Abstract
Despite decades of research on the fate of phenolic compounds when water is disinfected with hypochlorous acid (HOCl), there is still considerable uncertainty regarding the formation mechanisms and identity of ring cleavage products, especially at higher chlorine doses. This study focuses on the formation of electrophilic ring cleavage products - a class of compounds that poses potential health risks at relatively low concentrations -from the reactions of phenols with chlorine. By monitoring the formation of products of reactions between ring cleavage products and the model nucleophile N-α-acetyl-lysine, we identified the α,β-unsaturated dialdehyde 2-butene-1,4-dial (BDA) and its chlorinated analogue chloro-2-butene-1,4-dial (Cl-BDA) after chlorination of phenol, para- and ortho-substituted chlorophenols (2-Cl, 4-Cl, 2,4-diCl-, 2,6-diCl and 2,4,6-triCl-phenol) and 3,5-di-Cl-catechol. Maximum yields of BDA were observed when chlorine was present in large excess (HOCl:phenol ratios of 30:1 to 50:1) with yields ranging from 18% for phenol to 46% for 3,5-diCl-catechol. BDA and Cl-BDA formation was also observed during chlorination of brominated phenols. For methyl-substituted phenols, the presence of methyl substituents in both positions ortho to the hydroxy group inhibited BDA and Cl-BDA formation, but chlorination of cresols and 2,3-dimethylphenol yielded methyl- and dimethyl-BDA species. This study provides new insights into the formation of reactive and toxic electrophiles during chlorine disinfection. It also provides evidence for the importance of phenoxy radicals produced by one-electron transfer reactions initiated by chlorine in the production of dicarbonyl ring cleavage products.
Introduction
The widespread adoption of chlorine disinfection in drinking water treatment resulted in significant decreases in the incidences of waterborne diseases such as cholera and typhoid fever.1–3 Unfortunately, chlorine reacts with organic and inorganic water constituents to produce a variety of disinfection by-products (DBPs) that pose potential health risks.4,5 More than 700 DBPs have been identified, including suspected and known carcinogens, including haloacetaldehydes and haloacetonitriles.6–8 However, despite decades of research, researchers have only been able to account for 40% of the halogenated DBP mass balance.9,10
Among the chemical moieties that serve as DBP precursors, phenols are of particular importance due to their widespread occurrence in natural organic matter (NOM) and anthropogenic chemicals (e.g., consumer products such as triclosan, additives that are released by plastic pipes like bisphenols and nonylphenol) coupled with their relatively high reactivity with hypochlorous acid (HOCl).10–13 The reaction of HOCl with phenolic compounds occurs through an electrophilic attack on an aromatic carbon to yield chlorine-substituted products.14–16 The main initial products reported from the reaction between phenol and chlorine are 2- and 4-chlorophenol, which are produced as a result of the preferential attack of HOCl at the ortho and para positions.17 Subsequent reactions with HOCl yield 2,4- and 2,6-dichlorophenol followed by 2,4,6-trichlorophenol.14,18–20 Under conditions typically encountered during drinking water disinfection, chlorophenols are frequently detected at concentrations between 1 and 10 µg/L.21–28 The presence of chlorophenol is primarily of concern due to their low taste and odor thresholds.14,16,29 In addition, 2,4,6-triCl-phenol is a probable human carcinogen.30,31
To reduce concentrations of chlorophenols produced during drinking water treatment, plant operators often increase the initial chlorine concentration.15,16 This process converts chlorinated phenols into ring cleavage products, including chloroform, trihalomethanes and chlorinated and non-chlorinated organic acids.20,32–38 Despite substantial efforts to understand the mechanisms of ring cleavage and the products produced during extended chlorination of phenolic compounds, the chemistry involved in the process is only partially understood.19 For example, researchers have reported that chlorination of meta-substituted phenols (e.g., resorcinol) results in their nearly quantitative conversion to chloroform.20,36 In contrast, chlorination of ortho- and para-substituted phenols produces much smaller amounts of ring cleavage products, with chloroform yields ranging from approximately 1 to 10%.20,36,37 This indicates the formation of other, so far unknown ring cleavage products. The importance of identifying these compounds is highlighted by inability of known transformation products to explain the mutagenicity observed with in vitro bioassays after phenol-containing water is chlorinated.39–42
Due to analytical challenges associated with the identification of low molecular weight aliphatic compounds, the ring cleavage products of phenolic compounds requires the development of more sensitive analytical methodologies. An approach that is increasingly used in toxicology to identify reactive and potentially toxic metabolites involves the detection of their reaction products (i.e., adducts) with biomolecules, such as DNA and proteins.43,44 In vivo, the reactions, which are referred to as direct molecular initiating events, have been associated with adverse outcome pathways, such as cancer and cardiovascular diseases.45,46 Among the reactive metabolites produced during oxidative water treatment, reactive electrophiles (e.g., aldehydes and epoxides) are of particular importance due to their reactivity with nucleophilic moieties in proteins, including cysteine, lysine and histidine, and primary and secondary amines in DNA.47–50 In toxicology, this has led to the development of in chemico methods to screen large numbers of chemicals for their binding to biomolecules.51,52 In addition, this approach has recently been applied to identify reactive transformation products formed from the reactions of phenolic compounds with hydroxyl radical (HO•).53
To gain insight into the nature and potential toxicity of ring cleavage products associated with the chlorination of phenols, we applied conditions typically employed during drinking water chlorination and used the amino acid N-α-acetyl-lysine to detect reactive electrophiles. N-α-acetyl-lysine was chosen as a target biomolecule because its nucleophilicity makes it a preferred reaction partner for many electrophiles.47,54 Experiments using various halogenated and non-halogenated phenols, alkylphenols and catechols and different chlorine doses were used to investigate the effect of phenol structure on reaction mechanisms and product yields.
Materials and Methods
Chemicals
Phenols and dichlorobenzoquinone were purchased from Sigma-Aldrich (St. Louis, MO) and Fisher Scientific (Fairlawn, NJ), respectively, at the highest available purity (>97%). N-α-acetyl-lysine (NAL) was purchased from Sigma-Aldrich (purity >98%). All other chemicals were obtained from Fisher Scientific. Working solutions of chlorine were prepared by diluting a commercial solution of sodium hypochlorite (NaOCl, 5% active chlorine, reagent grade, Fisher Scientific). Sodium hypochlorite was standardized by iodometry.55 2-Butene-1,4-dial (BDA) was synthesized in our laboratory as described below.
Chlorination experiments
Experiments to assess the transformation of phenols were conducted at different HOCl concentrations at a fixed initial phenol concentration of 0.1 mM (molar HOCl:phenol ratios varied from 1:10 to 40:1). To investigate the fate of ring cleavage products at very high HOCl doses, additional experiments were performed using HOCl:phenol ratios up to 200:1; SI Fig. S3). Experiments were performed in ultrapure water buffered at pH 8±0.1 using 50 mM borate. If not indicated otherwise, chlorination experiments were performed for 30 min, after which the remaining HOCl was quenched with excess thiosulfate (minimum HOCl:thiosulfate ratio 1:10). Afterwards the solutions were allowed to react for 15 min before NAL was added (100 µL, 1 mg mL−1, 0.5 mM final conc.). Samples were stored in the dark at room temperature for 24h prior to analysis of NAL adducts using LC/MS/MS and LC-HRMS (discussed below). Control experiments were conducted in the absence of HOCl, phenols and/or NAL to verify that the observed products were attributable to the reactions of the ring cleavage products with NAL. Additional experiments with H2O2 as alternative quenching agent gave similar results, indicating that thiosulfate had negligible impacts on the formation of transformation products. All experiments were performed at room temperature (20±3°C).
