Abstract
Interoceptive sensibility (IS) refers to the subjective experience of perceiving and being aware of one’s internal body sensations, and is typically evaluated using self-report questionnaires or confidence ratings. Here we evaluated IS in 81 patients with OCD and 76 controls using the Multidimensional Scale of Interoceptive Awareness (MAIA), which contains 8 subscales assessing adaptive and maladaptive responses to sensation. Compared to controls, OCD patients showed hyperawareness of body sensations. Patients also demonstrated a more maladaptive profile of IS characterized by greater distraction from and worry about unpleasant sensations, and reduced tendency to experience the body as safe and trustworthy. These findings were independent of medication status and comorbidities in the patient group. Correlational analyses showed that subscales of the MAIA were differentially associated with OCD symptom dimensions. These findings indicate that patients with OCD show abnormality of IS that is independent of confounding factors related to medication and comorbidities and associated with different OCD symptom dimensions. Future work would benefit from examining neural correlates of these effects and evaluating whether dimensions of IS are impacted by treatments for the disorder.
Introduction
Interoception is the process by which individuals detect, integrate, and interpret signals from within the body (Khalsa et al., 2018; Tsakiris & Critchley, 2016). Physical sensations from visceral and somatic sources provide an integrated sense of the physiological condition of the body that is critically important for maintaining homeostasis (Craig, 2002; Critchley & Harrison, 2013; Khalsa et al., 2018; Paulus & Stein, 2010). These sensations motivate behavior and influence perception, attention, decision-making, and emotional processes (Craig, 2002; Singer, Critchley, & Preuschoff, 2009; Tsakiris & Critchley, 2016). It is, therefore, not surprising that altered interoceptive processes have been reported in many psychiatric conditions, including anxiety, depression, addiction, psychosis, and anorexia (Ardizzi et al., 2016; T. A. Brown et al., 2017; Khalsa et al., 2018; McKay, Kim, Mancusi, Storch, & Spankovich, 2018; Paulus & Stein, 2010; Tsakiris & Critchley, 2016).
Obsessive-compulsive disorder (OCD) is a chronic and often debilitating disorder that occurs in 2 to 3% of the global population (Kessler, Petukhova, Sampson, Zaslavsky, & Wittchen, 2012; Ruscio, Stein, Chiu, & Kessler, 2010). OCD is characterized by the presence of obsessions (i.e. recurrent, intrusive urges or thoughts that cause distress) and/or compulsions (i.e. time-consuming and repetitive behaviors or mental rituals that are performed to reduce anxiety caused by obsessions). While there is a wide variety of idiosyncratic obsessions and compulsions experienced in OCD, more common symptoms include fears of contamination with washing/cleaning compulsions, fears of being responsible for harm with checking behaviors, taboo or disturbing thoughts involving sexual or religious themes, and concerns over symmetry and the need for things to be “just right” with ordering and arranging compulsions (Abramowitz, Franklin, Schwartz, & Furr, 2003; Denys, de Geus, van Megen, & Westenberg, 2004; Pinto et al., 2007).
Studies examining whether OCD patients correctly perceive what is going on in their body (“interoceptive accuracy”) have yielded mixed results. Interoceptive accuracy is frequently assessed using heartbeat detection tasks where participants count the number of times their heart beats over a period of several seconds, which is then compared to actual heart rate output from electrocardiogram or plethysmography to determine accuracy. Two studies recently assessing interoceptive accuracy in this manner reported both increased (Yoris et al., 2017) and decreased (Schultchen, Zaudig, Krauseneck, Berberich, & Pollatos, 2019) detection accuracy in patients with OCD compared to controls. Although it is unclear why these studies produced different findings, it is possible that variations of the heartbeat detection tasks utilized – with Yoris et al (2017) having participants tap their hand in synchrony with their own heartbeat and Schultchen et al. (2019) having participants silently count their heartbeats – contributed to this discrepancy.
In addition to interoceptive accuracy, one can also measure an individual’s perceived sensitivity to, and awareness of, internal body sensations (“interoceptive sensibility”; IS), which is typically evaluated using self-report questionnaires or confidence ratings (Garfinkel & Critchley, 2013). Interoceptive accuracy is not necessarily correlated with IS (Call, Ambrosini, Picconi, Mehling, & Committeri, 2015; Ferentzi, Horvath, & Koteles, 2019; Garfinkel & Critchley, 2013; Garfinkel, Seth, Barrett, Suzuki, & Critchley, 2015; Meessen et al., 2016), and to our knowledge no prior studies have investigated IS in OCD patients. Several questionnaires that have been used to evaluate IS include the Body Perception Questionnaire (Porges, 1993), Body Awareness Questionnaire (Shields, Mallory, & Simon, 1989), and the Self Awareness Questionnaire (Longarzo et al., 2015). These questionnaires generally evaluate the tendency to perceive, notice, and be aware of body sensations. However, IS is a multifaceted construct that also includes how sensations are interpreted, regulated, and used to inform behavior (Mehling, 2016; Mehling et al., 2012). The different ways in which individuals respond to body sensations may be relevant for psychiatric disorders and treatment. For example, experiencing emotional distress or worry about uncomfortable or painful body sensations is thought to be maladaptive and is generally associated with anxiety, hypochondriasis, and somatization disorders (Mehling, 2016; Porges, 1993). By contrast, the tendencies to experience the body as safe and trustworthy and to regulate emotional responses through attention to the body are more adaptive and are incorporated into mindfulness approaches to improving mental health (Hanley, Mehling, & Garland, 2017; Tsur, Berkovitz, & Ginzburg, 2016). As such, a multidimensional approach to characterizing IS in OCD may help further our understanding of interoception in the disorder particularly in relation to adaptive and maladaptive responses to the body.
Even though there is no published work on IS in OCD, past studies examining anxiety sensitivity (AS) in OCD may provide insight into interoception in the disorder. AS involves fear of anxiety-related body sensations (for example, rapid heart rate, shortness of breath, sweating) in relation to beliefs regarding the negative physical, cognitive, and social consequences arising from the sensations (Blakey & Abramowitz, 2018; Blakey, Abramowitz, Reuman, Leonard, & Riemann, 2017; Reiss & McNally, 1985). AS is associated with OCD symptom severity in individuals diagnosed with the disorder (Blakey & Abramowitz, 2018; Calamari, Rector, Woodard, Cohen, & Chik, 2008). Greater fear of the physical consequences of anxiety-related hyperarousal symptoms (“AS-Physical”) is associated with increased contamination and symmetry/ordering/“not-just-right” symptoms in both OCD patients (Poli, Melli, Ghisi, Bottesi, & Sica, 2017) and non-clinical college students (Wheaton, Mahaffey, Timpano, Berman, & Abramowitz, 2012).
