Abstract
Efficient approaches that enable the synthesis of analogs of natural product antibiotics are needed to keep up with the emergence of multiply-resistant strains of pathogenic organisms. One promising candidate in this area is fidaxomicin, which boasts impressive in vitro anti-tubercular activity but has poor systemic bioavailability. We designed a flexible synthetic route to this target to enable the exploration of new chemical space and the future development of analogs with superior pharmacokinetics. We developed a robust approach to each of the key macrocyclic and sugar fragments, their union via stereoselective glycosylation, and a convergent late-stage macrolide formation with fully glycosylated fragments. Although we were able to demonstrate that the final Suzuki cross-coupling and ring-closing metathesis steps enabled macrocycle formation in the presence of the northern resorcylic rhamnoside and southern novioside sugars, these final steps were hampered by poor yields and the formation of the unwanted Z-macrocycle as the major stereoisomer.
Keywords: Lipiarmycin, Tiacumicin, Macrolide antibiotics, Ring closing metathesis
1. Introduction
Fidaxomicin (1) is an FDA approved macrolide antibiotic [1] with modest in vitro efficacy against Gram-positive bacteria and notably potent activity against Clostridium difficile (MIC5o = 31 μg/mL) [2] and Mycobacterium tuberculosis (MIC50 = 15 μg/mL) including drug resistant strains (MIC50 = 8–45 μg/mL) [3]. A recently obtained cryo-EM structure of fidaxomicin bound to RNA-polymerase [4] has provided insight on the binding mode and biochemically important structural features of fidaxomicin.
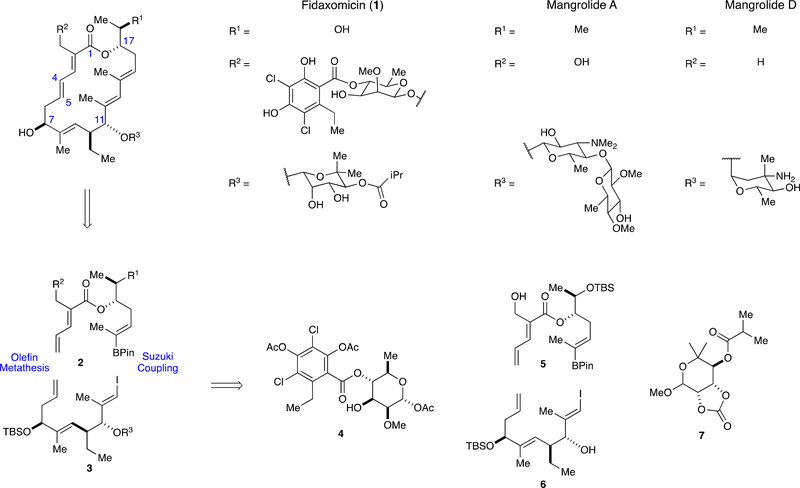
Despite its promising anti-tubercular activity, approved clinical use of fidaxomicin is limited to treatment of C. difficile associated colitis [5]. Unfortunately, fidaxomicin has poor oral bioavailablity and is rapidly cleared following alternative dosing modes [6]. These limitations preclude its use for the systemic treatment of tuberculosis infections. Thus, a fidaxomicin analog with improved bioavailability could represent a new class of antibiotics for the treatment of systemic bacterial infections, including tuberculosis and multidrug-resistant tuberculosis. Semisynthetic approaches have been severely hampered by the instability of the macrolide, which is readily available by fermentation [7], to standard acid mediated glycolysis methods [8]. Several groups have reported the structure and bioactivity of biosynthetically prepared analogs [9]. However, a robust medicinal chemistry program would enable access to a wider variety of structural modifications than is generally possible using bioengineering approaches. In this context, fidaxomicin has gained significant attention as a synthetic target in recent years [10,11]. Four syntheses of the macrolide aglycone and two syntheses of the natural product have been reported since 2015 [12,13]. Additionally, we and others have completed syntheses of the structurally related mangrolides A and D, which share the macrolactone core of fidaxomicin (see Scheme 1) [14]. Here we report our approach to the synthesis of fully glycosylated northern and southern fragments of fidaxomicin, and explore their union via Suzuki cross-coupling and ring-closing metathesis.
Scheme 1.
Structures of fidaxomicin, mangrolide A, and mangrolide D and retrosynthetic strategy towards Fidaxomicin.
