Abstract
Objective:
Statins are a class of drugs that competitively bind to the active site of HMG-CoA reductase enzyme, thereby inhibiting the initial steps in cholesterol synthesis. Originally approved for use in lowering serum cholesterol, a risk factor for developing atherosclerosis and coronary heart disease, statins have subsequently been noted to have myriad extrahepatic effects, including potential effects on cognition, diabetes, breast cancer, bone, and muscle. This narrative review assesses the current state of the science regarding the risks and benefits of statin therapy in women in order to identify areas where additional research is needed.
Methods:
Basic and clinical studies were identified by searching PubMed with particular attention to inclusion of female animals, women, randomized controlled trials and sex-specific analyses.
Results:
Statin therapy is generally recommended to reduce the risk of cardiovascular disease. However, none of the current clinical guidelines offer sex-specific recommendations for women due to lack of understanding of sex-differences and underlying mechanisms of disease processes. In addition, conclusions regarding efficacy of treatments do not consider lipid solubility for the drug, dosing, duration of treatment, interactions with estrogen or comorbidities. Pleiotropic effects of statins are often derived from secondary analysis of studies with cardiovascular events as primary outcomes and remain unproven.
Conclusions:
Many of the trials that have established the efficacy and safety of statins were conducted predominantly or entirely in men, with results extrapolated to women. Additional research is needed to guide clinical recommendations specific to women.
Keywords: statins, women, heart disease, diabetes, cognition
Introduction
Statins refer to a class of drugs originally approved by the United States Food and Drug Administration (FDA) for use in lowering serum cholesterol, in particular low density lipoprotein cholesterol, to reduce the risk for atherosclerosis. Similar to many other drugs, FDA approval of statins was primarily based on studies performed in men. With their widespread clinical use, it has become clear that statins have myriad effects on other systems, including the brain, breast, and musculoskeletal system. Moreover, statins are a heterogeneous class of medications that are not bioequivalent. Their tissue distribution is variable, and depends on the relative degree of lipophilicity, with the more lipophilic statins exerting greater extrahepatic effects.[1, 2] While simvastatin has been studied more than other statins, sweeping generalizations about effects of other statins outside of lipid-lowering should be made with caution. Until recently, many of the statin trials predominantly involved men with the results extrapolated to women and without regard to potential interactions of the drugs with sex-steroid action or metabolism. This narrative review examines the current state of the science regarding statin therapy in women from a theoretical and contextual point of view focused on sex-specific randomized controlled trial data, where available, in order to identify areas where additional research is needed.
Methods
For this narrative review, a search of PubMed was conducted for randomized controlled trials through March 10, 2019 that focused on sex-specific data on statins where available, including meta-analyses of such. When such data were not available, observational and other data were discussed. Search terms included statin therapy in men and women and individual search terms related to statins and genetic variations in metabolism, lipophilic and hydrophilic formulations, estrogen metabolism, coronary heart disease (CHD), stroke, mortality, dementia, diabetes, myotoxicity, osteoporosis and cancer. Current professional guidelines relating to statin use were considered.
Mechanism of action, pharmacogenomics, and potential impact of sex steroids
Statins are a class of drugs that share structural homology with 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). Thus, these drugs competitively bind to the active site of HMG-CoA reductase (HMGCR) inhibiting the conversion of HMG-CoA to mevalonic acid, which is an early step in the cholesterol synthesis pathway (Figure 1).[3, 4] Most endogenous production of cholesterol occurs in the liver.[5, 6] When the concentration of cholesterol in the serum is decreased due to reduction in endogenous synthesis, hepatic expression of LDL receptors is upregulated leading to increased uptake of LDL cholesterol, which further lowers the plasma concentration of cholesterol.[7] It is important to note that inhibition of HMGCR impacts not only cholesterol synthesis, but also the synthesis of other intermediate compounds in the pathway, such as isoprenoid intermediates. Decreased synthesis of these other compounds may contribute to the pleiotropic effects of statins as well as statin-associated adverse events.[8]
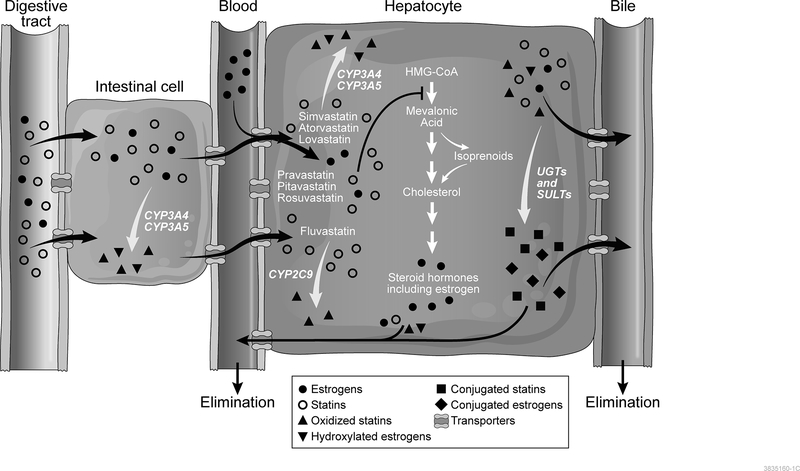
Figure 1. Mechanism of Action of Statins and Potential Interactions with Estrogen.
Orally administered statins (open circles) enter intestinal cells passively or through active transport. Simvastatin, atorvastatin, and lovastatin may undergo oxidative metabolism by CYP3A4/5 enzymes (triangles) within the intestinal cell. Next, statins are transported through the bloodstream into hepatocytes. OATP1B1 is one of several transporters responsible for hepatic uptake of statins. In the liver, statins competitively bind to the active site of HMG-CoA reductase, inhibiting the conversion of HMG-CoA to mevalonic acid (depicted by blunt-ended arrow), which is an early step in the cholesterol synthesis pathway (shown in center of hepatocyte). Simvastatin, lovastatin, and atorvastatin are metabolized by CYP3A4/5, while fluvastatin is metabolized primarily by CYP2C9 (metabolites depicted by triangles). Pravastatin, pitavastatin, and rosuvastatin undergo limited cytochrome P450-mediated metabolism. In addition, statins may also undergo glucuronidation or sulfation mediated by polymorphic glucuronosyltransferase and sulfotransferase enzymes (metabolites shown as squares). Finally, statins may be transported out of the hepatocyte and into bile for elimination or into the bloodstream for renal elimination.
Estrogens (filled circles) may be administered orally or transdermally, or may be endogenously produced. Orally administered estrogens must pass through the intestinal cells where CYP3A4/5-mediated metabolism may occur (metabolites shown as inverted triangles). Transdermally administered estrogens and endogenous estrogens enter the hepatocytes from the blood where they may be metabolized by CYP3A4/5 and/or undergo sulfate and glucuronide conjugation (diamonds), similar to statins. Sulfate- and glucuronide-conjugated estrogens may use the same transporters as statins, including OATP1B1.
Statins are orally administered and undergo extensive first-pass metabolism resulting in low systemic bioavailability (Figure 1). The drugs enter intestinal cells passively or through active transport. Several statins, such as simvastatin, atorvastatin, and lovastatin, may be partially metabolized by CYP3A4/5 enzymes within the intestinal cell. Statins are then transported through the portal bloodstream and into hepatocytes. SLCO1B1, a polymorphic gene encoding OATP1B1, is one of several transporters that mediates hepatic uptake of statins. Although all statins are transported by OATP1B1, the impact of the genetic variants differs by statin, due in part to the contribution of other OATP transporters.[9] The most commonly studied single-nucleotide polymorphism, c.521T>C (p.Val174Ala; rs4149056) reduces the statin transport activity of OATP1B1 and has been associated with statin-related myotoxicity, particularly in individuals treated with simvastatin, with an odds ratio of 4.5 per copy of the c.521C allele.[10] Other variants in SLCO1B1 as well as ATP-binding cassette transporters may alter the bioavailability and distribution, but the clinical significance of these variants remains less clear than that of the SLCO1B1 c.521T>C variant.