The relationship between symmetry/ordering/“not-just-right” symptoms and AS is consistent with other findings associating this symptom dimension in OCD with behavioral and neural aspects of interoception. Patients with symmetry/ordering/“not-just-right” symptoms report urges to engage in repetitive behaviors in order to resolve feelings of incompleteness or discomfort arising from the sensation that things are “not just right” (Coles, Frost, Heimberg, & Rheaume, 2003; Taylor, 2014). These symptoms are part of a larger category of OCD symptoms called “sensory phenomena” (Leckman, Walker, Goodman, Pauls, & Cohen, 1994), which are aversive or distressing sensations or perceptions that drive repetitive behaviors in addition to, or instead of, fear of harm (Cohen & Leckman, 1992; Ferrao et al., 2012). Sensory phenomena, including symmetry/ordering/“not-just-right” symptoms, are common in Tourette’s disorder (Leckman et al., 1994; Miguel et al., 2000), where repetitive behaviors are frequently driven by the buildup of a physical urge (premonitory urge) (Leckman, Walker, & Cohen, 1993; Reese et al., 2014). Prior work has shown that a premonitory urge to tic is associated with increased interoceptive awareness (in the form of greater accuracy during heartbeat detection) (Ganos et al., 2015), and there is overlap between neural mechanisms of interoception and those associated with sensory phenomena in OCD including the insula and somatosensory cortex (C. Brown et al., 2019). These data, along with past work on AS, suggest that greater sensitivity to body sensations may be related to sensory-based symptoms in OCD.
In the present study, we investigated different aspects of IS in a sample of patients with OCD and healthy controls, and evaluated the associations between IS and OCD symptom dimensions. Based upon the literature described above, we hypothesized that patients would demonstrate a more maladaptive profile of IS compared to controls, which may be more prominent in OCD dimensions associated with more physical symptoms/sensory phenomena (i.e. the symmetry/ordering/“not-just-right” symptom dimension).
Methods
Subjects and Procedure
The study protocol was approved by the Institutional Review Boards at the Icahn School of Medicine at Mount Sinai (ISMMS), Nathan Kline Institute for Psychiatric Research (NKI), and New York University School of Medicine (NYUSoM). All subjects provided written informed consent prior to the initiation of data collection and received monetary compensation for their time. The dataset described in this paper is part of a larger set of neuroimaging studies including a clinical drug trial.
Subjects were recruited at three locations between May 2014 and September 2019. Eighty-eight patients with OCD participated within that time frame (25 were recruited at ISMMS, 19 at NKI, and 44 at NYUSoM). Eighty-two healthy controls also completed the study (69 were recruited at ISMMS, 6 at NKI, and 7 at NYUSoM). Data from 7 OCD patients and 6 controls were excluded (7 patients and 2 controls were excluded as outliers for having scores that exceeded 1.5 times of the interquartile range (IQR) below the 25th percentile (i.e. lower than 25th percentile - 1.5*IQR) or above the 75th percentile (i.e. higher than 75th percentile + 1.5*IQR) on any subscale of the MAIA; 1 control dropped out of the study and 3 controls met study exclusion criteria after questionnaire completion). Final data were analyzed from 81 OCD patients and 76 controls. All patients met DSM-5 criteria for OCD and were excluded for lifetime presence of a bipolar disorder, schizophrenia spectrum disorder, or moderate or severe alcohol or substance use disorder. These exclusionary criteria were applied because they would affect ability to participate in the larger neuroimaging studies from which this sample was drawn. Twenty-six out of the 81 patients (32%) had no Axis 1 comorbidities; the remaining 55 patients (68%) had at least one current comorbid Axis I disorder including generalized anxiety disorder (n = 29), panic disorder (n = 16), excoriation disorder (n = 10), social anxiety disorder (n = 9), agoraphobia (n = 9), attention deficit hyperactivity disorder (n = 9), and body dysmorphic disorder (n = 8). Less frequent current comorbidities included illness anxiety disorder (n = 5), alcohol use disorder (mild, n = 5), trichotillomania (n = 5), hoarding disorder (n = 4), Tourette’s disorder (n = 3), substance use disorder (mild, n = 3), major depressive disorder (n = 3), binge eating disorder (mild, n = 3), persistent tic disorder (n = 2), somatic symptom disorder (n = 2), and post-traumatic stress disorder (n = 2). Thirty-eight of the 81 patients (47%) were not taking psychotropic medications; the remaining 43 patients (53%) were taking antidepressants targeting monoaminergic neurotransmission (n = 39), benzodiazepines as needed (n = 10), stimulants (n = 4), atypical antipsychotics (n = 5), anticonvulsants (n = 4), and psychoactive antihypertensives (n = 2). Diagnoses were made by a trained rater using the Mini International Neuropsychiatric Interview (M.I.N.I., (Sheehan et al., 1998).
IS was measured using the MAIA (Mehling, 2016; Mehling et al., 2012), which includes 32 questions assessing 8 dimensions of IS previously identified through factor analysis: 1) tendency to notice or become aware of body sensations (“noticing”; 4 items), 2) tendency to not distract oneself from sensations of pain or discomfort (“not-distracting”; 3 items), 3) tendency to not worry about or experience emotional distress in response to sensations of pain or discomfort (“not-worrying”; 3 items), 4) ability to sustain and control attention to body sensations (“attentional control”; 7 items), 5) awareness of the link between emotion and body sensations (“emotional awareness”; 5 items), 6) ability to regulate negative emotion through attention to body sensations (“self-regulation”; 4 items), 7) tendency to listen to body sensations for insight into emotion and guide behavior (“listening”; 3 items), and 8) tendency to experience the body as safe and trustworthy (“trusting”; 3 items). The MAIA was designed to distinguish between adaptive and maladaptive forms of IS (Mehling, 2016). Noticing and emotional awareness subscales are considered ambiguous and can be classified as either adaptive or maladaptive (Mehling et al., 2009). Higher scores on attentional control, self-regulation, listening, and trusting subscales reflect a mindful style of attention and are considered adaptive, while increased distraction (i.e. lower scores on not-distracting) and worrying (i.e. lower scores on not-worrying) over unpleasant body sensations are considered non-mindful and maladaptive (Goubert, Crombez, Eccleston, & Devulder, 2004; Mehling, 2016; Mehling et al., 2009). The MAIA has construct validity with other measures of body awareness, mindfulness, and emotion regulation (Mehling, 2016; Mehling et al., 2012), and has overall acceptable internal-consistency reliability with Cronbach’s alpha ranging between 0.66 to 0.82 for the subscales. Alphas for five out of eight subscales (emotional awareness, listening, trusting, attentional control, and self-regulation) were greater than 0.70, and all item-subscale correlations were between 0.35 to 0.74 (Mehling et al., 2012).
To determine if IS was related to severity of OCD symptom dimensions, OCD patients completed the DOCS (Abramowitz et al., 2010), which is a self-report scale where patients rate ‘time occupied’, ‘avoidance’, ‘distress’, ‘interference’, and ‘difficulty disregarding/resisting’ for four categories of OCD symptoms: 1) concerns about germs and contamination; 2) concerns about responsibility for harm, injury, or bad luck; 3) unacceptable or taboo thoughts (e.g. about sex, immorality, or violence); and 4) concerns about symmetry, completeness, and the need for things to be “just right”.
Data Analysis
Primary analyses focused on the difference between OCD patients and controls on each of the 8 MAIA subscales, and, within patients, the relationship between MAIA scores and severity of OCD symptom dimensions. Correlational analyses explored associations between MAIA subscales and demographics variables (age, education, and sex) within the entire sample. Analyses-of-variance (ANOVAs) examined group differences between the subscales; analyses-of-covariance (ANCOVAs) included covariates specifying sex, age, and/or education for MAIA subscales that were significantly correlated with these variables. Shapiro-Wilk tests of normality conducted on the residuals from ANOVAs/ANCOVAs indicated that all subscales except not-distracting and not-worrying exhibited a non-normal error distribution. Although the ANOVA framework is typically considered appropriate when sample sizes are sufficiently large (e.g., n > 50) even when errors are non-normal (Pek, Wong, & Wong, 2017), we also report replications from non-parametric Mann-Whitey U tests.