2. Results and discussion
Our synthesis was designed to be as convergent and modular as possible to expedite subsequent syntheses of natural product analogs. As outlined in Scheme 1, we envisioned unification of northern and southern fragments 2 and 3 by a sequential Suzuki cross coupling and ring closing metathesis (RCM) sequence. This strategy was adapted from our longstanding work on the mangrolide family [15]. The Gademann group also utilized these disconnections in their synthesis of both fidaxomicin and the mangrolides [12c,14c,14d]. However, where they appended the rhamnoside unit post macrolactone formation, we endeavored to pursue the unification of fully glycosylated northern and southern fragments. This would, in principle, allow us to reduce the number of linear manipulations at the end of the sequence. To that end we needed to develop a β-selective rhamnosylation of northern macrolide fragment 5 with a fully functionalized resorcyl rhamnoside 4. Southern fragment 3 on the other hand, would be accessible via the previously described Koenigs-Knorr glycosylation of southern macrolide fragment 6 with novioside 7 [13a]. Our initial objective was to develop robust, scalable routes towards fragments 4–7 and explore their unification via stereoselective glycosylation.
Our forward synthesis began with the preparation of resorcylated D-rhamnoside donor 4 (Scheme 2A). In an adaption of the procedure reported by Gademann and coworkers for the corresponding anomeric methyl ether, the anomeric position of D-mannose was derivatized as a benzyl ether, followed by masking the 3- and 4-hydroxyl groups as the butanediacetal 8 in 55% yield for the two steps [16]. A Garegg-Samuelsson iodination/hydrogenolysis sequence removed the 6-hydroxyl group to provide rhamnoside 9 in good yield. Methylation of the remaining alcohol proceeded with excellent yield to give 10 and was followed by final cleavage of the butanediacetal under mild hydrolytic conditions to provide up to 9 g of key rhamnoside 11 in a single batch.
Scheme 2.
Synthesis of orsellinic rhamnoside 4. Reagents and conditions: (a) BnOH, HCl; (b) (MeO)3CH, CSA, butanedione, MeOH; then Et3N (55%, 2 steps); (c) I2, PPh3, imidazole, THF (84%); (d) H2, Pd/C, MeOH, Et3N (90%); (e) NaH, Mel, THF (>95%); (f) TFA, DCM, H2O; Et3N (69%); (g) BnOH, PPh3, DIAD, THF; (h) pyridine, Tf2O (13a: 61%, 2 steps; 13b: 65%, 2 steps); (i) Pd(PPh3)4, LiCl, dioxane, Et3N, tributyl(vinyl)tin; (j) NiCl2, NaBH4, MeOH (14a: 66%, 2 steps; 14b: 58%, 2 steps); (k) 14b, 11, 300 nm UV light (15: 37%; 16: 20%); (l) 14a, 11, NaH, THF (15: 31%; 16: 52%); (m) NaH, THF (54%); (n) H2, Pd(OH)2, MeOH; (o) SO2Cl2, EtOAc; (p) Ac2O, DMAP, EtOAc (66%, 3 steps).
We have previously described both base mediated and photochemical methods for the preparation of hindered salicylate and resorcylate esters and amides using dioxinones similar to 14a and 14b [17]. In order to explore these approaches for the introduction of the homoorsellinate residue we prepared both 14a and 14b starting with trihydroxybenzoic acid derivatives 12a and 12b (Scheme 2B). Selective etherification of the para-phenol under Mitsunobu conditions, followed by triflation of the remaining ortho-phenol under standard conditions efficiently provided 13a and 13b. [17c,18] Subsequent Stille cross-coupling with tributyl(vinyl)tin proceeded in excellent yields, and the resultant styrenes could be selectively reduced in the presence of the benzyl ether using a NiCl2/sodium borohydride system [19] to provide 14a or 14b on multigram scale.
Sodium hydride promoted acylation of 11 with 14a proceeded with moderate thermodynamic selectivity for the desired 4-acyl isomer 16 (31% yield of 15, 52% yield of 16, Scheme 2C) [17a,20]. Resubmission of 15 to the sodium hydride acylation conditions provided additional 16. By contrast, photochemical acylation of 11 with 14b proceeded in lower yield, and afforded a kinetic mixture of acylated products 15 and 16 in which the undesired regioisomer 15 dominated (37% and 20% isolated yield respectively) [17b,c]. Using the superior sodium hydride mediated acylation of rhamnoside 11 with 14a, we prepared up to a gram of 16 in one batch. In preparation for glycosylation of the northern fragment (vide infra), intermediate 16 was sequentially debenzylated via hydrogenolysis, chlorinated using sulfuryl chloride, and carefully tris-acetylated to afford the fully functionalized rhamnoside 4 in good overall yield.
