Introduction
Humans are exposed to a wide range of air pollutants known to be associated with adverse health effects. These pollutants can be associated with a wide range of activities and sources, including: the burning of fossil fuels, traffic-related emissions, industrial activities, agricultural activities, and wildfires, as well as a diverse group of pollutants that originate indoors (Figure 1). For an individual, their totality of exposure is determined by concentrations of pollutants in the various microenvironments where they spend time (home, school, work, etc.), and their time activity patterns (how much time is spent in each of these spaces). Indoor exposures are strongly influenced by the characteristics of the building, its location, and ambient sources, due to the exchange of air between indoor and outdoor spaces. Because of these relationships, and due to the fact that most individuals spend the majority of their time indoors, the majority of human exposure to pollutants of outdoor origin actually occurs indoors. This review will focus on ambient pollutants, those which are emitted or formed in the atmosphere and primarily measured by outdoor monitoring stations or models that depend on outdoor metrics.
Figure 1.
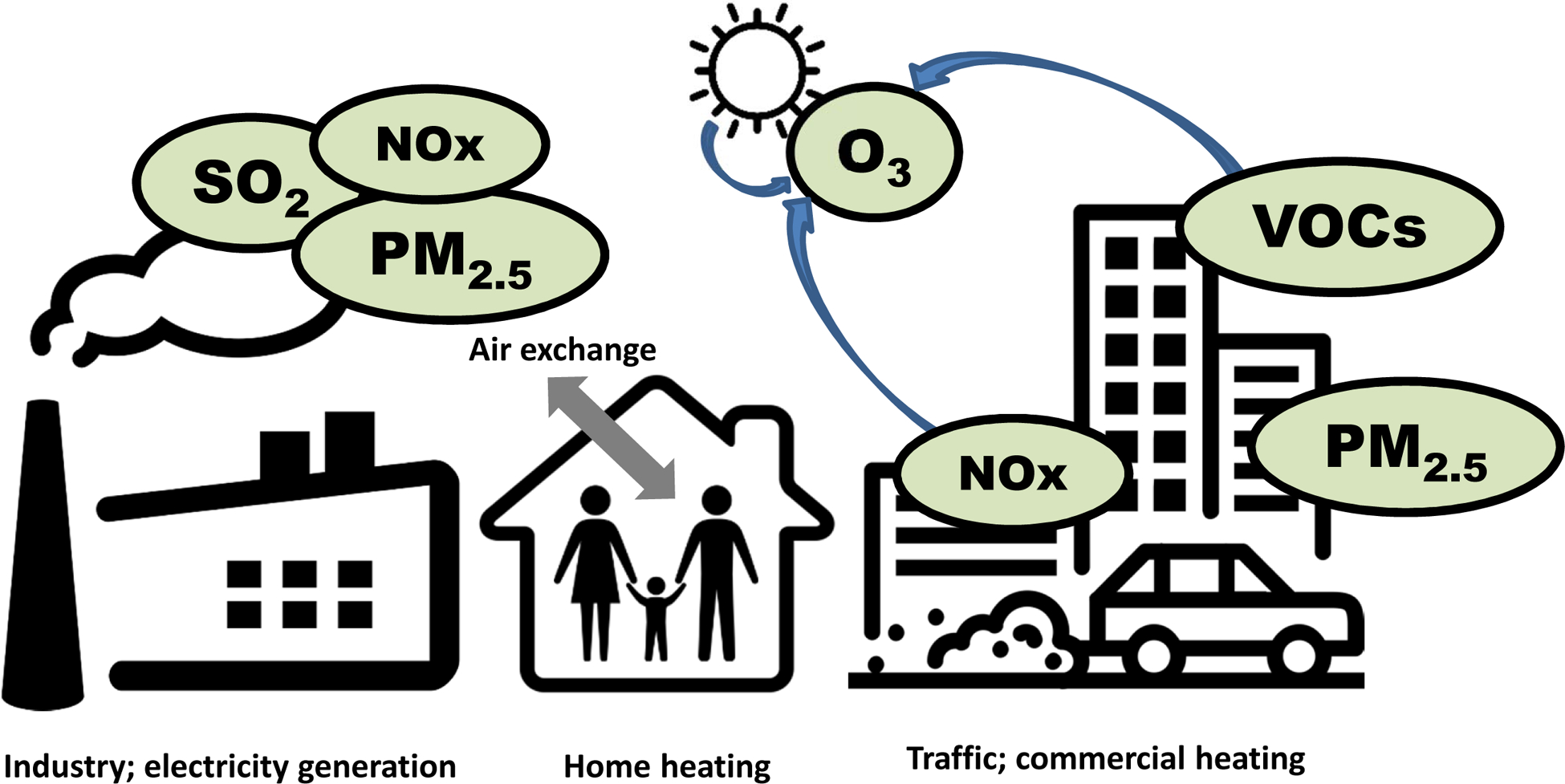
Key sources of ambient air pollutants. Schematic of source apportionment for major ambient/outdoor air pollutants. SO2, sulfur dioxide; NOx, oxides of nitrogen; PM2.5, particulate matter less than 2.5 microns in diameter; O3, ozone; VOCs, volatile organic compounds.