Within the liver, simvastatin, lovastatin, and atorvastatin are metabolized by CYP3A4/5, while fluvastatin is metabolized primarily by CYP2C9.[11] These enzymes show significant interindividual variability in activity, due to genetic variation as well as to induction and inhibition mediated by other endogenous and exogenous substances. This variability may impact medication efficacy and contribute to adverse drug reactions. Several variants, which vary in frequency among populations, have been described in CYP3A4 and CYP3A5 that are known to lead to reduced function due to alternative splicing.[12, 13, 14] Although several of these variants have been associated with variability in statin response,[12, 15, 16] studies did not account for sex as a biological variable by evaluating men and women separately, so it remains unclear whether any interaction between genetic variation and sex may impact statin response and adverse effects. Similarly, CYP2C9 has over 60 known alleles, the prevalence of which vary by population, but the impact of these variants on statin metabolism and potential interaction with sex has not been studied.[12] Pravastatin, pitavastatin, and rosuvastatin undergo limited cytochrome P450-mediated metabolism.[4] In addition, statins may also undergo glucuronidation or sulfation mediated by polymorphic glucuronosyltransferase and sulfotransferase enzymes.[13] Finally, statins may be transported out of the hepatocyte and into bile for elimination or into the bloodstream for renal elimination.[4, 13]
Estrogens are metabolized in the liver by many of the same cytochrome P450 enzymes and undergo sulfate and glucuronide conjugation, similar to statins (Figure 1). In addition, estrone-3-sulfate and estradiol 17β-D-glucuronide are also known substrates for OATP1B1. Because of this common pathway, statins could compete with estrogens for the same enzymes and transporters, resulting in drug-hormone interactions. In support of this hypothesis, many clinical studies suggest that women tend to metabolize medications more quickly than men, particularly substrates of CYP3A4 corresponding to higher levels of CYP3A4 protein in hepatic tissue.[14] In an in vitro study, estradiol induced expression of CYP3A4 in human hepatocytes, while progesterone induced expression of CYP3A4 and CYP3A5. These effects were most readily observed at high concentrations approaching those occurring during pregnancy.[15] High estrogen levels in pregnancy also alter lipid metabolism leading to hyperlipidemia in the second and third trimesters that is thought to provide energy for fetal growth and development.[16] In addition, female sex has been associated with increased likelihood of statin-related myotoxicity, particularly among carriers of the SLCO1B1 c.521C allele, also suggesting that estrogen may compete with statins for this transporter.[17]
Another link between estrogen and lipid metabolism is evidenced by the finding that polymorphisms in ESR1 encoding the estrogen receptor were associated with triglyceride and high-density lipoprotein cholesterol (HDL).[18] Further studies to elucidate the interactions among estrogens, lipid metabolism, and statins are warranted.
In contrast, in a subgroup analysis from the Estrogen in the Prevention of Atherosclerosis Trial of postmenopausal women randomized to receive oral estradiol versus placebo,[19] no significant differences in DHEA, androstenedione, testosterone, estrone, and estradiol levels were observed between the untreated and the statin treated groups (mean change from baseline to on-trial levels), whether or not the women received estradiol. The majority of the treated participants were using pravastatin, and whether these results apply to use of other statins remains to be determined.
Potential benefits and risks of statin therapy in women
Cardiovascular disease
Statins are the most commonly used medications for lowering serum lipids to reduce primary and secondary risk for cardiovascular disease (CVD) and all-cause mortality in women and men. This practice is based on theory established from basic studies using experimental animal models for the development of atherosclerotic plaque with lipid peroxidation leading to endothelial dysfunction, invasion of macrophages into the damaged region, ultimately leading to intima-media thickening, necrosis and calcification.[20] This model was developed using male animals with the assumption that the progression of atherosclerotic disease was the same in females, although this has yet to be substantiated as plaque development is diffuse in women.[21, 22] In addition, atherosclerosis is often described as an inflammatory disease. Immunological activation differs between males and females with a potential genetic contribution to predisposition to atherosclerosis associated with immune activation regulated by genes on the Y chromosome.[23, 24] Current prescribing guidelines for statin therapy for primary and secondary prevention of cardiovascular disease are based on evaluation of serum concentrations of low-density lipoprotein along with the presence of existing atherosclerosis and/or diabetes.[25–28] However, they are not sex-specific. Indeed, careful examination of randomized control trial (RCT) data and meta-analyses show that the effects of statin therapy on CVD and all-cause mortality differ depending upon sex and use in primary versus secondary prevention.
Statin therapy in primary prevention of coronary heart disease
In a large sex-specific meta-analysis in which primary and secondary CHD prevention trials were analyzed independently, statin therapy reduced CHD in primary prevention in men but not in women,[29] even with inclusion of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) trial in which 5,356 women were enrolled and followed for more than 5 years.[30] Statin therapy did not significantly reduce all-cause mortality in women (or men) in primary prevention (Table 1).[29] These results were confirmed in another sex-specific meta-analysis of statins and CHD prevention that included RCTs conducted in individuals with diabetes mellitus without a history of CVD (Table 1).[31] Also included in this meta-analysis were MEGA and the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER), another primary CHD prevention RCT with a cohort of 6,801 women.[32] Even though this latter meta-analysis included a larger number of women at greater CHD risk than the previously mentioned sex-specific meta-analysis, the reduction in CHD risk in women was also statistically nonsignificant; all-cause mortality was null and no different than that reported in other meta-analyses (Table 1).
Table 1.
Comparison of Primary Prevention of Statin Therapy on Coronary Heart Disease and All-Cause Mortality in Women and Men
| Outcome | Women HR (95% CI) | N | Men HR (95% CI) | N |
|---|---|---|---|---|
| Coronary heart disease | ||||
| Walsh and Pignone27 | 0.89 (0.69–1.09) | 11,435 | ||
| Petretta et al.23 | 0.95 (0.78–1.16) | 13,346 | 0.55 (0.41–0.75) | 28,346 |
| Brugts et al.25 | 0.79 (0.56–1.13) | 20,817 | 0.72 (0.61–0.86) | 26,921 |
| Hope-330 | 0.83 (0.64–1.09)a | 5,871 | 0.72 (0.58–0.90)* | 6,831 |
| Total mortality | ||||
| Walsh and Pignone27 | 0.95 (0.62–1.46) | 11,435 | ||
| Petretta et al.23 | 0.96 (0.81–1.13) | 11,849 | 0.93 (0.83–1.04) | 20,426 |
| Brugts et al.25 | 0.91 (0.76–1.08) | 20,817 | 0.95 (0.86–1.06) | 26,921 |
| Hope-330 | 0.93 (0.80–1.08)b | 5,871 |
Cardiovascular death, nonfatal myocardial infarction or nonfatal stroke
Women and men combined
HR = hazard ratio; CI = confidence interval
Statin therapy in secondary prevention of coronary heart disease
Whereas evidence fails to support the effectiveness of statin therapy for primary prevention in women, individual RCTs show that statin therapy reduces CHD in secondary prevention. In a large meta-analysis in which secondary (eight RCTs; n=8,272 women) and primary (six RCTs; n=11,435 women) prevention trials with statin therapy were analyzed separately in women, data show a significant reduction in CHD events in secondary (hazard ratio (HR), 0.80; 95% confidence interval (CI), 0.71–0.91) but not in primary (HR, 0.89; 95% CI, 0.69–1.09) prevention.[33] All-cause mortality in women was neither reduced in secondary (HR, 1.00, 95% CI, 0.77–1.29) nor primary (HR, 0.95, 95% CI, 0.62–1.46) prevention (Table 1).[33]
Statin therapy and mortality