In addition to the primary analysis of group effects, we conducted 2 × 2 between-subjects ANOVAs to determine whether the presence of Axis I comorbidities (+/−) and/or current use of psychotropic medication (+/−) were related to subscale scores in OCD patients. To determine whether IS was differentially related to OCD symptom dimensions, Pearson’s correlations were performed between MAIA subscale scores and severity scores for each of the 4 symptom dimensions on the DOCS, with partial correlations controlling for sex, age, and/or education where appropriate.
Results
Demographics and clinical information
There were no significant group differences in demographic variables, including age (MControls = 31.0, SDControls = 10.1; MOCD = 34.1, SDOCD = 12.6), years of education (MControls = 15.9, SDControls = 2.0; MOCD = 15.8, SDOCD = 2.0), and sex (Controls: 37 males, 39 females; OCD: 28 males, 55 females), all p > .05 (Table 1). Clinical information for the patient sample is also shown in Table 1.
Table 1:
Demographics and clinical information (76 Controls, 81 patients with OCD).
| Without covariates |
With covariates |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls |
OCD |
Group comparisons (ANOVA) | Covariate | Controls |
OCD |
Group comparisons (ANCOVA) | |||||
| Mean | SD | Mean | SD | EMM | SE | EMM | SE | ||||
| Age (years) | 31.0 | 10.1 | 34.1 | 12.6 | t (151.3) = 1.73^, p = .09 | ||||||
| Education (years) | 15.9 | 2.0 | 15.8 | 2.0 | t (155) = −0.05, p = .96 | ||||||
| Biological Sex | 37M/39F | 28M/53F | χ2 (1) = 3.2, p = .07 | ||||||||
| Y-BOCS | - | - | 24.1 | 6.1 | - | ||||||
|
MAIA
subscales (Average score) |
|||||||||||
| Noticing | 2.5 | 1.3 | 3.7 | 0.94 | F(1,155) = 42.5** | Age | 2.6 | 0.13 | 3.7 | 0.12 | F(1,154) = 39.3** |
| Not-distracting | 3.2 | 1.2 | 2.4 | 0.94 | F(1,155) = 23.1** | Sex, Education | 3.2 | 0.12 | 2.4 | 0.11 | F(1,153) = 21.6** |
| Not-worrying | 3.2 | 0.90 | 2.4 | 1.0 | F(1,155) = 30.8** | ||||||
| Emotional awareness | 2.8 | 1.3 | 3.8 | 0.94 | F(1,155) = 33.4** | Age | 2.8 | 0.13 | 3.8 | 0.12 | F(1,154) = 31.1** |
| Listening | 2.2 | 1.4 | 2.6 | 1.5 | F(1,155) = 4.0* | ||||||
| Trusting | 3.9 | 0.87 | 3.3 | 1.3 | F(1,155) = 13.3** | ||||||
| Attention control | 3.0 | 1.1 | 3.0 | 0.94 | F (1,155) = 0.15, p = .7 | ||||||
| Self-regulation | 3.1 | 1.1 | 2.8 | 1.2 | F (1,155) = 2.3, p = 0.1 | ||||||
Note: Demographics variables (age, biological sex, education) were included as covariates for subscales that were significantly associated with these variables. Marginal means are presented for subscales where covariates were applied. Significant group differences are indicated with asterisks.
Abbreviations: MAIA, Multidimensional Assessment of Interoceptive Awareness; ANOVA, analysis of variance; ANCOVA, analysis of covariance; EMM, estimated marginal means.
Levene’s test of homogeneity of variance across the groups for age was not assumed, and degrees of freedom were adjusted using Satterthwaite’s approximation.
p < .05,
p < .001.
IS in relation to age, education, and sex in the entire sample
Within the entire sample, noticing and emotional awareness subscales were both positively correlated with age (Pearson’s r = .18, p = .017 and r = .16, p = .043, respectively). Not-distracting was negatively correlated with education (r = −.19, p = .012) and greater among males than females (t(165) = 2.19, p = .030). No other subscale scores were related to age, education, or biological sex.
Group differences in IS dimensions
ANOVAs comparing OCD patients with controls revealed significant differences on 6 of the 8 subscales. Compared to controls, OCD patients reported increased noticing, distracting (lower scores on not-distracting), worrying (lower scores on not-worrying), emotional awareness, listening, and decreased trusting of their body sensations, all p < .05 (Table 1). Patients and controls did not differ in attentional control and self-regulation. Mann-Whitney U tests confirmed these differences using non-parametric analyses (p ≤ .002 for all subscales except listening, where p = .050). Group differences in noticing, emotional awareness, and not-distracting remained even after including relevant demographic variables as covariates in ANCOVA analyses; noticing (controlling for age), emotional awareness (controlling for age), and not-distracting (controlling for sex and education), p < .001 (Table 1, Figure 1).
Figure 1:
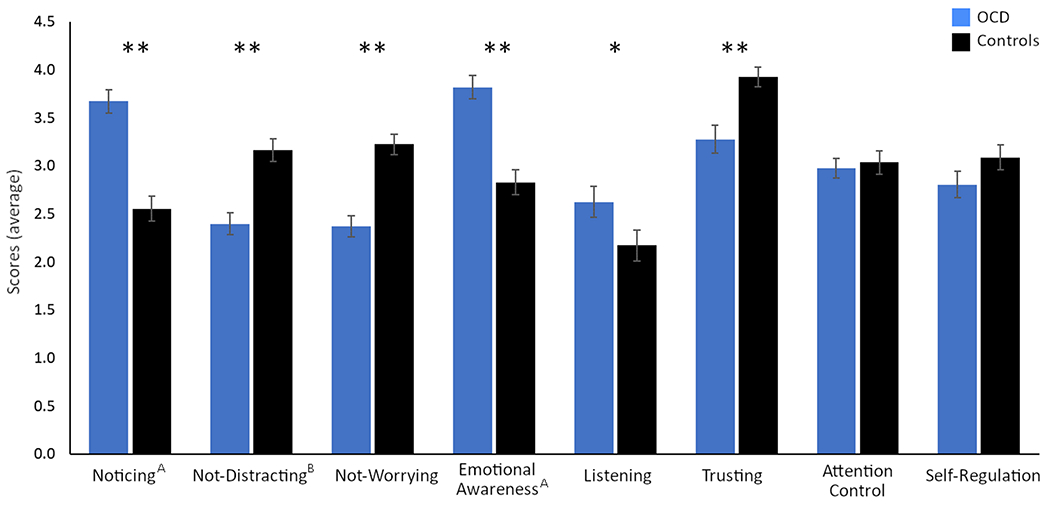
Average scores on all eight subscales of the MAIA in 76 healthy controls and 81 patients with OCD. Demographic variables (age, biological sex, education) were included as covariates for subscales that were significantly associated with these variables. Marginal means are presented for subscales where covariates were applied. Significant group differences are indicated with asterisks.
*p < .05, **p < .001.
A Covariates include age.
B Covariates include biological sex and education.