LC-MS/MS analysis
Analysis of NAL adducts was conducted using LC-MS/MS analysis as described previously.53 Briefly, chromatographic separation was achieved using a Hydro-RP column using 0.1% acetic acid and methanol as eluents. Positive electrospray ionization (ESI+) mass spectrometry was used for detection of NAL adducts. Precursor ion scan mode was used (precursor ion: m/z 84) to facilitate adduct identification. BDA was quantified by using a standard addition method (5–7 levels; max. added concentration at least one order of magnitude above the sample concentration). The stock solution of BDA (1 M) was obtained by hydrolysis of 2,5-dimethoxy-2,5-dihydrofuran in water as reported previously.53,56
LC-HRMS analysis
For determination of accurate masses of observed adducts, a LTQ Orbitrap XL HRMS system (Thermo Scientific) was used. The Orbitrap was coupled to an Agilent 1260 HPLC system (quaternary pump, autosampler). Chromatographic separation was achieved using the same method as described for the LC-MS/MS. Mass ranges for full-scan MS experiments were set to 220–600 m/z, the injection volume was 50 μL. Internal calibration was used to ensure accurate mass determinations with a resolution of 100,000 (mass accuracy < 1 ppm). High Energy Collisional Dissociation (HCD) was used to obtain MS2 spectra of the main adducts. An isolation window of 2 m/z was used for the precursor ion selection and normalized collision energy was set to 35%. Further details are provided in Prasse et al.53
Results & Discussion
Identification of N-α-acetyl-lysine adducts
For phenol, three NAL adducts were detected when chlorine was present in large excess relative to phenol (molar chlorine:phenol ratio > 10:1; Fig. 1; SI, Fig. S1). Two of the adducts had the same mass (m/z 255) while the third had a mass of m/z 289. Analysis by HRMS yielded the formula C12H19O4N2 for the two adducts with m/z 255 (255.13402; Δm = 0.33 ppm). These adducts, which have previously been observed in experiments in which phenol was exposed to UV light and hydroxyl radical, were confirmed to be BDA.53 Quantification using standard addition indicated a maximum BDA yield of 18% under the investigated experimental conditions (chlorine-to-phenol ratio 30:1; Figs. 2 and 3A), thus demonstrating the important contribution of these previously unknown transformation products to the ring cleavage products produced when phenol reacts with chlorine. Analysis of adduct m/z 289 using HRMS indicated substitution of one hydrogen with a chlorine atom (C12H18O4N2Cl; exact mass: 289.09506; Δm = 0.35 ppm), which was also evident from the observed isotope pattern (SI, Fig. S2) and the loss of HCl in MS2 fragmentation experiments (Fig. 1). Due to the high similarity of the fragmentation pattern with m/z 255, we conclude that the adduct with m/z 289 was most likely 2-chloro-2-butene-1,4-dial (Cl-BDA).
Fig. 1.
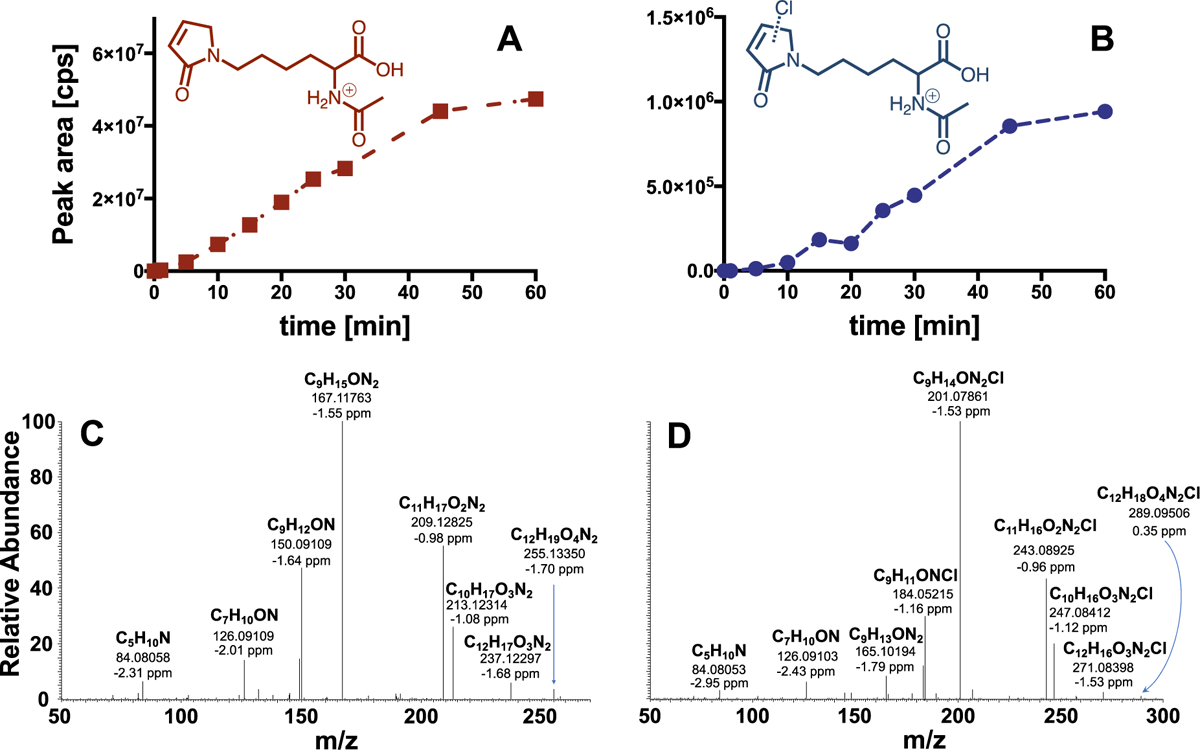
Kinetics of the formation of NAL-adducts (A) m/z 255 and (B) m/z 289 during reaction of phenol with chlorine (molar HOCl:phenol ratio 10:1; HOCl: 1 mM; phenol 0.1 mM). LC/MS/MS in precursor ion scan mode was used for identification of adducts while the chemical structures of the adducts were derived from HRMS analysis. Results of MS2 experiments with (C) m/z 255 and (D) m/z 289 show cleavage of H2O, cleavage of C2H2O and H2CO2. In addition, cleavage of NH3 from m/z 201 and 165 in MS2 spectrum of m/z 289 yielding m/z 184 and 148, respectively (i.e., before and after HCl elimination), and from m/z 167 in MS2 spectrum of m/z 255 yielding m/z 150 was observed. See SI for Cl isotope pattern observed for m/z 289 (Fig. S2).
Fig. 2.
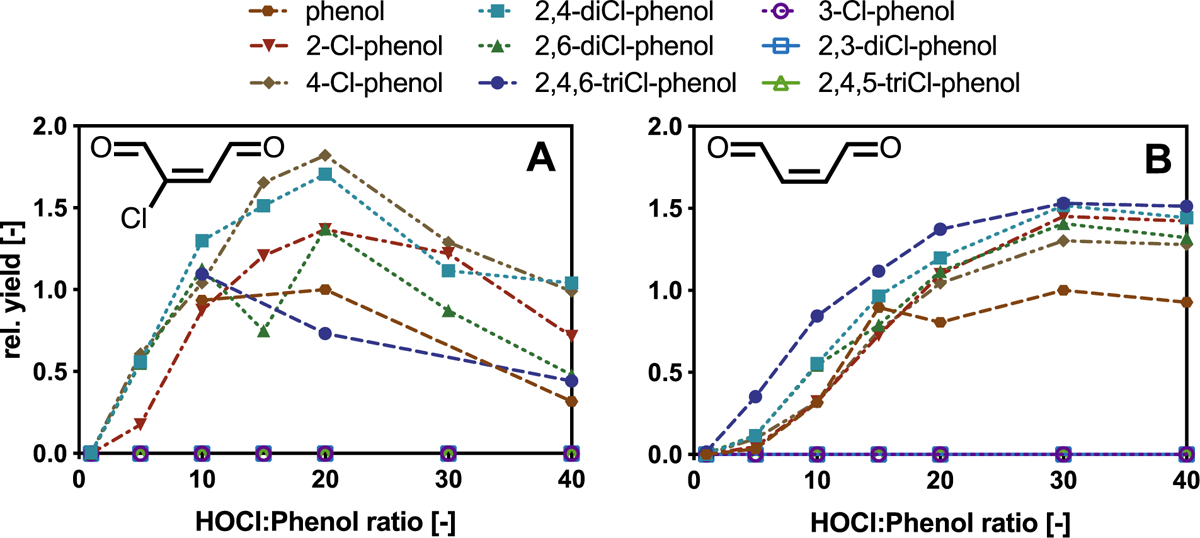
Yields of (A) Cl-BDA and (B) BDA in experiments with chlorinated phenols relative to those obtained for phenol (rel. yield). Conditions: borate buffer (50 mM); pH 8; incubation time: 30 min; initial phenol concentration = 0.1 mM. The lines are shown to guide the eye. Cl-BDA and BDA were absent in experiments with 3-Cl-phenol, 2,3-diCl-phenol and 2,4,5-triCl-phenol.