Individual primary CHD prevention trials and sex-specific meta-analyses consistently show that statin therapy does not reduce CHD or all-cause mortality in women without preexisting CVD. The one possible exception is JUPITER in which CHD was lower (HR, 0.54, 95% CI, 0.37–0.80) in women in a post-hoc analysis. However, JUPITER was stopped early after only a median follow-up of 1.9 years, and it is unclear whether findings from JUPITER were due to premature cessation of the trial, the unique characteristics of the cohort (women aged ≥60 years with low-density lipoprotein cholesterol <130 mg/dL and high-sensitivity C-reactive protein ≥2 mg/dL), trial biases and flaws[34, 35] or due to the subjective nature of certain components of the primary endpoint.[32] The primary cardiovascular endpoint in JUPITER was a composite of “hard endpoints” [nonfatal myocardial infarction (MI), nonfatal stroke, or confirmed death resulting from cardiovascular causes] and “soft endpoints,” whose occurrence rely on medical decisions (arterial revascularization or hospitalization for unstable angina pectoris). In men, all the hard and soft components of the composite primary endpoint were significantly lower with statin therapy than in the placebo arm. In women, only the soft subjective endpoints (revascularizations and hospitalizations) were significantly lower and clearly drove the primary endpoint to statistical significance because none of the hard endpoints in the women differed significantly between statin therapy and placebo (P>.10).[32] All-cause mortality was not statistically different between statin therapy and placebo in women (P=.12) or men (P=.08).[32] Including JUPITER in meta-analyses along with other primary prevention trials does not alter the conclusion that statin therapy has a null effect on CHD events and all-cause mortality in primary CHD prevention in women.[31]
Further evidence that statin therapy has a null effect on CHD and all-cause mortality in primary prevention in women derives from the recent Heart Outcomes Prevention Evaluation (HOPE)-3 trial, a large RCT of 5,874 women and 6,831 men at intermediate risk (at least one or two cardiovascular risk factors) for CVD.[36] The first co-primary endpoint was the composite of cardiovascular death, nonfatal MI or nonfatal stroke, and the second co-primary endpoint additionally included revascularizations, heart failure, and resuscitated cardiac arrest. After a median follow-up of 5.6 years, statin therapy relative to placebo had a statistically non-significant effect on the first co-primary endpoint in women (HR, 0.83; 95% CI, 0.64–1.09). Relative to placebo, statin therapy also had a statistically non-significant effect on the secondary co-primary endpoint in women (HR, 0.82; 95% CI, 0.64–1.06) even though this endpoint contained the soft subjective endpoint of revascularizations. Although both JUPITER and HOPE-3 involved the same statin (rosuvastatin), the results in women on the subjective endpoint differed. On the other hand, relative to placebo, statin therapy significantly reduced both HOPE-3 co-primary endpoints in men. Statin therapy had no effect on all-cause mortality in HOPE-3 in women and men (HR, 0.93; 95% CI, 0.80–1.08).
Statin therapy and stroke prevention
Statin therapy in the primary or secondary prevention of ischemic stroke is less clear than CHD. Unlike CHD, there are no published meta-analyses examining the effect of statins in primary prevention of stroke in women and men independently. However, in the majority of primary prevention RCTs, including the large MEGA trial, with women and men combined, there is no statistically significant reduction in stroke with statin therapy.[30, 37, 38] In JUPITER, stroke was not significantly reduced in women (HR, 0.75; 95% CI, 0.45–1.5), whereas, in men, stroke was reduced (HR, 0.40; 95% CI, 0.26–0.73).[39] In a sex-stratified meta-analysis of secondary prevention trials, statins had no significant effect on stroke (RR, 0.92; 95% CI, 0.76–1.10) and all-cause mortality (RR, 0.82; 95% CI, 0.76–1.13) in women, whereas, in men, stroke (RR, 0.81; 95% CI, 0.72–0.92) and all-cause mortality (RR, 0.79; 95% CI, 0.72–0.87) were significantly reduced.[40] On the other hand, statins may increase the risk for hemorrhagic stroke.[41, 42]
Summary
Careful examination of the source trials used in meta-analyses clearly show that in primary prevention, statins do not statistically significantly reduce CHD in women.[29, 31] Additionally, in truly sex-specific meta-analyses that separate primary prevention trials from secondary prevention trials, statins do not statistically significantly reduce CHD in primary prevention in women although there appears to be CHD reduction in secondary prevention.[33] Importantly, all-cause mortality is not influenced by statin therapy in women in primary or secondary prevention.[29, 31, 33] Conclusions from sex-specific meta-analyses are supported by three primary prevention trials that included the largest cohorts of women, MEGA, JUPITER and HOPE-3.[30, 32, 36] Cumulated data in women also show that statins do not reduce stroke in secondary prevention and are also unlikely to reduce stroke in primary prevention.[40] The underlying mechanisms that contribute to these sex-disparate effects of statins for primary prevention of cardiovascular disease in women require additional study. In addition, future clinical studies of statin use need to include sex-specific analysis and to consider hormonal status, e.g., current estrogen usage, as the combined use of estrogen and statins has been shown to reduce all-cause mortality unlike statins alone.[43]
Alzheimer’s disease and dementia
There are two key concepts that need to be considered when evaluating the potential impact of the use of statins on brain function and cognition. First, is the possibility of a direct effect of the drug on cellular processes within the brain,[44] including but not limited to mediating production of amyloid-β,[45] modulating production of tau-protein,[46] angiogenesis and neuronal sprouting,[47] modulating endothelial, inducible and neuronal isoforms of nitric oxide synthase,[48] and modulating inflammatory processes associated with leukocyte-endothelial interactions,[49] which may lead to changes in permeability of the blood brain barrier.[50] Second, is the possibility that the association of statin use with incidence of Alzheimer’s or other forms of dementia is secondary to peripheral effects of statins on lipid metabolism and reduction in cardiovascular risk.
Critical to the first possibility is the need for the drugs to cross the blood brain barrier and to alter neuronal processes. Whether the statin is lipophilic or hydrophilic will determine, in part, access to the brain tissue and the relative efficacy of various pleiotropic effects.[51–53] Direct evidence that statins affect cellular neuronal processes is derived from basic studies where the drugs are applied to cultured cells. Given that amyloid-β is associated with development of Alzheimer’s disease, and taking into consideration that the enzymes required for production of amyloid-β function best in a high cholesterol environment, reducing production of cholesterol by statins would, in theory, decrease production of amyloid-β and subsequently slow or prevent development of Alzheimer’s disease. Experimental models using neuronal cell cultures and animals provide supporting evidence for this hypothesis.[45]
Statin therapy and primary prevention of Alzheimer’s disease and dementia
Evidence to support this hypothesis in humans was derived from a cross-sectional, retrospective analysis comparing the prevalence of Alzheimer’s disease from hospital records of patients using statins to reduce serum cholesterol without any relation to dementia.[54] Lovastatin and pravastatin were the most frequently used statins (lovastatin being lipophilic and pravastatin being hydrophilic), and overall, the prevalence of a diagnosis of Alzheimer’s disease was 60–73% lower in statin users than in the patient population of persons > 60 years of age. Two of the hospitals in this study were Veterans’ Administration facilities; thus, most participants were men. Yet, at the third site, 55% of participants were female. At this site, the reduction in prevalence of diagnosed Alzheimer’s disease was greater in women (96.3 %) than in men (53%).[54] In contrast, in a small cohort of Catholic clergy (n=929) in which women represented about 70% of the participants, no association was found between statin use (included lipophilic and hydrophilic formulations) and changes in scores on cognitive tests or measures of Alzheimer’s pathology at autopsy. Specifically, on subanalysis of those with amyloid pathology, there were no significant differences in amyloid load as a continuous measure in users of all statins, more lipophilic, or more hydrophilic statin formulations.[55] Consistent with these results, in a cross sectional study (n = 1160; about 50% women; average age 79 years), there were no differences in neuroimaging markers of Alzheimer’s pathology (tau or β amyloid) between statin users who had used statins for over 10 years (range 10–37 years) and non-users regardless of age, sex, and type of statin.[56] A difference in fractional anisotropy of the genu of the corpus callosum between the groups reflected the burden of cardiovascular disease in the statin users. However, using a medical and pharmacy claims sample of Medicaid beneficiaries (399,979 statin users evaluated by dose), and using clinical codes to identify a diagnosis of Alzheimer’s disease, a reduction in Alzheimer’s disease risk varied by the type of statin, sex, and ethnicity, with a greater reduction in risk in women compared to men.[57] Differences among conclusions may reflect the variability in study populations, including age, dose, type and duration of statin therapy, as well as the stage of disease when statin therapy was initiated.
