Abstract
Aquafeed manufacturers have reduced, but not fully eliminated, fishmeal and fish oil and are seeking cost competitive replacements. We combined two commercially available microalgae, to produce a high-performing fish-free feed for Nile tilapia (Oreochromis niloticus)—the world’s second largest group of farmed fish. We substituted protein-rich defatted biomass of Nannochloropsis oculata (leftover after oil extraction for nutraceuticals) for fishmeal and whole cells of docosahexaenoic acid (DHA)-rich Schizochytrium sp. as substitute for fish oil. We found significantly better (p < 0.05) growth, weight gain, specific growth rate, and best (but not significantly different) feed conversion ratio using the fish-free feed compared with the reference diet. Fish-free feed also yielded higher (p < 0.05) fillet lipid, DHA, and protein content (but not significantly different). Furthermore, fish-free feed had the highest degree of in-vitro protein hydrolysis and protein digestibility. The median economic conversion ratio of the fish-free feed ($0.95/kg tilapia) was less than the reference diet ($1.03/kg tilapia), though the median feed cost ($0.68/kg feed) was slightly greater than that of the reference feed ($0.64/kg feed) (p < 0.05). Our work is a step toward eliminating reliance on fishmeal and fish oil with evidence of a cost-competitive microalgae-based tilapia feed that improves growth metrics and the nutritional quality of farmed fish.
Subject terms: Environmental impact, Ecology, Evolution, Zoology
Introduction
Aquaculture, the world’s most efficient producer of edible protein, continues to grow faster than any other major food sector in the world, in response to the rapidly increasing global demand for fish and seafood1,2. Feed inputs for aquaculture production represent 40–75% of aquaculture production costs and are a key market driver for aquaculture production1. The aquafeed market is expected to grow 8–10% per annum and is production of compound feeds is projected to reach 73.15 million tonne (mt) in 20252–9.
Ocean-derived fishmeal (FM) and fish oil (FO) in aquafeeds has raised sustainability concerns as the supply of wild marine forage fish will not meet growing demand and will constrain aquaculture growth1,2,10,11. Moreover, competition for FM and FO from pharmaceuticals, nutraceuticals, and feeds for other animals6,12 further exacerbates a supply–demand squeeze2,13. The use of forage fish (such as herrings, sardines, and anchovies) for FMFO production also affects human food security because approximately 16.9 million of the 29 mt of forage fish that is caught globally for aquaculture feed is directed away from human consumption every year14. More than 90 percent of these fish are considered food grade and could be directly consumed by humans, especially food insecure people in developing countries15.
Although more prevalent in aquafeeds for high-trophic finfish and crustaceans, FM and FO is also routinely incorporated (inclusion rates of 3–10%) in aquafeeds for low-trophic finfish like tilapia to enhance growth1,6,16–18. Tilapia (dominated by Oreochromis niloticus)—the world’s second top group of aquaculture organisms—is cultured in such large volumes and is such an integral part of human diets across the world, that even low inclusion rates of FMFO in aquafeeds for this species is a substantial portion of global demand of forage fish (Supplementary Table S1)19.
The aquafeed industry reduces reliance on FM and FO by using grain and oilseed crops (e.g., soy, corn, canola), however, terrestrial plant ingredients have low digestibility, anti-nutritional factors, and deficiencies in essential amino acids (lysine, methionine, threonine, and tryptophan)16,20. Crop oils also lack long-chain omega-3s (n-3s), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), important for human health21,22. Elevated levels of n-6 (e.g. linoleic acid) fatty acids from crop oils23,24 changes the long-chain n-3/n-6 ratio in tilapia flesh25 that is passed on to human consumers26–28, resulting in increased production of pro-inflammatory eicosanoids (via arachidonic acid), which has led nutritionists to doubt the health benefits of farmed tilapia21,25.
Alternatives to terrestrial crops have been too costly for broad adoption by aquafeed manufacturers (Sarker et al.15). However, nutritional disadvantages and poor fillet quality have prompted researchers to investigate marine microalgae as potential FMFO replacements in fish feeds due to balanced essential amino acids, minerals, vitamins, and long-chain n-3 fatty acids17,29–38. The peer-reviewed literature, however, lacks information on how using marine microalgae in fish-free diets affects growth, feed conversion and fillet quality of tilapia. There also are limited published data on the market price of fish-free diets made with alternative ingredients that show potential for economies of scale.
We conducted research to develop a new aquafeed formula by combining the protein-rich (50%) defatted marine microalgal co-products (under-utilized left-over biomass of Nannochloropsis oculata after EPA oil extraction for human supplement) with another DHA-rich (30% of total fatty acids) marine microalga (Schizochytrium sp.), increasingly available at commercial scale, to fully replace FMFO (fish-free) in tilapia aquafeeds. This study builds on our recent microalgae aquafeeds research. Sarker et al. replaced 33% of FM with under-utilized N. oculata defatted biomass in a tilapia diet that achieved final weight, weight gain, percent weight gain, specific growth rate, and protein efficiency ratio values comparable to the reference diet containing FM and FO17. Furthermore, it was previously reported that Schizochytrium sp. is a highly digestible source of nutrients for tilapia and can fully replace FO in tilapia feed30,33.
To examine the commercial viability of using marine microalgae to replace both FM and FO, we conducted a nutritional feeding experiment to compare three microalgal diets to a reference diet containing FM and FO levels found in commercial tilapia feed. Microalgal diets included defatted N. oculata to replace 33%, 66% or 100% of FM, and whole cell Schizochytrium sp. to replace 100% of FO (33NS, 66NS, 100NS). We measured effects of the four diets on growth metrics, in vitro protein digestibility, feed conversion ratio (FCR), protein efficiency ratio (PER), and fillet deposition of n-3 long-chain polyunsaturated fatty acids (LC PUFAs) and minerals. Furthermore, we conducted a hedonic analysis to estimate the market price of defatted N. oculata meal and whole cell Schizochytrium sp., feed costs, and the economic feed conversion ratio (ECR).
Materials and methods
The experimental design and fish use protocol were approved by the Institutional Animal Care and Use Committee (IACUC) of Dartmouth College. Also, we conducted all experiments in accordance with relevant guidelines and regulations. We euthanized the fish by single cranial pithing in the nutritional feeding experiment.
Diet formulation for nutritional feeding experiment
We incorporated N. oculata defatted biomass to replace different percentages of FM and whole cell Schizochytrium sp. to replace all FO in three tilapia experimental diets for a nutritional feeding trial. These three diet formulations were based on our previous digestibility data for N. oculata defatted biomass and whole cell Schizochytrium sp.17,30,33, and a prior study showing potential to replace all FO with whole cell Schizyochytrium sp.30. We compared these three experimental diets to a reference diet (served as control diet) containing FMFO at levels found in commercial tilapia feed. All diets were iso-nitrogenous (37% crude protein) and iso-energetic (12 kJ/g). Microalgae inclusion diets used N. oculata defatted biomass to replace 33% (33NS), 66% (66NS), and 100% (100NS) of the FM and whole cell Schizochytrium sp. to replace all FO in the test diets (33NS, 66NS, 100NS). Thus N. oculata comprised 3%, 5% and 8% of the diet by weight, respectively, and Schizochytrium sp. made up 3.2% of the diet by weight. We produced the diets in accordance with our previous work17,30,36. We obtained dried Schizochytrium sp. from ALGAMAC, Aquafauna Bio-marine, Inc., Hawthorne, CA, USA; and menhaden FO from Double Liquid Feed Service, Inc., Danville, IL, USA. Qualitas Health Inc., which markets EPA-rich oil extracted from N. oculata as a human supplement39 and seeks uses for tons of under-utilized defatted biomass from its large-scale production facilities, donated the N. oculata defatted biomass. Supplementary Table S8 reports proximate compositions and amino acid profiles of N. oculata defatted biomass and Schizochytrium sp.; total fatty acid profile by percentage of the defatted biomass and Schizochytrium sp ingredients reported in Supplementary Table S9; and macromineral and trace element composition of both ingredients reported in Supplementary Table S10. The formula, proximate analysis, and amino acid profiles of four dietary treatments reported in Table 1. The fatty acid profiles reported in Supplementary Table S11 and the macrominerals and trace elements of the four experimental diets reported in Supplementary Table S7.
Table 1.
Formulation (g/100 g diet) and essential amino acids (% in the weight of diet) of four experimental diets for juvenile tilapia.
| Ingredient (%) | Referencea | Diet | ||
|---|---|---|---|---|
| 33NSb | 66NSc | 100NSd | ||
| Fish meale | 7 | 4.69 | 2.38 | 0 |
| N. oculata defatted biomass | 0 | 3 | 5.5 | 8 |
| Schyzochytrium | 0 | 6.2 | 6.2 | 6.2 |
| Corn gluten meal | 30 | 30 | 30 | 30 |
| Soybean meal | 30 | 30 | 30 | 30 |
| Wheat flour | 20 | 20 | 20 | 20 |
| CaH2PO4 | 0.75 | 0.75 | 0.75 | 0.75 |
| Mineral mixf | 1 | 1 | 1 | 1 |
| Vitamin mixg | 1 | 1 | 1 | 1 |
| Fish oil | 3.2 | 0 | 0 | 0 |
| l-Lysine HCl | 0 | 0.5 | 0.54 | 0.6 |
| dl-Methionine | 0 | 0.18 | 0.2 | 0.2 |
| Carboxymethyl cellulose | 5.07 | 0.7 | 0.43 | 0.27 |
| Choline chloride | 2 | 2 | 2 | 2 |
| Proximate composition (%) | ||||
| Moisture | 22.74 | 19.02 | 20.06 | 18.85 |
| Protein | 35.01 | 38.38 | 37.84 | 36.78 |
| Fat | 5.31 | 5.56 | 4.96 | 5.6 |
| Fiber | 1.57 | 1.78 | 1.6 | 1.34 |
| Ash | 5.19 | 5.26 | 5.14 | 4.85 |
| Carbohydrates | 31.75 | 31.78 | 32 | 33.92 |
| Energy (kJ g−1) | 11.4 | 11.9 | 11.7 | 12.0 |
| Amino acids (% in the weight of diet as is) | ||||
| Arginine | 2.05 | 2.25 | 2.13 | 2.20 |
| Histidine | 1.64 | 1.70 | 1.73 | 1.72 |
| Isoleucine | 2.83 | 2.95 | 3.11 | 3.08 |
| Leucine | 0.61 | 0.67 | 0.65 | 0.68 |
| Lysine | 6.46 | 6.98 | 6.82 | 7.06 |
| Methionine | 1.42 | 1.43 | 1.34 | 1.25 |
| Phenylalanine | 0.75 | 0.76 | 0.71 | 0.81 |
| Threonine | 0.2 | 0.16 | 0.11 | 0.05 |
| Valine | 1.05 | 1.16 | 1.09 | 1.28 |
aReference: no replacement of fish meal (FM) and fish oil (FO).