Fig. 3.
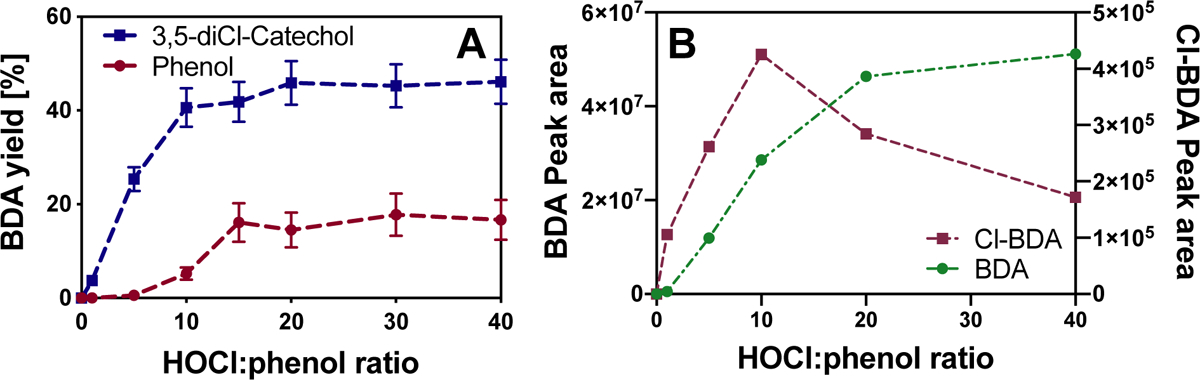
(A) BDA yields in experiments with phenol and 3,5-diCl-catechol at different molar HOCl:phenol ratios in borate buffer (50 mM) at pH 8 (incubation time: 30 min; initial concentration of phenol and 3,5-diCl-catechol: 0.1 mM). Error bars were derived from 95% confidence intervals of linear regressions obtained from standard addition method. (B) HOCl dose-dependent formation of BDA and Cl-BDA in chlorination experiments with 2,4,6-triCl-phenol.
Our results indicate the existence of a previously unrecognized ring cleavage pathway that results in the formation of chlorinated and non-chlorinated C4-unsaturated dicarbonyls from the reaction of phenol with chlorine. The yields of BDA increased up to a HOCl:phenol ratio of 50:1 after which concentrations decreased (SI, Fig. S3). A similar trend was observed for the formation of the ring cleavage product Cl-BDA, but the highest yield was observed at a HOCl:phenol ratio of 20:1 (Figure 2A). Cl-BDA formation at lower HOCl:phenol ratios compared to BDA suggests that BDA is formed from a reaction of Cl-BDA with HOCl (Figs. 2 and 3). In addition, the results also showed that BDA is eventually transformed by chlorine into products that are not detectable by this method. It is worth noting that the formation of Cl-BDA and BDA in kinetic experiments, which appears to occur simultaneously (Fig. 1), is likely attributable to Cl-BDA concentrations below the detection limit and/or transformation of Cl-BDA to BDA in the initial phase of the experiments when chlorine excess is highest.
Potential transformation pathways leading to the formation of α,β-unsaturated dicarbonyl compounds
To further elucidate the pathway through which phenols produce α,β-unsaturated ring cleavage products, additional experiments were conducted with chlorinated phenols. The results revealed that BDA and Cl-BDA are also formed when 2-Cl-, 4-Cl-phenol, 2,4-diCl-, 2,6-diCl-phenol and 2,4,6-triCl-phenol react with chlorine (Fig. 2). In contrast, reactions of 3-Cl-phenol, 2,3-diCl-phenol and 2,4,5-triCl-phenol with chlorine yielded little or no BDA or Cl-BDA, indicating that chlorine substituents in the meta position interfere with the reaction. In general, these results suggest that the formation of BDA and Cl-BDA involves a similar initial reaction in which electrophilic substitution produces o- and p-substituted chlorophenols with 2,4,6-triCl-phenol as the common precursor that reacts further with HOCl to produce ring cleavage products.14 This is also supported by the dose dependent formation of BDA which increases in the order phenol < 2-/4-Cl-phenol < 2,4-/2,6-diCl-phenol < 2,4,6-triCl-phenol (Fig. 2).
Previous studies on the chlorination of 2,4,6-triCl-phenol indicated that 2,6-dichlorobenzoquinone (DCBQ) formed with yield up to 18% at pH ≤ 6.57–59 In contrast, at circumneutral pH values (i.e., under conditions typical for drinking water disinfection), DCBQ yields were less than 0.5% for phenol and its chlorinated analogues.60 To assess the possibility that DCBQ acted as an intermediate in the formation of BDA and Cl-BDA, we exposed DCBQ to varying concentrations of chlorine at pH 8 and did not detect any product (results not shown). Therefore, we conclude that the formation of BDA and Cl-BDA occurs via a different reaction pathway.
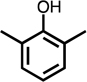
To gain insight into the role of substituents on the transformation pathway, experiments were conducted with various substituted phenolic and non-phenolic aromatic compounds (Table 1). The absence of BDA and Cl-BDA formation during chlorination of benzene, toluene and chlorobenzene under the same conditions as discussed for phenol revealed the critical role of the phenolic moiety and agrees with the low reactivity of these compounds with HOCl. Similarly, the results from experiments with p-methyl-anisole, which reacts rapidly with HOCl,61,62 also showed that the hydroxy group needs to be freely available (i.e., unsubstituted), for dicarbonyl compound production to occur.
Table 1.
Formation of C4-dicarbonyl compounds during chlorination of various substituted phenols and non-phenolic aromatic compounds.
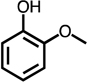
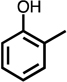
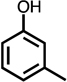
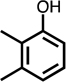
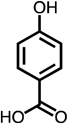
| C4-dialdehyde | catechol | guaiacol | o-cresol | m-cresol | p-cresol | 2,3-CH3-phenol | p-OH-benzoic acid | |
|---|---|---|---|---|---|---|---|---|
 |
 |
 |
 |
 |
 |
|||
| BDA | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | ✓ | |
| Cl-BDA | 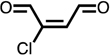 |
✓ | ✓ | ✕ | ✕ | ✕ | ✕ | ✓ |
| methyl-BDA | ✕ | ✕ | ✓ | ✓ | ✓ | ✕ | ✕ | |
| dimethyl-BDA | ✕ | ✕ | ✕ | ✕ | ✕ | ✓ | ✕ | |
| methyl-Cl-BDA | 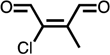 |
✕ | ✕ | ✕ | ✓ | ✕ | ✕ | ✕ |
|
Monoaromatic and phenolic compounds for which no C4- or methyl-C4-dicarbonyl formation was observed: | ||||||||
| benzene | toluene | Cl-benzene | p-CH3-anisole | 2,6-CH3-phenol | 2,4,6-CH3-phenol | resorcinol | hydroquinone | |
 |
 |
 |
||||||
For substituted phenols, the presence of methyl-substituents at both carbon atoms in the α-position relative to the hydroxy group (2,6-dimethyl- and 2,4,6-trimethyl-phenol) prevented the formation of BDA and Cl-BDA, indicating an involvement of the α-carbons in the reaction mechanism. In contrast, the presence of only one α-carbon methyl substituent (o-cresol) led to the formation of methyl-BDA (Table 1; SI, Fig. S4). The same adduct was previously observed in the reaction of cresols with hydroxyl radicals.53 Although methyl-BDA was detected for all cresols, the formation of chlorinated methyl-BDA (Cl-methyl-BDA) was only observed for m-cresol (SI, Fig. S5 and S6; see below for mechanistic explanation). For 2,3-dimethylphenol the formation of an adduct with m/z 283, attributable to the presence of a dimethylated BDA species was observed (Table 1, SI, Fig. S7). Finally, the formation of BDA and Cl-BDA from p-hydroxy benzoic acid chlorination was consistent with previous studies showing that p-hydroxy benzoic acids react with HOCl by replacement of the carboxy group, yielding 2,4,6-triCl-phenol,63,64 which subsequently underwent ring cleavage through reactions with chlorine. The presence of a second hydroxy group on the phenolic compound in either the meta (resorcinol) or the para position (hydroquinone) prevented the formation of BDA and Cl-BDA.