Statin therapy and secondary prevention of dementia
As encouraging as these data from observational studies may seem, the one prospective study that evaluated the efficacy and safety of atorvastatin to treat mild to moderate Alzheimer’s disease did not show improvement in cognitive performance over the 72 weeks of the trial. Although the study included about 50% women, the data were not disaggregated by sex.[58] The negative outcomes of this study may relate to the stage of disease at the time of initiation of therapy, type of statin, dose and duration of therapy.
Alzheimer’s disease, which may have a genetic component,[59] is but one form of dementia. The evidence that statin use may reduce dementia due to other causes is equivocal. A small, case-controlled study which did not distinguish between Alzheimer’s disease and other forms of dementia found a lower risk of developing dementia with the use of statins. The aggregated data were adjusted for sex and cardiovascular risk factors.[60] However, in a study that limited analysis to risk for dementia, there was no significant effect of statin use on risk in men or women.[61] A similar conclusion was supported by data from a large population study (n=2,004,692) using the QResearch database in England and Wales where no significant association was found between the use of statins and incidence of dementia in women.[62] Alternatively, another study showed greater reduction in the risk of dementia in statin use in Taiwanese women compared to men.[63] Collectively, these data point to the need for further investigation into how race/ethnicity and cultural factors influence development and progression of dementia.
Summary
While statins will continue to be prescribed to lower cholesterol as a cardiovascular risk factor, the evidence that the use of statins may reduce the risk for Alzheimer’s disease in women requires further study. In addition, there is a need for additional studies to evaluate the associations of statins and cognitive decline and dementia in women. None of the existing reports relating statin use to dementia in women have considered how hormonal status might influence statin efficacy through competition with estrogen for binding sites and regulatory pathways affected by statins or genetic variants in enzymatic targets for statins or amyloid deposition.[64–66] In addition, factors affecting a woman’s estrogen exposure throughout life may independently affect risk for dementia and Alzheimer’s disease, such as premature ovarian insufficiency or bilateral oophorectomy prior to the age of natural menopause,[67] and use of menopausal hormone therapy.[68–74] In addition, female specific risks for CVD such as a history of hypertensive pregnancy disorders including preeclampsia need to be considered.[75, 76]
Diabetes
Several large placebo-controlled trials and meta-analyses have confirmed that statins have a modest diabetogenic effect.[32, 77–83] However, the evidence is limited in terms of sex differences in this risk. The risk does seem to be higher in individuals who have pre-existing risk factors for diabetes, including elevated body mass index and metabolic syndrome,[83–85] and it is hypothesized that statin therapy perhaps simply accelerates the development of diabetes in predisposed individuals.[84] Statins are thought to impact both insulin sensitivity and β-cell secretion, but the precise mechanisms underlying the diabetogenic potential are not known.[86–89]
The risk of statin-induced diabetes seems to be a medication class effect,[85] and the absolute risk has been estimated to be around 0.2% per year in the major trials. In a large meta-analysis of 13 clinical trials, hydrophilic and lipophilic statins were associated with similar risk for incident diabetes (OR 1.08, 0.98–1.20 versus 1.10, 0.99–1.22 respectively), but the risk was higher in older individuals.[82] In another meta-analysis of 5 statin trials with 32,752 participants followed for a mean duration of 4.9 years, intensive-dose statin therapy was associated with a greater risk of incident diabetes compared to moderate-dose statin therapy, with 2 additional cases in the intensive therapy group per 1000 patient years (18.9±5.2 versus 16.9±5.5 cases per 1000 patient years). For the participants receiving high-dose therapy in comparison to those receiving moderate-dose therapy, the odds ratio was 1.12 (95% CI 1.04–1.22) for development of diabetes, and 0.84 (95% CI 0.75–0.94) for cardiovascular events.[90] A recent meta-analysis of 17 trials revealed that pravastatin was associated with the lowest risk of incident diabetes, atorvastatin with intermediate risk, and rosuvastatin with a 25% increased risk.[79] The relationship between duration of statin therapy and risk of incident diabetes is not clear, but based on data from 20 major statin trials, length of treatment does not seem to affect the risk.[84, 91]
Only a few studies have explored the sex differences in the diabetogenic potential of statin therapy. The JUPITER trial, a large primary prevention trial utilizing rosuvastatin, enrolled 6801 women ≥ 60 years of age and 11,001 men ≥ 50 years of age. There was a higher incidence of physician-reported diabetes in women treated with rosuvastatin versus placebo (1.53 versus 1.03 per 100 person years, respectively; HR: 1.49; 95% CI, 1.11–2.01; P=0.008) compared with men (1.36 versus 1.20 per 100 person years, respectively; HR: 1.14; 95% CI, 0.91–1.43; P=0.24). However, the test for heterogeneity of diabetes by sex was not found to be significant (P for heterogeneity=0.16).[32]
Statin use was also associated with an increased risk of incident diabetes in postmenopausal women participating in the Women’s Health Initiative (WHI) trial, (HR, 1.71; 95% CI, 1.61–1.83), compared to non-users. The association was noted for all statin classes, and was somewhat decreased, but remained significant after adjustment for confounders.[92]
In a recent analysis using the prospective population-based Rotterdam Study, that included 9535 individuals (41.7% males), with a mean age of 64.3 years, ever use of statin therapy was associated with a 38% higher risk of incident diabetes. However, in the sex-stratified analyses, this risk was significant only in males (HR, 1.52; 95% CI: 1.07–2.16), but not in females (HR, 1.28; 95% CI: 0.93–1.74).[93]
Summary
Based on the limited evidence, it is not clear whether statin therapy poses a greater risk of diabetes in women compared to men. Moreover, it is unclear whether the increased risk of diabetes in patients treated with statins confers an additional cardiovascular risk. In men, given the clear and significant benefit of statin therapy in reduction of cardiovascular events, the small absolute increase in the risk of diabetes may be outweighed by the cardiovascular benefit of statin use.[82] The evidence is not as clear in women.
Cancer
Breast cancer
Theories that statins may have anticancer properties are supported mechanistically by reduction in the downstream products of the mevalonate pathway,[94] with evidence for inhibition of angiogenesis, cancer cell proliferation and migration, and induction of apoptosis.[95–101] In contrast, mutation in p53, a tumor suppressor protein, up-regulates the mevalonate pathway and is associated with increased cancer risk.[102, 103] Although genetic variants impacting statin metabolism (and potentially circulating estrogen levels) have been associated with breast cancer risk,[104] the findings are not consistent. The Women’s Health Initiative observational study failed to demonstrate modification of the relationship between statins and breast cancer risk by genetic polymorphisms.[105] In population-based studies, statins have been associated with reduced cancer-specific mortality in individuals with cancer,[106] and specifically in women with cancer,[107] but not with reduced incidence of cancer in men or women without cancer.[108] Most of the female-specific data are culled from larger studies on statin use, and information on cancers that affect both men and women (e.g., colon cancer), are not commonly disaggregated by sex. Thus, detailed data on statins in women with cancer are largely limited to breast cancer.
Statin therapy and breast cancer incidence
When considering cancer incidence or outcomes, it is important to note that none of the existing statin trials was designed to examine these particular endpoints.[1] At least 4 randomized controlled trials have shown signals for increased risk of breast cancer in women randomized to statin therapy relative to placebo.[109–112] Although the number of women included in these trials was small, and none of these trials were designed to study breast cancer specifically, all cancer and breast cancer were monitored safety events. As such, these findings lend credence for concern. In contrast, meta-analyses have examined the association between statin use and breast cancer incidence,[106, 113–120] three of which were specific to breast cancer,[113, 118, 119] and none showed a relationship between statin use and incidence of breast cancer.