bReplacement of 33% of FM with N. oculata and 100% of FO with Schizochytrium sp.
cReplacement of 66% of FM with N. oculata and 100% of FO with Schizochytrium sp.
dReplacement of 100% of FM with N. oculata and 100% of FO with Schizochytrium sp.
eOmega Protein, Inc. Houston, Texas 77042, as manufacturer specification, the guaranteed gross composition analysis: crude.
protein, 60%; crude fat, 6%; fiber, 2%.
fMineral premix (mg kg−1 dry diet unless otherwise stated):ferrous sulphate, 0.13; NaCl, 6.15; copper sulphate, 0.06; manganese , sulphate, 0.18; potassium iodide, 0.02; zinc sulphate, 0.3; carrier (wheat middling or starch).
gVitamin premix (mg kg−1 dry diet unless otherwise stated):vitamin A (as acetate), 7500 IU kg−1 dry diet; vitamin D3 (as cholecalcipherol), 6000 IU kg−1 dry diet; vitamin E (as dl-a-tocopherylacetate), 150 IU kg−1 dry diet; vitamin K (as menadione Na-bisulphate), 3; vitamin B12 (as cyanocobalamin), 0.06; ascorbic acid (as ascorbyl polyphosphate), 150; d-biotin, 42; choline (as chloride), 3000; folic acid, 3; niacin (as nicotinic acid), 30; pantothenic acid, 60; pyridoxine, 15; riboflavin, 18; thiamin, 3.
Experimental design and sampling to evaluate tilapia growth on N. oculata defatted biomass and Schizochytrium sp. Diets
We conducted the feeding experiment using a completely randomized design of four diets × three replicates tanks in recirculating aquaculture systems (RAS). Four hundred eighty Nile tilapia (mean initial weight 34.5 ± 2.06 g) were put into randomized groups of 40, bulk weighed, and transferred to a tank. Tilapia had been acclimated to the FMFO containing reference diet for 7 days prior to distribution. The initial stocking density remained within levels recommended to avoid physiological stress on tilapia (< 0.25 lbs/gal in 80 gallon RAS tanks). We carefully monitored water quality daily to maintain favorable conditions for tilapia across all RAS tanks and kept the water temperature at 28.7 ± 0.25 °C, pH at 7.1 ± 0.1, dissolved oxygen at 6.1 ± 0.15 mg/L, total ammonia nitrogen at 0.26 ± 0.1 mg/L, and nitrite nitrogen at 0.3 ± 0.01 mg/L17,30.
We administered feed at a rate of 8% of body weight until day 60, 6% until day 121, and 4% until day 183, with feedings performed twice per day at 09:00 and 15:30 h. We measured fish biomass monthly by randomly selecting 10 fish as a weight sample to adjust feeding rates for growth and we bulk weighed all fish every other month for sampling events (day 0, 60, 121, and 185). We withheld feed for 24 h prior to the weighing procedure to reduce handling stress on fish.
Biological sampling and tissue collection
We randomly selected and weighed 10 individual fish from the total starting stock at the beginning of the experiment, then euthanized (by single cranial pithing17, and stored fish tissues at – 20 °C for future biochemical analysis. At day 121 of the experiment, we euthanized 6 fish per tank, and 6 additional fish at day 185, the terminus of the trial. Half of the fish sampled on day 121 and day 185 were filleted, and half were kept whole and then stored at − 20 °C for further processing17,30. All samples from the initial sampling, day 121, and day 185 were freeze dried at − 20 °C, then fully homogenized. Both whole body and fillet samples were sent to New Jersey Feed Laboratory, Inc (Ewing, NJ, USA) for full proximate, energy, amino acid, and fatty acid profiles.
Analytical procedure and calculation
We quantified final weight, weight gain, weight gain percentage, FCR, SGR, PER, and survival rate for each of the dietary treatments. Each of these parameters were calculated as follows: weight gain = (final weight − initial weight/initial weight) × 100; FCR, FCR = feed intake/weight gain; protein efficiency ratio; SGR (%/day) = 100 × ln final wet weight (g) − ln initial wet weight (g))/Time (days), PER = weight gain (g)/protein fed (g); and survival rate (%) = (final number of fish/initial number of fish) × 10017,34,40.
The trace mineral content of each of the experimental diets, sampled fish fillets, and whole bodies was analyzed by the Department of Earth Science at Dartmouth College17. Each 100 mg sample was acid digested in 0.5 mL 9:1 HNO3/HCl in open vessel digestion with heating at 105 °C for 1 h. Samples were diluted to 10 mL in DI water prior to analysis. All measurements were recorded gravimetrically. Digested samples were run by ICP-MS analysis using an Agilent 7700 × with collision (He) and reaction (H2) gases. The methodology and quality control followed EPA method 6020a.
Degree of protein hydrolysis and in-vitro protein digestibility
We performed an in-vitro digestibility assessment according to the method prescribed in Yasumaru and Lemos to measure the degree of protein hydrolysis of our experimental diets in the presence of tilapia stomach crude enzyme extract and intestine crude enzyme extract41. A 50 g sample from each of the four diets was ground via mortar and pestle until all materials could fit through a 0.5 mm food sieve. We allotted 80 mg by protein basis of each diet with 25 mL DI water in a 50 mL reaction vessel immersed in a water bath held at 25 °C. The reaction mixture, containing diet and DI water, was adjusted to pH 2.0 with 0.1 M HCl using a Hannah instrument HI-901C1 potentiometric auto titrator, set to dose 0.3 mL HCl every 2 min for 30 min until pH equilibrium was reached. After equilibrium, we introduced 200 µL stomach crude enzyme extract prepared according to Yasumaru and Lemos with storage solution modifications sourced from Chaijaroen and Thongruang41,42. After crude enzyme extract introduction, we made minor pH changes adding 0.1 M HCl or 0.01 M NaOH by hand when necessary. Once we introduced the crude enzyme extract, we initiated a predetermined program on the auto titrator to dose 0.025–0.075 mL in proportion to the change in pH measured. This program dosed accordingly every 3-min interval to keep the pH at 2.0 for 1 h. The program was paused, when necessary, to prevent over adjusting the solution during the titration. After the 1-h stomach digestion period, we recorded the total volume dosed. We then adjusted the reaction mixture pH to 8.0, using 0.1 M NaOH, and allowed the auto titrator to dose 0.025 mL 0.1 M NaOH for approximately 1 h to allow the mixture to reach equilibrium. Once pH equilibrium was reached, we introduced 250 µL intestinal crude enzyme extract, prepared in the same way as the stomach crude enzyme extract. Minor adjustments to pH were made by hand using 0.01 M NaOH or 0.1 M HCl. Then we initiated the auto titrator method to dose 0.01–0.025 mL 0.1 M NaOH proportional to the measured change in pH, in order to hold the pH at 8.0 for 1 h, and recorded the total volume dosed. All diets were run in triplicate41,43. We quantified the degree of protein hydrolysis in the stomach using the following equation:
| 1 |
where DH is the degree of hydrolysis, V is the volume of the acid consumed (mL), N is the normality of the acid (H+ available for release × Molarity), E is the mass of the substrate protein (g), P is the number of peptide bonds cleaved (mol g protein−1) and when amino acid composition is unknown, (8.0), and FpH is the correction factor for pH 2.0 at 25 °C (1.08).
We quantified the degree of protein hydrolysis in the intestine using the following equation:
| 2 |
where B is the volume of alkali consumed (mL), Nb is the normality of the alkali (alkali groups × Molarity), a is the average degree of dissociation of the a-NH2 groups (1/a = 1.50 for pH 8.0 at 25 °C), MP is the mass of substrate protein (g), and Htot is the total number of peptide bonds in the protein substrate [7.6–9.2 meqv g protein−1] according to the source of protein44.
After calculating the degree of protein hydrolysis, we determined the in vitro protein digestibility using a prediction equation model as reported by Yasumaru and Lemos and Tibbets41,43. The degree of protein hydrolysis was used as input in the following equation to determine in vitro protein digestibility, IPD = (3.5093DH + 70.248).
Economic analysis of fish-free feed formulated with microalgae blends
We obtained commodity and market prices for the formulated feed ingredients from a variety of sources (Supplementary Tables S5 and S12). We conducted non-parametric bootstraps in RSTUDIO (v.1.2.5033) based on 10,000 replicates using the adjusted bootstrap percentile method to estimate the median and 95% confidence intervals.
We conducted a hedonic analysis in RSTUDIO to estimate the price of defatted N. oculata meal and whole cell Schizochytrium sp. The general methodology of hedonic analysis is described in Maisashvili et al.45. We used mixed-effects linear models using maximum likelihood methods46,47.
Following Maisashvili et al., we selected crude protein, ether extract, methionine, and lysine as the key input variables in our defatted N. oculata meal model45. We used the following regression formula:
| 3 |
where yt is the vector of feed ingredient prices observed at time t, CP is a vector of independent variables reflecting the crude protein content of the corresponding feed ingredients, Met is a vector of independent variables reflecting the methionine content of the corresponding feed ingredients, Lys is a vector of independent variables reflecting the lysine content of the corresponding feed ingredients, EE is a vector of independent variables reflecting the ether extract content of the corresponding feed ingredients, β0 is the fixed-effect intercept, β1 is the fixed-effect coefficient of CP2, β2 is the fixed-effect coefficient of Met2, β3 is the fixed-effect coefficient of Lys2, β4 is the fixed-effect coefficient of EE, b0,CP is the random-effect intercept of CP at time t, b0,EE is the random-effect intercept of EE at time t, b1 is the random-effect coefficient of CP at time t, b2 is the random-effect coefficient of EE at time t, ε is the residual error, and t is the time period (2010–2019).
We selected the top fatty acids present in both the commodity oils (vegetable and fish) and in Schizochytrium sp. that did not require an extrapolation. Thus, we used the following regression formula:
| 4 |
where yt is the vector of oil ingredient prices observed at time t, 20:5n-3 is a vector of independent variables reflecting the EPA content of the corresponding oil ingredients, 14:0 is a vector of independent variables reflecting the myristic acid content of the corresponding oil ingredients, 16:1n-7 is a vector of independent variables reflecting the palmitoleic acid content of the corresponding oil ingredients, 16:0 is a vector of independent variables reflecting the palmitic acid content of the corresponding oil ingredients, β0 is the fixed-effect intercept, β1 is the fixed-effect coefficient of 20:5n-32, β2 is the fixed-effect coefficient of 14:02, β3 is the fixed-effect coefficient of 16:1n-72, β4 is the fixed-effect coefficient of 14:0, β5 is the fixed-effect coefficient of 16:0, b0,14:0 is the random-effect intercept of 14:0 at time t, b0,16:0 is the random-effect intercept of 16:0 at time t, b1 is the random-effect coefficient of 14:0 at time t, b2 is the random-effect coefficient of 16:0 at time t, ε is the residual error, and t is the time period (2010–2019).