Our synthesis of northern macrolide fragment 5 initiates with a stereoselective synthesis of borylated homopropargyl alcohol 19 and tracks with that of previous reports [12a,13a]. As shown in Scheme 3A, Sharpless epoxidation and silylation of 1-butene-3-ol yielded enantiomerically enriched 17 (38% yield for 2 steps) [21]. Epoxide opening with propynyl lithium occurred as expected to afford the desired alkyne 18 in high yield [12a]. We found that regio- and stereoselective borylation of the alkyne was most efficient using conditions developed by the Carretero group [22], providing the needed secondary alcohol 19 with high yield and excellent selectivity on gram scale.
Scheme 3.
Synthesis of northern fragment borylated ester 5. Reagents and conditions: (a) Ti(OiPr)4, d-DIPT, DCM, TBHP, 4 Å MS; (b) TBSCl, imidazole (38%, 2 steps); (c) propyne, nBuLi, BF3-OEt2 (90%); (d) CuCl, PCy3, NaOtBu, B2Pin2, MeOH, PhMe (81%); (e) rac-BINAP, Rh(COD)2OTf, 21, Ph3CCOOH, H2, DCE; then MnO2, DCM (70%); (f) MeOTf, MeCN; then KOTMS, Et2O (82%); (g) 19, Et3N, DMAP, 2,4,6-trichlorobenzoyl chloride, PhMe (60%); (h) Br2, CHCl3 (93%); (i) dihydropyran, PPTS, DCM (84%); (j) TBAF, HMPA (85%, >20:1 dr); (k) Pd2(dba)3, P(2-furyl)3, tributyl(vinyl)tin, THF (93%); (l) LiOH, H2O, THF, EtOH (>95%); (m) 19, Et3N, DMAP, 2,4,6-trichlorobenzoyl chloride, THF; (n) PPTS, EtOH (45%, 2 steps).
To prepare the dienoic acid 23, we adapted the hydrogen mediated C–C coupling method developed by the Krische group (Scheme 3B) [23]. Known enyne 20 [24], available in two steps from propargyl alcohol, was coupled with aldehyde 21 to provide the allylic alcohol with excellent regio- and stereoselectivity for the desired E-diene. The resulting crude alcohol was then oxidized to dienone 22 in 70% yield for the two step sequence. Activation of the N-methyl imidazole with methyl triflate was followed by potassium trimethylsilanolate mediated hydrolysis to generate the dienoic acid 23 in good yield (82%, 2 steps) [25]. It is worth noting that this alternative synthesis of dienoic acid 23 (6 steps, E/Z > 20:1, 49% overall yield) compares favorably to those previously reported for this or related dienoates [12a-c]. Acid 23 was esterified with alcohol 19 using previously described Yamaguchi conditions to generate known bis-silyl ester 24 [13a,14b]. Conditions to selectively cleave the primary TBS group in the context of an intact macrocycle have been reported [8b,13a]. Unfortunately, we were unable to identify conditions to effect a selective deprotection of the primary silyl ether on the borylated ester 24, and therefore pursued the synthesis of dienoic acid 28 according to a protocol adapted from Zhu and coworkers (Scheme 3C) [12b].
In the event, ethyl 2-(hydroxymethyl)acrylate was dibrominated and protected as the THP ether, giving dibromide 25 in excellent yield for the two-step process (78% overall) [26,27]. Stereoselective elimination of the α-bromide with tetrabutylammonium fluoride in HMPA provided acryloyl bromide 26 in 85% yield and excellent stereoselectivity (>20:1 E/Z). Stille coupling of vinyl bromide 26 with tributyl (vinyl)tin yielded dienoate 27 in 93% yield [28]. Using this route, we were able to prepare multigram quantities of 27 in 62% overall yield over four steps from commercially available ethyl 2-(hydroxymethyl)acrylate. Hydrolysis of ester 27 afforded the dienoic acid 28 (>95%), which was esterified as before with alcohol 19. This time, the THP-protected primary alcohol could be selectively removed with ethanolic PPTS to yield the target northern fragment alcohol 5 in 45% yield (2 steps) with no observable degradation of the TBS or BPin functionalities.