Statin therapy and breast cancer recurrence and mortality
The risk of breast cancer recurrence was found to be reduced in statin users in multiple studies, though not all results reached statistical significance.[121–127] A meta-analysis of 8 observational cohort studies examined the association of statin use and risk of breast cancer recurrence and death and found a significantly reduced risk of breast cancer recurrence in statin users (OR, 0.79; 95% CI 0.74–0.85), with no considerable heterogeneity across studies.[125]
The relationship between mortality and statin use in women with breast cancer has been examined in several cohort studies with mixed findings.[107, 126–128] Several meta-analyses have demonstrated an association between statin use and reduced breast cancer-specific mortality.[106, 119, 125, 129] A recent meta-analysis of seven studies demonstrated statin use to be associated with reduced breast cancer-specific mortality (HR 0.73; 95% CI 0.59–0.92) and all-cause mortality (HR 0.72, 95% CI 0.58–0.89). Five of the studies in the meta-analysis included HR values and CIs according to statin solubility (lipophilic versus hydrophilic) and showed lipophilic statin use to be associated with significantly reduced breast cancer-specific (HR, 0.57; 95% CI 0.46–0.70) and all-cause mortality (HR, 0.57; 95% CI 0.48–0.69).[129] Hydrophilic statins were associated with reduced all-cause mortality (HR, 0.79; 95% CI 0.65–0.97), but not breast cancer-specific mortality (HR 0.8=94; 95% CI 0.76–1.17).[129] Interestingly, the reduced mortality was only noted in groups followed for less than 4 years. The analysis was limited by high heterogeneity among the included studies.
Ovarian cancer
The association of statin use and ovarian cancer risk was examined in the Women’s Health Initiative study and demonstrated an increased risk of ovarian cancer, primarily with pravastatin, a hydrophilic statin (HR: 1.30, 95% CI 1.04–1.62).[130] In contrast, a large case-control study involving 2044 women with epithelial ovarian cancer and 2100 frequency-matched controls utilized logistic regression controlling for potential confounders and matching factors to examine the relationship between statin use and risk of epithelial ovarian cancer. The authors noted that statin users had a significantly lower risk of serous and non-serous epithelial ovarian cancers versus controls (OR, 0.68; 95% CI 0.54–0.85).[131]
Summary
Breast cancer risk signals in certain randomized controlled trials are concerning, although meta-analyses have failed to demonstrate a statistically significant elevated breast cancer risk associated with statin use. Data on the association between ovarian cancer risk and statin use are limited. Additional study with randomized controlled trials specifically designed to examine the effects of statin therapy on breast and other hormone sensitive cancers is needed, ideally considering factors such as hormone receptor status (in the case of breast cancer), tumor cell type, stage and grade to help clarify potential relationships.
Musculoskeletal health
Statin-related myotoxicity
Myotoxicity, defined broadly for the purposes of this manuscript as any muscle-related toxicity ranging from muscle aches to rhabdomyolysis associated with muscle enzyme elevation, is a well-known potential adverse effect associated with statin therapy with greater risk in women and the elderly.[10, 132] A large population study in England and Wales involving 46% women found that statins were associated with moderate or serious myopathy in men and in women, with no significant differences between individual statins in either men or women.[62] A small prospective observational Swedish study involving 192 statin users (50% women) reported that women were more likely to experience statin-related myotoxicity (17% of women versus 12% of men), although 20% of women reporting myopathy were concomitantly taking a medication with the potential to inhibit CYP3A4 and increase concentrations of some statins.[133] Similarly, a case-control study involving 125 cases and 481 controls reported on multivariate analysis that female sex and an SLCO1B1 genetic variant were the only factors associated with muscle toxicity.[17] In contrast, the Prediction of Muscular Risk in Observational conditions (PRIMO) study investigating muscular symptoms in 7924 hyperlipidemic patients on high dose statin therapy (about 35% women) reported no sex differences in muscle pain on univariate analysis, but low body fat mass and regular physical activity were linked to a higher incidence of muscle pain.[134] Taken together, the data suggest that women may be a somewhat greater risk for statin-related myotoxicity compared to men, but the reasons for this, whether related to lower body mass, genetic variations affecting drug metabolism and potential interactions with estrogen, concomitant use of medications affecting statin metabolism, or other effect, are unclear and require further study.
Statin therapy and osteoporosis
The data regarding the effect of statin therapy on bone health are equivocal. While several observational cohort or case control studies have reported higher bone density and reduced fracture risk in male and female statin users compared to non-users, post-hoc analyses of some major randomized trials have not replicated these results.[135–142] Statin therapy also did not improve bone density or reduce fracture risk over a 4-year follow-up in the Women’s Health Initiative observational study, the largest observational study on this issue.[143] These inconsistencies might be explained on the basis of differences in study design, statin class, timing of initiation, dosing and duration of therapy, sex/race/ethnicity of participants, lack of sex-specific analysis, concurrent use of other medications, including menopausal hormone therapy, and inadequate adjustment for unexplored confounders.[144]
Conclusions
Statins are heterogeneous compounds which affect multiple body systems in addition to their known lipid-lowering properties and appear to impact women differently than men. Additional studies of effects of statins in women are needed with regard to important chronic diseases of aging such as CVD, Alzheimer’s disease and dementia, diabetes, cancer, and osteoporosis. Studies need to take into account not only the type of statin, dose, length of use, and co-morbid conditions, but also female-specific factors such as early loss of ovarian function and pregnancy-associated diseases, hormonal status including ongoing use and type of hormone therapies, and genetic variants affecting statin metabolism in order to fully establish a benefit-risk profile for women.
Supplementary Material
Acknowledgments
Sources of funding: This study was funded in part by NIH grants awarded to Drs. Hodis and Miller.
Footnotes
Conflicts of interest/financial disclosures: Dr. Faubion consults for AMAG, Mithra Pharmaceuticals, Procter & Gamble. The other authors have no conflicts to disclose.
