Abstract
Several members of the chemokine family are involved in regulation of fibrosis. This review manuscript discusses the role of the chemokines in the pathogenesis of myocardial fibrosis. The CC chemokine CCL2 exerts fibrogenic actions through recruitment and activation of monocytes and macrophages expressing its receptor, CCR2. Other CC chemokines may also contribute to fibrotic remodeling by recruiting subsets of fibrogenic macrophages. CXC chemokines containing the ELR motif may exert pro-fibrotic actions, through recruitment of activated neutrophils and subsequent formation of neutrophil extracellular traps (NETs), or via activation of fibrogenic monocytes. CXCL12 has also been suggested to exert fibrogenic actions through effects on fibroblasts and immune cells. In contrast, the CXCR3 ligand CXCL10 was found to reduce cardiac fibrosis, inhibiting fibroblast migration. Chemokines are critical links between inflammation and fibrosis in myocardial disease and may be promising therapeutic targets for patients with heart failure accompanied by prominent inflammation and fibrosis.
Keywords: chemokine, fibrosis, fibroblast, macrophage, heart failure, myocardial infarction, extracellular matrix
Introduction
Fibrotic remodeling is a common pathologic abnormality found in most myocardial diseases. The adult mammalian heart has negligible regenerative capacity; thus, following myocardial infarction the myocardium heals through formation of a collagen-based scar, resulting in reparative fibrosis. In many other pathophysiologic conditions, including pressure overload, metabolic disease and certain genetic cardiomyopathies, increased deposition of extracellular matrix proteins may occur in the absence of significant cardiomyocyte death, resulting in interstitial or perivascular fibrosis. Excessive deposition of fibrous tissue in the cardiac interstitium may promote both systolic and diastolic dysfunction. Moreover, fibrotic changes may play an important role in the pathogenesis of arrhythmias and conduction defects. Although, activated fibroblasts and myofibroblasts are the main cellular effectors of fibrosis, producing large amounts of extracellular matrix proteins, other cell types, including immune cells, vascular cells and cardiomyocytes may contribute to the fibrotic response, by secreting fibrogenic growth factors and matricellular proteins[1]. In many myocardial conditions, fibroblast activation is triggered by an inflammatory response, involving recruitment of fibrogenic leukocytes in the remodeling myocardium[2].
Chemokines are a family of chemotactic cytokines with a critical role in leukocyte trafficking in homeostasis and disease. Based on their structure, chemokines can be classified into four subfamilies (CC, CXC, CX3C and XC), depending on the number of aminoacids between their first two highly-conserved cysteine residues. In CC chemokines, the first two cysteines are adjacent, whereas CXC and CX3C chemokines have one and three non-conserved aminoacids respectively between the two cysteines (hence the CXC and CX3C designations). XC chemokines have only one cysteine residue near the N-terminus. This structural classification has important functional implications, determining which leukocyte populations are recruited by each subfamily. CC chemokines predominantly recruit mononuclear cells. In contrast, a subgroup of CXC chemokines that contain the ELR sequence (glutamic acid-leucine-arginine) immediately preceding the CXC motif, serve primarily as neutrophil chemoattractants. In injured and inflamed tissues, chemokines bind to glycosaminoglycans on the endothelial surface, or in the extracellular matrix and signal by interacting with G-protein-coupled seven-transmembrane chemokine receptors[3].
The pro-inflammatory actions of chemokines have been implicated in the pathogenesis of tissue fibrosis in the heart[4] and in other organs[5]. Although actions on immune cells are likely responsible for most of the effects of the chemokines in fibrosis, evidence suggests that certain members of the chemokine family may also exert direct actions on fibroblasts (Figure 1). This review manuscript summarizes recent progress in understanding the role of chemokines in myocardial fibrosis.
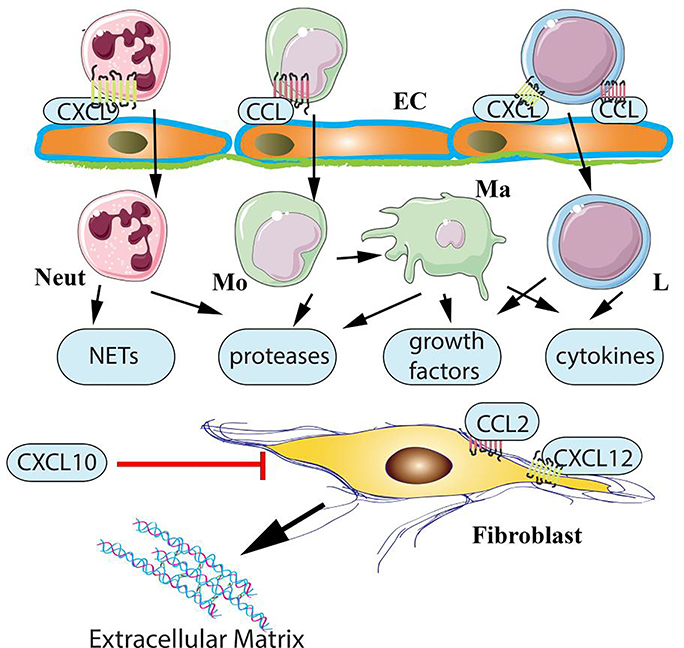
Figure 1: Chemokine actions in cardiac fibrosis.
Both CC (CCL) and CXC (CXCL) chemokines have been implicated in the pathogenesis of cardiac fibrosis. CC chemokines, such as CCL2, may promote fibrosis through recruitment of fibrogenic monocytes and activation of macrophages that produce growth factors and cytokines. CXC chemokines containing the ELR motif may promote fibrosis by recruiting neutrophils. Activated neutrophils may stimulate fibroblasts by generating neutrophil extracellular traps (NETs) or by secreting proteases and growth factors. Effects of ELR+ CXC chemokines on recruitment of fibrogenic mononuclear cells have also been suggested. Both CC and CXC chemokines may be involved in chemoattraction of lymphocytes with pro-fibrotic properties. Although the effects of chemokines in regulation of fibrosis are generally attributed to leukocyte recruitment, direct actions of some members of the family on fibroblasts cannot be excluded. The CXCR3 ligand CXCL10 exerts anti-fibrotic actions, inhibiting fibroblast migration through CXCR3-independent effects that may involve proteoglycans (Neut, neutrophil; Mo, monocyte; Ma, macrophage; L, lymphocyte; EC, endothelial cells).
Immune cells in cardiac fibrosis
The idea that chronic inflammation may promote fibrotic tissue remodeling is not new [6]. Although activated fibroblasts and myofibroblasts are the central effector cells in tissue fibrosis [7],[8], serving as the main source of extracellular matrix proteins, their activation involves in many cases recruitment of immune cells that synthesize large amounts of fibrogenic growth factors, such as Transforming Growth Factor (TGF)-β [9]. Macrophages are recruited and activated following injury and secrete fibrogenic cytokines, growth factors and matricellular proteins [10]. Lymphocytes also infiltrate injured tissues and may stimulate fibrogenic cascades [11]. Mast cells accumulate and degranulate in many fibrotic conditions and may contribute to fibroblast activation by releasing their fibrogenic granular contents, including growth factors, matrix metalloproteinases and the mast cell-specific proteases tryptase and chymase [12],[13]. It has been suggested that certain leukocyte subsets may exhibit characteristics of fibroblast progenitors and may be directly involved in the pathogenesis of fibrosis by converting to activated myofibroblasts [14],[15]. Although, in the injured heart, the contribution of circulating cells to the fibroblast population is likely to be limited [16],[17], there is no doubt that immune cell populations can play a critical role in cardiac fibrosis by secreting fibroblast-activating mediators [2]. Recruitment and activation of fibrogenic immune cells in injured and remodeling tissues, including the myocardium, is dependent on induction of chemokines [4]
Induction of chemokines in fibrotic hearts
Based on their patterns of expression, chemokines can be divided into homeostatic and inflammatory groups. Homeostatic chemokines are constitutively expressed and are implicated in cell homing in lymphoid organs. Inflammatory chemokines, on the other hand, show low levels of expression in normal tissues, and are markedly upregulated following injury regulating recruitment of leukocytes. Some members of the chemokine family (such as CXCL12/Stromal cell-Derived factor (SDF)-1 have both homeostatic and inflammatory roles, showing both constitutive expression, and induction in inflamed tissues. Fibrotic conditions are associated with induction of several inflammatory chemokines.