We next turned our focus to the preparation of southern fragment 6 (Scheme 4). Our work on mangrolide A disclosed a route to polyketide fragment 6 utilizing a 2,3-Wittig rearrangement [29] to establish the C10–C11 stereochemistry and a late stage bis-functionalization of an alkyne to prepare the required E-vinyl iodide [14b,15]. A related rearrangement has subsequently been utilized by the Roulland group to elegantly set this same stereochemical diad [12d]. As shown in scheme 4A, our route commenced with L-lactate-derived aldehyde 29. Wittig homologation of the aldehyde afforded Z-olefin 30 in 83% yield (Z/E > 20:1). Methanolysis (pTSOH) of the THP-ether was followed by alkylation with propargyl bromide 31 (KOtBu, THF) to afford [2,3]-Wittig precursor 32 in 76% (2 steps). Deprotonation with nBuLi in THF induced a smooth rearrangement to the corresponding alcohol which was trapped in situ with methoxymethyl chloride to provide 33 in 88% yield and with excellent stereoselectivity. The relative stereochemistry was predicted to result from [2,3]-rearrangement occurring via cyclic TTS-A. The stereochemistry was unambiguously assigned via comparison of fragment 6 resulting from this route, to the identical material reported previously by us and others [12c,14a]. Careful selective ozonolysis of the alkene in 33 (Me2S work-up) was followed by homologation of the resultant aldehyde with stabilized ylide 34 to afford E-configured α,β-unsaturated ester 35 in 79% yield (2 steps). To set the stage for subsequent alkyne functionalization, ester 35 was reduced, followed by a TIPS deprotection, and TES protection sequence. The resultant terminal alkyne 36, obtained in 76% for this 3-step sequence, was subjected to a one-pot stannocupration/methylation/iodination to yield the vinyl iodide with simultaneous cleavage of the TES ether in 80% yield [30]. Periodinane mediated oxidation of the primary alcohol gave aldehyde 37. A diastereoselective Brown allylation was followed by TBS protection of the resultant alcohol to afford intermediate 38 in 65% yield and excellent stereoselectivity (>20:1 dr). A final boron trifluoride mediated cleavage of the methoxymethyl ether afforded target secondary alcohol 6 in 77% yield.
Scheme 4.
Two alternative syntheses of southern fragment 6. Reagents and conditions: (a) nPrPPh3Br, nBuLi, THF (83%); (b) pTsOH, MeOH; (c) 31, tBuOK, THF, (76%, 2 steps); (d) nBuLi, THF; then MOMCl (88%); (e) O3, DCM; then Me2S (93%); (f) 34, toluene (85%); (g) DIBAL, DCM; (h) TBAF, THF; (i) TESOTf, lutidine, DCM (76%, 3 steps); (j) CuCN, (Bu3Sn)2, nBuLi, Mel; then I2, THF (80%); (k) DMP, DCM (91%); (l) (–)-Ipc2B-allyl, Et2O; then H2O2, NaOH; (m) TBSOTf, lutidine, DCM (65%, 2 steps); (n) BF3-OEt2, Me2S (77%); (o) [Ir(COD)Cl]2, (R)-Cl-MeO-BIPHEP, Cs2CO3, mNO2BzOH, allyl acetate, THF (57%, >20:1 dr).
During the course of our synthesis of mangrolide D, we developed an alternative approach to 6 utilizing an Oppolzer sultam mediated aldol reaction (Scheme 4B) [12a,14a]. For this work, we exploited primary alcohol 39 to explore a Krische-type iridium catalyzed allylation from the alcohol oxidation state to further streamline this synthetic sequence. Gratifyingly, allylic alcohol 39 directly provided homoallylic alcohol 40 with excellent stereoselectivity (>20:1 dr) using the reported conditions [31]. A straightforward protection/deprotection sequence then revealed key intermediate 6, which was identical to material produced according to Scheme 4A and previous syntheses [12c,14a].