References
- 1.Ahern TP, et al. , Statins and breast cancer prognosis: evidence and opportunities. Lancet Oncol, 2014. 15(10): p. e461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamelin BA and Turgeon J, Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharmacol Sci, 1998. 19(1): p. 26–37. [DOI] [PubMed] [Google Scholar]
- 3.Istvan ES and Deisenhofer J, Structural mechanism for statin inhibition of HMG-CoA reductase. Science, 2001. 292(5519): p. 1160–4. [DOI] [PubMed] [Google Scholar]
- 4.Schachter M, Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol, 2005. 19(1): p. 117–25. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Cholesterol metabolism in man. West J Med, 1978. 128(1): p. 13–25. [PMC free article] [PubMed] [Google Scholar]
- 6.Shitara Y and Sugiyama Y, Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther, 2006. 112(1): p. 71–105. [DOI] [PubMed] [Google Scholar]
- 7.Brown MS and Goldstein JL, A receptor-mediated pathway for cholesterol homeostasis. Science, 1986. 232(4746): p. 34–47. [DOI] [PubMed] [Google Scholar]
- 8.Buhaescu I and Izzedine H, Mevalonate pathway: a review of clinical and therapeutical implications. Clin Biochem, 2007. 40(9–10): p. 575–84. [DOI] [PubMed] [Google Scholar]
- 9.Niemi M, Transporter pharmacogenetics and statin toxicity. Clin Pharmacol Ther, 2010. 87(1): p. 130–3. [DOI] [PubMed] [Google Scholar]
- 10.Group, S.C., et al. , SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med, 2008. 359(8): p. 789–99. [DOI] [PubMed] [Google Scholar]
- 11.Bottorff M and Hansten P, Long-term safety of hepatic hydroxymethyl glutaryl coenzyme A reductase inhibitors: the role of metabolism-monograph for physicians. Arch Intern Med, 2000. 160(15): p. 2273–80. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, et al. , Decreased warfarin clearance associated with the CYP2C9 R150H (*8) polymorphism. Clin Pharmacol Ther, 2012. 91(4): p. 660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whirl-Carrillo M, et al. , Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther, 2012. 92(4): p. 414–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanger UM and Schwab M, Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther, 2013. 138(1): p. 103–41. [DOI] [PubMed] [Google Scholar]
- 15.Choi SY, Koh KH, and Jeong H, Isoform-specific regulation of cytochromes P450 expression by estradiol and progesterone. Drug Metab Dispos, 2013. 41(2): p. 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiznitzer A, et al. , Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: a population-based study. Am J Obstet Gynecol, 2009. 201(5): p. 482 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakar NS, et al. , Genetic and Clinical Factors Are Associated With Statin-Related Myotoxicity of Moderate Severity: A Case-Control Study. Clin Pharmacol Ther, 2018. 104(1): p. 178–187. [DOI] [PubMed] [Google Scholar]
- 18.Smiderle L, et al. , ESR1 polymorphisms and statin therapy: a sex-specific approach. Pharmacogenomics J, 2016. 16(6): p. 507–513. [DOI] [PubMed] [Google Scholar]
- 19.Peck A, et al. , Effect of statins on estrogen and androgen levels in postmenopausal women treated with estradiol. Climacteric, 2011. 14(1): p. 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libby P, et al. , Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol, 2009. 54(23): p. 2129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pepine CJ, Ischemic heart disease in women. J Am Coll Cardiol, 2006. 47(3 Suppl): p. S1–3. [DOI] [PubMed] [Google Scholar]
- 22.Shaw LJ, et al. , Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol, 2006. 47(3 Suppl): p. S4–S20. [DOI] [PubMed] [Google Scholar]
- 23.Bloomer LD, et al. , Male-specific region of the Y chromosome and cardiovascular risk: phylogenetic analysis and gene expression studies. Arterioscler Thromb Vasc Biol, 2013. 33(7): p. 1722–7. [DOI] [PubMed] [Google Scholar]
- 24.Winham SJ, de Andrade M, and Miller VM, Genetics of cardiovascular disease: Importance of sex and ethnicity. Atherosclerosis, 2015. 241(1): p. 219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grundy SM, et al. , 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol, 2018. [Google Scholar]
- 26.Gulati M and Merz CN, New cholesterol guidelines and primary prevention in women. Trends Cardiovasc Med, 2015. 25(2): p. 84–94. [DOI] [PubMed] [Google Scholar]
- 27.Jellinger PS, et al. , American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr Pract, 2017. 23(Suppl 2): p. 1–87. [DOI] [PubMed] [Google Scholar]
- 28.Mosca L, Sex, statins, and statistics. Lancet, 2015. 385(9976): p. 1368–9. [DOI] [PubMed] [Google Scholar]
- 29.Petretta M, et al. , Impact of gender in primary prevention of coronary heart disease with statin therapy: a meta-analysis. Int J Cardiol, 2010. 138(1): p. 25–31. [DOI] [PubMed] [Google Scholar]
- 30.Mizuno K, et al. , Usefulness of pravastatin in primary prevention of cardiovascular events in women: analysis of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA study). Circulation, 2008. 117(4): p. 494–502. [DOI] [PubMed] [Google Scholar]
- 31.Brugts JJ, et al. , The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ, 2009. 338: p. b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mora S, et al. , Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation, 2010. 121(9): p. 1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh JM and Pignone M, Drug treatment of hyperlipidemia in women. JAMA, 2004. 291(18): p. 2243–52. [DOI] [PubMed] [Google Scholar]
- 34.de Lorgeril M, et al. , Cholesterol lowering, cardiovascular diseases, and the rosuvastatin-JUPITER controversy: a critical reappraisal. Arch Intern Med, 2010. 170(12): p. 1032–6. [DOI] [PubMed] [Google Scholar]
- 35.Kaul S, Morrissey RP, and Diamond GA, By Jove! What is a clinician to make of JUPITER? Arch Intern Med, 2010. 170(12): p. 1073–7. [DOI] [PubMed] [Google Scholar]
- 36.Yusuf S, et al. , Cholesterol Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N Engl J Med, 2016. 374(21): p. 2021–31. [DOI] [PubMed] [Google Scholar]
- 37.Downs JR, et al. , Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA, 1998. 279(20): p. 1615–22. [DOI] [PubMed] [Google Scholar]
- 38.Shepherd J, et al. , Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med, 1995. 333(20): p. 1301–7. [DOI] [PubMed] [Google Scholar]
- 39.Everett BM, et al. , Rosuvastatin in the prevention of stroke among men and women with elevated levels of C-reactive protein: justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER). Circulation, 2010. 121(1): p. 143–50. [DOI] [PubMed] [Google Scholar]
- 40.Gutierrez J, et al. , Statin therapy in the prevention of recurrent cardiovascular events: a sex-based meta-analysis. Arch Intern Med, 2012. 172(12): p. 909–19. [DOI] [PubMed] [Google Scholar]
- 41.Amarenco P, et al. , Results of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial by stroke subtypes. Stroke, 2009. 40(4): p. 1405–9. [DOI] [PubMed] [Google Scholar]
- 42.Amarenco P, et al. , High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med, 2006. 355(6): p. 549–59. [DOI] [PubMed] [Google Scholar]
- 43.Berglind IA, et al. , Hormone therapy and risk of cardiovascular outcomes and mortality in women treated with statins. Menopause, 2015. 22(4): p. 369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shinohara M, et al. , Possible modification of Alzheimer’s disease by statins in midlife: interactions with genetic and non-genetic risk factors. Front Aging Neurosci, 2014. 6: p. 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fassbender K, et al. , Simvastatin strongly reduces levels of Alzheimer’s disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci U S A, 2001. 98(10): p. 5856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meske V, et al. , Blockade of HMG-CoA reductase activity causes changes in microtubule-stabilizing protein tau via suppression of geranylgeranylpyrophosphate formation: implications for Alzheimer’s disease. Eur J Neurosci, 2003. 17(1): p. 93–102. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, et al. , Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol, 2003. 53(6): p. 743–51. [DOI] [PubMed] [Google Scholar]
- 48.Vaughan CJ, Delanty N, and Basson CT, Do statins afford neuroprotection in patients with cerebral ischaemia and stroke? CNS Drugs, 2001. 15(8): p. 589–96. [DOI] [PubMed] [Google Scholar]
- 49.Honjo M, et al. , Statin inhibits leukocyte-endothelial interaction and prevents neuronal death induced by ischemia-reperfusion injury in the rat retina. Arch Ophthalmol, 2002. 120(12): p. 1707–13. [DOI] [PubMed] [Google Scholar]
- 50.Kirsch C, et al. , Brain cholesterol, statins and Alzheimer’s Disease. Pharmacopsychiatry, 2003. 36 Suppl 2: p. S113–9. [DOI] [PubMed] [Google Scholar]