As inputs to Eqs. (3) and (4), we used the mean annual prices for 12 meal ingredients and 7 oil ingredients from January 2010 to December 2019 (see Supplementary Table S12 for details about the commodities and data sources). Although some studies have used shorter time horizons for their hedonic models (e.g. 2 years)48, we followed other studies that used longer time horizons (e.g. 10 years) in their hedonic models49 and economic analysis of agricultural commodities to capture variability50. We incorporated a freight component to calculate the costs to bring these commodities to the Port of Shanghai, China. To account for the multi-modal components of the freight costs of U.S. commodities, we applied modal transport shares (e.g. rail, truck, barge) of grain commodities (e.g. corn, wheat, soybeans, sorghum, and barley) to the distances between the grain production sites and U.S. ports (see Supplementary Table S13 and Supplementary Methods for further details). We used a shipping route distance calculator to estimate the international shipping distances (Supplementary Table S14). We obtained the nutritional composition of the feed commodities from Archer Daniel Midlands and Feedinamics (Supplementary Table S15). We obtained the fatty acid profiles of the oils used in the feed from the literature (Supplementary Table S16). For the terrestrial-plant-based oils, we used the fatty acid values reported in Dubois et al.51. For FO, we used the fatty acid values reported in Sarker et al.30. We scaled the vectors of independent variables (Supplementary Tables S15 and S16) with the parameters provided in Supplementary Tables S17 and S18, for defatted N. oculata and whole cell Schizochytrium sp., respectively. We assessed the goodness of fit using graphical methods and diagnostic tests (see Supplementary Methods, Supplementary Tables S19 and S20, and Supplementary Figs. S2–S7 for further details).
We estimated the price of defatted N. oculata meal with Eq. (3), the scaled parameters (Supplementary Table S21), the fixed-effect coefficients (Supplementary Table S22), and the random-effect coefficients (Supplementary Table S23). We estimated the price of whole cell Schizochytrium sp. with Eq. (4), the scaled parameters (Supplementary Table S24), the fixed-effect coefficients (Supplementary Table S25), and the random-effect coefficients (Supplementary Table S26). To convert the estimated price of Schizochytrium sp. oil to whole cell Schizochytrium sp., we multiplied the price by the fraction of lipids in Schizochytrium (0.54).
We calculated the costs of all ingredients of formulated reference feed and experimental feeds (which combined N. oculata defatted biomass with Schizochytrium sp.) to determine the diet costs in USD per kg (Supplementary Table S27). The price of each diet was determined by multiplying the respective contributions of each feed ingredient by their respective costs per kg and summing the values obtained for all of the ingredients in each of the formulated diets. Finally, we estimated the production cost of tilapia ($/kg fish) via ECR to compare among the four experimental tilapia feeds (which combined defatted biomass with Schizochytrium sp.). We estimated fish production cost as ECR using the equation of Piedecausa52:
| 5 |
where ECR is the economic conversion ratio, and FCR is the feed conversion ratio.
Statistical analysis
Statistical analysis (ANOVA) was performed according to Sarker et al.17 to determine the significant differences in proximate and amino acid content, fatty acid profile, final weight, weight gain, weight gain percentage, in vitro protein digestibility, FCR, SGR, PER, survival rate, and ECR for each of the treatments. When significant differences were found, we compared the treatment means using Tukey’s test of multiple comparisons (posthoc), with a 95% confidence interval. The IBM Statistical Package for the Social Sciences (SPSS) program for Windows (v. 21.0, Armonk, NY, USA) was used for all statistical methods.
Data and code availability
The datasets and RSTUDIO files used in the economic analysis including the hedonic regression analyses (used to estimate the price of defatted N. oculata meal and whole cell Schizochytrium), bootstrap confidence intervals of feed ingredient prices, and the ECR for Fig. 2 are available at the following link: 10.6071/M3VD5V.
Figure 2.
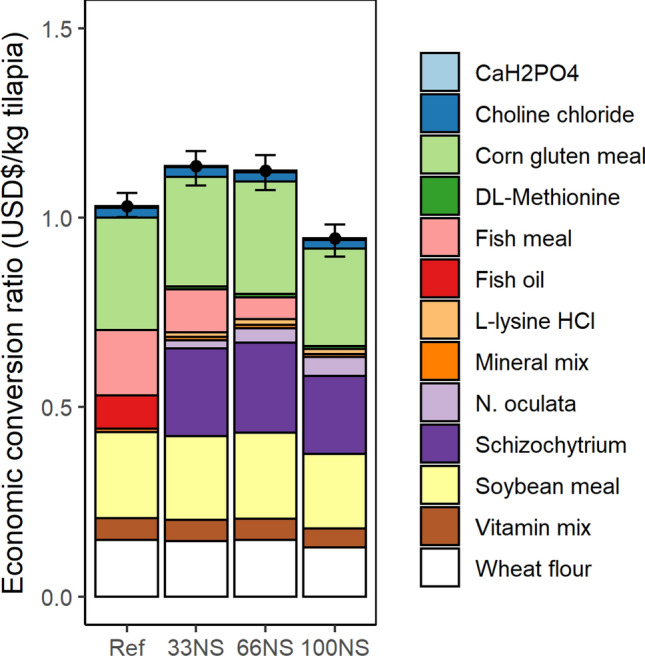
Economic conversion ratio of the reference (Ref) and experimental diets disaggregated by ingredient. The experimental diets include a replacement of fishmeal (FM) with defatted biomass of N. oculata (N) to replace 33%, 66% or 100% of FM; and whole cell Schizochytrium sp. (S) to replace 100% of fish oil. The error bars represent the 95% confidence interval.
Results
Growth, nutrient utilization and proximate composition of tilapia carcass
Fish fed the fish-free diet for 184 days displayed significantly better (p < 0.05) final weight, weight gain, percent weight gain and specific growth rate than fish fed the reference diet, which contained FM and FO levels typically found in commercial tilapia diets (Table 2). Growth rates were linear throughout the experiment and weights measured for the fish-free diet diverged from those for the reference diet by day 128 (Supplementary Fig. S1). Tilapia fed fish-free feed showed an improved food conversion ratio and protein use efficiency ratio though differences among diets and were not statistically significant. We detected no difference in survival rate among all diets and all fish appeared healthy (no visual signs of illness or deformities) at the end of the experiment. The whole-body proximate composition (Supplementary Table S2) did not significantly differ across the dietary treatments; lipid contents ranged from 2 to 5% and protein contents ranged from 13 to 17% across the four treatments.
Table 2.
Results from feeding tilapia iso-nitrogenous, iso-caloric, iso-energetic diets that replaced different percentages of fish meal with N. oculata defatted biomass and of fish oil with Schizochytrium sp. whole cells.
| Dieta | ANOVA | |||||
|---|---|---|---|---|---|---|
| Referenceb | 33NSc | 66NSd | 100NSe | F value | P value | |
| Initial Wt. (g) | 33.3 ± 1.7 | 35.5 ± 2.2 | 34.9 ± 2.1 | 34.4 ± 2.2 | 0.2 | 0.88 |
| Final Wt. (g) | 139.9 ± 4.5f | 196.1 ± 23.6f,g | 168.9 ± 19.9f,g | 207.3 ± 9.8g | 4 | 0.05 |
| Wt. gain (g)h | 106.6 ± 13.1f | 160.6 ± 21.4f,g | 135.8 ± 4.6f,g | 172.9 ± 8.4g | 4.7 | 0.03 |
| Wt. gain (%)i | 318.8 ± 28.0f | 447.8 ± 34.6f,g | 392.6 ± 27.7f,g | 504.3 ± 27.3g | 7.2 | 0.01 |
| FCRj | 1.61 ± 0.1 | 1.57 ± 0.1 | 1.60 ± 0.1 | 1.40 ± 0.1 | 3 | 0.09 |
| SGRk | 0.62 ± 0.05f | 0.81 ± 0.04f,g | 0.74 ± 0.04f,g | 0.87 ± 0.03g | 6.5 | 0.01 |
| PERl | 1.23 ± 0.1 | 1.1 ± 0.0 | 1.1 ± 0.1 | 1.3 ± 0.02 | 2.9 | 0.1 |
| Survival (%)m | 93.3 ± 1.7 | 93.33 ± 0.8 | 97.5 ± 1.4 | 90.8 ± 5.5 | 0.8 | 0.49 |
aValues are means ± standard errors of three replicate groups (n = 3).
bReference: no replacement of fish meal (FM) and fish oil (FO).
cReplacement of 33% of FM with N. oculata and 100% of FO with Schizochytrium sp.
dReplacement of 66% of FM with N. oculata and 100% of FO with Schizochytrium sp.
eReplacement of 100% of FM with N. oculata and 100% of FO with Schizochytrium sp.
f, gMean values not sharing a superscript letter in the same row differ significantly (P < 0.05) from Tukey’s HSD test.
hWeight (Wt.) gain (g) = final Wt. − initial Wt.
iWt. gain (%) = (final Wt. − initial Wt.)/initial Wt. × 100.
jFeed conversion ratio (FCR) = feed intake (g)/Wt. gain (g).
kSpecific growth rate (SGR) (%/day) = 100% × (ln final wet Wt. (g) − ln initial wet Wt. (g))/Time (days).
lProtein efficiency ratio (PER) = Wt. gain (g)/protein fed (g).
mSurvival (%) = (Final number of fish/Initial number of fish) × 100%.
Fillet proximate and amino acid composition
We detected the highest crude protein, lipid, and ash content in the fillet tissue of tilapia fed the fish-free feed (100NS), with the only significant difference (p < 0.05) being crude lipid (Supplementary Table S3). Crude protein contents ranged from 18–24% among the four dietary treatments. Nile tilapia fillets from the fish-free feed treatment had significantly higher lipid content (1.8%) compared to fillets from the reference (0.8%), 33NS, (0.9%), and 66NS (0.9%) feeds. The fillet amino acid composition, except for methionine and histidine, did not differ across the diets (Supplementary Table S4). We detected significantly lower (p < 0.05) methionine and histidine content in the 33NS diet compared to other diets. Methionine and histidine content in the 66NS diet was the highest when compared to the fish-free and reference diets, but was not significantly different.