Injury rapidly upregulates chemokine expression in the myocardium, inducing a wide range of inflammatory CC and CXC chemokines, in cardiac endothelial cells, macrophages, fibroblasts and cardiomyocytes (Table 1) [18]. Increased chemokine levels have been consistently documented in experimental models of cardiac fibrosis [1],[19], and in patients with fibrotic cardiomyopathic conditions [20]. Several mechanisms have been implicated in activation of the chemokine system in injured and remodeling hearts. First, in myocardial diseases associated with cardiomyocyte death, necrotic cells release damage-associated molecular patterns (DAMPs), stimulating Toll-like receptor (TLR) signaling responses, and promoting downstream activation of the Nuclear Factor (NF)-κB system and chemokine transcription [21]. Second, activation of the inflammasome results in release of active Interleukin (IL)-1β, stimulating chemokine expression [22]. Third, injury-associated release and activation of proteases generates extracellular matrix fragments that induce chemokine expression in many different cell types. Fourth, mechanical stress may activate neurohumoral signals (such as angiotensin II), thus stimulating pro-inflammatory signaling, resulting in induction of chemokines [23]. Neurohumoral activation of calcium (Ca2+) /calmodulin (CaM)-dependent kinase IIδ has been suggested to promote induction of CCL2 in pressure overload models [24],[25]. Fifth, oxidative stress has been extensively implicated in induction of the chemokine response following myocardial injury [26].
Table 1:
Chemokines in cardiac fibrosis
| Chemokine | Chemokine receptors | Cellular source in models of cardiac fibrosis | Cellular targets and effects | References |
|---|---|---|---|---|
| CCL2 | CCR2, CCR4 | Macrophages, endothelial cells and fibroblasts have been reported as major sources of CCL2 in remodeling hearts. CCL2 upregulation in stressed and injured cardiomyocytes has also been demonstrated. | The fibrogenic actions of CCL2 likely involve recruitment of proinflammatory and fibrogenic CCR2+ monocytes and macrophages. May directly activate fibroblasts; however, the in vivo significance of such effects is unclear. Has been suggested to recruit fibroblast progenitors. May also promote arteriogenesis. | [27],[30],[32],[33],[24],[25] |
| CCL3 | CCR1, CCR4, CCR5 | In a model of myocarditis, macrophages and dendritic cells were the main source of CCL3. | May stimulate recruitment of pro-inflammatory leukocytes that may promote fibrosis. In myocarditis, CCL3 was found to play a critical role in virus-induced inflammation. | [99],[100],[101] |
| CCL4 | CCR5, CCR8 | CCL4 is upregulated in infarcted and remodeling hearts; however its cellular localization has not been systematically studied. Macrophages, endothelial cells and fibroblasts are likely cellular sources. | Although CCL4 is upregulated in injured and fibrotic hearts, its role in myocardial biology remains unknown. | [48] |
| CCL5 | CCR5, CCR3, CCR1 | Although many cell types (including macrophages, platelets, endothelial cells and fibroblasts) are known to produce CCL5, its cellular localization in fibrotic hearts has not been studied. | In a model of infarction, CCL5 was found to promote fibrosis, likely by accentuating inflammatory injury. May form heteromers with α-defensin, stimulating leukocyte recruitment. CCR5-mediated recruitment of anti-inflammatory leukocyte subsets has been suggested to restrain inflammation following infarction. | [47],[46],[48] |
| CCL24 | CCR3 | In myocarditis, macrophages were the main cellular source of CCL24. CCL24 levels were markedly increased in regenerating neonatal mouse hearts. However, its cellular localization has not been studied. | Although CCL24 has been implicated in fibrosis in other organs, whether it is involved in myocardial fibrosis is unknown. In eosinophilic myocarditis. CCL24 may promote fibrosis by recruiting eosinophils. | [53],[52] |
| CXCL8 | CXCR1, CXCR2 | Leukocytes, endothelial cells were found to be major sources of CXCL8 in infarcted and remodeling hearts. Fibroblasts and lymphocytes are also capable of secreting CXCL8. | May promote fibrosis by recruiting and activating neutrophils. Fibrogenic actions of CXCL8-activated neutrophils may involve formation of neutrophil extracellular traps (NETs). CXCL8 may also exert angiogenic actions. | [102],[103] |
| CXCL1 and other CXCR2 ligands | CXCR2 | Macrophages, vascular cells, fibroblasts and mast cells can produce CXCR2 ligands. | CXCR2 ligands may promote fibrosis through recruitment of fibrogenic leukocyte subsets. CXCR2-mediated inflammation may indirectly promote fibrosis by increasing systemic blood pressure. | [23],[56],[55] |
| CXCL4 | CXCR3 | Platelets, macrophages | Although the involvement of CXCL4 in cardiac fibrosis has not been systematically studes, exogenous CXCL4 was found to increase MMP activity and to inhibit macrophage phagocytosis. CXCL4 may also be involved in lymphocyte recruitment. | [63], [65] |
| CXCL10 | CXCR3, PG | Endothelial cells, leukocytes | CXCL10 exerts anti-fibrotic actions, inhibiting fibroblast migration and has also been suggested to have angiostatic properties. May also be involved in recruitment of lymphocytes. | [60],[61],[62] |
| CXCL12 | CXCR4, CXCR7 | Vascular cells, leukocytes, fibroblasts and cardiomyocytes | CXCL12 exerts a wide range of actions on all cell types involved in cardiac remodeling. Effects on inflammatory leukocyte recruitment, angiogenesis (through recruitment of progenitors), and cardiomyocyte survival have been reported. Fibrogenic actions may involve direct effects on fibroblast migration, or activation of fibrogenic macrophages. | [71],[72],[73],[74],[75],[76],[77],[78],[104] |
| CX3CL1 | CX3CR1 | Endothelial cells, macrophages | In a model of viral myocarditis, CX3CR1 was found to inhibit fibrosis; however, the cellular basis for these effects is unclear. In other tissues both fibrogenic and anti-fibrotic actions of CX3CL1 have been reported. Although macrophages are the most likely cellular targets of CX3CL1 in fibrotic and remodeling hearts, some experimental studies have suggested actions on many different cell types, including cardiomyocytes, fibroblasts and endothelial cells. | [83], [84] |
The role of the chemokines in cardiac fibrosis
CC chemokines
The CCL2/CCR2 axis
CCL2/monocyte chemoattractant protein (MCP)-1 is the best-studied member of the CC chemokine family in myocardial disease. CCL2 is markedly upregulated in experimental models of ischemic and non-ischemic cardiac fibrosis [27],[28],[29] and is overexpressed in myocardial samples from patients with heart failure [20]. Studies using genetic loss-of-function approaches or pharmacologic inhibition in mouse models support the notion that CCL2 and its main receptor CCR2 play a critical role in myocardial fibrosis. In a mouse model of reperfused myocardial infarction, CCL2 disruption attenuated myofibroblast infiltration [30]. In a model of hypertensive fibrosis administration of an anti-CCL2 antibody reduced fibrotic remodeling [29]. In a model of ischemic non-infarctive cardiomyopathy induced through brief repetitive ischemia/reperfusion, CCL2 loss attenuated interstitial fibrosis and improved dysfunction [27]. Moreover, in models of diabetic cardiomyopathy, genetic and pharmacologic inhibition of CCR2 arttenuated fibrosis [31].