Both electrophilic ring cleavage products were detected in experiments with catechol (i.e., 1,2-dihydroxybenzene). Results from experiments with 3,5-diCl-catechol, the hydroxy-analogue of 2,4,6-triCl-phenol further revealed that the presence of a second hydroxy group in ortho position substantially promotes the formation of BDA and Cl-BDA with BDA yields up to 46% (Fig. 3A). The results further indicate that Cl-BDA could be a potential precursor of BDA as Cl-BDA is formed at lower chlorine doses compared to BDA (Fig. 3B).
Under conditions representative of drinking water treatment (i.e., circumneutral pH, high HOCl:phenol ratios), the distribution of ring cleavage products exhibits trends that suggest the existence of at least two main transformation pathways. For meta-substituted phenols (e.g., resorcinol, 3-chlorophenol) high yields of chloroform20,36,65,66 along with low yields of α,β-unsaturated dicarbonyls are observed. In contrast, ortho- and para-substituted phenols exhibit lower yields of chloroform and higher yields of α,β-unsaturated dicarbonyls.
Implications for the underlying reaction mechanism
The formation of chlorinated and non-chlorinated α,β-unsaturated dicarbonyl compounds from the reactions of chlorine with phenolic compounds has not been previously reported and cannot be explained by reaction pathways postulated in literature.65,67,68 However, a potentially relevant reaction pathway involving the formation of a radical intermediate via one-electron transfer has been indicated previously based on the detection of chlorinated phenyl-phenols when phenol and alkylphenols react with chlorine.69–72 Furthermore, the formation of BDA has been reported previously for the reaction of phenol with hydroxyl radicals (•OH), which also suggests the potential involvement of phenoxy radical intermediates, which can form from BDA via an endoperoxide intermediate.53,73 Even though the relevance of free radical intermediates in aqueous chlorination of phenolic compounds has not been studied in detail, several reaction mechanisms could potentially contribute to their formation. In particular, the reaction of phenols with HOCl via single electron transfer can result in the formation of the HOCl•− radical anion, which quickly decomposes into •OH and chloride.74 This process would likely be accelerated by the reaction of phenol with reactive intermediates, in particular ClO•, which is formed from the scavenging of •OH by OCl−.75 However, the presence of the radical scavenger t-BuOH did not significantly influence the formation of BDA (results not shown). In addition, while •OH production was demonstrated for the reaction of various hydroxyphenol species with chlorine,76 •OH yields in experiments with 2,4,6-triCl-phenol were only very low (results not shown). Alternatively, formation of phenoxy radical intermediates has also been postulated for the reaction with dichlorine monoxide (Cl2O).77 Cl2O is formed in water via nucleophilic attack of OCl− on HOCl and its formation is favorable at elevated HOCl concentrations as used in parts of this study.78,79 In the case of Cl2O, formation of phenoxy radical intermediates can be attributed to the decomposition of Cl2O to Cl• and ClO•.77 However, these experiments have so far only been performed in organic solvents and the relevance of this mechanism is controversial as other studies were not able to detect the formation of radical intermediates.77 Another mechanism that is supported by the high BDA yields observed for 3,5-diCl-catechol (Fig. 3 A) involves dihydroxyphenols, in particular catechol and hydroquinone, for which the formation of phenoxy-radicals is also possible via direct oxidation by HOCl. This would give rise to quinone species80, which then react with a second catechol or hydroquinone molecule by comproportionation.81,82 Overall, our results strongly suggest the involvement phenoxy radical intermediates that lead to the formation of Cl-BDA and BDA. However, additional studies are necessary to confirm the underlying reaction mechanisms.
Formation of α,β-unsaturated dicarbonyl compounds from chlorination of bromophenols
In order to investigate whether also the chlorination of bromophenols, which are of increasing concern as drinking water contaminants especially in coastal regions, results in the formation of α,β-unsaturated dicarbonyl compounds, additional chlorination experiments were performed with 2-Br, 4-Br, 2,4-diBr, 2,6-diBr-, 2,4,6-triBr-phenol (Fig. 4). Similar to their chlorinated analogues, formation of BDA was observed in chlorination experiments with all investigated bromophenols. In contrast, Cl-BDA formation was only observed for 2-Br, 4-Br-, 2,6-diBr-phenol. Furthermore, chlorination of 4-Br-, 2,4-diBr- and 2,4,6-triBr-phenol also gave rise to the formation of a brominated BDA species (Br-BDA; see SI, Figs. S8 and S9 for details).
Fig. 4.
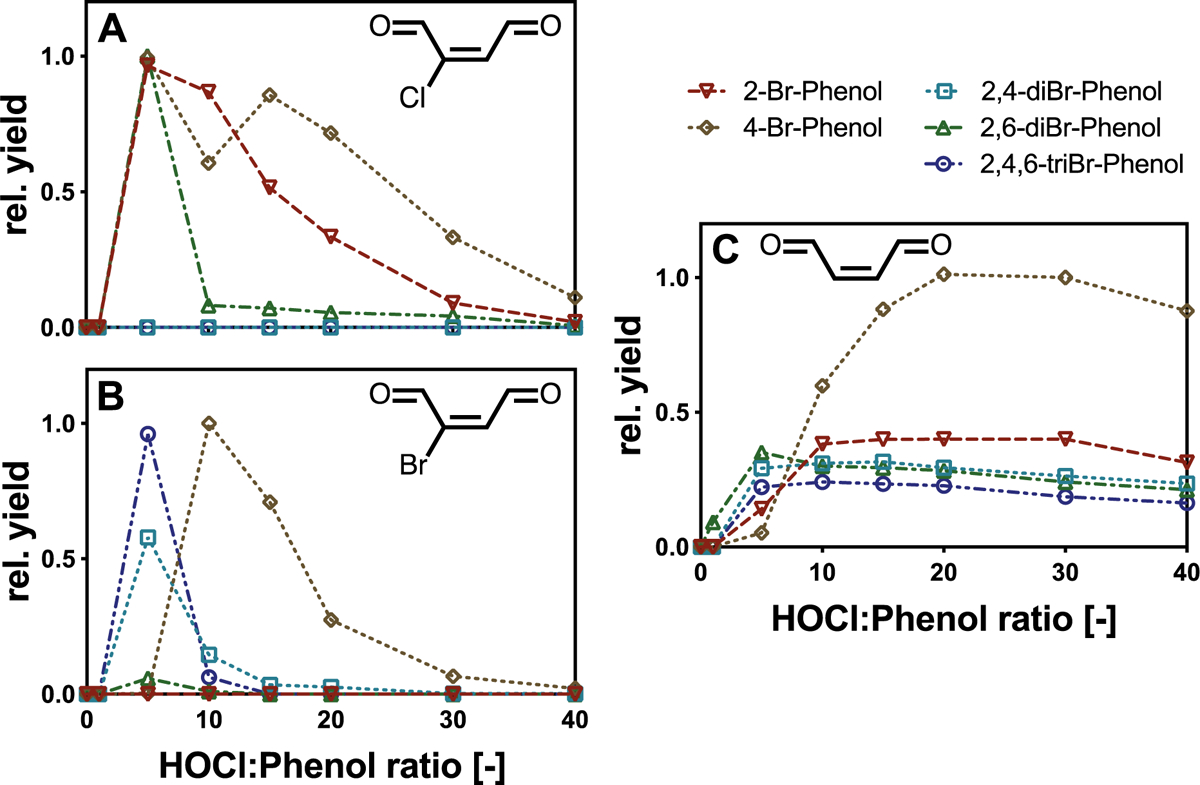
Yields of (A) Cl-BDA, (B) Br-BDA and (C) BDA in chlorination experiments with bromophenols at various molar HOCl:phenol ratios. Yields are given relative to the highest yields of all three compounds observed in experiments with 4-Br-phenol (rel. yield). Experimental conditions: 50 mM borate buffer, pH8; initial concentration of bromophenols: 0.1 mM; chlorination time: 30 min.