Effects of comorbidities and medication on IS
Among the 43 medicated OCD patients, 34 had at least one Axis 1 comorbidity and 9 did not. Among the 38 unmedicated OCD patients, 21 had at least one comorbidity and 17 did not. Six two-way between-subjects ANOVAs were conducted to evaluate the effects of medication status and comorbidity on MAIA subscales that showed group differences. A main effect of comorbidity was found for the trusting subscale, such that patients with an Axis I comorbidity reported a reduced tendency to experience their body as safe and trustworthy than those without a comorbidity (F(1,77) = 14.27, p < .001) (Figure 2). There were no other significant main effects, although there was a trend towards medicated patients showing increased noticing than unmedicated patients (F(1,77) = 3.82, p = .054).
Figure 2:
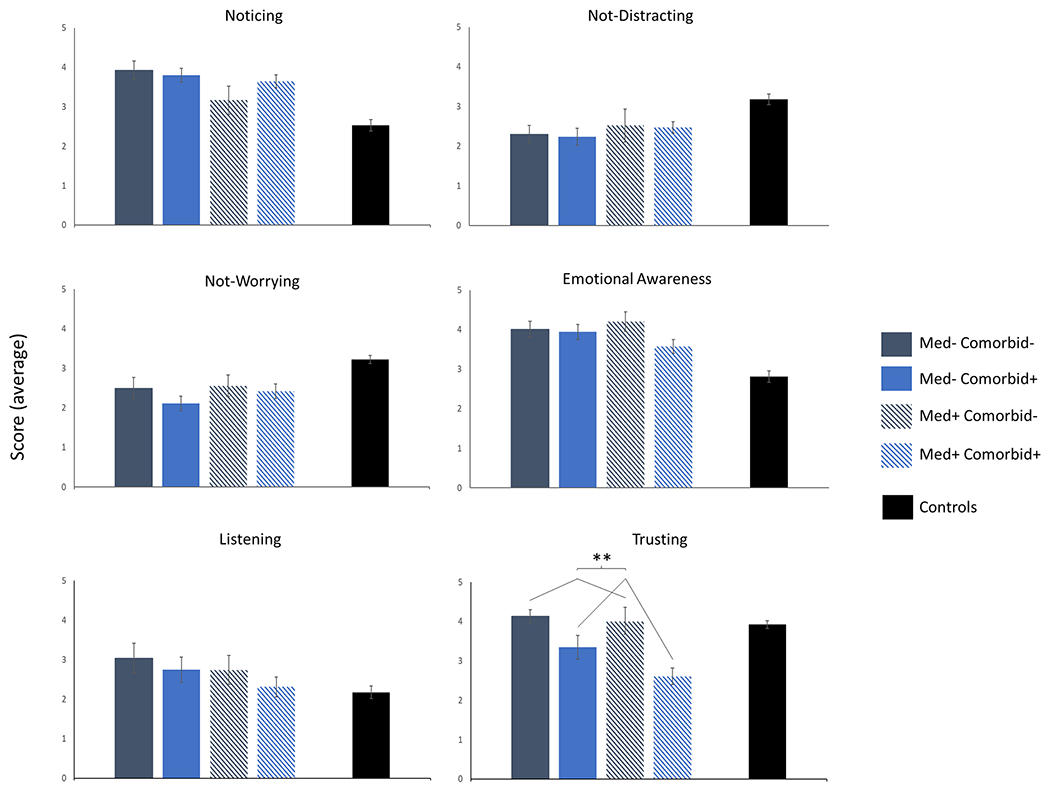
Average scores in OCD patients on the six subscales of the MAIA that showed group differences based on medication and comorbidity status.
Sample sizes: Med− Comorbid− (n = 17), Med− Comorbid+ (n = 21), Med+ Comorbid− (n = 9), Med+ Comorbid+ (n = 34), Controls (n = 76).
Abbreviations: Med−, unmedicated; Med+, medicated; Comorbid−, without Axis 1 comorbidity; Comorbid+, with at least an Axis 1 comorbidity.
** p < .001 for the main effect of comorbidity.
Relationship between IS and obsessive-compulsive symptom dimensions
Within OCD patients, older age was associated with increased distracting (r = −.23, p = 0.042) and increased trusting (r = .28, p = .010), and females reported greater noticing than males (t(79) = −2.7, p = .010). These demographic factors were used as covariates for correlations with the relevant subscales.
There was a positive correlation between noticing and symptoms related to symmetry, completeness, and “not-just-right” experiences (partial r = .27 [controlling for sex], p = .014) as well as for concerns about responsibility for harm (partial r = .24 [controlling for sex], p = .030) (Figure 3). Greater worrying was also associated with increased concerns about responsibility for harm (r = −.32, p = .004) and with increased concerns about germs and contamination (r = −.23, p = .043). Finally, greater distracting was associated with more symptoms related to unacceptable and taboo thoughts (partial r = −.25 [controlling for age], p = .027). No other subscales were significantly correlated with severity scores of the different OCD dimensions.
Figure 3:
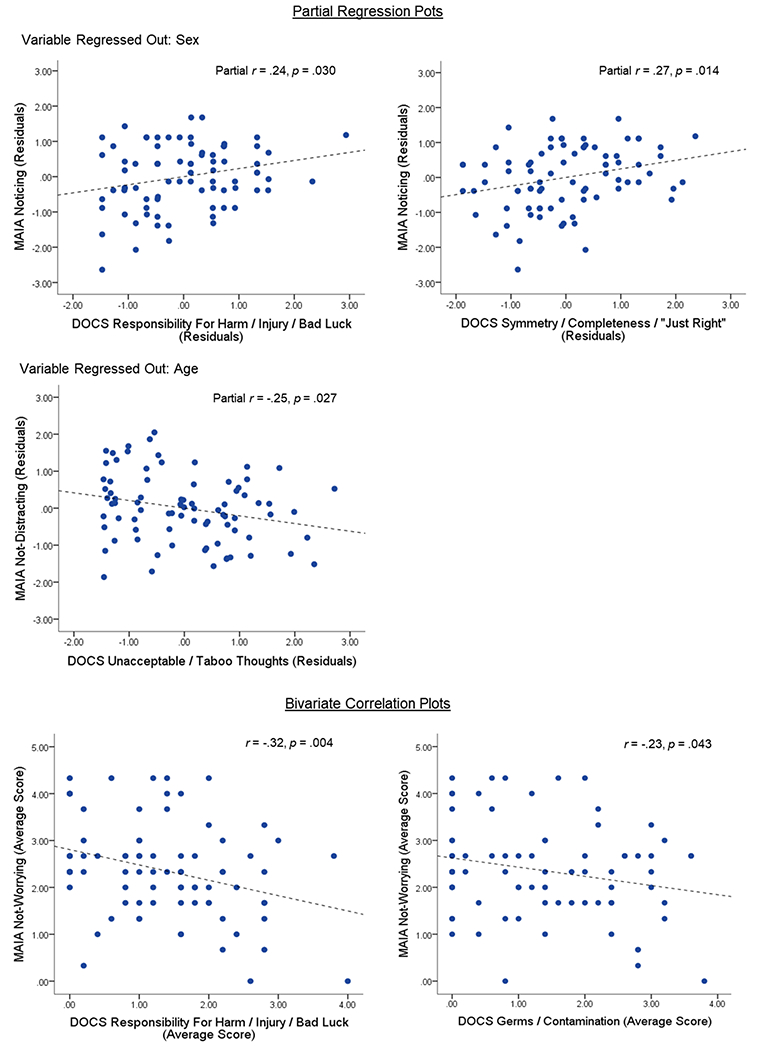
Scatterplots showing the associations between MAIA subscales and obsessive-compulsive symptom dimensions using the DOCS within the patient sample (n = 81). Only significant correlations are presented (all p < .05). Partial regression plots display the relationship between MAIA subscale and DOCS dimension after regressing out effects of demographic variables where necessary.