Which cellular mechanisms mediate the fibrogenic actions of CCL2/CCR2? CCL2-mediated cardiac fibrosis is predominantly attributed to recruitment and activation of CCR2+ monocytes and macrophages, resulting in release of fibrogenic mediators, such as TGF-β and osteopontin, capable of activating cardiac fibroblasts [27],[30],[32],[33]. The mammalian heart contains a resident macrophage population, derived predominantly from yolk sac and fetal monocyte progenitors [34]. In normal hearts, these macrophages have the capability to self-renew; however, following infarction, the cardiac macrophage population markedly expands through recruitment of abundant CCR2+ monocytes [35].[36]. Thus, myocardial injury enriches the heart with a wide range of macrophage phenotypes with distinct functional properties. Some of these cells have been suggested to exert cardioprotective actions [35]; others may contribute to phagocytosis of dead cells and repair of the infarcted heart[37], whereas some subsets may exert pro-inflammatory[38], fibrogenic, or angiogenic actions. Much like remodeling mouse hearts, human failing hearts also contain CCR2+ and CCR2-negative macrophage subsets with distinct functional properties [39]. Single cell transcriptomic analysis may contribute to identification of specific fibroblast-activating macrophages in remodeling hearts.
Whether in addition to its effects on monocytes and macrophages, CCL2 induces cardiac fibrosis through actions on other cell types remains poorly documented. Lymphocytes have been implicated in the pathogenesis of cardiac fibrosis [40]; however, the potential involvement of CCL2 in their recruitment remains unknown. Although some studies have suggested that CCL2 may directly stimulate fibroblast activation [41], in mouse cardiac fibroblasts, CCL2 stimulation had no significant effects on profibrotic gene expression profile and proliferative activity[27].
The potential role of other CC chemokines in cardiac fibrosis
Induction of several other members of the CC chemokine subfamily (including CCL3, CCL4, CCL5, CCL12 and CCL24) has been reported in experimental models of cardiac fibrosis [42],[43],[44,45]. These chemokines may recruit distinct subpopulations of leukocytes, thus contributing to the pathogenesis of cardiac fibrosis. CCL5 and CCL3 may stimulate fibrosis through recruitment of monocytes and lymphocytes expressing the CCR5 receptor. In the ischemic myocardium, CCL5 was found to form heteromers with neutrophil-derived a-defensin, that bind to CCR5 mediating monocyte recruitment [46]. CCL5 neutralization in a model of myocardial infarction attenuated collagen deposition; however the effects on fibrotic remodeling were indirect, related to attenuated infarct size due to reduced inflammatory injury [47]. Other studies have suggested that CC chemokine-induced leukocyte infiltration may also play a role in suppression of post-infarction inflammation through recruitment of anti-inflammatory monocyte and lymphocyte subsets. In a model of myocardial infarction, CCR5 was implicated in recruitment of regulatory T cells in the infarcted myocardium, suppressing inflammation and attenuating adverse matrix remodeling [48],[49]. Evidence suggesting direct effects of CCR5 ligands in non-infarctive cardiac fibrosis is lacking. In a model of hypertension induced through administration of desoxycorticosterone acetate (DOCA) and angiotensin II, global loss of CCR5 did not affect myocardial fibrosis [50].
CCL24 has been implicated in activation of fibrogenic pathways in the lung and skin [51]; however, its potential role in cardiac fibrosis remains unknown. As a potent eosinophil chemoattractant, CCL24 may be involved in the pathogenesis of fibrosis in eosinophilic myocarditis [52]. The marked induction of CCL24 in regenerating neonatal hearts [53] adds an intriguing layer of complexity to the possible actions of this CC chemokine in myocardial disease.
CXC chemokines in cardiac fibrosis
ELR+ CXC chemokines
ELR+ CXC chemokines act predominantly as neutrophil chemoattractants, signaling through the CXCR1 and CXCR2 receptors. CXCL8/Interleukin-8 is the prototypical ELR+ CXC chemokine in humans and acts as a potent neutrophil chemoattractant, binding CXCR1 with high affinity. In contrast, rodents lack a CXCL8 homologue, but have several ELR+ CXC chemokines with similar functional properties. In addition to their role in neutrophil recruitment, ELR+ CXC chemokines have also been suggested to play a role in angiogenesis [54] and fibrosis. Several recent investigations have demonstrated that disruption of CXCR2 signaling may attenuate cardiac fibrosis, presumably through attenuation of leukocyte infiltration. CXCL1 and CXCL2 are upregulated in spontaneously hypertensive rat hearts, and CXCR2 inhibition attenuates cardiac fibrosis, hypertrophy and dysfunction [55]. However, the fibrogenic and pro-hypertrophic actions of CXCR2 may be indirect, involving effects on blood pressure regulation. CXCL1, one of the CXCR2 ligands has been reported to contribute to the development of angiotensin-induced cardiac fibrosis [23]. The fibrogenic effects of CXCR2 ligands have been attributed to recruitment of fibrogenic monocyte subpopulations [23],[56]. Neutrophils, may also contribute to the fibrogenic actions of ELR+ CXC chemokines through secretion of proteases that generate fibrogenic matrix fragments, or through release of fibrogenic growth factors and cytokines. Neutrophils can also release their decondensed chromatin and form large extracellular DNA networks, called neutrophil extracellular traps (NETs). NETosis has been implicated in fibrogenic activation in the heart and other organs [57],[58],[59]. However, considering the short life span of neutrophils in the injured myocardium, their relative role as cellular effectors of fibrosis is unclear.
CXCR3 ligands: the role of CXCL10
The CXCR3 ligands (CXCL9, CXCL10 and CXCL11) are the best characterized group of ELR-negative CXC chemokines. These chemokines do not stimulate neutrophil chemotaxis, but have been implicated in recruitment of lymphocyte subsets. Moreover, CXCL10 has been suggested to exert direct actions on fibroblasts and endothelial cells that may have important implications in the regulation of cardiac fibrosis. Evidence in both large animal models and rodents suggests that CXCL10/interferon-γ-inducible protein (IP)-10 is consistently induced following cardiac injury [60],[61]. Global loss-of-function studies in mice suggested that CXCL10 may exert anti-fibrotic actions. CXCL10-mediated inhibition of fibrosis may involve recruitment of anti-fibrotic leukocyte subpopulations, or direct de-activating effects on cardiac fibroblasts [60],[61]. In vitro experiments in cardiac fibroblasts showed that CXCL10 inhibits growth-factor-mediated fibroblast migration [61], through interactions with proteoglycans that were independent of CXCR3 [62].
CXCL4/platelet factor (PF)-4 has also been implicated in cardiac remodeling [63] through effects that may involve, at least in part, interactions with CXCR3 [64]. Exogenous infusion of CXCL4 perturbed repair of the infarcted heart, inhibiting macrophage phagocytosis and increasing MMP levels [63]. Unfortunately, very limited information is available on the role of endogenous CXCL4 in fibrotic remodeling of the heart. Pharmacologic inhibition experiments supported the notion that heterophilic interactions between CXCL4 and CCL5 may contribute to NET formation, accentuating ischemic inflammatory injury [65].
CXCL12/SDF-1
CXCL12/SDF-1 is a multifunctional ELR-negative chemokine with a critical role in cardiovascular development [66] and in angiogenesis [67,68]. CXCL12 is induced following myocardial injury, and has been suggested to play an important role in regulation of cardiomyocyte survival, inflammation and neovessel formation in healing infarcts [69],[70]. A growing body of evidence suggests that CXCL12 may be implicated in the pathogenesis of fibrosis in several different organs. Several studies have suggested that CXCL12 may exert fibrogenic actions through activation of its main receptor, CXCR4. CXCR4 inhibition attenuated cardiac fibrosis in a genetic model of murine cardiomyopathy due to cardiac-specific overexpression of the stress kinase MSt1 [71], and in models of diabetic fibrotic cardiomyopathy [72] and cardiorenal syndrome [73]. The cellular basis for the fibrogenic actions of CXCL12 remains poorly understood. CXCL12-induced fibrosis has been attributed to direct effects on fibroblast migration, to recruitment of fibroblast progenitors [74], or to activation of fibrogenic macrophages [75]. In vitro studies suggest direct activating effects of CXCL12 on fibroblasts. CXCL12 stimulation promotes proliferation and induces collagen synthesis in cardiac fibroblasts [76]. It has been suggested that CXCR4-mediated activation of a migratory phenotype in cardiac fibroblasts may not necessarily require CXCL12, but may also involve chemokine-independent interactions of the receptor with high-mobility group box-1 (HMGB1) [77], a DAMP released in the injured myocardium. On the other hand, some CXCL12 actions may be CXCR4-independent, involving the CXCR7 receptor. In a model of cardiac fibrosis induced through isoproterenol infusion, administration of a CXCR7 inhibitor attenuated cardiac fibrosis [78].
