | 69.7% | % SUD | 31.1% | ||
| IE valve site | % | IE valve site | % | ||
| TV only | 25 | 37.9% | TV only | 9 | 7.8% |
| MV only | 12 | 18.2% | MV only | 18 | 15.5% |
| AV only | 16 | 24.2% | AV only | 28 | 24.1% |
| Multiple valves | 11 | 16.7% | Multi | 22 | 19.0% |
| ACHD | 1 | 1.5% | ACHD | 7 | 6.0% |
| CIED | 1 | 1.5% | CIED | 18 | 15.5% |
| OHT Rx for IE | 2 | 1.7% | |||
| Cult neg | 2 | 1.7% | |||
| Total IE | 66 | 100.0% | Total IE | 106 | 100.0% |
| Eval by CTS @UWMC | 21 | No. of King County | 53 | 45.7% | |
| Underwent CTS | 14 | 66.7% | CTS | 68 | 64.2% |
| CTS with SUD | 11 | 78.6% | CTS with SUD | 26 | 38.2% |
| HMC | N | UWMC | N | ||
| SUD YES | 46 | SUD YES | 33 | ||
| ≈TOTAL | Median‡ | ≈TOTAL | Median† | ||
| Total/Indirect Charges at HMC | $4,883,841/$849,646 | $80,477/$14,454 | Total/indirect charges at UWMC | $10,562,761/$1,870,385 | $255,602/$47,214 |
| SUD NO | 20 | SUD NO | 64 | ||
| ≈TOTAL | Median‡ | ≈TOTAL | Median† | ||
| Total/indirect charges at HMC | $2,738,833/$419,242 | $90,461/$16,301 | Total/indirect charges at UWMC | $14,876,929/$2,273,113 | $199,465/$29,105 |
| ‡t test SUD vs. no SUD | P = 0.34 | †t test SUD vs. no SUD | P = 0.026 |
ACHD indicates adult congenital heart disease; AV, aortic valve; CIED, cardiac implanted electrophysiology device; CTS, cardiothoracic surgery; MV, mitral valve; OHT, orthotopic heart transplant; TV, tricuspid valve.
*HMC patients who underwent CTS at UWMC are included in both groups.
†HMC SUD vs. no SUD.
‡UWMC SUD vs. no SUD.
A total of 166 patients were identified who met criteria for true active endocarditis; 41 patients were excluded, lacking Duke Li criteria for current IE.
At HMC, SUD was seen in 70% of patients versus 31% of patients at UWMC, and there was a higher percentage of women at HMC compared with UWMC.
Isolated tricuspid valve endocarditis was seen in 38% of cases at HMC but only 8% of cases at UWMC.
Left-sided endocarditis or multiple valve involvement was similar in both groups, but device infection was more common at UWMC. Cardiac surgery (all at UWMC) was undertaken in 21% of HMC IE cases, and 64% of UWMC total cases; of these, 32% were in those with SUD. Referral from non-UW Medicine providers for endocarditis surgery was seen in 54% of cases at UWMC.
Both total and indirect median costs for endocarditis care at UWMC were significantly higher in individuals with SUD (t test, 2 tailed: P = 0.014, P = 0.026, respectively), largely due to more complex surgery and to extended postoperative care. At HMC, total and indirect costs were not significantly different for SUD versus non-SUD, with the majority of cases being managed without surgical intervention.
Our discussions in the ethics forums, as well as our discussions with addiction medicine, led our group to prioritize urgent addiction medicine consultation at the time of IE diagnosis, and certainly before consideration of cardiac surgery, both to initiate therapy and to ensure that postoperative therapy would continue. The multidisciplinary Addiction Medicine team includes nursing, social work, and peer-support specialists working in an screening, brief intervention, and referral to treatment model. Formal addiction specialist physician consultation was then provided. This, of course, required full and frank discussion with the patient, family, and primary medical team.
Consensus on Disparate Clinical Profiles
Our multidisciplinary group determined that candidacy for cardiac surgery in IE be determined initially based on the American College of Cardiology/American Heart Association criteria for IE cardiac surgery, realizing that difficult decisions and some moral distress to proceed with such surgery,26 would arise in some IE cases. We felt that the approach of “what would it take?” to proceed with surgery, would engage the patient and family, primary care team and consultants, and help us to address social, psychiatric, and limiting medical comorbidities to achieve realistic goals in individual patients. The common elements in setting up the remaining components of the pathway are illustrated in Figure 2. These elements serve the organizational requirements of IE diagnosis and Addiction medicine consultation regardless of need for surgery, as well as to achieve consensus regarding indications and urgency for IE cardiac surgery in a clinical “team huddle.”