Fillet macro minerals and trace elements composition
We did not find any significant differences in macromineral composition in fillets across all diets (Table 3). Fillet trace element composition also did not significantly differ across the dietary treatments, except for selenium, which differed significantly (p < 0.05) between the reference and 33NS diets but not among the reference, fish-free and 66 NS diets. We detected the lowest level of arsenic in fish fillet of fish-free feed. Other trace elements—boron, mercury, lead and molybdenum—were at non-detectable levels in all fish fillets.
Table 3.
Macro minerals and trace elements content (wet weight basis) of fillet from Nile tilapia after 184 days on the experimental diets.
| Macro minerals (%) | Referenceb | Filleta | ANOVA | |||
|---|---|---|---|---|---|---|
| 33NSc | 66NSd | 100NSe | F Value | P Value | ||
| Phosphorus | 11.92 ± 1.89 | 10.85 ± 0.78 | 12.98 ± 0.24 | 9.53 ± 0.84 | 1.75 | 0.23 |
| Calcium | 7.37 ± 2.45 | 6.43 ± 1.01 | 8.53 ± 0.73 | 3.08 ± 0.84 | 2.65 | 0.12 |
| Magnesium | 1.28 ± 0.14 | 1.19 ± 0.06 | 1.37 ± 0.06 | 1.28 ± 0.12 | 0.58 | 0.64 |
| Potassium | 18.19 ± 2.33 | 16.78 ± 0.47 | 18.83 ± 0.99 | 17.65 ± 1.55 | 0.33 | 0.8 |
| Sulfur | 10.87 ± 0.84 | 10.21 ± 0.3 | 11.65 ± 0.62 | 10.51 ± 0.26 | 1.25 | 0.35 |
| Trace elements (mg kg−1) | ||||||
| Copper | 1.74 ± 0.11 | 1.47 ± 0.17 | 1.8 ± 0.21 | 1.65 ± 0.2 | ||
| Iron | 17.43 ± 1.58 | 14.28 ± 1.07 | 19.51 ± 1.29 | 16.83 ± 1.15 | 2.8 | 0.1 |
| Manganese | 0.91 ± 0.13 | 0.75 ± 0.07 | 0.87 ± 0.08 | 0.67 ± 0.05 | 1.67 | 0.24 |
| Selenium | 0.95 ± 0.08f | 0.63 ± 0.05g | 0.84 ± 0.04f,g | 0.75 ± 0.04f,g | 6.73 | 0.01 |
| Zinc | 42.28 ± 10.92 | 32.91 ± 0.59 | 38.69 ± 1.3 | 33.46 ± 2.36 | 0.62 | 0.61 |
| Arsenic | 0.21 ± 0.04 | 0.14 ± 0.03 | 0.27 ± 0.03 | 0.14 ± 0.02 | ||
| Boron | NDh | ND | ND | ND | ||
| Aluminum | ND | ND | ND | 0.01 | ||
| Mercury | ND | ND | ND | ND | ||
| Lead | ND | ND | ND | ND | ||
| Molybdenum | 0.07 ± 0 | 0.06 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | ||
aValues are means ± standard errors of three replicate groups (n = 3); each replicate involving pooled whole tissues of 5 fish.
bReference: no replacement of fish meal (FM) and fish oil (FO).
cReplacement of 33% of FM with N. oculata and 100% of FO with Schizochytrium sp.
dReplacement of 66% of FM with N. oculata and 100% of FO with Schizochytrium sp.
eReplacement of 100% of FM with N. oculata and 100% of FO with Schizochytrium sp.
f, gMean values not sharing a superscript letter in the same row differ significantly (P < 0.05) from Tukey’s HSD test.
hNot detectable (ND) (< 0.000 µg/g).
Fillet fatty acid (% of total fatty acids) content
The fillet of tilapia fed the experimental diets was similar to the dietary fatty acid content of the corresponding feed. Across diets, the concentrations of total n-3 PUFA, n-6 PUFA, n-3 LC PUFA, and n-6 LC PUFA, were not significantly different (Table 4). We also found that the total saturated fatty acid (SFA), most of the SFA fractions, total mono-unsaturated fatty acids (MUFA), and most MUFA fractions did not differ across diets. Fish fed the reference diet displayed the highest (p < 0.05) concentrations of 16:1n-7 which corresponds to the 16:1n-7 content in experimental diets. In the fillet of fish fed the reference and fish-free feed, we detected similar MUFA fractions of 16:1n-9, 18:1n-7, and 20:1n-9. Total PUFAs were significantly higher (p < 0.05) in tilapia fillet fed microalgae inclusion diets (33NS, 66NS, and 100NS) compared to the reference diet. Many of the individual PUFAs did not vary greatly among dietary treatments. However, n-6 fatty acids, 18:3n-6, 20:3n-6, 22:4n-6, and 22:5n-6 showed significant differences (p < 0.05) between the diets. Among n-3 PUFAs, we detected significantly higher (p < 0.05) 22:6n-3 DHA in tilapia fed microalgae inclusion diets compared to reference diet. The highest EPA content in the reference diet reflected the higher EPA supplied by this diet. The reference diets had the highest concentrations of 20:3n-6, and 22:4n-6 compare to the three other treatments. In contrast, tilapia fed the reference diet had significantly (p < 0.05) decreased concentrations of 22:5n-6 compared to fish fed microalgae inclusion diets. The n-3/n-6 PUFA ratios did not differ significantly between all four dietary treatments. The n-3/n-6 LC PUFA ratio was highest in the fish-free and reference diets.
Table 4.
Fatty acid content of fillets from Nile tilapia after 156 days on the experimental diets.
| Fillet (% TFA)a | Referenceb | 33NSc | 66NSd | 100NSe | F value | P value |
|---|---|---|---|---|---|---|
| 14:00 | 3.75 ± 0.09 | 2.8 ± 0.06 | 3.57 ± 0.53 | 4.3 ± 0.47 | 2.96 | 0.09 |
| 15:00 | 0.39 ± 0.02 | 0.26 ± 0.01 | 0.36 ± 0.07 | 0.38 ± 0.05 | 1.55 | 0.27 |
| 16:00 | 19.71 ± 0.38f,g | 16.38 ± 0.12g | 22.32 ± 1.84f,g | 23.8 ± 2.03f | 5.49 | 0.02 |
| 17:00 | 0.45 ± 0.04f | 0.26 ± 0.02g | 0.36 ± 0.03f,g | 0.31 ± 0.03g | 6.74 | 0.01 |
| 18:00 | 7.2 ± 0.06f | 5.26 ± 0.08g | 7.43 ± 0.42f | 6 ± 0.35f,g | 13.38 | 0 |
| 20:00 | 0.36 ± 0.03 | 0.26 ± 0.01 | 0.31 ± 0.04 | 0.32 ± 0.02 | 2.73 | 0.11 |
| 22:00 | 0.14 ± 0.07 | 0.08 ± 0.04 | 0.14 ± 0.02 | 0.12 ± 0 | 0.49 | 0.69 |
| 24:00 | 0.2 ± 0.05 | 0.15 ± 0.02 | 0.25 ± 0.06 | 0.09 ± 0.05 | 1.18 | 0.57 |
| Total SFAi | 32.2 ± 2.41 | 25.45 ± 2 | 34.74 ± 2.72 | 35.32 ± 2.88 | 2.01 | 0.19 |
| 16:1n-9 | 0.53 ± 0.05f | 0.34 ± 0.01g | 0.39 ± 0.04f | 0.42 ± 0.03f | 5.3 | 0.02 |
| 16:1n-7 | 3.93 ± 0.05f | 1.64 ± 0.03h | 2.2 ± 0.44f,g,h | 3.4 ± 0.5f,g | 9.99 | 0 |
| 18:1n-9 | 19.03 ± 0.39 | 12.91 ± 0.08 | 16.3 ± 2.11 | 18.06 ± 1.98 | 3.41 | 0.07 |
| 18:1n-7 | 3.15 ± 0.13f | 1.82 ± 0.04g | 2.51 ± 0.18f | 2.65 ± 0.21f | 12.34 | 0 |
| 20:1n-9 | 1.23 ± 0.07f | 0.67 ± 0.02g | 0.83 ± 0.11f,g | 0.92 ± 0.08f | 9.88 | 0 |
| 20:1n-7 | 0.04 ± 0.04 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1 | 0.44 |
| 22:1n-11 | 0.19 ± 0.11 | 0.02 ± 0.02 | 0.21 ± 0.04 | 0.14 ± 0.08 | ||
| 22:1n-9 | 0.05 ± 0.05 | 0.04 ± 0.04 | 0.23 ± 0.14 | 0.14 ± 0.14 | 0.74 | 0.55 |
| 24:1n-9 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 2.29 | 0.155 |
| Total MUFAj | 28.15 ± 2.29 | 17.44 ± 1.56 | 22.67 ± 1.96 | 25.73 ± 2.18 | 3.6 | 0.06 |
| 18:2n-6 | 13.13 ± 0.22 | 9.66 ± 0.14 | 10.95 ± 0.51 | 11.61 ± 0.51 | ||
| 18:3n-6 | 0.52 ± 0.09f | 0.16 ± 0.02g | 0.18 ± 0.03f,g | 0.21 ± 0.06f | 9.27 | 0 |
| 20:2n-6 | 0.72 ± 0.07 | 0.59 ± 0.03 | 0.68 ± 0.05 | 0.62 ± 0.11 | 0.68 | 0.58 |
| 20:3n-6 | 0.96 ± 0.01f | 0.43 ± 0.02g | 0.51 ± 0.05g | 0.5 ± 0.09g | 22.31 | 0 |
| 20:4n-6 ARAk | 2.92 ± 0.15 | 1.8 ± 0.03 | 2.53 ± 0.65 | 1.68 ± 0.3 | 2.62 | 0.12 |
| 22:4n-6 | 0.9 ± 0.04f | 0.38 ± 0.02g | 0.43 ± 0.11g | 0.42 ± 0.13g | 7.8 | 0 |
| 22:5n-6 | 1.22 ± 0.02g | 4.98 ± 0.13f | 6.15 ± 1.64f | 4.79 ± 1.19f | 4.4 | 0.04 |
| Total n-6 PUFAl | 20.37 ± 1.73 | 18 ± 1.34 | 21.43 ± 1.54 | 19.83 ± 1.58 | 0.15 | 0.92 |
| 18:3n-3 ALAm | 0.73 ± 0.07 | 0.52 ± 0.02 | 0.5 ± 0.05 | 0.58 ± 0.07 | 3.54 | 0 |
| 18:4n-3 | 0.22 ± 0.02 | 0 ± 0 | 0.0 ± 0.0 | 0 ± 0 | ||
| 20:3n-3 | 0.16 ± 0 | 0.1 ± 0.01 | 0.07 ± 0.04 | 0.18 ± 0.04 | 3.12 | 0.08 |
| 20:4n-3 | 0.29 ± 0.02f | 0.12 ± 0.01f,g | 0.08 ± 0.04g | 0.12 ± 0.06f,g | 5.38 | 0.02 |
| 20:5n-3 EPAn | 1.47 ± 0.27f | 0.33 ± 0.02† | 0.46 ± 0.1g | 0.35 ± 0.08g | 13.63 | 0.02 |
| 22:5n-3 | 2.92 ± 0.11 | 0.77 ± 0.02 | 0.94 ± 0.2 | 1.05 ± 0.24 | ||
| 22:6n-3 DHAo | 7.02 ± 0.47g | 10.17 ± 0.15f | 11.99 ± 2.7f | 10.59 ± 2.7f | 36.45 | 0 |
| Total n-3 PUFAp | 12.81 ± 0.94 | 12.01 ± 1.41 | 14.07 ± 1.67 | 12.87 ± 1.46 | 8 | 0 |
| Total PUFA | 33.18 ± 2.67g | 30.01 ± 2.75f | 35.5 ± 3.21f | 32.7 ± 3.04f | 0.86 | 0.5 |
| Total n-6 LCPUFAq | 6.72 ± 0.29 | 8.18 ± 0.23 | 10.3 ± 2.5 | 8.01 ± 1.82 | 0.94 | 0.46 |
| Total n-3 LCPUFAr | 11.86 ± 0.87 | 11.49 ± 0.21 | 13.54 ± 3.08 | 12.29 ± 3.12 | 0.16 | 0.91 |
| n-3/n-6 PUFA ratios | 0.63 ± 0.54 | 0.67 ± 1.05 | 0.66 ± 1.08 | 0.65 ± 0.92 | 0.07 | 0.97 |
| n-3/n-6 LCPUFA ratio | 1.76 ± 3f | 1.4 ± 0.91g | 1.31 ± 1.23g | 1.53 ± 1.71f | 8.41 | 0 |
aTotal fatty acids (TFA) (%); mean ± standard error for 3 replicates per diet (pooled whole tissues of 5 fish/replicate).