CX3CL1/Fractalkine
The CX3C chemokine CX3CL1/fractalkine is rapidly released following myocardial injury [79], and chemoattracts monocytes/macrophages expressing the CX3CR1 receptor. Considering the involvement of macrophages in tissue fibrosis, an important role for CX3CL1 in fibrotic remodeling has been suggested. However, in vivo studies investigating the role of the CX3CL1/CX3CR1 axis in fibrosis have produced conflicting results. In a model of hepatic fibrosis CX3CL1/CX3CR1 signaling was found to inhibit macrophage-driven fibrogenesis [80]. In contrast, studies in models of renal fibrosis suggested pro-fibrotic actions of the CX3CL1/CX3CR1 axis [81],. Although CX3CR1+ macrophages are abundant in the infarcted and remodeling myocardium [82], the role of CX3CL1/CX3CL1 in cardiac fibrosis has not been systematically studied. In a model of viral myocarditis, global loss of CX3CR1 accentuated inflammation and increased fibrosis [83]; however, the cellular basis for these effects is unclear. In experimental models of heart failure induce through myocardial infarction or left ventricular pressure overload, CX3CL1 was found to promote dysfunction. These detrimental actions were attributed to effects on cardiomyocyte function and fibroblast phenotype [84]. Moreover, in a complex model of unilateral nephrectomy followed by angiotensin II infusion, loss of CX3CR1 did not affect myocardial fibrosis [85].
Chemokines as therapeutic targets in cardiac fibrosis
Considering their role in tissue inflammation and fibrosis, several members of the chemokine family are attractive therapeutic targets in human fibrotic conditions. Early phase clinical trials using therapeutic approaches neutralizing the CCL2/CCR2 axis, or dual CCR2/CCR5 inhibition have suggested beneficial effects in patients with fibrosis-associated conditions, such as diabetic nephropathy [86],[87], HIV-associated fibrogenic activation [88] and non-alcoholic steatohepatitis [89],[90] (Table 2). In contrast, a phase 2 trial using an anti-CCL2 neutralizing antibody in patients with idiopathic pulmonary fibrosis did not show protective effects. Clinical studies examining the effects of chemokine inhibition in patients with cardiac fibrotic conditions have not been performed. A large amount of experimental evidence suggests that some members of the chemokine family may be attractive therapeutic targets for patients with heart failure associated with prominent inflammatory and fibrotic changes. CCL2/CCR2, the best studied chemokine/chemokine receptor pair in myocardial disease, has been implicated in the pathogenesis of both ischemic and non-ischemic cardiomyopathy, and may be a promising target for therapeutic intervention. However, a lot of additional information is needed to support the case for chemokine-based therapeutics in heart failure. Although emerging evidence supports the notion that inflammation may play an important role in heart failure with preserved ejection fraction (HFpEF) [91],[92] and experimental studies suggest that macrophages may contribute to diastolic dysfunction [93], whether CCL2 or other CC chemokines are involved in disease progression remains unknown. The pathophysiologic heterogeneity of human HFpEF that cannot be recapitulated by any animal model is a major challenge for successful clinical translation. Therapeutic implementation of chemokine targeting approaches will require identification of heart failure patients with prominent chemokine responses that may be causally involved in progression of adverse remodeling.
Table 2:
Targeting the chemokines in human fibrosis-associated conditions
| Condition | Anti-chemokine approach | Findings | Reference |
|---|---|---|---|
| Lupus nephritis | Inhibition of CCL2 (and other CC chemokines) through administration of bindarit | In a randomized double-blind clinical trial, treatment of acute lupus nephritis patients with bindarit reduced proteinuria. Fibrosis-related endpoints were not assessed. | [105] |
| Diabetic nephropathy (albuminuria) | Treatment with a CCR2 inhibitor (CCX140-B) | In a randomized double-blind placebo-controlled trial, CCR2 inhibition reduced proteinuria in patients with type 2 diabetes. Fibrosis-related endpoints were not assessed. | [86] |
| Diabetic nephropathy (albuminuria) | Treatment with emapticap pegol (NOX-E36), an L-aptamer that binds and inhibits CCL2. | In a phase IIa study, CCL2 inhibition was safe and well-tolerated and reduced the albumin to creatinine ratio. Fibrosis-related endpoints were not assessed. | [87] |
| Idiopathic pulmonary fibrosis (IPF) | Monoclonal anti-CCL2 neutralizing antibody (carlumab) | In a phase 2 trial, CCL2 inhibition did not affect forced vital capacity and the change in diffusing capacity in IPF patients. | [106] |
| HIV-infected patients | Oral CCR2/CCR5 antagonist (cenicriviroc) | In HIV-infected patients on stable antiretroviral therapy, cenicriviroc reduced plasma biomarkers of fibrosis | [88] |
| Hepatic fibrosis associated with HIV infection | Oral CCR2/CCR5 antagonist (cenicriviroc) | Cenicriviroc decreased the enhanced liver fibrosis index in HIV-infected patients. | [107] |
| Non-alcoholic steatohepatitis accompanied by liver fibrosis | Oral CCR2/CCR5 antagonist (cenicriviroc) | In a phase 2b randomized-controlled trial (CENTAUR) cenicriviroc was well tolerated and reduced fibrosis progression. | [89],[90] |
Moreover, chemokine targeting in heart failure patients may carry significant risks, related to the need for prolonged inhibition of pathways involved in responses to injury and repair. Some members of the chemokine family, including CCL2, have also been implicated in arteriogenesis and may play a role in formation of collateral vessels in patients with chronic ischemic heart disease [94]. Other chemokines, such as CXCL12 have been suggested to exert pro-survival actions on cardiomyocytes [95], while recruiting angiogenic progenitors and promoting angiogenesis [96]. Thus, in some cases the benefits of any anti-fibrotic effects of chemokine inhibition may be outweighed by the abrogation of important protective and reparative actions.
Design of therapeutic strategies targeting the chemokine system should also carefully consider and exploit the temporal and spatial patterns of chemokine induction and activity following myocardial injury (Figure 2). Following myocardial infarction, the rapid and intense upregulation of pro-inflammatory CC and CXC chemokines in the infarct plays an important role in reparative fibrosis by recruiting activated monocytes, activating phagocytic macrophages and promoting growth factors expression and release. As professional phagocytes clear the infarct from dead cells and matrix debris, the inflammatory response in the infarct zone is suppressed; this is a crucial event for cardiac repair. However, in large infarcts, extensive loss of contractile cardiomyocytes results in profound hemodynamic perturbations, chronic activation of neurohumoral pathways and a low-grade chemokine-driven inflammatory reaction in the non-infarcted remodeling myocardium [97],[98]. These inflammatory changes may play a major role in the pathogenesis of chronic post-infarction heart failure.