- 51.Bettermann K, et al. , Statins, risk of dementia, and cognitive function: secondary analysis of the ginkgo evaluation of memory study. J Stroke Cerebrovasc Dis, 2012. 21(6): p. 436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butterfield DA, Barone E, and Mancuso C, Cholesterol-independent neuroprotective and neurotoxic activities of statins: perspectives for statin use in Alzheimer disease and other age-related neurodegenerative disorders. Pharmacol Res, 2011. 64(3): p. 180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wood WG, et al. , Statins and neuroprotection: a prescription to move the field forward. Ann N Y Acad Sci, 2010. 1199: p. 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolozin B, et al. , Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol, 2000. 57(10): p. 1439–43. [DOI] [PubMed] [Google Scholar]
- 55.Arvanitakis Z, et al. , Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology, 2008. 70(19 Pt 2): p. 1795–802. [DOI] [PubMed] [Google Scholar]
- 56.Ramanan VK, et al. , Statins and Brain Health: Alzheimer’s Disease and Cerebrovascular Disease Biomarkers in Older Adults. J Alzheimers Dis, 2018. 65(4): p. 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zissimopoulos JM, et al. , Sex and Race Differences in the Association Between Statin Use and the Incidence of Alzheimer Disease. JAMA Neurol, 2017. 74(2): p. 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feldman HH, et al. , Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology, 2010. 74(12): p. 956–64. [DOI] [PubMed] [Google Scholar]
- 59.Farrer LA, et al. , Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA, 1997. 278(16): p. 1349–56. [PubMed] [Google Scholar]
- 60.Jick H, et al. , Statins and the risk of dementia. Lancet, 2000. 356(9242): p. 1627–31. [DOI] [PubMed] [Google Scholar]
- 61.Ancelin ML, et al. , Lipid lowering agents, cognitive decline, and dementia: the three-city study. J Alzheimers Dis, 2012. 30(3): p. 629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hippisley-Cox J and Coupland C, Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ, 2010. 340: p. c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chuang CS, et al. , Decreased prevalence of dementia associated with statins: a national population-based study. Eur J Neurol, 2015. 22(6): p. 912–8. [DOI] [PubMed] [Google Scholar]
- 64.Hodis HN and Mack WJ, Hormone therapy and risk of all-cause mortality in women treated with statins. Menopause, 2015. 22(4): p. 363–4. [DOI] [PubMed] [Google Scholar]
- 65.Moyer AM, et al. , SLCO1B1 genetic variation and hormone therapy in menopausal women. Menopause, 2018. 25(8): p. 877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moyer AM, et al. , Influence of SULT1A1 genetic variation on age at menopause, estrogen levels, and response to hormone therapy in recently postmenopausal white women. Menopause, 2016. 23(8): p. 863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rocca WA, et al. , Loss of Ovarian Hormones and Accelerated Somatic and Mental Aging. Physiology (Bethesda), 2018. 33(6): p. 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coker LH, et al. , Postmenopausal hormone therapy and subclinical cerebrovascular disease: the WHIMS-MRI Study. Neurology, 2009. 72(2): p. 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Espeland MA, et al. , Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA, 2004. 291(24): p. 2959–68. [DOI] [PubMed] [Google Scholar]
- 70.Espeland MA, et al. , Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med, 2013. 173(15): p. 1429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gleason CE, et al. , Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS-Cognitive and Affective Study. PLoS Med, 2015. 12(6): p. e1001833; discussion e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Resnick SM, et al. , Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology, 2009. 72(2): p. 135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shumaker SA, et al. , Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA, 2004. 291(24): p. 2947–58. [DOI] [PubMed] [Google Scholar]
- 74.Savolainen-Peltonen H, et al. , Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ, 2019. 364: p. l665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fields JA, et al. , Preeclampsia and cognitive impairment later in life. Am J Obstet Gynecol, 2017. 217(1): p. 74 e1–74 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raman MR, et al. , Influence of preeclampsia and late-life hypertension on MRI measures of cortical atrophy. J Hypertens, 2017. 35(12): p. 2479–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Casula M, et al. , Statin use and risk of new-onset diabetes: A meta-analysis of observational studies. Nutr Metab Cardiovasc Dis, 2017. 27(5): p. 396–406. [DOI] [PubMed] [Google Scholar]
- 78.Chan JC, et al. , Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA, 2009. 301(20): p. 2129–40. [DOI] [PubMed] [Google Scholar]
- 79.Navarese EP, et al. , Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol, 2013. 111(8): p. 1123–30. [DOI] [PubMed] [Google Scholar]
- 80.Rajpathak SN, et al. , Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care, 2009. 32(10): p. 1924–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ridker PM, et al. , Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med, 2008. 359(21): p. 2195–207. [DOI] [PubMed] [Google Scholar]
- 82.Sattar N, et al. , Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet, 2010. 375(9716): p. 735–42. [DOI] [PubMed] [Google Scholar]
- 83.Waters DD, et al. , Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J Am Coll Cardiol, 2011. 57(14): p. 1535–45. [DOI] [PubMed] [Google Scholar]
- 84.Newman CB, et al. , Statin Safety and Associated Adverse Events: A Scientific Statement From the American Heart Association. Arterioscler Thromb Vasc Biol, 2019. 39(2): p. e38–e81. [DOI] [PubMed] [Google Scholar]
- 85.Ward NC, Watts GF, and Eckel RH, Statin Toxicity. Circ Res, 2019. 124(2): p. 328–350. [DOI] [PubMed] [Google Scholar]
- 86.Betteridge DJ and Carmena R, The diabetogenic action of statins - mechanisms and clinical implications. Nat Rev Endocrinol, 2016. 12(2): p. 99–110. [DOI] [PubMed] [Google Scholar]
- 87.Brault M, et al. , Statin treatment and new-onset diabetes: a review of proposed mechanisms. Metabolism, 2014. 63(6): p. 735–45. [DOI] [PubMed] [Google Scholar]
- 88.Mach F, et al. , Adverse effects of statin therapy: perception vs. the evidence - focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur Heart J, 2018. 39(27): p. 2526–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mancini GB, et al. , Diagnosis, Prevention, and Management of Statin Adverse Effects and Intolerance: Canadian Consensus Working Group Update (2016). Can J Cardiol, 2016. 32(7 Suppl): p. S35–65. [DOI] [PubMed] [Google Scholar]
- 90.Preiss D, et al. , Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA, 2011. 305(24): p. 2556–64. [DOI] [PubMed] [Google Scholar]
- 91.Swerdlow DI, et al. , HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet, 2015. 385(9965): p. 351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Culver AL, et al. , Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med, 2012. 172(2): p. 144–52. [DOI] [PubMed] [Google Scholar]
- 93.Ahmadizar F, et al. , Undertreatment of hypertension and hypercholesterolaemia in children and adolescents with type 1 diabetes: long-term follow-up on time trends in the occurrence of cardiovascular disease, risk factors and medications use. Br J Clin Pharmacol, 2018. 84(4): p. 776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fritz G, Targeting the mevalonate pathway for improved anticancer therapy. Curr Cancer Drug Targets, 2009. 9(5): p. 626–38. [DOI] [PubMed] [Google Scholar]
- 95.Al-Haidari AA, Syk I, and Thorlacius H, HMG-CoA reductase regulates CCL17-induced colon cancer cell migration via geranylgeranylation and RhoA activation. Biochem Biophys Res Commun, 2014. 446(1): p. 68–72. [DOI] [PubMed] [Google Scholar]
- 96.Beckwitt CH, et al. , Statins attenuate outgrowth of breast cancer metastases. Br J Cancer, 2018. 119(9): p. 1094–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Campbell MJ, et al. , Breast cancer growth prevention by statins. Cancer Res, 2006. 66(17): p. 8707–14. [DOI] [PubMed] [Google Scholar]
- 98.Ghosh-Choudhury N, et al. , Simvastatin induces derepression of PTEN expression via NFkappaB to inhibit breast cancer cell growth. Cell Signal, 2010. 22(5): p. 749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koyuturk M, Ersoz M, and Altiok N, Simvastatin induces apoptosis in human breast cancer cells: p53 and estrogen receptor independent pathway requiring signalling through JNK. Cancer Lett, 2007. 250(2): p. 220–8. [DOI] [PubMed] [Google Scholar]