bReference: no replacement of fish meal (FM) and fish oil (FO).
cReplacement of 33% of FM with N. oculata and 100% of FO with Schizochytrium sp.
dReplacement of 66% of FM with N. oculata and 100% of FO with Schizochytrium sp.
eReplacement of 100% of FM with N. oculata and 100% of FO with Schizochytrium sp.
f,g,hMean values not sharing a superscript letter in the same row differ significantly (P < 0.05) from Tukey’s HSD test.
iSaturated fatty acids (SFA) is the sum of all fatty acids without double bonds.
jMonounsaturated fatty acids (MUFA) is the sum of all fatty acids with a single bond.
kArachidonic acid (ARA).
lOmega-6 (n-6) Polyunsaturated fatty acids (PUFAs) (sum of all fatty acids with ≥ 2 double bonds (18:2, 18:3, 20:2, 20:3, 20:4, 22:4, 22:5).
mAlpha-linolenic acid (ALA).
nEicosapentaenoic acid (EPA).
oDocosahexaenoic acid (DHA).
pOmega-3 (n-3) PUFAs (18:3, 18:4, 20:3, 20:4, 20:5, 22:5, 22:6).
qn-6 long-chain (LC) PUFA (20:2, 20:3, 20:4, 22:4, 22:5).
rn-3 LCPUFA(20:3, 20:4, 20:5, 22:5, 22:6).
sRatio calculated for total n-3 PUFA: total n-6 PUFA (n-3/n-6).
Amounts of major n-3 and n-6 PUFA (mg/g) in the fillet
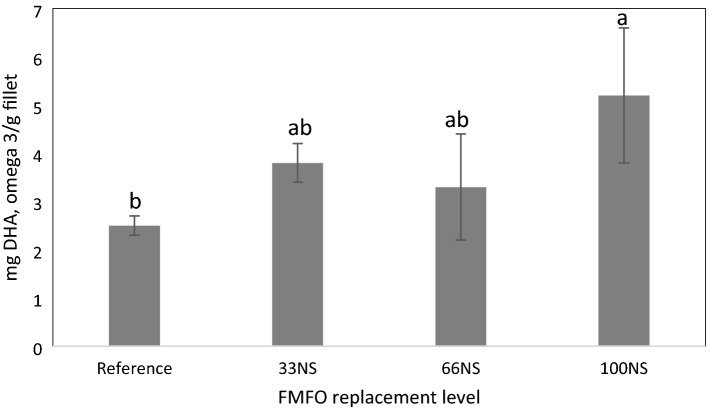
The amount of n-3 PUFAs, EPA and DHA did differ among diets (Supplementary Table S11). All diets that combined Schizochytrium with N. oculata defatted biomass enhanced the DHA deposition in the fillet. Tilapia fed fish-free feed, 100NS diet deposited a significantly higher (p < 0.05) amount of DHA (5.15 mg/g) than fish fed the reference diet which deposited DHA at 2.47 mg/g (Fig. 1). The EPA content of fish fed the reference diet was significantly higher (p < 0.05) compared to the other three diets and reflected the higher EPA supplied by this diet. The amounts of major n-6 PUFA deposition in the fish fillet (mg/g fillet) were not significantly different among diets.
Figure 1.
Docosahexaenoic acid (DHA), a key omega-3 fatty acid for human health, content in fish fillets fish fed the reference feed and three experiment diets. The experimental diets include a replacement of fishmeal (FM) with defatted biomass of N. oculata (N) to replace 33%, 66% or 100% of FM; and whole cell Schizochytrium sp. (S) to replace 100% of fish oil. Values are the mean of 3 replicates with pooled whole tissues of 5 fish per replicate. Values across the bars not sharing a common superscript were significantly different as determined by Tukey’s HSD test, P < 0.05. The error bars represent the standard error of the mean.
Degree of protein hydrolysis and in-vitro protein digestibility
We detected the highest degree of protein hydrolysis and in-vitro protein digestibility in the fish-free feed (100NS), although the difference was not statistically significant compared to the reference feed (Table 5).
Table 5.
Degree of protein hydrolysis and of in vitro protein digestibility of experimental feeds.
| Referenceb | 33NSc | 66NSd | 100NSe | |
|---|---|---|---|---|
| DH %f | 4.29 ± 0.3g,h | 3 ± 0.62g,h | 2.25 ± 1.06h | 5.64 ± 0.54g |
| IPD %i | 85.3 ± 0.9g,h | 80.8 ± 2.2g,h | 78.1 ± 3.7h | 87.7 ± 2.6g |
aMean ± standard error for 3 replicates per diet.
bReference: no replacement of fish meal (FM) and fish oil (FO).
cReplacement of 33% of FM with N. oculata and 100% of FO with Schizochytrium sp.
dReplacement of 66% of FM with N. oculata and 100% of FO with Schizochytrium sp.
eReplacement of 100% of FM with N. oculata and 100% of FO with Schizochytrium sp.
fDegree of protein hydrolysis (DH).
g,hMean values across the row not sharing a common superscript were significantly different P < 0.05 from Tukey’s HSD test.
iIn-vitro protein digestibility (IPD)43.
Economic analysis of fish-free feed formulated with microalgae blends
Here, we compared the estimated ingredient prices, the formulated feed prices and the ECR across experimental diets formulated with microalgae blends and the reference diet.
Results of the hedonic regression analysis show that the median price [and 95% confidence interval] is $0.44 [0.39, 0.49] and $2.38 [1.93, 2.57] per kg biomass for defatted N. oculata and whole cell Schizochytrium sp., respectively (Supplementary Table S5). While the median price of soybean meal is modestly greater (1.07 times) than the median price of defatted N. oculata, the median price of FM is nearly 3.5 times the median price of defatted N. oculata. In contrast to defatted N. oculata being much cheaper than FM, the median price of whole cell Schizochytrium sp. is roughly 1.4 times the median price of FO. Owing to this greater price of Schizochytrium sp. compared with FO, the median [and 95% confidence interval] price of the fish-free feed that combined defatted N. oculata meal with whole cell Schizochytrium sp. (100NS), at $0.68 [0.62, 0.73] per kg feed, was slightly greater than the reference diet at $0.64 [0.61, 0.68] per kg feed (Table 6).
Table 6.
Formulated feed cost, feed conversion ratio, and economic conversion ratio of tilapia production.
| Scenario | Formulated feed costa | Feed conversion ratiob | Economic conversion ratioa |
|---|---|---|---|
| ($/kg feed) | ($/kg tilapia) | ||
| Referencec | 0.64 [0.61, 0.68] | 1.61 ± 0.1 | 1.03 [0.95, 1.13]d,e |
| 33NSf | 0.72 [0.66, 0.78] | 1.57 ± 0.1 | 1.14[1.00, 1.27]e |
| 66NSg | 0.70 [0.64, 0.76] | 1.60 ± 0.1 | 1.12 [0.99, 1.25]e |
| 100NSh | 0.68 [0.62, 0.73] | 1.40 ± 0.1 | 0.95 [0.82, 1.07]d |
| F value | 3.00 | 13.49 | |
| P value | 0.09 | 0.002 |
aMedian [and 95% confidence interval].
bMean ± standard error for 3 replicates per diet.
cReference: no replacement of fish meal (FM) and fish oil (FO).
d,eMedian values throughout the column not sharing a common superscript were significantly different as determined by Tukey’s HSD test, P < 0.05.
fReplacement of 33% of FM with N. oculata and 100% of FO with Schizochytrium sp.
gReplacement of 66% of FM with N. oculata and 100% of FO with Schizochytrium sp.
hReplacement of 100% of FM with N. oculata and 100% of FO with Schizochytrium sp.
The ECR, defined as the price of the formulated feed in US dollars per kg tilapia weight gain, of the fish-free feed was smaller than ECR of the reference diet (Fig. 2 and Table 6), despite the slightly greater price of the fish-free feed (100NS) compared to reference diet. We detected significant differences (p < 0.05) in ECR across all diets. While not significantly different, the ECR of the fish-free feed (100NS) at $0.95 [0.90, 0.98]/kg tilapia was roughly 92% the ECR of the reference diet ($1.03 [1.00, 1.07]/kg tilapia) (Fig. 2). This can be explained by the smaller FCR of the fish-free feed (1.40 ± 0.06) compared with reference diet (1.61 ± 0.05).