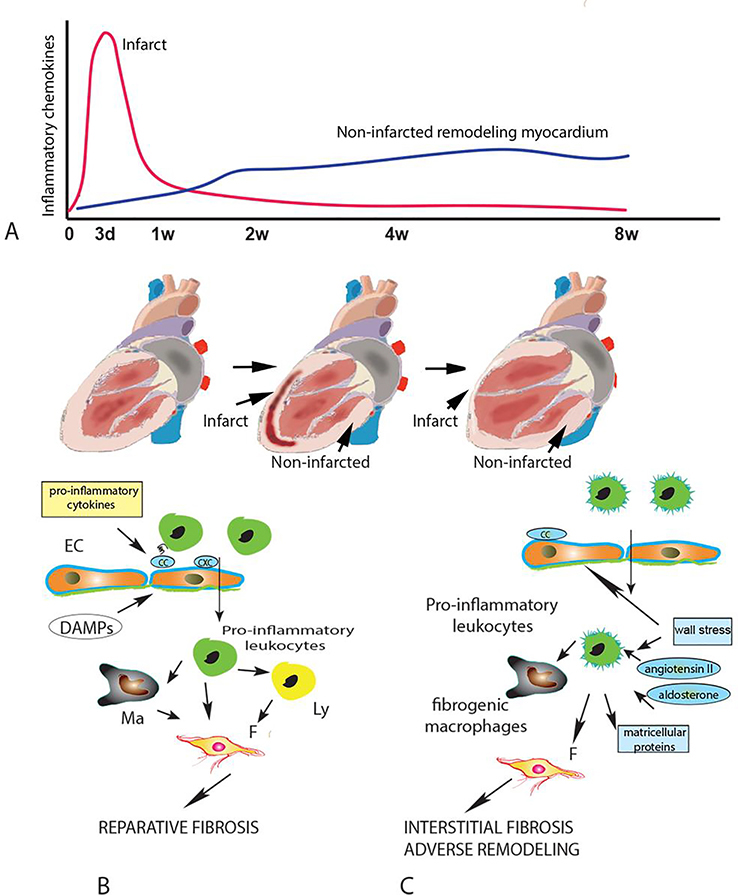
Figure 2: The spatiotemporal dynamics of chemokine actions in cardiac repair and post-infarction heart failure.
A. Following myocardial infarction, release of damage-associated molecular patterns (DAMPs) by dying cardiomyocytes and degraded extracellular matrix rapidly stimulates marked upregulation of pro-inflammatory CC and CXC chemokines in the infarct zone. As macrophages (Ma) clear the infarct from dead cells and matrix debris, the chemokine response is suppressed. However, in large infarcts, the profound hemodynamic perturbations caused by massive loss of contractile cardiomyocytes causes a low-grade chronic upregulation of pro-inflammatory chemokines in the non-infarcted myocardium. B. In the infarct, early induction of chemokines recruits pro-inflammatory leukocytes, resulting in expansion of phagocytic macrophages and fibroblast activation. The early chemokine response is important for reparative fibrosis. C. In the chronic remodeling phase, high intraventricular pressures increase wall stress and trigger neurohumoral activation, promoting low-level chemokine induction, followed by recruitment and activation of fibrogenic monocytes and macrophages that may cause interstitial fibrosis, contributing to the pathogenesis of adverse remodeling and post-infarction heart failure. EC, endothelial cell; Ly, lymphocyte.
Conclusions:
Our understanding of the role of the chemokines in fibrotic remodeling of the heart remains limited. Future studies need to focus on identification of specific chemokine/chemokine receptor pairs that regulate recruitment of fibrogenic leukocytes in the injured myocardium, on dissection of leukocyte-derived mediators responsible for chemokine-driven fibrosis, and on the potential role of direct actions of chemokine family members on fibroblasts. Moreover, we need to expand our knowledge on the patterns of expression and potential role of chemokines in human cardiac fibrosis. Targeting fibrogenic immune cells may hold promise as a therapeutic strategy in subpopulations of heart failure patients exhibiting prominent fibrotic responses.
Acknowledgments
SOURCES OF FUNDING: Dr Frangogiannis’ laboratory is supported by National Institutes of Health grants R01 HL76246, R01 HL85440, and R01 HL149407, and by U.S. Department of Defense grants PR151029 and PR181464.
Footnotes
CONFLICT OF INTEREST STATEMENT:
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest
** of outstanding interest
- 1.Frangogiannis NG: Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med 2019, 65:70–99. [DOI] [PubMed] [Google Scholar]
- 2.Okyere AD, Tilley DG: Leukocyte-Dependent Regulation of Cardiac Fibrosis. Front Physiol 2020, 11:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffith JW, Sokol CL, Luster AD: Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 2014, 32:659–702. [DOI] [PubMed] [Google Scholar]
- 4.Dobaczewski M, Frangogiannis NG: Chemokines and cardiac fibrosis. Front Biosci (Schol Ed) 2009, 1:391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roh YS, Seki E: Chemokines and Chemokine Receptors in the Development of NAFLD. Adv Exp Med Biol 2018, 1061:45–53. [DOI] [PubMed] [Google Scholar]
- 6.Frangogiannis NG: Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflamm Res 2004, 53:585–595. [DOI] [PubMed] [Google Scholar]
- 7.Humeres C, Frangogiannis NG: Fibroblasts in the Infarcted, Remodeling, and Failing Heart. JACC Basic Transl Sci 2019, 4:449–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pakshir P, Noskovicova N, Lodyga M, Son DO, Schuster R, Goodwin A, Karvonen H, Hinz B: The myofibroblast at a glance. J Cell Sci 2020, 133. [DOI] [PubMed] [Google Scholar]
- 9.Frangogiannis NG: Transforming Growth Factor (TGF)-beta in tissue fibrosis. J Exp Med 2020, 217:e20190103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wynn TA, Vannella KM: Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44:450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanton RM, Carrillo-Salinas FJ, Alcaide P: T-cell recruitment to the heart: friendly guests or unwelcome visitors? Am J Physiol Heart Circ Physiol 2019, 317:H124–H140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levick SP, Melendez GC, Plante E, McLarty JL, Brower GL, Janicki JS: Cardiac mast cells: the centrepiece in adverse myocardial remodelling. Cardiovasc Res 2012, 89:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frangogiannis NG, Perrard JL, Mendoza LH, Burns AR, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML: Stem cell factor induction is associated with mast cell accumulation after canine myocardial ischemia and reperfusion. Circulation 1998, 98:687–698. [DOI] [PubMed] [Google Scholar]
- 14.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A: Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1994, 1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 15.Haider N, Bosca L, Zandbergen HR, Kovacic JC, Narula N, Gonzalez-Ramos S, Fernandez-Velasco M, Agrawal S, Paz-Garcia M, Gupta S, et al. : Transition of Macrophages to Fibroblast-Like Cells in Healing Myocardial Infarction. J Am Coll Cardiol 2019, 74:3124–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, SC JL, Aronow BJ, Tallquist MD, et al. : Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun 2016, 7:12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore-Morris T, Cattaneo P, Guimaraes-Camboa N, Bogomolovas J, Cedenilla M, Banerjee I, Ricote M, Kisseleva T, Zhang L, Gu Y, et al. : Infarct Fibroblasts Do Not Derive From Bone Marrow Lineages. Circ Res 2018, 122:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen B, Frangogiannis NG: Chemokines in Myocardial Infarction. J Cardiovasc Transl Res 2020. [DOI] [PubMed] [Google Scholar]
- 19.Dewald O, Frangogiannis NG, Zoerlein M, Duerr GD, Klemm C, Knuefermann P, Taffet G, Michael LH, Crapo JD, Welz A, et al. : Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proc Natl Acad Sci U S A 2003, 100:2700–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frangogiannis NG, Shimoni S, Chang SM, Ren G, Shan K, Aggeli C, Reardon MJ, Letsou GV, Espada R, Ramchandani M, et al. : Evidence for an active inflammatory process in the hibernating human myocardium. Am J Pathol 2002, 160:1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng Y, Chen H, Cai J, Zou L, Yan D, Xu G, Li D, Chao W: Cardiac RNA induces inflammatory responses in cardiomyocytes and immune cells via Toll-like receptor 7 signaling. J Biol Chem 2015, 290:26688–26698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suetomi T, Miyamoto S, Brown JH: Inflammation in nonischemic heart disease: initiation by cardiomyocyte CaMKII and NLRP3 inflammasome signaling. Am J Physiol Heart Circ Physiol 2019, 317:H877–H890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Zhang YL, Lin QY, Liu Y, Guan XM, Ma XL, Cao HJ, Liu Y, Bai J, Xia YL, et al. : CXCL1-CXCR2 axis mediates angiotensin II-induced cardiac hypertrophy and remodelling through regulation of monocyte infiltration. Eur Heart J 2018, 39:1818–1831. [DOI] [PubMed] [Google Scholar]