FIGURE 2.
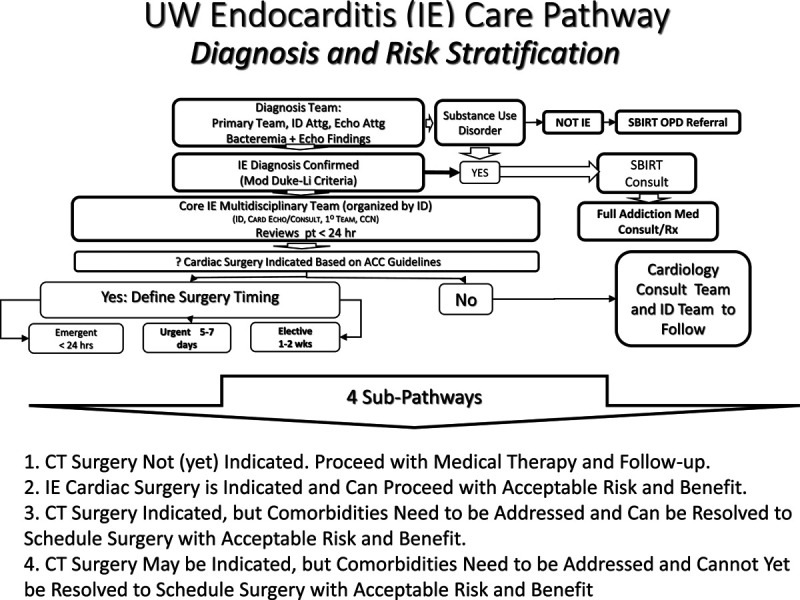
Initial diagnostic and therapeutic profiles common to all patients with IE. Subpathway categories 1–4 are listed. CT indicates cardiothoracic.
Based on the above chart review, we identified four subpathways for clinical care (Table 2) based on the severity of the patient’s endocarditis, its complications and confounding comorbidities. A higher proportion of medically-treated IE at HMC versus UWMC (41.7% vs. 23.4%) reflects the higher incidence of tricuspid valve IE at HMC. A higher proportion of delayed or deferred surgery at HMC versus UWMC (41.7% vs. 22.4%) reflects a higher incidence of SUD, including neurologic, renal, and behavioral disorders, as well as patient refusal of surgery. At UWMC, a higher proportion of patients proceeding directly to cardiac surgery reflects referral from a large number of non-UW Medicine providers in the Pacific Northwest and Alaska, both urgency as well as resolution of barriers to cardiac surgery prior to admission or transfer, contributed to this pattern.
TABLE 2.
Assignment of management pathways determined by chart review. See text for interpretation of these results.
| Subpathways: With SUD, HMC + UWMC | % | N | Subpathways: No SUD, HMC + UWMC | % | N |
|---|---|---|---|---|---|
| 1: Medical therapy | 41.7 | 30 | 1: Medical therapy | 23.4 | 25 |
| 2: Cardiac surgery direct | 15.3 | 11 | 2: Cardiac surgery direct | 43.0 | 46 |
| 2 CIED: Device explant | 1.4 | 1 | 2 CIED: Device explant | 15.0 | 16 |
| 3: Surgery delayed by comorbidities | 26.4 | 19 | 3: Surgery delayed by comorbidities | 13.1 | 14 |
| 4: Surgery prohibitive or declined by patient | 15.3 | 11 | 4: Surgery prohibitive or declined by patient | 9.3 | 10 |
The empirically derived subpathways are as follows:
Cardiac surgery not (yet) indicated. Proceed with Medical Therapy and Follow-up. Example: an individual on chronic hemodialysis with methicillin-sensitive S. aureus and isolated tricuspid valve IE, without significant tricuspid regurgitation (Fig. 3).
Cardiac surgery is indicated and can proceed with acceptable risk and benefit. Example: an otherwise healthy individual with IE who develops severe mitral regurgitation in the setting of S. viridans IE and chronic mitral valve prolapse (Fig. 4).