Abbreviations: MAIA, Multidimensional Assessment of Interoceptive Awareness; DOCS. Dimensional Obsessive-Compulsive Scale.
Discussion
The present study compared dimensions of IS between patients with OCD and controls and probed for relationships with OCD symptom dimensions. Patients not only reported an increased tendency to notice and be aware of body sensations and increased awareness of the link between emotion and the body – two IS dimensions not considered to be intrinsically adaptive or maladaptive (Mehling, 2016) - when compared to controls, they also showed a more maladaptive profile characterized by greater distraction from and worry about uncomfortable or painful sensations and reduced tendency to experience the body as safe and trustworthy. In terms of the purported adaptive aspects of IS, patients with OCD showed greater tendency to listen to the body for insight and guidance, although this effect was weaker than those found for other IS dimensions, and no significant group differences were found for the ability to control attention and regulate emotion through attention to the body. IS effects were not related to the presence of comorbidities or medication in the OCD group, with the exception of the trusting dimension, where patients with comorbidities reported significantly less trusting of the body than those without comorbidities. We also found that certain dimensions of IS were preferentially related to OCD symptom dimensions, with the noticing/awareness dimensions related to both symmetry/“just-right” symptoms as well as responsibility for harm symptoms. An increased tendency to worry about uncomfortable sensations was also related to responsibility for harm symptoms, as well as to contamination symptoms. Contrary to expectations, severity of symptoms related to unacceptable and taboo thoughts were also related to IS, such that increased tendency to distract from sensation was associated with greater severity of these symptoms. Overall, these data indicate that OCD patients show abnormal subjective IS characterized by a pattern of increases in maladaptive and other aspects of interoception.
Although few studies have investigated interoception in OCD, one prior study did find better interoceptive accuracy in the form of improved heartbeat detection accompanied with overactive electrophysiological markers of interoception in patients with OCD (Yoris et al., 2017), consistent with the present findings of increased noticing and awareness of body sensations. However, another study found that OCD patients were less accurate than controls when detecting their heartbeat (Schultchen et al., 2019). The current study cannot resolve this discrepancy as interoceptive accuracy and sensibility (i.e. subjective sensitivity to sensations) are often not related (Garfinkel et al., 2015; Meesen et al., 2016). However, the present findings of increased IS in OCD patients is consistent with studies in other internalizing disorders reporting greater subjective sensitivity to body sensations (T. A. Brown et al., 2017; Limmer, Kornhuber, & Martin, 2015; Olatunji, Deacon, Abramowitz, & Valentiner, 2007).
When examining the relationship between IS and OCD symptoms, there was a positive relationship between the noticing/awareness dimension of IS and severity of symptoms in the symmetry, completeness, and need for things to be “just right” category. Symptoms related to this symptom dimension are among the primary symptoms that comprise “sensory phenomenon” (SP). Approximately 70% of OCD patients experience some form of SP, with 30–40% of patients reporting aversive or uncomfortable body sensations (tactile or muscular/skeletal) that immediately precede or accompany their compulsions (Ferrão et al., 2012; Lee et al., 2009; Shavitt et al., 2014). OCD patients with touching, counting, and other repeating behaviors often report sensory-based urges preceding or occurring at the same time as these repetitive behaviors (Lee et al., 2009; Shavitt et al., 2014). Ordering and arranging compulsions are frequently performed until the patient achieves the sensation or perception that things are “just right” (Ferrao et al., 2012; Rosario et al., 2009). It has been suggested that some of these experiences could be related to altered interoceptive processes (C. Brown et al., 2019; Stern, 2014) subserved by activation of the insula (Aziz et al., 1997; Craig, 2002; Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004; Ibanez, Gleichgerrcht, & Manes, 2010); the present findings are consistent with prior work showing that anxiety sensitivity is related to symmetry/ordering/“not-just-right” symptoms (Poli et al., 2017; Wheaton et al., 2012) and further support our hypothesis that IS may be related to at least some sensory-based symptoms in OCD.
In addition to the relationship between the noticing dimension of IS and symmetry/ordering/“not-just-right” symptoms, both noticing and worrying dimensions were associated with greater responsibility for harm symptoms. These symptoms primarily involve fear of causing a bad event or harm to oneself or someone else, with repeated checking that the dreaded event did not occur. These data are consistent with the idea that interoception is also related to fear processing (Katkin, Wiens, & Öhman, 2001; Van Diest, 2019), highlighting the fact that aspects of IS are related to a variety of types of OCD symptoms.
OCD patients who showed increased tendencies to distract from unpleasant body sensations also had more symptoms related to taboo and ‘unacceptable’ thoughts and images related to violence, immorality, and sex. Some research suggests that distraction is maladaptive and counter-productive (Goubert et al., 2004; Mehling, 2016), while other notes therapeutic benefits to distraction that help regulate aversive emotion (Bjureberg et al., 2018; Najmi, Riemann, & Wegner, 2009). Drawing information from the field of mindfulness, interoception can be adaptive when one applies a meta-cognitive attitude when processing internal body sensations (Craig, 2002; Mehling et al., 2012) and utilizes the information derived from the body to update expectations about sensations (Craig, 2009; Duquette & Ainley, 2019; Gibson, 2019). Interestingly, the accompanying cognitive state influences whether distraction is beneficial or not (Wolgast & Lundh, 2017): Distraction appears to be beneficial if it is done for the purpose of temporarily postponing an experience and being willing to encounter the avoided state in the near future (“distracting-acceptance”) (Wolgast & Lundh, 2017). In contrast, distraction for the purpose of avoiding a distressed emotion or state altogether (“distracting-avoidance”) is considered maladaptive (Wolgast & Lundh, 2017). Given this context, we speculate that the use of distraction from uncomfortable sensations in OCD might align more with distracting-avoidance and could therefore be considered maladaptive.
The internal-consistency reliability of the MAIA subscales are generally acceptable. Psychometric analysis for the MAIA (Mehling et al., 2012) found that Cronbach’s alpha fell below 0.70 for three subscales (noticing, not-distracting, not-worrying), suggesting lower internal consistency (Taber, 2018). However, the Cronbach alpha is sensitive to the number of items within the scale (Cortina, 1993), and item-scale correlations can provide additional information on internal consistency (Hinkle, Jurs, & Wiersma, 1988). For all subscales on the MAIA, items correlate reasonably well with their respective subscales (all item-scale correlations > .30) (Nunnally, 1994), suggesting that the internal consistency reliability of the MAIA are within acceptable limits. In the time since this study was conducted, the MAIA-2 has been released, which shows some improvements on the scale psychometrics (Mehling, Acree, Stewart, Silas, & Jones, 2018).