- 24.Suetomi T, Willeford A, Brand CS, Cho Y, Ross RS, Miyamoto S, Brown JH: Inflammation and NLRP3 Inflammasome Activation Initiated in Response to Pressure Overload by Ca(2+)/Calmodulin-Dependent Protein Kinase II delta Signaling in Cardiomyocytes Are Essential for Adverse Cardiac Remodeling. Circulation 2018, 138:2530–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willeford A, Suetomi T, Nickle A, Hoffman HM, Miyamoto S, Heller Brown J: CaMKIIdelta-mediated inflammatory gene expression and inflammasome activation in cardiomyocytes initiate inflammation and induce fibrosis. JCI Insight 2018, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson MD, Canugovi C, Vendrov AE, Hayami T, Bowles DE, Krause KH, Madamanchi NR, Runge MS: NADPH Oxidase 4 Regulates Inflammation in Ischemic Heart Failure: Role of Soluble Epoxide Hydrolase. Antioxid Redox Signal 2019, 31:39–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, Kraemer D, Taffet G, Rollins BJ, Entman ML: Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation 2007, 115:584–592. [DOI] [PubMed] [Google Scholar]
- 28.Koyanagi M, Egashira K, Kitamoto S, Ni W, Shimokawa H, Takeya M, Yoshimura T, Takeshita A: Role of monocyte chemoattractant protein-1 in cardiovascular remodeling induced by chronic blockade of nitric oxide synthesis. Circulation 2000, 102:2243–2248. [DOI] [PubMed] [Google Scholar]
- 29.Kuwahara F, Kai H, Tokuda K, Takeya M, Takeshita A, Egashira K, Imaizumi T: Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension 2004, 43:739–745. [DOI] [PubMed] [Google Scholar]
- 30.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG: CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 2005, 96:881–889. [DOI] [PubMed] [Google Scholar]
- 31.Tan X, Hu L, Shu Z, Chen L, Li X, Du M, Sun D, Mao X, Deng S, Huang K, et al. : Role of CCR2 in the Development of Streptozotocin-Treated Diabetic Cardiomyopathy. Diabetes 2019, 68:2063–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakai N, Wada T, Furuichi K, Shimizu K, Kokubo S, Hara A, Yamahana J, Okumura T, Matsushima K, Yokoyama H, et al. : MCP-1/CCR2-dependent loop for fibrogenesis in human peripheral CD14-positive monocytes. J Leukoc Biol 2006, 79:555–563. [DOI] [PubMed] [Google Scholar]
- 33.Patel B, Bansal SS, Ismahil MA, Hamid T, Rokosh G, Mack M, Prabhu SD: CCR2(+) Monocyte-Derived Infiltrating Macrophages Are Required for Adverse Cardiac Remodeling During Pressure Overload. JACC Basic Transl Sci 2018, 3:230–244.*This study suggests a critical role for CCR2+monocyte-derived macrophages in fibrosis and remodeling of the pressure-overloaded myocardium.
- 34.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, et al. : Embryonic and Adult-Derived Resident Cardiac Macrophages Are Maintained through Distinct Mechanisms at Steady State and during Inflammation. Immunity 2014, 40:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dick SA, Macklin JA, Nejat S, Momen A, Clemente-Casares X, Althagafi MG, Chen J, Kantores C, Hosseinzadeh S, Aronoff L, et al. : Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol 2019, 20:29–39.*A study documenting protective effects of the resident cardiac macrophage population following injury.
- 36.Heidt T, Courties G, Dutta P, Sager H, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, et al. : Differential Contribution of Monocytes to Heart Macrophages in Steady-State and After Myocardial Infarction. Circ Res 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen B, Huang S, Su Y, Wu YJ, Hanna A, Brickshawana A, Graff J, Frangogiannis NG: Macrophage Smad3 Protects the Infarcted Heart, Stimulating Phagocytosis and Regulating Inflammation. Circ Res 2019, 125:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao HM, Weinheimer C, et al. : Tissue Resident CCR2- and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury. Circ Res 2019, 124:263–278.*A study suggesting distinct functional roles for CCR2+ and CCR2- resident cardiac macrophages following injury/
- 39.Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, Epelman S, Kreisel D, Liu Y, Itoh A, et al. : The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med 2018, 24:1234–1245.**A seminal study identifying and characterizing CCR2+ and CCR2- macrophages in remodeling human hearts.
- 40.Nevers T, Salvador AM, Velazquez F, Ngwenyama N, Carrillo-Salinas FJ, Aronovitz M, Blanton RM, Alcaide P: Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J Exp Med 2017, 214:3311–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kruglov EA, Nathanson RA, Nguyen T, Dranoff JA: Secretion of MCP-1/CCL2 by bile duct epithelia induces myofibroblastic transdifferentiation of portal fibroblasts. Am J Physiol Gastrointest Liver Physiol 2006, 290:G765–771. [DOI] [PubMed] [Google Scholar]
- 42.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG: Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol 2004, 164:665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lubos N, van der Gaag S, Gercek M, Kant S, Leube RE, Krusche CA: Inflammation shapes pathogenesis of murine arrhythmogenic cardiomyopathy. Basic Res Cardiol 2020, 115:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma Y, Chiao YA, Clark R, Flynn ER, Yabluchanskiy A, Ghasemi O, Zouein F, Lindsey ML, Jin YF: Deriving a cardiac ageing signature to reveal MMP-9-dependent inflammatory signalling in senescence. Cardiovasc Res 2015, 106:421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaya Z, Goser S, Buss SJ, Leuschner F, Ottl R, Li J, Volkers M, Zittrich S, Pfitzer G, Rose NR, et al. : Identification of cardiac troponin I sequence motifs leading to heart failure by induction of myocardial inflammation and fibrosis. Circulation 2008, 118:2063–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alard JE, Ortega-Gomez A, Wichapong K, Bongiovanni D, Horckmans M, Megens RT, Leoni G, Ferraro B, Rossaint J, Paulin N, et al. : Recruitment of classical monocytes can be inhibited by disturbing heteromers of neutrophil HNP1 and platelet CCL5. Sci Transl Med 2015, 7:317ra196. [DOI] [PubMed] [Google Scholar]
- 47.Montecucco F, Braunersreuther V, Lenglet S, Delattre BM, Pelli G, Buatois V, Guilhot F, Galan K, Vuilleumier N, Ferlin W, et al. : CC chemokine CCL5 plays a central role impacting infarct size and post-infarction heart failure in mice. Eur Heart J 2012, 33:1964–1974. [DOI] [PubMed] [Google Scholar]
- 48.Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG: CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol 2010, 176:2177–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saxena A, Dobaczewski M, Rai V, Haque Z, Chen W, Li N, Frangogiannis NG: Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Am J Physiol Heart Circ Physiol 2014, 307:H1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krebs C, Fraune C, Schmidt-Haupt R, Turner JE, Panzer U, Quang MN, Tannapfel A, Velden J, Stahl RA, Wenzel UO: CCR5 deficiency does not reduce hypertensive end-organ damage in mice. Am J Hypertens 2012, 25:479–486. [DOI] [PubMed] [Google Scholar]