Cardiac surgery indicated, but comorbidities need to be addressed and can be resolved to schedule surgery with acceptable risk and benefit. Example: a person with SUD with a non-hemorrhagic hemispheric stroke and E. faecalis aortic valve IE, moderate to severe aortic insufficiency without abscess or heart failure, agreeable to and enrolled in an addiction medicine program; surgery scheduled within 4 to 6 weeks with close follow-up (Fig. 5).
Cardiac surgery may be indicated, but comorbidities that are impediments to surgery cannot readily be resolved to schedule surgery with acceptable risk and benefit. Example: a marginally housed person with SUD and poor social support who presents with 10 to 18 mm vegetations on aortic and mitral valves due to methicillin-resistant S. aureus. Mitral leaflet perforation and moderate to severe regurgitation are present. Patient has eloped to an encampment 3 times to inject drugs and refuses addiction medicine treatment (Fig. 6).
FIGURE 3.
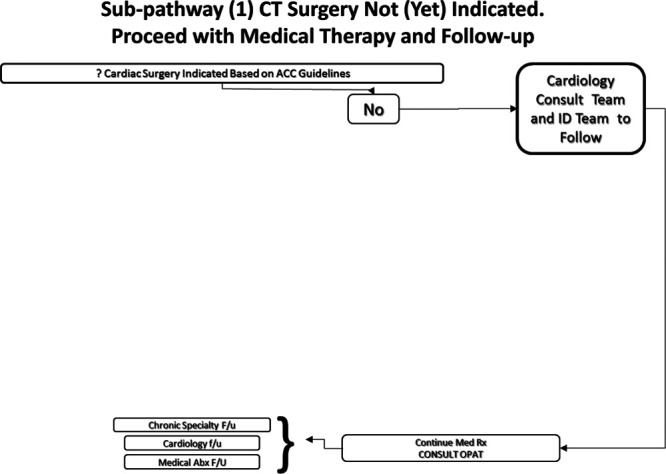
Subpathway.1 Cardiothoracic (CT) surgery not (yet) indicated. Proceed with medical therapy and follow-up. OPAT indicates outpatient antibiotic therapy service.
FIGURE 4.
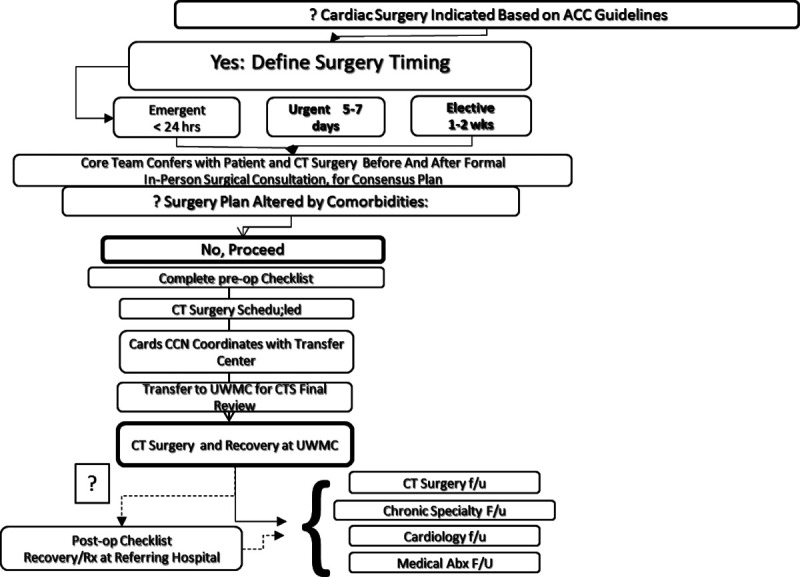
Subpathway.2 IE cardiothoracic (CT) surgery is indicated and can proceed with acceptable risk and benefit.
FIGURE 5.
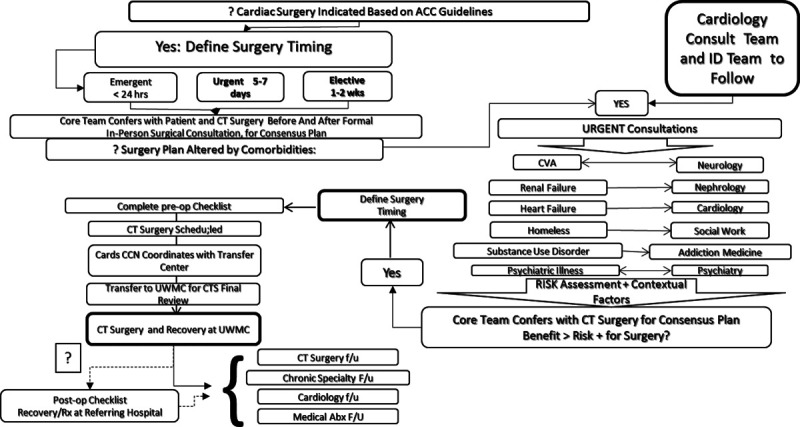
Subpathway.3 Cardiothoracic (CT) surgery is indicated, but comorbidities need to be addressed and can be resolved to schedule surgery with acceptable risk and benefit, based on patient stability and resolution of barriers to surgery.