The detection of Cl-BDA and Br-BDA in experiments with 4-Br-phenol suggests a reaction mechanism that involves the cleavage of the aromatic ring at different positions (Fig. 4). Chlorination of 4-bromophenol also led to the highest relative yields of both Cl-BDA and Br-BDA compared to the other brominated phenols (Fig. 4). The fact that BDA yields were substantially higher and the dose-dependent formation of all three carbonyl compounds exhibit similar behavior suggests the role of Cl-BDA and Br-BDA as BDA precursors. Furthermore, chlorination of 2-Br-phenol exclusively led to the formation of Cl-BDA whereas Br-BDA was the only halogenated BDA species observed for 2,4-diBr-phenol (Fig. 4). In addition, for 2,6-diBr-phenol (i.e., with bromine atoms in both o-positions), Br-BDA was detected in much lower yields compared to Cl-BDA. As such, the obtained results, clearly demonstrate the relevance of α,β-unsaturated dicarbonyl compounds as important DBPs in the chlorination of bromophenols with the formation of halogenated BDA species strongly depending on the position of the Br substituents (Fig. 5).
Figure 5:
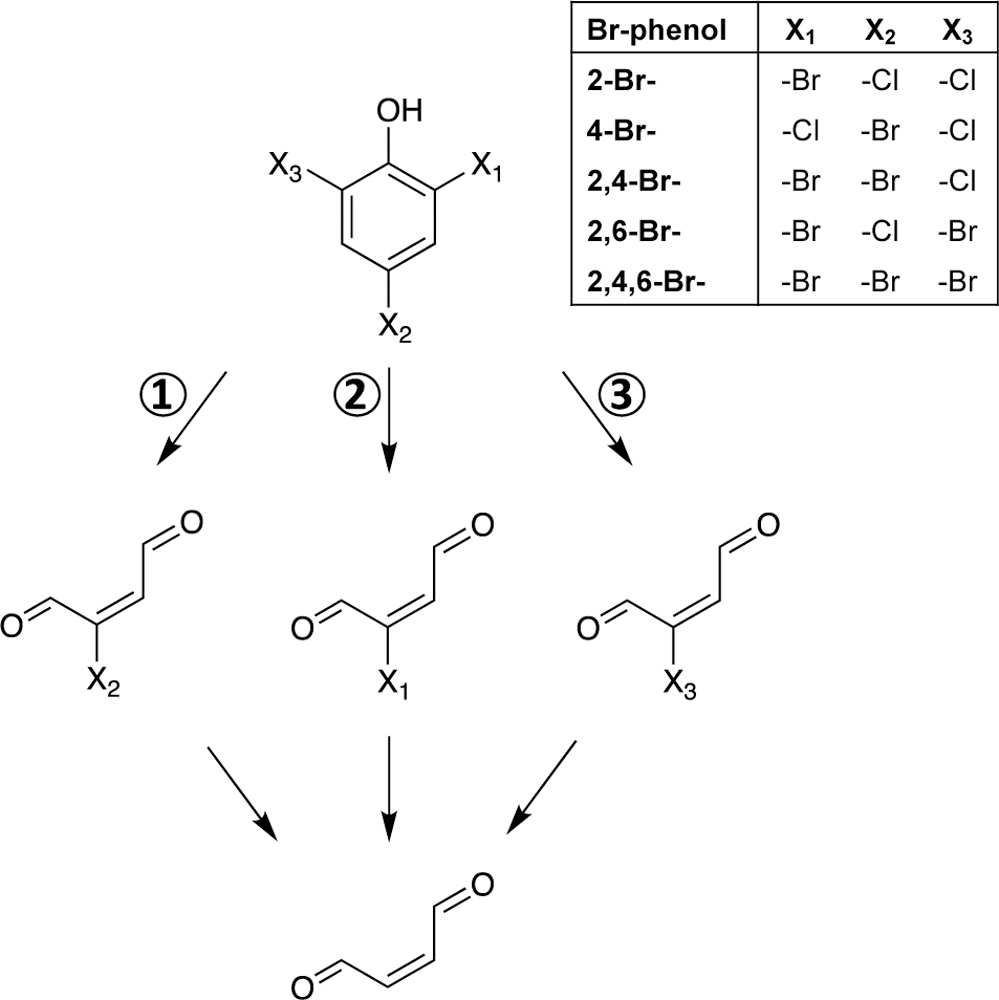
General transformation pathway of 2,4,6-trihalophenols during chlorination of bromophenols and subsequent formation of halogenated BDA and BDA. The results indicate the involvement of pathway 1 for all bromophenols while pathways 2 and 3 were not observed for 2-Br- and 2,4-diBr-phenol, respectively.
Practical Implications
The results of this study indicate that BDA, Cl-BDA, Br-BDA and their methyl-substituted analogues are important ring cleavage products of the reactions of phenols with HOCl under conditions typical of water treatment systems, with maximum BDA yields of up to 46% for 3,5-diCl-catechol. BDA, which is also formed during the CYP450-mediated metabolism of furan, is acutely toxic to liver cells and is highly reactive towards proteins and DNA.83–88 It exhibits a strong positive response in the Ames assay and induces strand breaks and cross-links in DNA.88,89 Similar effects have also been observed for methylated BDA species.90,91 The toxicity of the halogenated BDA compounds has not been studied. However, based on their reactivity with NAL, similar modes of action and toxicity are likely. Additional research is needed to assess the in vivo toxicity of these compounds from drinking water exposure.
Due to the widespread occurrence of phenolic compounds in drinking water sources, unsaturated C4-dicarbonyl compounds are likely present in chlorinated drinking water. Additional research is needed to quantify the concentrations of these C4-dicarbonyl compounds after water is disinfected with chlorine in drinking water treatment plants and in distribution systems. Phenol, chlorophenols and bromophenols occur in source waters and in finished drinking water at concentrations as high as 10 µg L−1.17,21,27,92–94 On the basis of results from this study, concentrations of α,β-unsaturated dialdehydes in a similar concentration range (i.e., several micrograms per liter) could occur when chlorine is employed for disinfection and to mitigate taste and odor issues associated with the presence of chlorophenols. The concentrations of these compounds will be affected by chlorine dose and the presence of organic and inorganic compounds that can react with dicarbonyls (e.g., nucleophilic compounds like proteins associated with biofilms). Additional research is needed on the biostability of α,β-unsaturated dicarbonyl compounds, their formation relative to other DBPs and the relevance of phenolic groups present in NOM as precursors of α,β-unsaturated dicarbonyl compounds formed during drinking water chlorination.
Supplementary Material
Acknowledgements
We thank Zhuoyue Zhang and Eva Maria Rodriguez Franco for assistance with some of the laboratory experiments. This research was supported by the US National Institute for Environmental Health Sciences Superfund Research Program (Grant P42 ES004705) at the University of California, Berkeley and internal funding from Johns Hopkins University.
Footnotes
Supporting Information
Additional information on detected NAL adducts including high-resolution mass spectrometry results showing isotope patterns of chlorinated and brominated BDA adduct species and MS2 fragmentation data of identified NAL adducts, yields of BDA at high chlorine doses (PDF).