- 51.Mor A, Segal Salto M, Katav A, Barashi N, Edelshtein V, Manetti M, Levi Y, George J, Matucci-Cerinic M: Blockade of CCL24 with a monoclonal antibody ameliorates experimental dermal and pulmonary fibrosis. Ann Rheum Dis 2019, 78:1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diny NL, Hou X, Barin JG, Chen G, Talor MV, Schaub J, Russell SD, Klingel K, Rose NR, Cihakova D: Macrophages and cardiac fibroblasts are the main producers of eotaxins and regulate eosinophil trafficking to the heart. Eur J Immunol 2016, 46:2749–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z, Cui M, Shah AM, Ye W, Tan W, Min YL, Botten GA, Shelton JM, Liu N, Bassel-Duby R, et al. : Mechanistic basis of neonatal heart regeneration revealed by transcriptome and histone modification profiling. Proc Natl Acad Sci U S A 2019, 116:18455–18465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, et al. : The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem 1995, 270:27348–27357. [DOI] [PubMed] [Google Scholar]
- 55.Zhang YL, Geng C, Yang J, Fang J, Yan X, Li PB, Zou LX, Chen C, Guo SB, Li HH, et al. : Chronic inhibition of chemokine receptor CXCR2 attenuates cardiac remodeling and dysfunction in spontaneously hypertensive rats. Biochim Biophys Acta Mol Basis Dis 2019, 1865:165551. [DOI] [PubMed] [Google Scholar]
- 56.Zhang YL, Cao HJ, Han X, Teng F, Chen C, Yang J, Yan X, Li PB, Liu Y, Xia YL, et al. : Chemokine Receptor CXCR-2 Initiates Atrial Fibrillation by Triggering Monocyte Mobilization in Mice. Hypertension 2020, 76:381–392. [DOI] [PubMed] [Google Scholar]
- 57.Martinod K, Witsch T, Erpenbeck L, Savchenko A, Hayashi H, Cherpokova D, Gallant M, Mauler M, Cifuni SM, Wagner DD: Peptidylarginine deiminase 4 promotes age-related organ fibrosis. J Exp Med 2017, 214:439–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chrysanthopoulou A, Mitroulis I, Apostolidou E, Arelaki S, Mikroulis D, Konstantinidis T, Sivridis E, Koffa M, Giatromanolaki A, Boumpas DT, et al. : Neutrophil extracellular traps promote differentiation and function of fibroblasts. J Pathol 2014, 233:294–307. [DOI] [PubMed] [Google Scholar]
- 59.Weckbach LT, Grabmaier U, Uhl A, Gess S, Boehm F, Zehrer A, Pick R, Salvermoser M, Czermak T, Pircher J, et al. : Midkine drives cardiac inflammation by promoting neutrophil trafficking and NETosis in myocarditis. J Exp Med 2019, 216:350–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frangogiannis NG, Mendoza LH, Lewallen M, Michael LH, Smith CW, Entman ML: Induction and suppression of interferon-inducible protein 10 in reperfused myocardial infarcts may regulate angiogenesis. FASEB J 2001, 15:1428–1430. [DOI] [PubMed] [Google Scholar]
- 61.Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, Veeranna V, Tager AM, Luster AD, Frangogiannis NG: Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res 2009, 105:973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saxena A, Bujak M, Frunza O, Dobaczewski M, Gonzalez-Quesada C, Lu B, Gerard C, Frangogiannis NG: CXCR3-independent actions of the CXC chemokine CXCL10 in the infarcted myocardium and in isolated cardiac fibroblasts are mediated through proteoglycans. Cardiovasc Res 2014, 103:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lindsey ML, Jung M, Yabluchanskiy A, Cannon PL, Iyer RP, Flynn ER, DeLeon-Pennell KY, Valerio FM, Harrison CL, Ripplinger CM, et al. : Exogenous CXCL4 infusion inhibits macrophage phagocytosis by limiting CD36 signalling to enhance post-myocardial infarction cardiac dilation and mortality. Cardiovasc Res 2019, 115:395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mueller A, Meiser A, McDonagh EM, Fox JM, Petit SJ, Xanthou G, Williams TJ, Pease JE: CXCL4-induced migration of activated T lymphocytes is mediated by the chemokine receptor CXCR3. J Leukoc Biol 2008, 83:875–882. [DOI] [PubMed] [Google Scholar]
- 65.Vajen T, Koenen RR, Werner I, Staudt M, Projahn D, Curaj A, Sonmez TT, Simsekyilmaz S, Schumacher D, Mollmann J, et al. : Blocking CCL5-CXCL4 heteromerization preserves heart function after myocardial infarction by attenuating leukocyte recruitment and NETosis. Sci Rep 2018, 8:10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T: Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996, 382:635–638. [DOI] [PubMed] [Google Scholar]
- 67.Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G: Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood 2002, 99:2703–2711. [DOI] [PubMed] [Google Scholar]
- 68.Salcedo R, Wasserman K, Young HA, Grimm MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ, Oppenheim JJ: Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: In vivo neovascularization induced by stromal-derived factor-1alpha. Am J Pathol 1999, 154:1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doring Y, Pawig L, Weber C, Noels H: The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease. Front Physiol 2014, 5:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Penn MS, Pastore J, Miller T, Aras R: SDF-1 in myocardial repair. Gene Ther 2012, 19:583–587. [DOI] [PubMed] [Google Scholar]
- 71.Chu PY, Joshi MS, Horlock D, Kiriazis H, Kaye DM: CXCR4 Antagonism Reduces Cardiac Fibrosis and Improves Cardiac Performance in Dilated Cardiomyopathy. Front Pharmacol 2019, 10:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chu PY, Walder K, Horlock D, Williams D, Nelson E, Byrne M, Jandeleit-Dahm K, Zimmet P, Kaye DM: CXCR4 Antagonism Attenuates the Development of Diabetic Cardiac Fibrosis. PLoS One 2015, 10:e0133616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chu PY, Zatta A, Kiriazis H, Chin-Dusting J, Du XJ, Marshall T, Kaye DM: CXCR4 antagonism attenuates the cardiorenal consequences of mineralocorticoid excess. Circ Heart Fail 2011, 4:651–658. [DOI] [PubMed] [Google Scholar]
- 74.Li X, Weng X, Shi H, Gao R, Wang P, Jia D, Zhang S, Dong Z, Sun X, Yang J, et al. : Acetaldehyde dehydrogenase 2 deficiency exacerbates cardiac fibrosis by promoting mobilization and homing of bone marrow fibroblast progenitor cells. J Mol Cell Cardiol 2019, 137:107–118. [DOI] [PubMed] [Google Scholar]
- 75.Chu PY, Mariani J, Finch S, McMullen JR, Sadoshima J, Marshall T, Kaye DM: Bone marrow-derived cells contribute to fibrosis in the chronically failing heart. Am J Pathol 2010, 176:1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jackson EK, Zhang Y, Gillespie DD, Zhu X, Cheng D, Jackson TC: SDF-1alpha (Stromal Cell-Derived Factor 1alpha) Induces Cardiac Fibroblasts, Renal Microvascular Smooth Muscle Cells, and Glomerular Mesangial Cells to Proliferate, Cause Hypertrophy, and Produce Collagen. J Am Heart Assoc 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Di Maggio S, Milano G, De Marchis F, D’Ambrosio A, Bertolotti M, Palacios BS, Badi I, Sommariva E, Pompilio G, Capogrossi MC, et al. : Non-oxidizable HMGB1 induces cardiac fibroblasts migration via CXCR4 in a CXCL12-independent manner and worsens tissue remodeling after myocardial infarction. Biochim Biophys Acta Mol Basis Dis 2017, 1863:2693–2704. [DOI] [PubMed] [Google Scholar]
- 78.Menhaji-Klotz E, Hesp KD, Londregan AT, Kalgutkar AS, Piotrowski DW, Boehm M, Song K, Ryder T, Beaumont K, Jones RM, et al. : Discovery of a Novel Small-Molecule Modulator of C-X-C Chemokine Receptor Type 7 as a Treatment for Cardiac Fibrosis. J Med Chem 2018, 61:3685–3696. [DOI] [PubMed] [Google Scholar]
- 79.Boag SE, Das R, Shmeleva EV, Bagnall A, Egred M, Howard N, Bennaceur K, Zaman A, Keavney B, Spyridopoulos I: T lymphocytes and fractalkine contribute to myocardial ischemia/reperfusion injury in patients. J Clin Invest 2015, 125:3063–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karlmark KR, Zimmermann HW, Roderburg C, Gassler N, Wasmuth HE, Luedde T, Trautwein C, Tacke F: The fractalkine receptor CX(3)CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology 2010, 52:1769–1782. [DOI] [PubMed] [Google Scholar]