FIGURE 6.
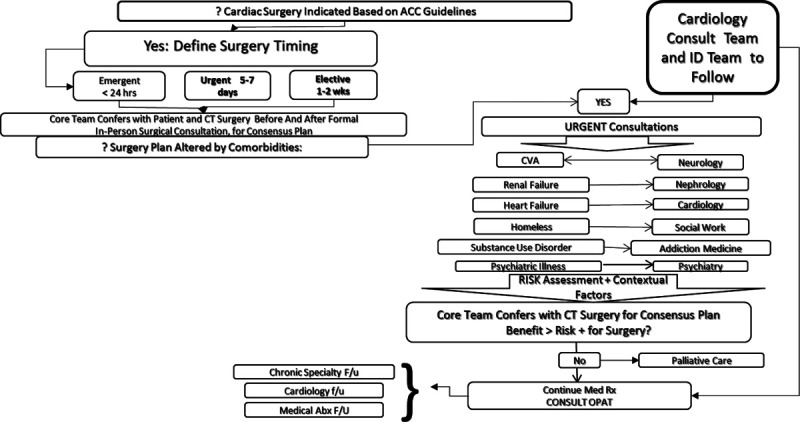
Subpathway.4 Cardiothoracic (CT) surgery may be indicated, but comorbidities that are impediments to surgery cannot readily be resolved to schedule surgery with acceptable risk and benefit. Patient is reassessed at appropriate intervals.
It must be emphasized that these 4 pathways are to be used as guidelines for patient care and must admit to modification based on new data or a change in patient condition.
Implementation Rollout: Linkage and Education
Our integrated clinical pathways (Supplement 2, available at http://links.lww.com/HPC/A217) gave us the language and structure to present our recommendations to caregivers at key forums within the medical centers: a follow-up ethics forum, 5-noon conferences, 3 internal web-based document libraries, as well as ongoing individual IE consultations. IE discovery and care became linked to IE clinical pathway presentation and discussion.
CONCLUSIONS
The process of development and implementation of our endocarditis integrated clinical pathway has provided our institutions with a more uniform approach to the care of both local patients and those referred from a distance. The consensus-driven and evidence-based criteria we adopted have facilitated communication among stakeholders and engaged our consultants in a framework that is mutually productive: our consultants are also those whose patients may enter the IE population. We are in the process of assessing clinical outcomes with the pathways to further refine their utility.
LIMITATIONS
The appropriate application and optimal timing of cardiac surgery in IE remain undefined in specific high-risk populations: poststroke; in persons with SUD who develop recurrent endocarditis; in those with left-sided endocarditis with large vegetations > 10 mm but significant comorbidity, and in those with significant cognitive impairment or lack of social support which can limit recovery and benefit. Our pathways provide a real-time approach to call out and address, if not resolve, these uncertainties. The inclusion of patients referred to UWMC from non-UW Medicine providers may be subject to selection bias, as variations in external management and perceived surgical candidacy likely differ from UW Medicine patterns. Extending these pathways to referring institutions is our goal.
ACKNOWLEDGMENTS
The workgroup would like to thank colleagues who contributed valuable time and insight in the development and refinement of pathway ideas and implementation: Paul Pottinger, MD, Chetan Seshadri, MD, and Jenell Stewart, MD (all of the Division of Allergy and Infectious Disease) as well as Laboratory Medicine, Addiction Medicine and the Screening, Brief Intervention, and Referral to Treatment (SBIRT) team members, Echocardiography Lab sonographers, James Kirkpatrick, MD, and the UW Medicine Ethics Committee.
DISCLOSURES
Nothing to disclose.
Supplementary Material
Footnotes
This content was not previously presented.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.critpathcardio.com).