- 100.Rao S, et al. , Lovastatin mediated G1 arrest in normal and tumor breast cells is through inhibition of CDK2 activity and redistribution of p21 and p27, independent of p53. Oncogene, 1998. 17(18): p. 2393–402. [DOI] [PubMed] [Google Scholar]
- 101.Wang T, et al. , Simvastatin-induced breast cancer cell death and deactivation of PI3K/Akt and MAPK/ERK signalling are reversed by metabolic products of the mevalonate pathway. Oncotarget, 2016. 7(3): p. 2532–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Freed-Pastor WA, et al. , Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell, 2012. 148(1–2): p. 244–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kotler E, et al. , A Systematic p53 Mutation Library Links Differential Functional Impact to Cancer Mutation Pattern and Evolutionary Conservation. Mol Cell, 2018. 71(1): p. 178–190 e8. [DOI] [PubMed] [Google Scholar]
- 104.Lee E, et al. , The association of polymorphisms in hormone metabolism pathway genes, menopausal hormone therapy, and breast cancer risk: a nested case-control study in the California Teachers Study cohort. Breast Cancer Res, 2011. 13(2): p. R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bock CH, et al. , The effect of genetic variants on the relationship between statins and breast cancer in postmenopausal women in the Women’s Health Initiative observational study. Breast Cancer Res Treat, 2018. 167(3): p. 741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mei Z, et al. , Effects of statins on cancer mortality and progression: A systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. Int J Cancer, 2017. 140(5): p. 1068–1081. [DOI] [PubMed] [Google Scholar]
- 107.Nielsen SF, Nordestgaard BG, and Bojesen SE, Statin use and reduced cancer-related mortality. N Engl J Med, 2012. 367(19): p. 1792–802. [DOI] [PubMed] [Google Scholar]
- 108.Cholesterol Treatment Trialists, C., et al. , Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One, 2012. 7(1): p. e29849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clearfield M, et al. , Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS): efficacy and tolerability of long-term treatment with lovastatin in women. J Womens Health Gend Based Med, 2001. 10(10): p. 971–81. [DOI] [PubMed] [Google Scholar]
- 110.Sacks FM, et al. , The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med, 1996. 335(14): p. 1001–9. [DOI] [PubMed] [Google Scholar]
- 111.Shepherd J, et al. , Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet, 2002. 360(9346): p. 1623–30. [DOI] [PubMed] [Google Scholar]
- 112.Strandberg TE, et al. , Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S). Lancet, 2004. 364(9436): p. 771–7. [DOI] [PubMed] [Google Scholar]
- 113.Bonovas S, et al. , Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol, 2005. 23(34): p. 8606–12. [DOI] [PubMed] [Google Scholar]
- 114.Browning DR and Martin RM, Statins and risk of cancer: a systematic review and metaanalysis. Int J Cancer, 2007. 120(4): p. 833–43. [DOI] [PubMed] [Google Scholar]
- 115.Dale KM, et al. , Statins and cancer risk: a meta-analysis. JAMA, 2006. 295(1): p. 74–80. [DOI] [PubMed] [Google Scholar]
- 116.Kuoppala J, Lamminpaa A, and Pukkala E, Statins and cancer: A systematic review and meta-analysis. Eur J Cancer, 2008. 44(15): p. 2122–32. [DOI] [PubMed] [Google Scholar]
- 117.Matsushita Y, et al. , Pravastatin use and cancer risk: a meta-analysis of individual patient data from long-term prospective controlled trials in Japan. Pharmacoepidemiol Drug Saf, 2010. 19(2): p. 196–202. [DOI] [PubMed] [Google Scholar]
- 118.Undela K, Srikanth V, and Bansal D, Statin use and risk of breast cancer: a meta-analysis of observational studies. Breast Cancer Res Treat, 2012. 135(1): p. 261–9. [DOI] [PubMed] [Google Scholar]
- 119.Wu QJ, et al. , Statin use and breast cancer survival and risk: a systematic review and meta-analysis. Oncotarget, 2015. 6(40): p. 42988–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.He Y, et al. , Statins and Multiple Noncardiovascular Outcomes: Umbrella Review of Meta-analyses of Observational Studies and Randomized Controlled Trials. Ann Intern Med, 2018. 169(8): p. 543–553. [DOI] [PubMed] [Google Scholar]
- 121.Ahern TP, et al. , Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst, 2011. 103(19): p. 1461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boudreau DM, et al. , Comparative safety of cardiovascular medication use and breast cancer outcomes among women with early stage breast cancer. Breast Cancer Res Treat, 2014. 144(2): p. 405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chae YK, et al. , Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer Invest, 2011. 29(9): p. 585–93. [DOI] [PubMed] [Google Scholar]
- 124.Kwan ML, et al. , Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat, 2008. 109(3): p. 573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mansourian M, et al. , Statins Use and Risk of Breast Cancer Recurrence and Death: A Systematic Review and Meta-Analysis of Observational Studies. J Pharm Pharm Sci, 2016. 19(1): p. 72–81. [DOI] [PubMed] [Google Scholar]
- 126.Nickels S, et al. , Mortality and recurrence risk in relation to the use of lipid-lowering drugs in a prospective breast cancer patient cohort. PLoS One, 2013. 8(9): p. e75088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brewer TM, et al. , Statin use in primary inflammatory breast cancer: a cohort study. Br J Cancer, 2013. 109(2): p. 318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Murtola TJ, et al. , Statin use and breast cancer survival: a nationwide cohort study from Finland. PLoS One, 2014. 9(10): p. e110231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu B, et al. , The relationship between statins and breast cancer prognosis varies by statin type and exposure time: a meta-analysis. Breast Cancer Res Treat, 2017. 164(1): p. 1–11. [DOI] [PubMed] [Google Scholar]
- 130.Desai P, et al. , An analysis of the association between statin use and risk of endometrial and ovarian cancers in the Women’s Health Initiative. Gynecol Oncol, 2018. 148(3): p. 540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Akinwunmi B, et al. , Statin therapy and association with ovarian cancer risk in the New England Case Control (NEC) study. Int J Cancer, 2019. 144(5): p. 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nguyen KA, et al. , A comprehensive review and meta-analysis of risk factors for statin-induced myopathy. Eur J Clin Pharmacol, 2018. 74(9): p. 1099–1109. [DOI] [PubMed] [Google Scholar]
- 133.Skilving I, et al. , Statin-induced myopathy in a usual care setting-a prospective observational study of gender differences. Eur J Clin Pharmacol, 2016. 72(10): p. 1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bruckert E, et al. , Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther, 2005. 19(6): p. 403–14. [DOI] [PubMed] [Google Scholar]
- 135.An T, et al. , Efficacy of statins for osteoporosis: a systematic review and meta-analysis. Osteoporos Int, 2017. 28(1): p. 47–57. [DOI] [PubMed] [Google Scholar]
- 136.Meier CR, et al. , Statin drugs and the risk of fracture. JAMA, 2000. 284(15): p. 1921–2. [PubMed] [Google Scholar]
- 137.Morse LR, et al. , Wheelchair use and lipophilic statin medications may influence bone loss in chronic spinal cord injury: findings from the FRASCI-bone loss study. Osteoporos Int, 2016. 27(12): p. 3503–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pedersen TR, et al. , Follow-up study of patients randomized in the Scandinavian simvastatin survival study (4S) of cholesterol lowering. Am J Cardiol, 2000. 86(3): p. 257–62. [DOI] [PubMed] [Google Scholar]
- 139.Pena JM, et al. , Statin therapy and risk of fracture: results from the JUPITER randomized clinical trial. JAMA Intern Med, 2015. 175(2): p. 171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reid IR, et al. , Effect of pravastatin on frequency of fracture in the LIPID study: secondary analysis of a randomised controlled trial. Long-term Intervention with Pravastatin in Ischaemic Disease. Lancet, 2001. 357(9255): p. 509–12. [DOI] [PubMed] [Google Scholar]
- 141.Rejnmark L, et al. , Hip fracture risk in statin users--a population-based Danish case-control study. Osteoporos Int, 2004. 15(6): p. 452–8. [DOI] [PubMed] [Google Scholar]
- 142.Scranton RE, et al. , Statin use and fracture risk: study of a US veterans population. Arch Intern Med, 2005. 165(17): p. 2007–12. [DOI] [PubMed] [Google Scholar]
- 143.LaCroix AZ, et al. , Statin use, clinical fracture, and bone density in postmenopausal women: results from the Women’s Health Initiative Observational Study. Ann Intern Med, 2003. 139(2): p. 97–104. [DOI] [PubMed] [Google Scholar]
- 144.Lin TK, et al. , Long-term effect of statins on the risk of new-onset osteoporosis: A nationwide population-based cohort study. PLoS One, 2018. 13(5): p. e0196713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.