References
- (1).Cantor KP Water Chlorination, Mutagenicity, and Cancer Epidemiology. Am. J. Public Health 1994, 84 (8), 1211–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Cutler DM; Miller G The Role of Public Health Improvements in Health Advances: The Twentieth-Century United States. Demography 2005, 42 (1), 1–22. [DOI] [PubMed] [Google Scholar]
- (3).McGuire MJ Eight Revolutions in the History of US Drinking Water Disinfection. J. Am. Water Works Assoc 2006, 98 (3), 123–149. [Google Scholar]
- (4).Sedlak DL; von Gunten U The Chlorine Dilemma. Science 2011, 331 (6013), 42–43. [DOI] [PubMed] [Google Scholar]
- (5).von Gunten U Oxidation Processes in Water Treatment: Are We on Track? Environ. Sci. Technol 2018, 52 (9), 5062–5075. [DOI] [PubMed] [Google Scholar]
- (6).Richardson SD; Ternes TA Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem 2018, 90 (1), 398–428. [DOI] [PubMed] [Google Scholar]
- (7).Richardson SD; Plewa MJ; Wagner ED; Schoeny R; Demarini DM Occurrence, Genotoxicity, and Carcinogenicity of Regulated and Emerging Disinfection by-Products in Drinking Water : A Review and Roadmap for Research. 2007, 636, 178–242. [DOI] [PubMed] [Google Scholar]
- (8).Chuang YH; Szczuka A; Mitch WA Comparison of Toxicity-Weighted Disinfection Byproduct Concentrations in Potable Reuse Waters and Conventional Drinking Waters as a New Approach to Assessing the Quality of Advanced Treatment Train Waters. Environ. Sci. Technol 2019, 53 (7), 3729–3738. [DOI] [PubMed] [Google Scholar]
- (9).Richardson SD Disinfection By-Products and Other Emerging Contaminants in Drinking Water. TrAC - Trends Anal. Chem 2003, 22 (10), 666–684. [Google Scholar]
- (10).Li XF; Mitch WA Drinking Water Disinfection Byproducts (DBPs) and Human Health Effects: Multidisciplinary Challenges and Opportunities. Environ. Sci. Technol 2018, 52 (4), 1681–1689. [DOI] [PubMed] [Google Scholar]
- (11).Aeschbacher M; Graf C; Schwarzenbach RP; Sander M Antioxidant Properties of Humic Substances. Environ. Sci. Technol 2012, 46, 4916–4925. [DOI] [PubMed] [Google Scholar]
- (12).Fiss EM; Rule KL; Vikesland PJ Formation of Chloroform and Other Chlorinated Byproducts by Chlorination of Triclosan-Containing Antibacterial Products. Environ. Sci. Technol 2007, 41 (7), 2387–2394. [DOI] [PubMed] [Google Scholar]
- (13).Tentscher PR; Bourgin M; von Gunten U Ozonation of Para-Substituted Phenolic Compounds Yields p-Benzoquinones, Other Cyclic α,β-Unsaturated Ketones, and Substituted Catechols. Environ. Sci. Technol 2018, 52 (8), 4763–4773. [DOI] [PubMed] [Google Scholar]
- (14).Burttschell RH; Rosen AA; Middleton FM; Ettinger MB Chlorine Derivatives of Phenol Causing Taste and Odor. J. Am. Waterworks Assoc 1959, 51 (2), 205–214. [Google Scholar]
- (15).Lee GF; Morris JC Kinetics of Chlorination of Phenol-Chlorophenolic Tastes and Odors. Int. J. Air Wat. Poll 1962, 6 (567), 419–431. [PubMed] [Google Scholar]
- (16).Ettinger MB; Ruchhoft CC Effect of Stepwise Chlorination on Taste- and Odor-Producing Intensity of Some Phenolic Compounds. J. Am. Water Work. Assoc 1951, 43 (7), 561–567. [Google Scholar]
- (17).Acero JL; Piriou P; Von Gunten U Kinetics and Mechanisms of Formation of Bromophenols during Drinking Water Chlorination: Assessment of Taste and Odor Development. Water Res 2005, 39 (13), 2979–2993. [DOI] [PubMed] [Google Scholar]
- (18).Ge F; Zhu L; Chen H Effects of pH on the Chlorination Process of Phenols in Drinking Water. J. Hazard. Mater 2006, 133 (1–3), 99–105. [DOI] [PubMed] [Google Scholar]
- (19).Núñez-Gaytán AM; Vera-Avila LE; De Llasera MG; Covarrubias-Herrera R Speciation and Transformation Pathways of Chlorophenols Formed from Chlorination of Phenol at Trace Level Concentration. J. Environ. Sci. Heal. - Part A Toxic/Hazardous Subst. Environ. Eng 2010, 45 (10), 1213–1222. [DOI] [PubMed] [Google Scholar]
- (20).Gallard H; von Gunten U Chlorination of Phenols: Kinetics and Formation of Chloroform. Environ. Sci. Technol 2002, 36 (5), 884–890. [DOI] [PubMed] [Google Scholar]
- (21).Simpson KL; Hayes KP Drinking Water Disinfection By-Products: An Australian Perspective. Water Res 1998, 32 (5), 1522–1528. [Google Scholar]
- (22).Fingler S; Drevenkar V; Tkalčević B; Šmit Z Levels of Polychlorinated Biphenyls, Organochlorine Pesticides, and Chlorophenols in the Kupa River Water and in Drinking Waters from Different Areas in Croatia. Bull. Environ. Contam. Toxicol 1992, 49 (6), 805–812. [DOI] [PubMed] [Google Scholar]
- (23).Hebert A; Forestier D; Lenes D; Benanou D; Jacob S; Arfi C; Lambolez L; Levi Y Innovative Method for Prioritizing Emerging Disinfection By-Products (DBPs) in Drinking Water on the Basis of Their Potential Impact on Public Health. Water Res 2010, 44 (10), 3147–3165. [DOI] [PubMed] [Google Scholar]
- (24).Jin M; Chen X; Pan B Simultaneous Determination of 19 Chlorophenols in Water by Liquid Chromatography-Mass Spectrometry with Solid-Phase Extraction. J. Liq. Chromatogr. Relat. Technol 2006, 29 (9), 1369–1380. [Google Scholar]
- (25).Nikolaou AD; Kostopoulou MN; Lekkas TD Organic By-Products of Drinking Water Chlorination. Glob. NEST Int. J 1999, 1 (3), 143–156. [Google Scholar]
- (26).Paasivirta J; Heinola K; Humppi T; Karjalainen A; Knuutinen J; Mantykoski K; Paukku R; Piilola T; Surma-Aho K; Tarhanen J; Welling J; Vihonen H; Sarkka J Polychlorinated Phenols, Guaiacols and Catechols in the Environment. Chemosphere 1985, 14 (5), 469–491. [Google Scholar]
- (27).Michalowicz J The Occurrence of Chlorophenols, Chlorocatechols and Chlorinated Methoxyphenols in Drinking Water of the Largest Cities in Poland. Polish J. Environ. Stud 2005, 14 (3), 327–333. [Google Scholar]
- (28).Michalowicz J; Stufka-Olczyk J; Milczarek A; Michniewicz M Analysis of Annual Fluctuations in the Content of Phenol, Chlorophenols and Their Derivatives in Chlorinated Drinking Waters. Environ. Sci. Pollut. Res 2011, 18 (7), 1174–1183. [DOI] [PubMed] [Google Scholar]
- (29).Young WF; Horth H; Crane R; Ogden T; Arnott M Taste and Odour Threshold Concentrations of Potential Potable Water Contaminants. Water Res 1996, 30 (2), 331–340. [Google Scholar]
- (30).Yin D; Zhu H; Hu P; Zhao Q Genotoxic Effect of 2,4,6-Trichlorophenol on P53 Gene in Zebrafish Liver. Environ. Toxicol. Chem 2009, 28 (3), 603–608. [DOI] [PubMed] [Google Scholar]
- (31).Huff J Long-Term Toxicology and Carcinogenicity of 2,4,6-Trichlorophenol. Chemosphere 2012, 89 (5), 521–525. [DOI] [PubMed] [Google Scholar]
- (32).Gallard H; von Gunten U Chlorination of Natural Organic Matter: Kinetics of Chlorination and of THM Formation. Water Res 2002, 36 (1), 65–74. [DOI] [PubMed] [Google Scholar]