Discussion
Our results demonstrate the feasibility of combining commercially available microalgal biomasses to formulate fish-free aquaculture feeds that are high-performing and show potential to become cost-competitive. This is the first report of successfully combining protein-rich-defatted biomass of one microalgal species with DHA-rich whole-cell biomass of another microalgal species to achieve full replacement of FM and FO ingredients in a tilapia feed formulation. This also is the first report of improved feed utilization metrics, including growth, weight gain, specific growth rate, and of beneficial DHA fatty acid profile in Nile tilapia fed a fish-free microalgal diet compared to a commercial feed formulation containing FM and FO. Production is increasing for both types of microalgal biomass used in the fish-free diet, indicating good potential to achieve economies of scale. Our estimate of the ECR for the fish-free diet supports the proposition that biomass from these microalgae will inevitably become cost competitive with FM and FO commodities.
Nutritional benefit of combining N. oculata defatted biomass and Schizochytrium in the fish-free diet
The combination of Schizochytrium sp. and defatted biomass of N. oculata in the fish-free feed exhibited two major benefits. First, fish fed the fish-free feed had improved growth consistent with our prior observations that Schizochytrium sp. is a highly digestible ingredient for tilapia33 and that elevated levels of Schizochytirum sp. led to improved growth, FCR, and PER30. Second, we found the highest in-vitro protein digestibility in the fish-free feed, suggesting that protein originating from defatted N. oculata biomass was the most digestible when in the presence of highly digestible Schizochytrium sp., presumably due to the latter triggering certain digestive enzymes, release and activity. Thus, the combination of defatted N. oculata biomass and Schizochytrium sp. appears to be better suited to the digestive enzymes present in tilapia digestive systems than conventional diets with FMFO; and the presence of Schizochytrium sp. may support more efficient digestion of the fish free-feed at the higher inclusion levels of N. oculata defatted biomass. However, further research is necessary to elucidate the digestive enzyme profiles present under different dietary regimes and to assess the differences in the digestibility of microalgal fish-free feeds compared to conventional feed with FMFO.
Other studies also point to benefits of including Schizochytrium in aquafeeds. Our prior study reported better digestibility, improved growth, fillet protein, and lipid content by Nile tilapia fed diets with inclusion of Schizochytrium in fish-free feed. Similar results were reported in a study that found dietary inclusion of Schizochytrium sp. stimulated muscle or tissue development of Atlantic salmon53. Our observations of beneficial effects of including Schizochytrium in fish-free feed on the growth of tilapia is also consistent with findings in shrimp and barramundi, which demonstrated an algal derived DHA stimulated growth performance54,55. Moreover, high levels of a micronutrient, such as the carotenoid, astaxanthin, and bioactive compounds, in DHA-rich Schizochytrium could contribute to the growth of fish30,55.
Significantly lower weight gain of tilapia fed the reference feed compared to fish-free feed also seems consistent with the fact the FM and FO in reference diet had limited dietary 22:6n-3 DHA. This would cause increased energy expenditure for de novo DHA biosynthesis, given that DHA biosynthesis is a rather expensive metabolic exercise. Such diversion of energy to DHA biosynthesis would reduce the growth performance of tilapia.
The human health benefit of using highly digestible 22:6n-3 DHA-rich Schizochytrium is reflected in this study, given that tilapia fed the fish-free feed yielded the highest amount of 22:6n-3 DHA in fillet—almost twice that of conventional feed (Supplementary Table S6). Results are consistent with our previous findings where increasing levels of Schizochytrium sp. corresponded to reduced levels of FO in tilapia feed and resulted in significant increases in fillet 22:6n-3 DHA deposition compared to a reference diet containing FMFO30.
Nile tilapia is not an oily fish like salmon, but nevertheless deserves efforts to improve nutritional value of farmed fish because it is produced in huge tonnages and is an important component of human diets in many parts of the world, especially Asia and Africa. Thus, improvement of tilapia nutritional value through increased levels of DHA could benefit a very large number of people, many of whom have low levels of n-3 LC-PUFA in their diets23. Our results support the relative ease of enhancing the n-3 LC PUFA composition of tilapia fillets, while also achieving a fish-free diet, by combining Schizochytrium sp. and N. oculata defatted biomass. Tilapia with elevated DHA levels after eating fish-free feed will have tremendous market potential56. Feed manufacturers can exploit this feature to market aquafeeds to aquaculturists aiming to cater to health-conscious consumers who are willing to pay a premium for DHA-enhanced tilapia fillets. Tilapia fed reference feed exhibited significantly increased amounts of 20:5n-3 EPA compared to microalgae-inclusion diets due to a higher concentration of 20:5n-3 EPA in the reference diet. Our results on fillet deposition of ALA, EPA and DHA can be explained by prior research and the relative abundance of these fatty acids in Schizochytrium sp.
Impacts of fish-free diet on macrominerals and trace elements
The literature has little data on the elemental composition of microalgae; and we found that most of the essential macrominerals and trace elements were at higher levels in N. oculata defatted biomass and Scizochytrium sp whole cells (Table 3) than in conventional terrestrial feed ingredients57. We found higher levels for most macrominerals in the Scizochytrium sp whole cells than N. oculata defatted biomass, and higher levels of trace elements in the N. oculata defatted biomass than in Scizochytrium sp whole cells (Table 3). Depositions of macrominerals and several trace elements in tilapia fillet were not significantly different among all dietary treatments (Table 3). We found non-detectable levels of boron, mercury, and lead in tilapia fillets across all diets. Moreover, most of the trace element concentration in fillet was lower than the concentration of all experimental diets. We previously suggested that these trace elements may be excreted and less absorbed by Nile tilapia58,59. We detected the lowest level (0.03 mg kg−1) of total arsenic in the fish-free microalgae feeds and the highest level (0.33 mg kg−1) in reference feed (Supplementary Table S7). However, the level of total arsenic in all the diets (0.03–0.33 mg kg−1) including reference feed was below the European Union level of 10 mg kg−1 set for in aquaculture feed60. High levels of arsenic have been previously reported in FOs, thus contributing considerably to higher arsenic levels in commercial aquaculture feeds61–63. The level of total arsenic in the fillet of tilapia did not differ across the diets (Table 3), and the levels were in the range between 0.14-0.21 mg kg−1 lower than reported values in Atlantic salmon fillet (0.3–1.1 mg kg−1)64.
Feed conversion ratio (FCR) considerations
FCR is a key driver of farming efficiency, economic and environmental performance. Improving the FCR of farmed tilapia through improved feed technology would help increase the cost effectiveness of fish-free diets. Tilapia farming can further reduce the FCR close to 1:1 by a variety of means including better feed formulations using highly digestible feed ingredients, use of appropriate pellet size for each life stage, and better on-farm feed management practices (e.g., storage and feeding rates). Extruded sinking pelleted feed could improve overall FCR; moreover, extrusion or enzymatic processing of under-utilized, defatted biomass of microalgae, such as N. oculata used in this study, could further improve the FCR of fish-free feed, and also help push feed formulated with microalgae towards being cost-competitive with conventional feed17,65.
Economic analysis of fish-free feed formulated with microalgae blends
Our estimate of the market price of defatted N. oculata meal is in good agreement with another study that used hedonic methods to estimate the of market price of defatted N. oculata meal45. However, key differences between our study and the study conducted by Maisashvili et al. is that we used more recent commodity prices (January 2010 to December 2019 instead of January 2005 to December 2012) and the list of commodities used in our analysis are more representative of tilapia feed ingredients instead of ingredients for carnivorous fish and shrimp feed. With respect to whole cell Schizochytrium sp., we are unaware of other studies using hedonic methods to estimate the implied market price of this ingredient. Nevertheless, our implied price results for whole cell Schizochytrium sp. are in general agreement with studies that have used alternative methods66,67.
The similar estimated costs of the fish-free feed (100NS) and reference diet suggest that using combinations of microalgal biomass, that are on track to achieve economies of scale, is a feasible strategy for achieving large-scale production of cost-competitive fish-free diets. An emerging path to economies of scale for the two microalgae used in this study is a biorefinery business model whereby oil rich fractions of the microalgal biomass are marketed as high-value products, such as omega-3 rich human supplements, and other fractions as lower-priced feed ingredients68,69. N. oculata contains an appreciable amount of the omega-3 fatty acid, EPA70. The projected global growth of over 14% in omega-3 fatty acids from microalgae in the near future will result in a large supply of defatted biomass67. Furthermore, the production of Schizochytrium sp., already at commercial-scale, is also anticipated to grow, as the projected compound annual growth rate of DHA from microalgae sources is expected to exceed 10% in the near future67.
In order for such high-performing fish-free feed for tilapia to succeed in the market, we acknowledge that Schizochytrium sp. needs to become cost-competitive with FO sources for aquaculture feeds. Analysts predict ongoing technological improvements and R&D efforts to produce Schizochytrium sp. will quickly make it a cost competitive substitute for FO due to lower production costs and higher market availability71,72. FO substitutes with Schizochytrium sp have emerged within the last year with new products from many agribusiness giants and animal nutrition companies (Corbion, BioMar, Archer Daniels Midland and Veramaris), presumably due to favorable economics and high production volumes. A commercial producer of Schizochytrium oil, Veramis, recently joined a global challenge to sell the most “fish-free” oil for aquafeed to reduce demand pressures on wild-caught stocks, the fish-free feed (F3) FO Challenge73. Alternative feed ingredients like natural marine algal oil have also recently been approved for use in the supply chain by the UK retailer, Tesco74. Given the proliferation of alternative feed ingredients by global industry leaders and stakeholders (aquafeed company, innovators, aquafarmers, investors, and aquaculture supply chain), market opportunities appear to be growing and evolving for using microalgal protein and oil for fish-free feed75,76.
Conclusion
Our results provide a framework for the development of fish-free feeds and the first evidence of a high performing feed for tilapia that combines two different marine microalgae. Defatted marine microalgae, a protein-rich biomass left over after extracting oil for other products, is currently under-utilized (often creating disposal problems even though it is food-grade), and is increasingly available as the algal-oil nutraceutical market grows. Advancing the use of microalgal defatted biomass in aquafeeds would improve the sustainability of aquaculture by reducing its reliance on FM extracted from forage fisheries. Combining under-utilized defatted biomass protein with DHA-rich marine microalga in the fish-free feed resulted in better tilapia growth compared with fish fed a conventional diet containing FMFO. Furthermore, tilapia fed the fish-free feed yielded the highest amount of DHA in the fillet, almost twice higher than in those fed conventional feed. Thus, feeding a DHA-rich, microalgae blended diet to farmed tilapia is a practical way to improve human health benefits of eating farmed tilapia. Moreover, these results suggest other kinds of microalgae combinations are possible and worthy of future investigation. Our fish-free formulation also shows potential cost-competitiveness, given that the ECR of the fish-free diet was slightly lower, though not significantly different, than the reference diet. The microalgal ingredients in our fish-free feed, thus, show potential to supply the expanding aquaculture industry with a stable and affordable supply of healthy protein and oil for fish-free feed, doing so without causing harm to oceans or food security of resource-poor people.