Preparation of the southern protected novioside sugar 7 commenced with commercially available D-lyxose (Scheme 5), Treatment of this material with HCl in a mixture of methanol and acetone simultaneously accomplished an isomerization to the furanose, anomeric methyl ketal formation, and ketalization of the cis-diol to provide compound 41 in 75% [32], The primary alcohol was then oxidized to the carboxylic acid and alkylated to form the corresponding methyl ester (~65% overall) [33], Addition of excess methylmagnesium bromide afforded tertiary alcohol 42 (>95%), By this four-step sequence up to 9,5 g (41% overall yield) of material was prepared in a single batch with no purifications, This method compared favorably to the previous six-step approach from O-methyl mannose reported by Kaufmann [13a], From 42 onwards our route to 3 followed the sequence reported by Kaufmann et al, [13a] with some minor modifications, Isomerization of 42 to the α- and β-pyranoses, 44a and 44b respectively, was performed in a mixture of trifluoroacetic acid and methanol, All four possible isomers of deprotected material were individually isolated and characterized along with recovered starting material 42 in the indicated quantities, In two subsequent iterations, furanose isomers 43a and 43b and recovered 42 were combined and resubmitted to the reaction conditions to generate a similar distribution of products, The total combined yield of isolated 44a and 44b was 70%, We next took advantage of an improved acylation protocol reported by Kaufmann [8b] to install the cyclic carbonate and the isobutyrate, which provided noviosides 7a and 7b from 44a and 44b respectively (~76–82% yield), Glycosylation of 6 with unstable glycosyl bromide 45 was performed using a slight modification of the previously reported method [13a], Thus treatment of 7b with HBr in acetic acid afforded the unstable glycosyl bromide 45, which was isolated but not purified [35], A Helferich type Koenigs-Knorr glycosylation of 6 with crude 45 provided the desired β-anomer 3 as the major product and with better than 2:1 stereoselectivity [36].
Scheme 5.
Synthesis of protected novioside 7 and fully glycosylated southern fragment 3, Reagents and conditions: (a) HCl, MeOH, acetone (75%); (b) TEMPO, KBr, NaHCO3, NaOCl, EtOAc, H2O (>95%); (c) Mel, K2CO3, DMF (66%); (d) MeMgBr, THF (>95%); (e) TFA, MeOH, H2O; (f) TFA, MeOH, H2O (70% yield of 44a and 44b after 3 cycles); (g) 44a or 44b, CDI, THF; (h) (iPrCO)2O, Et3N, DMAP, DCM (76% from 44a, 82% from 44b over 2 steps); (i) 7b, HBr, AcOH, DCM; (j) 6, HgBr2, HgO, 4 Å MS, DCM; then Et3N (67%, 2 steps, >2:1 β:α).
As shown in Scheme 6, glycosylation of the northern fragment 5 was best performed with the crude glycosyl bromide derived from acetate 4 (HBr, HOAc, DCM) and mediated by silver carbonate to yield the desired β-anomer 46 in 35% isolated yield (α/β of crude mixture >3:1) along with recovered alcohol 5 (44%). This approach differs from previously reported methods for the installation of this challenging β-mannose type glycosidic bond in the fidaxomicin literature [37]. Methanolysis of the phenolic acetates (K2CO3, MeOH) provided target fragment 2 (32%) along with an equal amount of monoacetate 47 (34%). The reaction time had to be kept short to avoid degradation of the sensitive pinacol ester. Purified 47 could be resubmitted to the same reaction conditions to furnish additional 2. In contrast to previous syntheses, we sought to explore macrolide formation using this fully glycosylated northern fragment 2. Gratifyingly, Suzuki coupling of this material with glycosylated southern fragment 3 using conditions described for coupling of a non-glycosylated northern fragment (cf. 24) provided the desired hindered diene 48, albeit with lower efficiency (38% versus published 88% for coupling of 3 with 24) [13a].
Scheme 6.
Synthesis of fully glycosylated northern fragment 2, cross-coupling with fully glycosylated southern fragment 5, and exploration of the final steps. Reagents and conditions: (a) 4, HBr, AcOH, DCM; then 5, Ag2CO3,4 Å MS, DCM (35% + 44% recovered 5, >3:1 β:α); (b) 46, K2CO3, MeOH (2: 35%; 47: 34%); (c) 47, K2CO3, MeOH (48%); (d) TlOEt, Pd(PPh3)4, THF, H2O (38%); (e) Grubbs II, PhMe; (f) NaH, ethylene glycol, THF; (g) HF·Et3N, THF.
All that remained was a penultimate ring closing olefin metathesis (RCM) and removal of the carbonate and silyl protecting groups. The ring closing metathesis of a mono-glycosylated (at C11) [13a], or a non-glycosylated [12b] seco-macrolide diene was reported to yield separable mixtures of E/Z-macrocycles with the desired natural E-isomer dominating (~2:1), and the unwanted Z-isomer recyclable after separation and resubjecting to the RCM conditions. Unfortunately, similar conditions with fully glycosylated seco-macrolide diene 48 led to an inseparable 1:2 mixture of double bond isomers with the unnatural Z-isomer dominating as determined by 1H NMR of the crude reaction product. Exploration of other RCM catalysts did not provide a satisfying solution. These results underscore the unpredictable influence of peripheral protecting groups and functionality on double bond geometry in the context of ring closing metathesis leading to larger ring systems [38,39].