Our findings may have implications for the treatment of OCD. The increased IS endorsed by patients in our sample, specifically the increased distress or worry over uncomfortable body sensations (not-worrying subscale), appear consistent with the clinical presentation of anxiety sensitivity (AS). This tendency to be more aware of sensations and to worry about sensations, coupled with the reduced experience of the body as safe and trustworthy, could potentially impede cognitive behavioral therapy if patients use aversive sensations as evidence to support maladaptive interpretations of intrusive thoughts (Blakey & Abramowitz, 2018). Furthermore, hypervigilance to physical sensations may intensify the distress/discomfort that patients experience during exposure and response prevention (ERP), and hinder patients’ confidence in their ability to deal with exposures, thereby increasing resistance (Blakey & Abramowitz, 2018). Moreover, the tendency of patients to use distraction as a coping mechanism for uncomfortable physical sensations may reduce the likelihood of enduring the distress required to successfully complete a course of ERP (Abramowitz, Blakey, Reuman, & Buchholz, 2018; Blakey & Abramowitz, 2018). Overall, our data provide support for the recommendations of Blakey & Abramowitz (2018) that clinicians should integrate interoceptive exposure into treatment and design concrete triggers to challenge OCD-related beliefs about body sensations (Blakey & Abramowitz, 2018; Craske et al., 2008).
There are several limitations to our study. First, the MAIA and DOCS are both self-report scales, and are therefore susceptible to individual biases, a limitation inherent to most self-report scales. In addition, although not an aim of this study, we did not assess interoceptive accuracy, and are therefore unable to draw conclusions regarding the relationships between objective and subjective interoception. Our study findings may be limited in generalizability as OCD patients with comorbid conditions such as bipolar disorder, schizophrenia spectrum disorder, and alcohol or substance use disorders were excluded from participation. Furthermore, even though the 79% of patients who were taking psychotropic medications were using serotonin reuptake inhibitors (SSRIs or SNRIs), others were using medications from other drug classes (e.g. tricyclic antidepressants, benzodiazepines, stimulants, atypical antipsychotics), which may limit reliable conclusions about medication effects on IS. Despite these limitations, to our knowledge this is the first study to report on IS in a sample of patients with OCD, where we found that patients were hyperaware of body sensations and had a more maladaptive profile of IS that was differentially related to OCD symptom dimensions. Additional efforts would help further elucidate IS in OCD, including work investigating neural mechanisms, evaluating the interactions between MAIA subscales, and testing whether dimensions of IS are impacted by treatments for the disorder.
Increased awareness of body sensations in OCD patients than controls.
OCD showed a more maladaptive profile of interoceptive sensibility than controls.
Aspects of interoceptive sensibility were differentially related to types of OCD symptoms.
Altered interoception in OCD was independent of medication and comorbidity.
Interoception may be related to sensory-based symptoms and harm avoidance in OCD.
Acknowledgements
This work is supported by NIMH grants R21/R33MH107589 and R01MH111794 (awarded to ERS).
Role of funding sources
Funding for this study was provided by the National Institute of Mental Health (NIH Grants R21/R33MH107589 and R01MH111794 to ERS). NIH had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
K.A. Collins is a paid independent rater for MedAvante-ProPhase. R.H. Tobe has received research support (through Nathan Kline Institute) from NIH, Roche Pharmaceuticals and Janssen Pharmaceuticals; Nathan Kline Institute has received all honoraria for Dr Tobe’s consulting with Roche Pharmaceuticals. In the last five years, D.V. Iosifescu has received consulting honoraria from Alkermes, Axsome, Centers for Psychiatric Excellence, Jazz, Lundbeck, Otsuka, Precision Neuroscience, Sage, Sunovion; he has received research support (through his academic institution) from Alkermes, Astra Zeneca, Brainsway, Litecure, Neosync, Otsuka, Roche, Shire. All other authors declare that they have no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramowitz JS, Blakey SM, Reuman L, & Buchholz JL (2018). New Directions in the Cognitive-Behavioral Treatment of OCD: Theory, Research, and Practice. Behav Ther, 49(3), 311–322. doi: 10.1016/j.beth.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Abramowitz JS, Deacon BJ, Olatunji BO, Wheaton MG, Berman NC, Losardo D, … Hale LR. (2010). Assessment of obsessive-compulsive symptom dimensions: Development and evaluation of the Dimensional Obsessive-Compulsive Scale. Psychol Assess, 22(1), 180–198. doi: 10.1037/a0018260 [DOI] [PubMed] [Google Scholar]
- Abramowitz JS, Franklin ME, Schwartz SA, & Furr JM (2003). Symptom Presentation and Outcome of Cognitive-Behavioral Therapy for Obsessive-Compulsive Disorder. Journal of Consulting and Clinical Psychology, 71(6), 1049–1057. doi: 10.1037/0022-006X.71.6.1049 [DOI] [PubMed] [Google Scholar]
- Ardizzi M, Ambrosecchia M, Buratta L, Ferri F, Peciccia M, Donnari S, … Gallese V. (2016). Interoception and positive symptoms in schizophrenia. Frontiers in Human Neuroscience, 10, 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz Q, Andersson JL, Valind S, Sundin A, Hamdy S, Jones AK, … Thompson DG. (1997). Identification of human brain loci processing esophageal sensation using positron emission tomography. Gastroenterology, 113(1), 50–59. doi: 10.1016/S0016-5085(97)70079-9 [DOI] [PubMed] [Google Scholar]
- Bjureberg J, Sahlin H, Hedman-Lagerlof E, Gratz KL, Tull MT, Jokinen J, …Ljotsson B. (2018). Extending research on Emotion Regulation Individual Therapy for Adolescents (ERITA) with nonsuicidal self-injury disorder: open pilot trial and mediation analysis of a novel online version. BMC Psychiatry, 15(1), 326. doi: 10.1186/s12888-018-1885-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakey SM, & Abramowitz JS (2018). Interoceptive exposure: an overlooked modality in the cognitive-behavioral treatment of OCD. Cognitive and Behavioral Practice, 25(1), 145–155. [Google Scholar]
- Blakey SM, Abramowitz JS, Reuman L, Leonard RC, & Riemann BC (2017). Anxiety sensitivity as a predictor of outcome in the treatment of obsessive-compulsive disorder. J Behav Ther Exp Psychiatry, 57, 113–117. doi: 10.1016/j.jbtep.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Brown C, Shahab R, Collins K, Fleysher L, Goodman WK, Burdick KE, & Stern ER (2019). Functional neural mechanisms of sensory phenomena in obsessive-compulsive disorder. J Psychiatr Res, 109, 68–75. doi: 10.1016/j.jpsychires.2018.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Berner LA, Jones MD, Reilly EE, Cusack A, Anderson LK, …Wierenga CE. (2017). Psychometric Evaluation and Norms for the Multidimensional Assessment of Interoceptive Awareness (MAIA) in a Clinical Eating Disorders Sample. Eur Eat Disord Rev, 25(5), 411–416. doi: 10.1002/erv.2532 [DOI] [PubMed] [Google Scholar]