- (33).Rook JJ Chlorination Reactions of Fulvic Acids in Natural Waters. Environ. Sci. Technol 1977, 11 (5), 478–482. 10.1021/es60128a014. [DOI] [Google Scholar]
- (34).Chang EE; Chiang PC; Chao SH; Lin YL Relationship between Chlorine Consumption and Chlorination By-Products Formation for Model Compounds. Chemosphere 2006, 64 (7), 1196–1203. [DOI] [PubMed] [Google Scholar]
- (35).Huang Y; Li H; Zhou Q; Li A; Shuang C; Xian Q; Xu B; Pan Y New Phenolic Halogenated Disinfection Byproducts in Simulated Chlorinated Drinking Water: Identification, Decomposition, and Control by Ozone-Activated Carbon Treatment. Water Res 2018, 146, 298–306. [DOI] [PubMed] [Google Scholar]
- (36).Gan W; Ge Y; Zhu H; Huang H; Yang X ClO 2 Pre-Oxidation Changes the Yields and Formation Pathways of Chloroform and Chloral Hydrate from Phenolic Precursors during Chlorination. Water Res 2019, 148, 250–260. [DOI] [PubMed] [Google Scholar]
- (37).Rook JJ Haloforms in Drinking Water. J. Am. Water Work. Assoc 1976, 68 (3), 168–172. [Google Scholar]
- (38).Zhai H; Zhang X Formation and Decomposition of New and Unknown Polar Brominated Disinfection Byproducts during Chlorination. Environ. Sci. Technol 2011, 45 (6), 2194–2201. [DOI] [PubMed] [Google Scholar]
- (39).Rapson WH; Nazar MA; Butsky VV Mutagenicity Produced by Aqueous Chlorination of Organic Compounds. Bull. Environ. Contam. Toxicol 1980, 24 (1), 590–596. [DOI] [PubMed] [Google Scholar]
- (40).Onodera S; Yoshimatsu K; Saitoh H; Uchida A Behavior of Mutagenic Formation from Phenolic Compounds in Water Disinfection with Chlorine and Their Mutagenic Potential Formation. Eisei kagaku 1998, 44 (4), 289–299. [Google Scholar]
- (41).Langvik V-A; Hormi O; Kronberg L; Tikkanen L; Holmbom B Formation of 3-Chloro-4-(Dichloromethyl)-5-Hydroxy-2(5H)-Furanone (MX) and Mutagenic Activity by Chlorination of Phenolic Compounds. Humic Subst. Aquat. Terr. Environ 1991, 33, 459–465. [Google Scholar]
- (42).Nazar MA; Rapson WH; Brook MA; May S; Tarhanen J Mutagenic Reaction Products of Aqueous Chlorination of Catechol. Mutat. Res 1981, 89, 45–55. [DOI] [PubMed] [Google Scholar]
- (43).Balbo S; Turesky RJ; Villalta PW DNA Adductomics. Chem. Res. Toxicol 2014, 27 (3), 356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Rappaport SM; Li H; Grigoryan H; Funk WE; Williams ER Adductomics: Characterizing Exposures to Reactive Electrophiles. Toxicol. Lett 2012, 213 (1), 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Ankley GT; Bennett RS; Erickson RJ; Hoff DJ; Hornung MW; Johnson RD; Mount DR; Nichols JW; Russom CL; Schmieder PK; et al. Adverse Outcome Pathways: A Conceptual Framework to Support Ecotoxicology Research and Risk Assessment. Environ. Toxicol. Chem 2010, 29 (3), 730–741. [DOI] [PubMed] [Google Scholar]
- (46).Vinken M The Adverse Outcome Pathway Concept : A Pragmatic Tool in Toxicology. Toxicology 2013, 312, 158–165. [DOI] [PubMed] [Google Scholar]
- (47).Lopachin RM; Gavin T Molecular Mechanisms of Aldehyde Toxicity: A Chemical Perspective. Chem. Res. Toxicol 2014, 27 (7), 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Nilsson AM; Bergström MA; Luthman K; Nilsson JLG; Karlberg AT A Conjugated Diene Identified as a Prohapten: Contact Allergenic Activity and Chemical Reactivity of Proposed Epoxide Metabolites. Chem. Res. Toxicol 2005, 18 (2), 308–316. [DOI] [PubMed] [Google Scholar]
- (49).Westberg EAC; Singh R; Hedebrant U; Koukouves G; Souliotis VL; Farmer PB; Segerbäck D; Kyrtopoulos S; Törnqvist MA Adduct Levels from Benzo[a]Pyrenediol Epoxide: Relative Formation to Histidine in Serum Albumin and to Deoxyguanosine in DNA in Vitro and in Vivo in Mice Measured by LC/MS-MS Methods. Toxicol. Lett 2015, 232 (1), 28–36. [DOI] [PubMed] [Google Scholar]
- (50).Peterson LA Reactive Metabolites in the Biotransformation of Molecules Containing a Furan Ring. Chem. Res. Toxicol 2013, 26, 6–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Gerberick GF; Troutman JA; Foertsch LM; Vassallo JD; Quijano M; Dobson RLM; Goebel C; Lepoittevin JP Investigation of Peptide Reactivity of Pro-Hapten Skin Sensitizers Using a Peroxidase-Peroxide Oxidation System. Toxicol. Sci 2009, 112 (1), 164–174. [DOI] [PubMed] [Google Scholar]
- (52).Natsch A; Emter R Reaction Chemistry to Characterize the Molecular Initiating Event in Skin Sensitization: A Journey to Be Continued. Chem. Res. Toxicol 2017, 30 (1), 315–331. [DOI] [PubMed] [Google Scholar]
- (53).Prasse C; Ford B; Nomura DK; Sedlak DL Unexpected Transformation of Dissolved Phenols to Toxic Dicarbonyls by Hydroxyl Radicals and UV Light. Proc. Natl. Acad. Sci. U.S.A 2018, 115 (10), 2311–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Uchida K Role of Reactive Aldehyde in Cardiovascular Diseases. Free Radic. Biol. Med 2000, 28 (12), 1685–1696. [DOI] [PubMed] [Google Scholar]
- (55).Rice EW; Baird RB; Eaton AD Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Assocation, American Water Works Assocation, Water Environment Federation, 2017. [Google Scholar]
- (56).Churchwell MI; Scheri RC; Von Tungeln LS; Gamboa da Costa G; Beland FA; Doerge DR Evaluation of Serum and Liver Toxicokinetics for Furan and Liver DNA Adduct Formation in Male Fischer 344 Rats. Food Chem. Toxicol 2015, 86, 1–8. [DOI] [PubMed] [Google Scholar]
- (57).Smith JG; Lee SF; Netzer A Chlorination in Dilute Aqueous Systems; 2,4,6-Trichlorophenol. Environ. Lett 1975, 10 (1), 47–52. [DOI] [PubMed] [Google Scholar]
- (58).Smith JG; Lee S-F; Netzer A Model Studies in Aqueous Chlorination: The Chlorination of Phenols in Dilute Aqueous Solution. Water Res 1976, 10, 985–990. [Google Scholar]
- (59).Svec P Chlorination of 2,4,6-Trichlorophenol in Acidic Aqueous Medium. Collect. Czechoslov. Chem. Commun 1984, 50, 1842–1851. [Google Scholar]
- (60).Kosaka K; Nakai T; Hishida Y; Asami M; Ohkubo K; Akiba M Formation of 2,6-Dichloro-1,4-Benzoquinone from Aromatic Compounds after Chlorination. Water Res 2017, 110, 48–55. [DOI] [PubMed] [Google Scholar]
- (61).Ogata Y; Kimura M; Kondo Y; Katoh H; Chen F-C Orientation in the Chlorination of Phenol and of Anisole with Sodium and T-Butyl Hypochlorites in Various Solvents. J. Chem. Soc. Perkin Trans 2 1984, 451–453. [Google Scholar]
- (62).De la Mare PBD; Ketley AD; Vernon CA The Kinetics and Mechanisms of Aromatic Halogen Substitution. Part I. Acid-Catalyzed Chlorination by Aqueous Solutions of Hypochlorous Acid. J. Chem. Soc 1954, 1290–1297. [Google Scholar]
- (63).Larson RA; Rockwell AL Chloroform and Chlorophenol Production by Decarboxylation of Natural Acids during Aqueous Chlorination. Environ. Sci. Technol 1979, 13 (3), 325–329. [Google Scholar]