While these results were a setback, we hoped to be able to separate the E/Z isomers at a later stage. At this point, we had only small amounts of crude RCM product mixtures available and subsequent reactions were performed on sub-milligram scale with product mixtures characterized primarily by crude NMR and mass spectrometry, but in insufficient quantities to support a more rigorous structural assignment. Nevertheless, the crude RCM mixture was subjected to carbonate cleavage and fluoride-mediated silyl ether cleavage as described [8b,13a]. Analysis of the crude reaction product by TLC and HPLC revealed three separable products. Each of these three products was isolated in low yield (<15%) by preparative RP-HPLC and independently characterized by 1H NMR and HRMS. Each consisted of an inseparable 2:1 Z/E mixture of olefin isomers and had a mass (m/z) consistent with that of authentic fidaxomicin. One product coeluted with commercial fidaxomicin in a co-injection experiment, and comparison of the 1H NMR of this material with that of authentic fidaxomicin supported its assignment as a 2:1 mixture of 4,5-Z-fidaxomicin and fidaxomicin. The remaining products appear to be constitutional isomers of fidaxomicin (same m/z), presumably isobutyrate migration products in the novioside sugar [40]. However, none of these materials was obtained in sufficient quantity to support a more rigorous structural assignment.
3. Conclusion
We have established robust synthetic routes to key intermediates 2–7 en route to fidaxomicin. In particular, the hydrogenative C–C coupling route (scheme 3B) to dienoate 23 represents a favorable route towards this compound. The [2,3]-Wittig rearrangement is a less contemporaneous, yet highly efficient approach to install the stereodiad embedded within the southern polyketide fragment 6. The catalytic Krische-type allylation from the alcohol oxidation stage represents and efficient alternative to classical reagent controlled allylations to install the homoallylic alcohol of fragment 6 with high diastereoselectivity. The preparation of novioside 7 from D-Lyxose represents a substantial improvement in both step count and efficiency over previous approaches. Koenigs-Knorr glycosylations towards both 2 and 3 were efficient and selective methods for the preparation of the corresponding glycosides. Finally, though we demonstrated that the cross-coupling and RCM of fully glycosylated fragments was possible, the yields were adversely impacted because the sugar decoration in the northern fragment reversed the E/Z-selectivity of the RCM macrocyclization, and these isomers were unfortunately no longer separable.
Supplementary Material
Acknowledgments
This work was supported by the NIH (grant GM1H329) and the Robert A. Welch Foundation (grant I-1422). J. K. De Brabander holds the Julie and Louis Beecherl, Jr., Chair in Medical Science. HRMS data were obtained from the Shimadzu Center for Advanced Analytical Chemistry (SCAAC) at UT Arlington.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tet.2020.131673.
References
- [1].(a) Erb W, Zhu J, Nat. Prod. Rep 30 (2013) 161–174; [DOI] [PubMed] [Google Scholar]; (b) McAlpine JB, Antibiot J. 70 (2017) 492–494. [DOI] [PubMed] [Google Scholar]
- [2].Goldstein EJC, Babakhani F, Citron DM, Clin. Infect. Dis 55 (2012) S143–S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kurabachew K, Lu SHJ, Krastel P, Schmitt EK, Suresh BL, Goh A, Knox JE, Ma NL, Jiricek J, Beer D, Cynamon M, Petersen F, Dartois V, Keller T, Dick T, Sambandamurthy VK, J. Antimicrob. Chemother 62 (2008) 713–719. [DOI] [PubMed] [Google Scholar]
- [4].Lin W, Das K, Degen D, Mazumder A, Duchi D, Wang D, Ebright YW, Ebright RY, Sineva E, Gigliotti M, Srivastava A, Mandal S, Jiang Y, Liu Y, Yin R, Zhang Z, Eng ET, Thomas D, Donadio S, Zhang H, Zhang C, Kapanidis AN, Ebright RH, Mol. Cell 70 (2018) 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Venugopal AA, Johnson S, Clin. Infect. Dis 54 (2012) 568. [DOI] [PubMed] [Google Scholar]
- [6].(a) Australian Therapeutic Goods Administration, Australian Public Assessment Report for Fidaxomicin, 2013. https://www.tga.gov.au/node/781;; (b) Shue YK, Sears PS, Shangle S, Walsh RB, Lee C, Gorbach SL, Okumu F, Preston RA, Antimicrob. Agents Chemother 52 (2008) 1391–1395; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sears P, Crook DW, Louie TJ, Miller MA, Weiss K, Clin. Infect. Dis 55 (2012) S116–S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Malcangi A, Trione G, Tiacumicin Production, US 8728796B2, 2014.