- Calamari JE, Rector NA, Woodard JL, Cohen RJ, & Chik HM (2008). Anxiety sensitivity and obsessive—compulsive disorder. Assessment, 15(3), 351–363. [DOI] [PubMed] [Google Scholar]
- Calì G, Ambrosini E, Picconi L, Mehling WE, & Committeri G (2015). Investigating the relationship between interoceptive accuracy, interoceptive awareness, and emotional susceptibility. Frontiers in psychology, 6, 1202–1202. doi: 10.3389/fpsyg.2015.01202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, & Leckman JF (1992). Sensory phenomena associated with Gilles de la Tourette’s syndrome. J Clin Psychiatry, 53(9), 319–323. [PubMed] [Google Scholar]
- Coles ME, Frost RO, Heimberg RG, & Rheaume J (2003). “Not just right experiences”: perfectionism, obsessive-compulsive features and general psychopathology. Behaviour Research and Therapy, 41(6), 681–700. doi: 10.1016/S0005-7967(02)00044-X [DOI] [PubMed] [Google Scholar]
- Cortina JM (1993). What is coefficient alpha? An examination of theory and applications. Journal of Applied Psychology, 75(1), 98–104. doi: 10.1037/0021-9010.78.1.98 [DOI] [Google Scholar]
- Craig AD (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655. [DOI] [PubMed] [Google Scholar]
- Craig AD (2009). How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10(1). [DOI] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, & Baker A (2008). Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy, 46(1), 5–27. doi: 10.1016/j.brat.2007.10.003 [DOI] [PubMed] [Google Scholar]
- Critchley HD, & Harrison NA (2013). Visceral influences on brain and behavior. Neuron, 77(4), 624–638. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, & Dolan RJ (2004). Neural systems supporting interoceptive awareness. Nat Neurosci, 7(2), 189–195. doi: 10.1038/nn1176 [DOI] [PubMed] [Google Scholar]
- Denys D, de Geus F, van Megen HJGM, & Westenberg HGM (2004). Symptom Dimensions in Obsessive-Compulsive Disorder: Factor Analysis on a Clinician-Rated Scale and a Self-Report Measure. Psychopathology, 37(4), 181–189. doi: 10.1159/000079509 [DOI] [PubMed] [Google Scholar]
- Duquette P, & Ainley V (2019). Working With the Predictable Life of Patients: The Importance of “Mentalizing Interoception” to Meaningful Change in Psychotherapy. Frontiers in psychology, 10, 2173–2173. doi: 10.3389/fpsyg.2019.02173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferentzi E, Horvath A, & Koteles F (2019). Do body-related sensations make feel us better? Subjective well-being is associated only with the subjective aspect of interoception. Psychophysiology, 56(4), e13319. doi: 10.1111/psyp.13319 [DOI] [PubMed] [Google Scholar]
- Ferrão YA, Shavitt RG, Prado H, Fontenelle LF, Malavazzi DM, de Mathis MA, . .. do Rosario MC. (2012). Sensory phenomena associated with repetitive behaviors in obsessive-compulsive disorder: an exploratory study of 1001 patients. Psychiatry Research, 197(3), 253–258. [DOI] [PubMed] [Google Scholar]
- Ganos C, Garrido A, Navalpotro I, Ricciardi L, Martino D, Edwards M, … Bhatia K. (2015). Premonitory Urge to Tic in Tourette’s Is Associated With Interoceptive Awareness. Movement Disorders, 30. doi: 10.1002/mds.26228 [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, & Critchley HD (2013). Interoception, emotion and brain: new insights link internal physiology to social behaviour. Commentary on:: “Anterior insular cortex mediates bodily sensibility and social anxiety” by Terasawa et al. (2012). Soc Cogn Affect Neurosci, 5(3), 231–234. doi: 10.1093/scan/nss140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Seth AK, Barrett AB, Suzuki K, & Critchley HD (2015). Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol Psychol, 104, 65–74. doi: 10.1016/j.biopsycho.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Gibson J (2019). Mindfulness, Interoception, and the Body: A Contemporary Perspective. Frontiers in psychology, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubert L, Crombez G, Eccleston C, & Devulder J (2004). Distraction from chronic pain during a pain-inducing activity is associated with greater post-activity pain. Pain, 110(1– 2), 220–227. doi: 10.1016/j.pain.2004.03.034 [DOI] [PubMed] [Google Scholar]
- Hanley AW, Mehling WE, & Garland EL (2017). Holding the body in mind: Interoceptive awareness, dispositional mindfulness and psychological well-being. J Psychosom Res, 99, 13–20. doi: 10.1016/j.jpsychores.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle DE, Jurs SG, & Wiersma W (1988). Applied statistics for the behavioral sciences (2nd ed.). Boston, MA, USA: Houghton Mifflin. [Google Scholar]
- Ibanez A, Gleichgerrcht E, & Manes F (2010). Clinical effects of insular damage in humans. Brain Structure and Function, 214(5–6), 397–410. [DOI] [PubMed] [Google Scholar]
- Katkin ES, Wiens S, & Ohman A (2001). Nonconscious fear conditioning, visceral perception, and the development of gut feelings. Psychol Sci, 12(5), 366–370. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, & Wittchen H-U (2012). Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res, 21(3), 169–184. doi: 10.1002/mpr.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, .. . Zucker N. (2018). Interoception and Mental Health: A Roadmap. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(6), 501–513. doi : 10.1016/j.bpsc.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Walker DE, & Cohen DJ (1993). Premonitory urges in Tourette’s syndrome. Am J Psychiatry, 150(1), 98–102. doi: 10.1176/ajp.150.1.98 [DOI] [PubMed] [Google Scholar]
- Leckman JF, Walker DE, Goodman WK, Pauls DL, & Cohen DJ (1994). “Just right” perceptions associated with compulsive behavior in Tourette’s syndrome. Am J Psychiatry, 151(5), 675–680. [DOI] [PubMed] [Google Scholar]
- Lee JC, Prado HS, Diniz JB, Borcato S, da Silva CB, Hounie AG, … do Rosario MC. (2009). Perfectionism and sensory phenomena: phenotypic components of obsessive-compulsive disorder. Compr Psychiatry, 50(5), 431–436. doi : 10.1016/j.comppsych.2008.11.007 [DOI] [PubMed] [Google Scholar]
- Limmer J, Kornhuber J, & Martin A (2015). Panic and comorbid depression and their associations with stress reactivity, interoceptive awareness and interoceptive accuracy of various bioparameters. J Affect Disord, 185, 170–179. doi: 10.1016/j.jad.2015.07.010 [DOI] [PubMed] [Google Scholar]
- Longarzo M, D’Olimpio F, Chiavazzo A, Santangelo G, Trojano L, & Grossi D (2015). The relationships between interoception and alexithymic trait. The Self-Awareness Questionnaire in healthy subjects. Front Psychol, 6, 1149. doi: 10.3389/fpsyg.2015.01149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay D, Kim SK, Mancusi L, Storch EA, & Spankovich C (2018). Profile Analysis of Psychological Symptoms Associated With Misophonia: A Community Sample. Behav Ther, 49(2), 286–294. doi: 10.1016/j.beth.2017.07.002 [DOI] [PubMed] [Google Scholar]
- Meessen J, Mainz V, Gauggel S, Dr Volz-Sidiropoulou E, Sutterlin S, & Forkmann T (2016). The Relationship Between Interoception and Metacognition: A Pilot Study. Journal of Psychophysiology, 30, 76–86. doi: 10.1027/0269-8803/a000157 [DOI] [Google Scholar]