Supplementary information
Acknowledgements
Funding for this work was provided by Agriculture and Food Research Initiative Competitive Grant no. 2016-67015-24619 from the USDA NIFA (to PKS); Dartmouth College from the Sherman Fairchild Professorship (to ARK), Dean of the Faculty, and Vranos family gift; University of California Santa Cruz, Dean of Social Sciences and Executive Vice Chancellor; and the National Sea Grant Aquaculture Federal Funding Opportunity, Social, Behavioral and Economic Research Needs in Aquaculture (NOAA-OAR-SG-2019-2005953). We thank Qualitas Health, Inc. for donating under-utilized Nannochloropsis oculata defatted biomass for this research. We thank the Department of Earth Sciences, Dartmouth for conducting analytical chemistry (macro minerals and trace elements).
Author contributions
Conceived and designed the experiments: P.K.S., A.R.K.; performed the experiments: D.S.F., H.M.N., P.K.S.; analyzed the data: P.K.S., B.M., C.G.; contributed reagents/materials/analysis tools: P.K.S., D.S.F., B.M., C.G.; wrote- original draft: P.K.S.; wrote- review and editing: P.K.S., A.R.K., B.M.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-75289-x.
References
- 1.FAO. The State of World Fisheries and Aquaculture 2018: Meeting the Sustainable Development Goals. (Food and Agriculture Organization (FAO) of the United Nations, 2018).
- 2.FAO. The State of World Fisheries and Aquaculture 2016: Contributing to Food Security and Nutrition for All. (Food and Agriculture Organization (FAO) of the United Nations, 2016).
- 3.Global Market Insights. Aquafeed Market Size by Application (Carp, Mollusks, Salmon, Crustaceans, Tilapia, Catfish) & Aquaculture Additives Market Size by Product (Amino Acids, Antibiotics, Vitamins, Feed Acidifiers), Competitive Analysis & Forecast, 2012–2022. 102 (2016).
- 4.Ekmekci H, Gül M. Economic structure and problems of trout enterprises: A case of fethiye. Turk. J. Agric. Food Sci. Technol. 2017;5:33–42. [Google Scholar]
- 5.Arru B, Furesi R, Gasco L, Madau F, Pulina P. The introduction of insect meal into fish diet: The first economic analysis on european sea bass farming. Sustainability. 2019;11:1697. doi: 10.3390/su11061697. [DOI] [Google Scholar]
- 6.Tacon, A. G. J., Hasan, M. R. & Metian, M. Demand and Supply of Feed Ingredients for Farmed Fish and Crustaceans: Trends and Prospects (Food and Agriculture Organization (FAO) of the United Nations, 2011).
- 7.Hasan M.R. Feeding global aquaculture growth. FAO Aquaculture Newsletter ii–iii (2017).
- 8.Hasan, M. R. Keynote presentation: Status of world aquaculture and global aquafeed requirement with special notes on Artemia. In Report of the FAO Expert Workshop on Sustainable Use and Management of Artemia Resources in Asia, Appendix 4, 16–17 (2016).
- 9.Pikitch EK, et al. The global contribution of forage fish to marine fisheries and ecosystems. Fish Fish. 2014;15:43–64. doi: 10.1111/faf.12004. [DOI] [Google Scholar]
- 10.Pauly D, Zeller D. Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat. Commun. 2016;7:10244–10244. doi: 10.1038/ncomms10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Checkley DM, Asch RG, Rykaczewski RRC. Climate, anchovy, and sardine. Ann. Rev. Mar. Sci. 2017;9:469–493. doi: 10.1146/annurev-marine-122414-033819. [DOI] [PubMed] [Google Scholar]
- 12.Klinger D, Naylor R. Searching for solutions in aquaculture: Charting a sustainable course. Annu. Rev. Environ. Resour. 2012;37:247–276. doi: 10.1146/annurev-environ-021111-161531. [DOI] [Google Scholar]
- 13.Chauton MS, Reitan KI, Norsker NH, Tveterås R, Kleivdal HT. A techno-economic analysis of industrial production of marine microalgae as a source of EPA and DHA-rich raw material for aquafeed: Research challenges and possibilities. Aquaculture. 2015;436:95–103. doi: 10.1016/j.aquaculture.2014.10.038. [DOI] [Google Scholar]
- 14.Cottrell RS, Blanchard JL, Halpern BS, Metian M, Froehlich HE. Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030. Nat. Food. 2020;1:301–308. doi: 10.1038/s43016-020-0078-x. [DOI] [Google Scholar]
- 15.Cashion T, Manach FL, Zeller D, Pauly D. Most fish destined for fishmeal production are food-grade fish. Fish Fish. 2017;18:837–844. doi: 10.1111/faf.12209. [DOI] [Google Scholar]
- 16.Li P, Mai K, Trushenski J, Wu G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids. 2009;37:43–53. doi: 10.1007/s00726-008-0171-1. [DOI] [PubMed] [Google Scholar]
- 17.Sarker PK. Towards sustainable aquafeeds: Evaluating substitution of fishmeal with lipid-extracted microalgal co-product (Nannochloropsis oculata) in diets of juvenile Nile tilapia (Oreochromis niloticus) PLoS ONE. 2018;13:0201315. doi: 10.1371/journal.pone.0201315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yones AM, El-Saidy DMSD, Abdel-Hakim NF. Effects of fish oil substitution with vegetable oils in diets of juvenile Nile tilapia, Oreochromis niloticus (L.) on growth performance, nutrients utilization and muscle fatty acids contents. Merit. Res. J. Food Sci. Technol. 2013;1:9–018. [Google Scholar]
- 19.Tacon AGJ. Trends in global aquaculture and aquafeed production: 2000–2017. Rev. Fish. Sci. Aquac. 2020;28:43–56. doi: 10.1080/23308249.2019.1649634. [DOI] [Google Scholar]
- 20.He J-Y, et al. Methionine and lysine requirements for maintenance and efficiency of utilization for growth of two sizes of tilapia (Oreochromis niloticus) Aquac. Nutr. 2013;19:629–640. doi: 10.1111/anu.12012. [DOI] [Google Scholar]
- 21.Turchini G, Torstensen B, Ng W-K. Fish oil replacement in finfish nutrition. Rev. Aquac. 2009;1:10–57. doi: 10.1111/j.1753-5131.2008.01001.x. [DOI] [Google Scholar]
- 22.Sarker PK, et al. Sustainability issues related to feeding salmonids: A Canadian perspective. Rev. Aquac. 2013;5:199–219. doi: 10.1111/raq.12013. [DOI] [Google Scholar]
- 23.Karapanagiotidis IT, Bell MV, Little DC, Yakupitiyage A, Rakshit SK. Polyunsaturated fatty acid content of wild and farmed Tilapias in Thailand: Effect of aquaculture practices and implications for human nutrition. J. Agric. Food Chem. 2006;54:4304–4310. doi: 10.1021/jf0581877. [DOI] [PubMed] [Google Scholar]
- 24.Teoh C-Y, Turchini GM, Ng W-K. Genetically improved farmed Nile tilapia and red hybrid tilapia showed differences in fatty acid metabolism when fed diets with added fish oil or a vegetable oil blend. Aquaculture. 2011;312:126–136. doi: 10.1016/j.aquaculture.2010.12.018. [DOI] [Google Scholar]
- 25.Ng W-K, Lim P-K, Sidek H. The influence of a dietary lipid source on growth, muscle fatty acid composition and erythrocyte osmotic fragility of hybrid tilapia. Fish Physiol. Biochem. 2001;25:301–310. doi: 10.1023/A:1023271901111. [DOI] [Google Scholar]
- 26.Simopoulos, A. P. The omega-6/omega-3 fatty acid ratio, genetic variation, and cardiovascular disease. Asia Pac. J. Clin. Nutr.17(Suppl 1), 131–134 (2008). [PubMed]
- 27.Weaver KL, et al. The content of favorable and unfavorable polyunsaturated fatty acids found in commonly eaten fish. J. Am. Diet. Assoc. 2008;108:1178–1185. doi: 10.1016/j.jada.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Alam MdA, et al. Evaluation of antioxidant compounds, antioxidant activities, and mineral composition of 13 collected purslane (Portulaca oleracea L.) accessions. BioMed Res. Int. 2014;2014:1–10. doi: 10.1155/2014/296063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bélanger-Lamonde A, et al. Algal and vegetable oils as sustainable fish oil substitutes in rainbow trout diets: An approach to reduce contaminant exposure. J. Food Qual. 2018;2018:1–12. doi: 10.1155/2018/7949782. [DOI] [Google Scholar]
- 30.Sarker PK, et al. Towards sustainable aquafeeds: Complete substitution of fish oil with marine microalga Schizochytrium sp. improves growth and fatty acid deposition in juvenile Nile tilapia (Oreochromis niloticus) PLoS ONE. 2016;11:0156684. doi: 10.1371/journal.pone.0156684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker AB, Berlinsky DL. Effects of partial replacement of fish meal protein by microalgae on growth, feed intake, and body composition of Atlantic cod. N.orth Am. J. Aquac. 2011;73:76–83. [Google Scholar]
- 32.Tibaldi E, et al. Growth performance and quality traits of European sea bass (D. labrax) fed diets including increasing levels of freeze-dried Isochrysis sp. (T-ISO) biomass as a source of protein and n-3 long chain PUFA in partial substitution of fish derivatives. Aquaculture. 2015;440:60–68. doi: 10.1016/j.aquaculture.2015.02.002. [DOI] [Google Scholar]
- 33.Sarker PK, Gamble MM, Kelson S, Kapuscinski AR. Nile tilapia (Oreochromis niloticus) show high digestibility of lipid and fatty acids from marine Schizochytrium sp. and of protein and essential amino acids from freshwater Spirulina sp. feed ingredients. Aquac. Nutr. 2016;22:109–119. doi: 10.1111/anu.12230. [DOI] [Google Scholar]
- 34.Kiron V, Phromkunthong W, Huntley M, Archibald I, De Scheemaker G. Marine microalgae from biorefinery as a potential feed protein source for Atlantic salmon, common carp and whiteleg shrimp. Aquac. Nutr. 2012;18:521–531. doi: 10.1111/j.1365-2095.2011.00923.x. [DOI] [Google Scholar]