- [8].(a) Jamison M, Ph.D. Dissertation, University of Texas Southwestern Medical Center, 2013; [Google Scholar]; (b) Kaufmann EB, Ph.D. Dissertation, University of Basel, 2017; [Google Scholar]; (c) Hattori H, Kaufmann E, Miyatake-Ondozabal H, Berg R, Gademann K, J. Org. Chem 83 (2018) 7180–7205. [DOI] [PubMed] [Google Scholar]
- [9].(a) Xiao Y, Li S, Niu S, Ma L, Zhang G, Zhang H, Zhang G, Ju J, Zhang C, J. Am. Chem. Soc 33 (2011) 1092–1105; [DOI] [PubMed] [Google Scholar]; (b) Niu S, Hu T, Li S, Xiao Y, Ma L, Zhang G, Zhang H, Yang X, Ju J, Zhang C, Chembiochem 12 (2011) 1740–1748; [DOI] [PubMed] [Google Scholar]; (c) Hochlowski JE, Jackson M, McAlpine JB, Rasmussen RR, Bromotiacumicin compounds (1998), US5767096A. [Google Scholar]
- [10].(For selected reviews, see): (a) Roulland E, Synthesis 50 (2018) 4189–4200; [Google Scholar]; (b) Dorst A, Gademann K, Helv. Chim. Acta 103 (2020), e2000038. [Google Scholar]
- [11].(For selected examples of syntheses of other complex antibiotics, see): (a) Wright PM, Seiple IB, Myers AG, Angew. Chem. Int. Ed 53 (2014) 8840–8869; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Seiple IB, Zhang Z, Jakubec P, Langlois-Mercier A, Wright PM, Hog DT, Yabu K, Allu SR, Fukuzaki T, Carlsen PN, Kitamura Y, Zhou X, Condakes ML, Szczypiński FT, Green WD, Myers AG, Nature 533 (2016) 338–345; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Charest M, Lerner CD, Brubaker JD, Siegel DR, Myers AG, Science 308 (2005) 395–398. [DOI] [PubMed] [Google Scholar]
- [12].(a) Glaus F, Altmann K-H, Angew. Chem. Int. Ed 54 (2015) 1937–1940; [DOI] [PubMed] [Google Scholar]; (b) Erb W, Grassot J-M, Linder D, Neuville L, Zhu J, Angew. Chem. Int. Ed 54 (2015) 1929–1932; [DOI] [PubMed] [Google Scholar]; (c) Miyatake-Ondozabal H, Kaufmann E, Gademann K, Angew. Chem. Int. Ed 54 (2015) 1933–1936; [DOI] [PubMed] [Google Scholar]; (d) Jeanne-Julien L, Masson G, Astier E, Genta-Jouve G, Servajean V, Beau JM, Norsikian S, Roulland E, Org. Lett 19 (2017) 4006–4009. [DOI] [PubMed] [Google Scholar]
- [13].(a) Kaufmann E, Hattori H, Miyatake-Ondozabal H, Gademann K, Org. Lett 17 (2015) 3514–3517; [DOI] [PubMed] [Google Scholar]; (b) Norsikian S, Tresse C, François-Eude M, Jeanne-Julien L, Masson G, Servajean V, Genta-Jouve G, Beau JM, Roulland E, Angew. Chem. Int. Ed 59 (2020) 6612–6616. [DOI] [PubMed] [Google Scholar]
- [14].(a) Gong J, Li W, Fu P, Macmillan J, De Brabander JK, Org. Lett 21 (2019) 2957–2961; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yu X, University of Texas Southwestern Medical Center, Ph.D. Dissertation, 2018; [Google Scholar]; (c) Hattori H, Roesslein J, Caspers P, Zerbe K, Miyatake-Ondozabal H, Ritz D, Rueedi G, Gademann K, Angew. Chem. Int. Ed 57 (2018) 11020–11024; [DOI] [PubMed] [Google Scholar]; (d) Hattori H, Hoff LV, Gademann K, Org. Lett 21 (2019) 3456–3459. [DOI] [PubMed] [Google Scholar]
- [15].De Brabander J, Small Molecule Compounds Selective against Gram-Negative Bacterial Infections, US 20150329582, 2015 Filed May 13, 2014. [Google Scholar]
- [16]. Our route differs from that of Gademann (ref. 13a) in that the deoxygenation sequence was carried out subsequent to masking of the cis-diol. [Google Scholar]