- (64).Sarkanen KV; Dence CW Reactions of P-Hydroxybenzyl Alcohol Derivatives and Their Methyl Ethers with Molecular Chlorine. J. Org. Chem 1960, 25 (5), 715–720. [Google Scholar]
- (65).Boyce SD; Hornig JF Reaction Pathways of THM Formation from the Halogenation of Dihydroxyaromatic Model Compounds for Humic Acid. Environ. Sci. Technol 1983, 17 (4), 202–211. [DOI] [PubMed] [Google Scholar]
- (66).Tretyakova NY; Lebedev AT; Petrosyan VS Degradative Pathways for Aqueous Chlorination of Orcinol. Environ. Sci. Technol 1994, 28 (4), 606–613. [DOI] [PubMed] [Google Scholar]
- (67).Heasley VL; Burns MD; Kemalyan NA; McKee TC; Schroeter H; Teegarden BR; Whitney SE; Wershaw RL Aqueous Chlorination of Resorcinol. Environ. Toxicol. Chem 1989, 8, 1159/1163. [Google Scholar]
- (68).Moye BCJ; Sternhell S The Degradation of Aromatic Rings: The Action of Hypochlorite on Phenols. Aust. J. Chem 1966, 19, 2107–2118. [Google Scholar]
- (69).Onodera S; Yamada K; Yamaji Y; Ishikura S Chemical Changes of Organic Compounds in Chlorinated Water - Formation of Polychlorinated Phenoxyphenols during the Reaction of Phenol with Hypochlorite in Dilute Aqueous Solution. J. Chromatogr 1984, 288, 91–100. [Google Scholar]
- (70).Onodera S; Takahashi M; Suzuki S Chemical Changes of Organic Compounds in Chlorinated Water. XIX. Production of Alkylpolychlorinated Phenoxyphenols (Predioxins) by Aqueous Chlorination of Alkylphenols. Japanese J. Toxicol. Environ. Heal 1993, 39 (1), 20–28. [Google Scholar]
- (71).Onodera S; Yamada K; Yamaji Y; Ishikura S; Su-, S. Chemical Changes of Organic Compounds in Chlorinated Water - Formation of Polychlorinated Methylphenoxymethyl-Phenols (Predioxins) during Chlorination of Methylphenols in Dilute Aqueous Solution. J. Chromatogr 1986, 354, 293–303. [Google Scholar]
- (72).Onodera S; Ogawa M; Yamawaki C; Yamagishi K; Suzuki S Production of Polychlorinated Phenoxyphenols (Predioxins) by Aqueous Chlorination of Organic Compounds. Chemosphere 1989, 19 (1–6), 675–680. [Google Scholar]
- (73).Pan X-M; Schuchmann MN; von Sonntag C Oxidation of Benzene by the OH Radical - a Product and Pulseradiolysis Study in Oxygenated Aqueous Solutions. J. Chem. Soc., Perkin Trans 2 1993, 289–297. [Google Scholar]
- (74).Klaening UK; Wolff T Laser Flash Photolysis of HClO, ClO-, HBrO, and BrO- in Aqueous Solution. Reaction of Cl- and Br-Atoms. Berichte der Bunsengesellschaft fuer Phys. Chemie 1985, 89, 243–245. [Google Scholar]
- (75).Buxton GV; Subhani MS Radiation Chemistry and Photochemsitry of Oxychlorine Ions. Part 1. - Radiolysis of Aqueous Solutions of Hypochlorite and Chlorite Ions. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1972, 68, 947–957. [Google Scholar]
- (76).Rodriguez Franco E; von Gunten U Generation of OH Radicals from the Chlorination of Hydroxyphenols and NOM Extracts. In prep
- (77).Renard JJ; Bolker HI The Chemistry of Chlorine Monoxide (Dichlorine Monoxide). Chem. Rev 1976, 76 (4), 487–508. [Google Scholar]
- (78).Busch M; Simic N; Ahlberg E Exploring the Mechanism of Hypochlorous Acid Decomposition in Aqueous Solutions. Phys. Chem. Chem. Phys 2019, 21 (35), 19342–19348. [DOI] [PubMed] [Google Scholar]
- (79).Lau SS; Abraham SM; Roberts AL Chlorination Revisited: Does Cl−serve as a Catalyst in the Chlorination of Phenols? Environ. Sci. Technol 2016, 50 (24), 13291–13298. [DOI] [PubMed] [Google Scholar]
- (80).Criquet J; Rodriguez EM; Allard S; Wellauer S; Salhi E; Joll CA; von Gunten U Reaction of Bromine and Chlorine with Phenolic Compounds and Natural Organic Matter Extracts - Electrophilic Aromatic Substitution and Oxidation. Water Res 2015, 85, 476–486. [DOI] [PubMed] [Google Scholar]
- (81).Guin PS; Das S; Mandal PC Electrochemical Reduction of Quinones in Different Media: A Review. Int. J. Electrochem 2011, 2011, 1–22. [Google Scholar]
- (82).Song Y; Buettner GR; Parkin S; Wagner BA; Robertson LW; Lehmler HJ Chlorination Increases the Persistence of Semiquinone Free Radicals Derived from Polychlorinated Biphenyl Hydroquinones and Quinones. J. Org. Chem 2008, 73 (21), 8296–8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Kedderis GL; Carfagna MA; Held SD; Batra R; Murphy JE; Gargas ML Kinetic Analysis of Furan Biotransformation by F-344 Rats in Vivo and in Vitro. Toxicol. Appl. Pharmacol 1993, 123, 274–282. [DOI] [PubMed] [Google Scholar]
- (84).Chen LJ; Hecht SS; Peterson LA Identification of Cis-2-Butene-1,4-Dial as a Microsomal Metabolite of Furan. Chem. Res. Toxicol 1995, 8 (7), 903–906. [DOI] [PubMed] [Google Scholar]
- (85).Byrns MC; Vu CC; Neidigh JW; Abad JL; Jones RA; Peterson LA Detection of DNA Adducts Derived from the Reactive Metabolite of Furan, Cis-2-Butene-1,4-Dial. Chem. Res. Toxicol 2006, 19, 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Gingipalli L; Dedon PC Reaction of Cis- and Trans-2-Butene-1,4-Dial with 2’-Deoxycytidine to Form Stable Oxadiazabicyclooctaimine Adducts. J. Am. Chem. Soc 1990, 123 (11), 2664–2665. [DOI] [PubMed] [Google Scholar]
- (87).Peterson LA Electrophilic Intermediates Produced by Bioactivation of Furan. Drug Metab. Rev 2006, 38 (4), 615–626. [DOI] [PubMed] [Google Scholar]
- (88).Peterson LA; Naruko KC; Predecki DP A Reactive Metabolite of Furan, Cis-2-Butene-1,4-Dial, Is Mutagenic in the Ames Assay. Chem. Res. Toxicol 2000, 13 (7), 531–534. [DOI] [PubMed] [Google Scholar]
- (89).Marinari UM; Ferro M; Sciaba L; Finollo R; Bassi AM; Brambilla G DNA-Damaging Activity of Biotic and Xenobiotic Aldehydes in Chinese Hamster Ovary Cells. Cell Biochem. Funct 1984, 2 (4), 243–248. [DOI] [PubMed] [Google Scholar]
- (90).Ravindranath V; Boyd MR Metabolic Activation of 2-Methylfuran by Rat Microsomal Systems. Toxicol. Appl. Pharmacol 1985, 78 (3), 370–376. [DOI] [PubMed] [Google Scholar]
- (91).Ravindranath V; Burka LT; Boyd MR Reactive Metabolites from the Bioactivation of Toxic Methylfurans. Science (80-. ). 1984, 224, 884–886. [DOI] [PubMed] [Google Scholar]
- (92).Peter A; von Gunten U Taste and Odour Problems Generated in Distribution Systems: A Case Study on the Formation of 2,4,6-Trichloroanisole. J. Water Supply Res. Technol. - AQUA 2009, 58 (6), 386–394. [Google Scholar]
- (93).Heitz A; Blythe J; Allpike B; Joll CA; Kagi R Plastic Tastes in Drinking Water: Factors Affecting the Chemistry of Bromophenol Formation. Water Sci. Technol. Water Supply 2002, 2 (5–6), 179–184. [Google Scholar]
- (94).Piriou P; Soulet C; Acero JL; Bruchet A; von Gunten U; Suffet IH Understanding Medicinal Taste and Odour Formation in Drinking Waters. Water Sci. Technol 2007, 55 (5), 85–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