- Mehling WE (2016). Differentiating attention styles and regulatory aspects of self-reported interoceptive sensibility. Philos Trans R Soc Lond B Biol Sci, 371(1708). doi: 10.1098/rstb.2016.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling WE, Acree M, Stewart A, Silas J, & Jones A (2018). The Multidimensional Assessment of Interoceptive Awareness, Version 2 (MAIA-2). PLoS One, 13(12), e0208034. doi: 10.1371/journal.pone.0208034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling WE, Gopisetty V, Daubenmier J, Price CJ, Hecht FM, & Stewart A (2009). Body awareness: construct and self-report measures. PLoS One, 4(5), e5614. doi : 10.1371/journal.pone.0005614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling WE, Price C, Daubenmier JJ, Acree M, Bartmess E, & Stewart A (2012). The Multidimensional Assessment of Interoceptive Awareness (MAIA). PLoS One, 7(11), e48230. doi: 10.1371/journal.pone.0048230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel EC, do Rosario-Campos MC, Prado HS, do Valle R, Rauch SL, Coffey BJ, … Leckman JF. (2000). Sensory phenomena in obsessive-compulsive disorder and Tourette’s disorder. J Clin Psychiatry, 61(2), 150–156. [DOI] [PubMed] [Google Scholar]
- Najmi S, Riemann BC, & Wegner DM (2009). Managing unwanted intrusive thoughts in obsessive-Compulsive disorder: Relative effectiveness of suppression, focused distraction, and acceptance. Behaviour Research and Therapy, 47(6), 494–503. doi : 10.1016/j.brat.2009.02.015 [DOI] [PubMed] [Google Scholar]
- Nunnally JC (1994). Psychometric theory 3E: Tata McGraw-hill education. [Google Scholar]
- Olatunji BO, Deacon BJ, Abramowitz JS, & Valentiner DP (2007). Body Vigilance in Nonclinical and Anxiety Disorder Samples: Structure, Correlates, and Prediction of Health Concerns. Behavior Therapy, 35(4), 392–401. doi : 10.1016/j.beth.2006.09.002 [DOI] [PubMed] [Google Scholar]
- Paulus MP, & Stein MB (2010). Interoception in anxiety and depression. Brain Struct Funct, 214(5–6), 451–463. doi: 10.1007/s00429-010-0258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pek J, Wong A, & Wong O (2017). Data Transformations for Inference with Linear Regression: Clarifications and Recommendations. Practical Assessment, 22. [Google Scholar]
- Pinto A, Eisen JL, Mancebo MC, Greenberg BD, Stout RL, & Rasmussen SA (2007). Taboo thoughts and doubt/checking: a refinement of the factor structure for obsessive-compulsive disorder symptoms. Psychiatry Res, 151(3), 255–258. doi: 10.1016/j.psychres.2006.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli A, Melli G, Ghisi M, Bottesi G, & Sica C (2017). Anxiety sensitivity and obsessive-compulsive symptom dimensions: Further evidence of specific relationships in a clinical sample. Personality and individual differences, 109, 130–136. doi : 10.1016/j.paid.2017.01.002 [DOI] [Google Scholar]
- Porges S (1993). Body perception questionnaire: Laboratory of Developmental Assessment University of Maryland. Pridobljeno, 15(12), 2009. [Google Scholar]
- Reese HE, Scahill L, Peterson AL, Crowe K, Woods DW, Piacentini J, … Wilhelm S (2014). The premonitory urge to tic: measurement, characteristics, and correlates in older adolescents and adults. Behav Ther, 45(2), 177–186. doi: 10.1016/j.beth.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss S, & McNally RJ (1985). Theoretical issues in behavior therapy In Reiss S & Bootzin RR (Eds.), The expectancy model of fear (pp. 107–121). London, England: Academic Pr. [Google Scholar]
- Rosario MC, Prado HS, Borcato S, Diniz JB, Shavitt RG, Hounie AG, … Miguel E. (2009). Validation of the University of Sao Paulo Sensory Phenomena Scale: initial psychometric properties. CNS Spectr, 14(6), 315–323. doi: 10.1017/s1092852900020319 [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Stein DJ, Chiu WT, & Kessler RC (2010). The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Molecular Psychiatry, 15(1), 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultchen D, Zaudig M, Krauseneck T, Berberich G, & Pollatos O (2019). Interoceptive deficits in patients with obsessive-compulsive disorder in the time course of cognitive-behavioral therapy. PLoS One, 14(5), e0217237. doi: 10.1371/journal.pone.0217237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavitt RG, de Mathis MA, Oki F, Ferrao YA, Fontenelle LF, Torres AR, …Simpson HB. (2014). Phenomenology of OCD: lessons from a large multicenter study and implications for ICD-11. J Psychiatr Res, 57, 141–148. doi : 10.1016/j.jpsychires.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, … Dunbar GC. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry, 59 Suppl 20, 22–33;quiz 34–57. [PubMed] [Google Scholar]
- Shields SA, Mallory ME, & Simon A (1989). The body awareness questionnaire: reliability and validity. Journal of personality Assessment, 53(4), 802–815. [Google Scholar]
- Singer T, Critchley HD, & Preuschoff K (2009). A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci, 13(8), 334–340. doi: 10.1016/j.tics.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Stern ER (2014). Neural Circuitry of Interoception: New Insights into Anxiety and Obsessive- Compulsive Disorders. Current Treatment Options in Psychiatry, 1(3), 235–247. doi: 10.1007/s40501-014-0019-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber KS (2018). The Use of Cronbach’s Alpha When Developing and Reporting Research Instruments in Science Education. Research in Science Education, 48(6), 1273–1296. doi: 10.1007/s11165-016-9602-2 [DOI] [Google Scholar]
- Taylor S (2014). Anxiety sensitivity: Theory, research, and treatment of the fear of anxiety: Routledge. [Google Scholar]
- Tsakiris M, & Critchley H (2016). Interoception beyond homeostasis: affect, cognition and mental health. Philos Trans R Soc Lond B Biol Sci, 371(1708), 20160002. doi: 10.1098/rstb.2016.0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsur N, Berkovitz N, & Ginzburg K (2016). Body Awareness, Emotional Clarity, and Authentic Behavior: The Moderating Role of Mindfulness. Journal of Happiness Studies, 17(4), 1451–1472. doi: 10.1007/s10902-015-9652-6 [DOI] [Google Scholar]
- Van Diest I (2019). Interoception, conditioning, and fear: The panic threesome. Psychophysiology, 56(8), e13421. doi: 10.1111/psyp.13421 [DOI] [PubMed] [Google Scholar]
- Wheaton MG, Mahaffey B, Timpano KR, Berman NC, & Abramowitz JS (2012). The relationship between anxiety sensitivity and obsessive-compulsive symptom dimensions. Journal of Behavior Therapy and Experimental Psychiatry, 43(3), 891–896. doi: 10.1016/j.jbtep.2012.01.001 [DOI] [PubMed] [Google Scholar]
- Wolgast M, & Lundh L-G (2017). Is Distraction an Adaptive or Maladaptive Strategy for Emotion Regulation? A Person-Oriented Approach. Journal of psychopathology and behavioral assessment, 39(1), 117–127. doi: 10.1007/s10862-016-9570-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoris A, Garcia AM, Traiber L, Santamaria-Garcia H, Martorell M, Alifano F, … Sedeno L. (2017). The inner world of overactive monitoring: neural markers of interoception in obsessive-compulsive disorder. Psychological Medicine, 47(11), 1957–1970. doi: 10.1017/S0033291717000368 [DOI] [PubMed] [Google Scholar]