REFERENCES
- 1.Murdoch DR, Corey GR, Hoen B, et al. ; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the international collaboration on endocarditis-prospective Cohort Study. Arch Intern Med. 2009;169:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol. 2017;69:325–344. [DOI] [PubMed] [Google Scholar]
- 3.Wang A, Gaca JG, Chu VH. Management considerations in infective endocarditis: a review. JAMA. 2018;320:72–83. [DOI] [PubMed] [Google Scholar]
- 4.Institute for Health Metrics Evaluation. Seattle, WA: University of Washington; Available at: https://vizhub.healthdata.org/gbd-compare/. [database online], Accessed January 22, 2020. [Google Scholar]
- 5.Baddour LM, Wilson WR, Bayer AS, et al. ; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435–1486. [DOI] [PubMed] [Google Scholar]
- 6.Fleischauer AT, Ruhl L, Rhea S, et al. Hospitalizations for endocarditis and associated health care costs among persons with diagnosed drug dependence - North Carolina, 2010-2015 MMWR Morb Mortal Wkly Rep. 2017;66:569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadri AN, Wilner B, Hernandez AV, et al. Geographic trends, patient characteristics, and outcomes of infective endocarditis associated with drug abuse in the United States From 2002 to 2016 J Am Heart Assoc. 2019;8:e012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg. 2015;100:875–882. [DOI] [PubMed] [Google Scholar]
- 9.Østerdal OB, Salminen PR, Jordal S, et al. Cardiac surgery for infective endocarditis in patients with intravenous drug use. Interact Cardiovasc Thorac Surg. 2016;22:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JB, Ejiofor JI, Yammine M, et al. Surgical outcomes of infective endocarditis among intravenous drug users. J Thorac Cardiovasc Surg. 2016;152:832–841.e1. [DOI] [PubMed] [Google Scholar]
- 11.Tookes H, Diaz C, Li H, et al. A cost analysis of hospitalizations for infections related to injection drug use at a county safety-net hospital in Miami, Florida. PLoS One. 2015;10:e0129360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodger L, Glockler-Lauf SD, Shojaei E, et al. Clinical characteristics and factors associated with mortality in first-episode infective endocarditis among persons who inject drugs. JAMA Netw Open. 2018;1:e185220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schranz AJ, Fleischauer A, Chu VH, et al. Trends in drug use-associated infective endocarditis and heart valve surgery, 2007 to 2017: a Study of Statewide Discharge Data. Ann Intern Med. 2019;170:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudasill SE, Sanaiha Y, Mardock AL, et al. Clinical outcomes of infective endocarditis in injection drug users. J Am Coll Cardiol. 2019;73:559–570. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura RA, Otto CM, Bonow RO, et al. ; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. [DOI] [PubMed] [Google Scholar]
- 16.Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100:524–527. [DOI] [PubMed] [Google Scholar]
- 17.Habib G, Lancellotti P, Antunes MJ, et al. ; ESC Scientific Document Group. 2015 ESC Guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–3128. [DOI] [PubMed] [Google Scholar]
- 18.Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169:1290–1298. [DOI] [PubMed] [Google Scholar]
- 19.Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112:1171–1176. [DOI] [PubMed] [Google Scholar]
- 20.Mestres CA, Paré JC, Miró JM; Working Group on Infective Endocarditis of the Hospital Clínic de Barcelona. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014) Rev Esp Cardiol (Engl Ed). 2015;68:363–368. [DOI] [PubMed] [Google Scholar]
- 21.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. [DOI] [PubMed] [Google Scholar]
- 22.Selton-Suty C, Célard M, Le Moing V, et al. ; AEPEI Study Group. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis. 2012;54:1230–1239. [DOI] [PubMed] [Google Scholar]
- 23.Sivak JA, Vora AN, Navar AM, et al. An approach to improve the negative predictive value and clinical utility of transthoracic echocardiography in suspected native valve infective endocarditis. J Am Soc Echocardiogr. 2016;29:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habib G, Badano L, Tribouilloy C, et al. ; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr. 2010;11:202–219. [DOI] [PubMed] [Google Scholar]
- 25.Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2013;26:921–964. [DOI] [PubMed] [Google Scholar]
- 26.DiMaio JM, Salerno TA, Bernstein R, et al. Ethical obligation of surgeons to noncompliant patients: can a surgeon refuse to operate on an intravenous drug-abusing patient with recurrent aortic valve prosthesis infection? Ann Thorac Surg. 2009;88:1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