- 35.Gong Y, Guterres HADS, Huntley M, Sørensen M, Kiron V. Digestibility of the defatted microalgae Nannochloropsis sp. and Desmodesmus sp. when fed to Atlantic salmon, Salmo salar. Aquac. Nutr. 2018;24:56–64. doi: 10.1111/anu.12533. [DOI] [Google Scholar]
- 36.Sørensen M. Nannochloropsis oceania-derived defatted meal as an alternative to fishmeal in Atlantic salmon feeds. PLoS ONE. 2017;12:1–20. doi: 10.1371/journal.pone.0179907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tocher DR, Betancor MB, Sprague M, Olsen RE, Napier JA. Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: Bridging the gap between supply and demand. Nutrients. 2019;11:89–89. doi: 10.3390/nu11010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beal CM, et al. Marine microalgae commercial production improves sustainability of global fisheries and aquaculture. Sci. Rep. 2018;8:1–8. doi: 10.1038/s41598-018-33504-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kagan ML, West AL, Zante C, Calder PC. Acute appearance of fatty acids in human plasma—A comparative study between polar-lipid rich oil from the microalgae Nannochloropsis oculata and krill oil in healthy young males. Lipids Health Dis. 2013;12:102. doi: 10.1186/1476-511X-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarker PK, Yossa R, Karanth S, Ekker M, Vandenberg GW. Influences of dietary biotin and avidin on growth, survival, deficiency syndrome and hepatic gene expression of juvenile Nile tilapia Oreochromis niloticus. Fish Physiol. Biochem. 2012;38:1183–1193. doi: 10.1007/s10695-012-9604-6. [DOI] [PubMed] [Google Scholar]
- 41.Yasumaru F, Lemos D. Species specific in vitro protein digestion (pH-stat) for fish: Method development and application for juvenile rainbow trout (Oncorhynchus mykiss) Aquaculture. 2014;426–427:74–84. doi: 10.1016/j.aquaculture.2014.01.012. [DOI] [Google Scholar]
- 42.Chaijaroen T, Thongruang C. Extraction, characterization and activity of digestive enzyme from Nile tilapia (Oreochromis niloticus) viscera waste. Int. Food Res. J. 2016;23:1432–1438. [Google Scholar]
- 43.Tibbetts SM, Yasumaru F, Lemos D. In vitro prediction of digestible protein content of marine microalgae (Nannochloropsis granulata) meals for Pacific white shrimp (Litopenaeus vannamei) and rainbow trout (Oncorhynchus mykiss) Algal Res. 2017;21:76–80. doi: 10.1016/j.algal.2016.11.010. [DOI] [Google Scholar]
- 44.Adler-Nissen J. Enzymatic Hydrolysis of Food Proteins. Amsterdam: Elsevier; 1986. [Google Scholar]
- 45.Maisashvili A, et al. The values of whole algae and lipid extracted algae meal for aquaculture. Algal Res. 2015;9:133–142. doi: 10.1016/j.algal.2015.03.006. [DOI] [Google Scholar]
- 46.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 47.Bates, D. et al. Linear Mixed-Effects Models using ‘Eigen’ and S4. (2020).
- 48.Carew R. A hedonic analysis of apple prices and product quality characteristics in British Columbia. Can. J. Agric. Econ. Can. Agroecon. 2000;48:241–257. doi: 10.1111/j.1744-7976.2000.tb00278.x. [DOI] [Google Scholar]
- 49.Costanigro M, McCluskey JJ, Mittelhammer RC. Segmenting the wine market based on price: Hedonic regression when different prices mean different products. J. Agric. Econ. 2007;58:454–466. doi: 10.1111/j.1477-9552.2007.00118.x. [DOI] [Google Scholar]
- 50.Ao J, Chen J. Price volatility, the maturity effect, and global oil prices: Evidence from Chinese commodity futures markets. J. Econ. Finance. 2020 doi: 10.1007/s12197-019-09497-1. [DOI] [Google Scholar]
- 51.Dubois V, Breton S, Linder M, Fanni J, Parmentier M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur. J. Lipid Sci. Technol. 2007;109:710–732. doi: 10.1002/ejlt.200700040. [DOI] [Google Scholar]
- 52.Piedecausa MA, Mazón MJ, García García B, Hernández MD. Effects of total replacement of fish oil by vegetable oils in the diets of sharpsnout seabream (Diplodus puntazzo) Aquaculture. 2007;263:211–219. doi: 10.1016/j.aquaculture.2006.09.039. [DOI] [Google Scholar]
- 53.Kousoulaki K, Mørkøre T, Nengas I, Berge RK, Sweetman J. Microalgae and organic minerals enhance lipid retention efficiency and fillet quality in Atlantic salmon (Salmo salar L.) Aquaculture. 2016;451:47–57. doi: 10.1016/j.aquaculture.2015.08.027. [DOI] [Google Scholar]
- 54.Glencross BD, Booth M, Allan GL. A feed is only as good as its ingredients—a review of ingredient evaluation strategies for aquaculture feeds. Aquac. Nutr. 2007;13:17–34. doi: 10.1111/j.1365-2095.2007.00450.x. [DOI] [Google Scholar]
- 55.Glencross B, Rutherford N. A determination of the quantitative requirements for docosahexaenoic acid for juvenile barramundi (Lates calcarifer) Aquac. Nutr. 2011;17:e536–e548. doi: 10.1111/j.1365-2095.2010.00795.x. [DOI] [Google Scholar]
- 56.Stoneham TR, et al. Production of omega-3 enriched tilapia through the dietary use of algae meal or fish oil: Improved nutrient value of fillet and offal. PLoS ONE. 2018;13:e0194241. doi: 10.1371/journal.pone.0194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Webster, C. Nutrient requirements and feeding of finfish for aquaculture—CABI.org. 1–27 (2002).
- 58.Norambuena F, et al. Algae in fish feed: Performances and fatty acid metabolism in juvenile atlantic salmon. PLoS ONE. 2015;10:1–17. doi: 10.1371/journal.pone.0124042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sinha, A. K., Kumar, V., Makkar, H. P. S., De Boeck, G., & Becker, K. Non-starch polysaccharides and their role in fish nutrition – A review. Food Chem. 127, 1409–1426 (2011).
- 60.European Unions. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed (2002).
- 61.Sloth JJ, Larsen EH, Julshamn K. Determination of organoarsenic species in marine samples using gradient elution cation exchange HPLC-ICP-MS. J. Anal. At. Spectrom. 2003;18:452–459. doi: 10.1039/b300508a. [DOI] [Google Scholar]
- 62.Sissener NH, et al. Surveillance of selected nutrients, additives and undesirables in commercial Norwegian fish feeds in the years 2000–2010. Aquac. Nutr. 2013;19:555–572. doi: 10.1111/anu.12007. [DOI] [Google Scholar]
- 63.Sele V, et al. Arsenic-containing fatty acids and hydrocarbons in marine oils—determination using reversed-phase HPLC-ICP-MS and HPLC-qTOF-MS. Talanta. 2014;121:89–96. doi: 10.1016/j.talanta.2013.12.049. [DOI] [PubMed] [Google Scholar]
- 64.Biancarosa I. Replacing fish meal with insect meal in the diet of Atlantic salmon (Salmo salar) does not impact the amount of contaminants in the feed and it lowers accumulation of arsenic in the fillet. Food Addit. Contam. Part. Chem. Anal. Control Expo. Risk Assess. 2019;36:1191–1205. doi: 10.1080/19440049.2019.1619938. [DOI] [PubMed] [Google Scholar]
- 65.Hasan, M. R. & Soto, S. Improving Feed Conversion Ratio and Its Impact on Reducing Greenhouse Gas Emissions in Aquaculture. 33–33 (Food and Agriculture Organization of the United Nations, 2017).
- 66.Ratledge, C. & Lippmeier, C. Microbial production of fatty acids. In Fatty Acids 237–278 (Elsevier, Amsterdam, 2017).
- 67.Bose A, O’Shea R, Lin R, Murphy JD. A perspective on novel cascading algal biomethane biorefinery systems. Bioresour. Technol. 2020;304:123027. doi: 10.1016/j.biortech.2020.123027. [DOI] [PubMed] [Google Scholar]
- 68.IEA. State of Technology Review—algae Bioenergy. An IEA Bioenergy Inter-task Strategic Project. (International Energy Agency, Paris, 2017).
- 69.Bryant HL, et al. The value of post-extracted algae residue. Algal Res. 2012;1:185–193. doi: 10.1016/j.algal.2012.06.001. [DOI] [Google Scholar]
- 70.Chua ET, Schenk PM. A biorefinery for Nannochloropsis: Induction, harvesting, and extraction of EPA-rich oil and high-value protein. Bioresour. Technol. 2017;244:1416–1424. doi: 10.1016/j.biortech.2017.05.124. [DOI] [PubMed] [Google Scholar]
- 71.Trushenski JT, Boesenberg J, Kohler CC. Influence of grow-out feed fatty acid composition on finishing success in Nile Tilapia. N. Am. J. Aquac. 2009;71:242–251. doi: 10.1577/A08-051.1. [DOI] [Google Scholar]
- 72.Ruiz J, et al. Towards industrial products from microalgae. Energy Environ. Sci. 2016;9:3036–3043. doi: 10.1039/C6EE01493C. [DOI] [Google Scholar]
- 73.Undercurrent News. Four firms in for ‘fish-free’ fish oil race. Undercurr. News. (2019).
- 74.Undercurrent News. Tesco moves to source salmon fed on alternative feed ingredients. Undercurr. News. (2019).
- 75.Nemo, L. Faux fish might help aquaculture keep feeding the world. Sci. Am. (2019).
- 76.Wright J. Aquafeed Moonshots at the F3 ‘Talent Show’. Portsmouth: Global Aquaculture Alliance; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets and RSTUDIO files used in the economic analysis including the hedonic regression analyses (used to estimate the price of defatted N. oculata meal and whole cell Schizochytrium), bootstrap confidence intervals of feed ingredient prices, and the ECR for Fig. 2 are available at the following link: 10.6071/M3VD5V.
Figure 2.

Economic conversion ratio of the reference (Ref) and experimental diets disaggregated by ingredient. The experimental diets include a replacement of fishmeal (FM) with defatted biomass of N. oculata (N) to replace 33%, 66% or 100% of FM; and whole cell Schizochytrium sp. (S) to replace 100% of fish oil. The error bars represent the 95% confidence interval.