- [17].(a) Bhattacharjee A, De Brabander JK, Tetrahedron Lett. 41 (2000) 8069–8073; [Google Scholar]; (b) Soltani O, De Brabander JK, Angew. Chem. Int. Ed 44 (2005) 1696–1699; [DOI] [PubMed] [Google Scholar]; (c) García-Fortanet J, Debergh JR, De Brabander JK, Org. Lett 7 (2005) 685–688. [DOI] [PubMed] [Google Scholar]
- [18].Gaddam J, Reddy GS, Marumudi K, Kunwar AC, Yadav JS, Mohapatra DK, Org. Biomol. Chem 17 (2019) 5601–5614. [DOI] [PubMed] [Google Scholar]
- [19].(a) Kumar Dey S, Ataur Rahman M, Alkhazim Alghamdi A, Reddy BVS, Yadav JS, Eur. J. Org Chem 2016 (2016) 1684–1687; [Google Scholar]; (b) Satoh T, Nanba K, Suzuki S, Chem. Pharm. Bull 19 (1971) 817–820. [Google Scholar]
- [20]. For a more detailed discussion of factors controlling thermodynamic equilibria in this system, see reference 8b. [Google Scholar]
- [21].Martin VS, Woodard SS, Katsuki T, Yamada Y, Ikeda M, Sharpless KB, J. Am. Chem. Soc 103 (1981) 6237–6240. [Google Scholar]
- [22].Moure AL, Arrayás RG, Cárdenas DJ, Alonso I, Carretero JC, J. Am. Chem. Soc 134 (2012) 7219–7222. [DOI] [PubMed] [Google Scholar]
- [23].Komanduri V, Krische MJ, J. Am. Chem. Soc 128 (2006) 16448–16449. [DOI] [PubMed] [Google Scholar]
- [24].Nishimura A, Tamai E, Ohashi M, Ogoshi S, Chem. Eur J 20 (2014) 6613–6617. [DOI] [PubMed] [Google Scholar]
- [25].Evans DA, Song H, Fandrick KR, Org. Lett 8 (2006) 3351–3354. [DOI] [PubMed] [Google Scholar]
- [26].Ben Ayed T, Amri H, Gaied MEM, Tetrahedron 41 (1991) 9621–9628. [Google Scholar]
- [27].Miyashita M, Yoshikoshi A, Grieco PA, J. Org. Chem 42 (1977) 3772–3774. [Google Scholar]
- [28].Friest JA, Broussy S, Chung WJ, Berkowitz D, Angew. Chem. Int. Ed 50 (2011) 8895–8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nakai T, Mikami K, Chem. Rev 86 (1986) 885–902. [Google Scholar]
- [30].Lipshutz BH, Ellsworth EL, Dimock SH, Reuter DC, Tetrahedron Lett. 30 (1989) 2065–2068. [Google Scholar]
- [31].(a) Kim IS, Nagi M-Y, Krische MJ, J. Am. Chem. Soc 130 (2008) 14891–14899; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Thorat RG, Harned AM, Chem. Commun 54 (2018) 241 −243. [DOI] [PubMed] [Google Scholar]
- [32].Petasis NA, Yao X, Raber JC, Nitrogen-containing Heterocycles, 2005. US 6927294B1. [Google Scholar]
- [33].Bosch MP, Campos F, Niubo I, Rosell G, Diaz JL, Brea J, Loza MI, Guerrero A, J. Med. Chem 47 (2004) 4041–4053 [DOI] [PubMed] [Google Scholar]
- [35]. We used purified 7b for this transformation, but Gademann has demonstrated that a mixture of 7a and 7b is also effective (ref. 13a). [Google Scholar]
- [36]. Stereoselectivity was estimated on the basis of crude NMR integration. The reported yield is of isolated β−3. Gademann reported an isolated yield of 48% of β−3 and 15% of α−3 (a 3:1 ratio). [Google Scholar]
- [37]. Roulland used an activated sulfoxide leaving group for their hydrogen bond directed glycosylation, and Gademann reported the activation of an N-phenyl trifluoroacetamide. [Google Scholar]
- [38].Martí-Centelles V, Pandey MD, Burguete MI, Luis SV, Chem. Rev 115 (2015) 8736–8834. [DOI] [PubMed] [Google Scholar]
- [39].Wu Y, Liao X, Wang R, Xie X, Brabander JK, J. Am. Chem. Soc 124 (2002) 3245–3253. [DOI] [PubMed] [Google Scholar]
- [40]. Such acyl migration products have been documented for natural fidaxomicin-related metabolites. See ref. 1a. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.