- 81.Shimizu K, Furuichi K, Sakai N, Kitagawa K, Matsushima K, Mukaida N, Kaneko S, Wada T: Fractalkine and its receptor, CX3CR1, promote hypertensive interstitial fibrosis in the kidney. Hypertens Res 2011, 34:747–752. [DOI] [PubMed] [Google Scholar]
- 82.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ: The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007, 204:3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muller I, Pappritz K, Savvatis K, Puhl K, Dong F, El-Shafeey M, Hamdani N, Hamann I, Noutsias M, Infante-Duarte C, et al. : CX3CR1 knockout aggravates Coxsackievirus B3-induced myocarditis. PLoS One 2017, 12:e0182643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xuan W, Liao Y, Chen B, Huang Q, Xu D, Liu Y, Bin J, Kitakaze M: Detrimental effect of fractalkine on myocardial ischaemia and heart failure. Cardiovasc Res 2011, 92:385–393. [DOI] [PubMed] [Google Scholar]
- 85.Ahadzadeh E, Rosendahl A, Czesla D, Steffens P, Prussner L, Meyer-Schwesinger C, Wanner N, Paust HJ, Huber TB, Stahl RAK, et al. : The chemokine receptor CX3CR1 reduces renal injury in mice with angiotensin II-induced hypertension. Am J Physiol Renal Physiol 2018, 315:F1526–F1535. [DOI] [PubMed] [Google Scholar]
- 86.de Zeeuw D, Bekker P, Henkel E, Hasslacher C, Gouni-Berthold I, Mehling H, Potarca A, Tesar V,H eerspink HJ, Schall TJ, et al. : The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol 2015, 3:687–696. [DOI] [PubMed] [Google Scholar]
- 87.Menne J, Eulberg D, Beyer D, Baumann M, Saudek F, Valkusz Z, Wiecek A, Haller H, Emapticap Study G: C-C motif-ligand 2 inhibition with emapticap pegol (NOX-E36) in type 2 diabetic patients with albuminuria. Nephrol Dial Transplant 2017, 32:307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bowler S, Siriwardhana C, Mitchell BI, D’Antoni ML, Ogata-Arakaki D, Souza S, Yee R, Gangcuangco LMA, Chow DC, Ndhlovu LC, et al. : Cenicriviroc, a dual CCR2 and CCR5 antagonist leads to a reduction in plasma fibrotic biomarkers in persons living with HIV on antiretroviral therapy. HIV Res Clin Pract 2019, 20:123–129. [DOI] [PubMed] [Google Scholar]
- 89.Friedman S, Sanyal A, Goodman Z, Lefebvre E, Gottwald M, Fischer L, Ratziu V: Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemp Clin Trials 2016, 47:356–365. [DOI] [PubMed] [Google Scholar]
- 90.Ratziu V, Sanyal A, Harrison SA, Wong VW, Francque S, Goodman Z, Aithal GP, Kowdley KV, Seyedkazemi S, Fischer L, et al. : Cenicriviroc Treatment for Adults with Nonalcoholic Steatohepatitis and Fibrosis: Final Analysis of the Phase 2b CENTAUR Study. Hepatology 2020, doi: 10.1002/hep.31108 Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 91.Schiattarella GG, Rodolico D, Hill JA: Metabolic Inflammation in Heart Failure with Preserved Ejection Fraction. Cardiovasc Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paulus WJ, Tschope C: A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013, 62:263–271. [DOI] [PubMed] [Google Scholar]
- 93.Hulsmans M, Sager HB, Roh JD, Valero-Munoz M, Houstis NE, Iwamoto Y, Sun Y, Wilson RM, Wojtkiewicz G, Tricot B, et al. : Cardiac macrophages promote diastolic dysfunction. J Exp Med 2018, 215:423–440.*This study demonstrates an important role for cardiac macrophages in regulating diastolic function, thus supporting their potential involvement in the pathogenesis of HFpEF.
- 94.Hoefer IE, van Royen N, Buschmann IR, Piek JJ, Schaper W: Time course of arteriogenesis following femoral artery occlusion in the rabbit. Cardiovasc Res 2001, 49:609–617. [DOI] [PubMed] [Google Scholar]
- 95.Saxena A, Fish JE, White MD, Yu S, Smyth JW, Shaw RM, DiMaio JM, Srivastava D: Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation 2008, 117:2224–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kanki S, Segers VF, Wu W, Kakkar R, Gannon J, Sys SU, Sandrasagra A, Lee RT: Stromal cell-derived factor-1 retention and cardioprotection for ischemic myocardium. Circ Heart Fail 2011, 4:509–518. [DOI] [PubMed] [Google Scholar]
- 97.Sager HB, Hulsmans M, Lavine KJ, Moreira MB, Heidt T, Courties G, Sun Y, Iwamoto Y, Tricot B, Khan OF, et al. : Proliferation and Recruitment Contribute to Myocardial Macrophage Expansion in Chronic Heart Failure. Circ Res 2016, 119:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, Prabhu SD: Dysfunctional and Proinflammatory Regulatory T-Lymphocytes Are Essential for Adverse Cardiac Remodeling in Ischemic Cardiomyopathy. Circulation 2019, 139:206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Toyozaki T, Saito T, Shiraishi H, Tsukamoto Y, Takano H, Nagai T, Hiroshima K, Ohwada H, Ishiyama S, Hiroe M: Macrophage inflammatory protein-1alpha relates to the recruitment of inflammatory cells in myosin-induced autoimmune myocarditis in rats. Lab Invest 2001, 81:929–936. [DOI] [PubMed] [Google Scholar]
- 100.Goser S, Ottl R, Brodner A, Dengler TJ, Torzewski J, Egashira K, Rose NR, Katus HA, Kaya Z: Critical role for monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha in induction of experimental autoimmune myocarditis and effective anti-monocyte chemoattractant protein-1 gene therapy. Circulation 2005, 112:3400–3407. [DOI] [PubMed] [Google Scholar]
- 101.Cook DN, Beck MA, Coffman TM, Kirby SL, Sheridan JF, Pragnell IB, Smithies O: Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science 1995, 269:1583–1585. [DOI] [PubMed] [Google Scholar]
- 102.Kukielka GL, Smith CW, LaRosa GJ, Manning AM, Mendoza LH, Daly TJ, Hughes BJ, Youker KA, Hawkins HK, Michael LH, et al. : Interleukin-8 gene induction in the myocardium after ischemia and reperfusion in vivo. J Clin Invest 1995, 95:89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ivey CL, Williams FM, Collins PD, Jose PJ, Williams TJ: Neutrophil chemoattractants generated in two phases during reperfusion of ischemic myocardium in the rabbit. Evidence for a role for C5a and interleukin-8. J Clin Invest 1995, 95:2720–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Segret A, Rucker-Martin C, Pavoine C, Flavigny J, Deroubaix E, Chatel MA, Lombet A, Renaud JF: Structural localization and expression of CXCL12 and CXCR4 in rat heart and isolated cardiac myocytes. J Histochem Cytochem 2007, 55:141–150. [DOI] [PubMed] [Google Scholar]
- 105.Ble A, Mosca M, Di Loreto G, Guglielmotti A, Biondi G, Bombardieri S, Remuzzi G, Ruggenenti P: Antiproteinuric effect of chemokine C-C motif ligand 2 inhibition in subjects with acute proliferative lupus nephritis. Am J Nephrol 2011, 34:367–372. [DOI] [PubMed] [Google Scholar]
- 106.Raghu G, Martinez FJ, Brown KK, Costabel U, Cottin V, Wells AU, Lancaster L, Gibson KF, Haddad T, Agarwal P, et al. : CC-chemokine ligand 2 inhibition in idiopathic pulmonary fibrosis: a phase 2 trial of carlumab. Eur Respir J 2015, 46:1740–1750. [DOI] [PubMed] [Google Scholar]
- 107.Sherman KE, Abdel-Hameed E, Rouster SD, Shata MTM, Blackard JT, Safaie P, Kroner B, Preiss L, Horn PS, Kottilil S: Improvement in Hepatic Fibrosis Biomarkers Associated With Chemokine Receptor Inactivation Through Mutation or Therapeutic Blockade. Clin Infect Dis 2019, 68:1911–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]