Any process that includes fossil fuel combustion is likely releasing pollutants that have been shown to cause health effects. Traffic-related emissions (including diesel), combustion related to electricity production (coal, natural gas, etc.), and other fuel burning can release particulate matter, oxides of nitrogen and other pollutants into the atmosphere. Once released, these pollutants can be transported, removed and/or chemically transformed.
Air pollution exposure has been implicated in myriad disease processes in almost every organ system1 and is currently the 5th highest risk factor for mortality worldwide2. A recent estimate shows that outdoor air pollution, mostly by driven by exposure to fine particulate matter (PM2.5, described below), leads to 3.3 (95% confidence interval 1.61–4.81) million premature deaths per year worldwide, with most of these deaths occurring in Asia3. These risks are in addition to the significant global burden of disease from household air pollution from the burning of solid fuels for cooking and heating4. In general, ambient air pollution levels have increased in low- and middle-income countries2, while notable progress has been made in the U.S.5 In addition to these global differences in exposure and health effects, many studies have highlighted the disproportionate burden of air pollution within low-income populations and communities of color in the U.S.6
We focus this article on key air pollutants (see Table 1) that have an extensive evidence base linking exposure to respiratory health effects with the exception of wildfire smoke which is addressed elsewhere in this issue. We aim to describe the most important ambient pollutants implicated in human health and disease and discuss the sources of ambient pollution. Recognizing that many of these individual pollutants are encountered as pollutant mixtures, we will outline the major respiratory risks of ambient pollution, as a whole and fractioned by pollutant or source where data is available. Focus will be on the inception, exacerbation and mortality related to chronic respiratory diseases and vulnerable populations. As the burden of air pollution on respiratory morbidity and mortality is high, clinicians must be aware of the potentially harmful exposures in order to properly advise vulnerable patients on avoidance strategies and/or affect change through public policy efforts.
Table 1.
Overview of ambient air pollutant sources and health effects. Data from Refs7, 19, 20, 27, 37, 39, 45, 55–57, 63, 64, 89, 96, 105, 130–133
| Key pollutants | Emission sources | Key health effects |
|---|---|---|
| Particulate matter (PM) |
|
|
| Ozone (O3) |
|
|
| Nitrogen dioxide (NO2) |
|
|
| Sulfur dioxide (SO2) |
|
|
| Traffic-related air pollution (TRAP) |
|
Particulate Matter –
The term particulate matter (PM) describes the mixture of solid and/or liquid particles suspended in air, which may or may not be visible. These particles are generated through mechanical or chemical processes and can be either directly emitted or can form through chemical reactions in the air. For example, PM can be generated from wildfires, combustion of fossil fuels (e.g., oil, gasoline, diesel), industrial sources, as well as from the suspension of road dust and soils. PM is often classified by the size fractions that determine the pattern of capture, deposition and transport in the lung, and thus directly affects the extent of human exposure and related health effects. For example, PM10 refers to particles that are smaller than 10 microns in diameter and PM2.5 refers to particles smaller than 2.5 microns in diameter. Both of these fractions are considered inhalable, with the smallest particles, such as PM2.5 or smaller fractions having a higher likelihood to be deposited deep in the lung. A recent systematic review7 summarized the literature that has estimated source apportionment of particulate matter, and found that globally 25% of urban ambient PM2.5 is generated by traffic, 15% by industrial activities, 20% by domestic fuel burning, 22% from unspecified sources of human origin, and 18% from natural dust and salt. Similarly, utilizing data from a national network of PM2.5 monitors with chemical speciation, Thurston et al.8 identified the major PM2.5 sources in the U.S. as (in no particular order) motor vehicle traffic, metals industry, crustal/soil particles, steel industry, coal combustion, oil combustion, salt particles and biomass burning. While motor vehicle emissions were ubiquitous, the relative contribution of these sources varied significantly by region.
While significant progress has been made in reducing PM2.5 in the U.S., exposure disparities across economic and racial lines are notable. A 2018 analysis of PM-emitting facilities in the United States showed that those living in poverty had 1.35 times higher burden than did the overall population, and that black populations had a 1.54 times higher burden than did the overall population9,10. A recent study focused on ambient monitoring data in Massachusetts showed that racial disparities in population-weighted concentrations increased over time, although overall exposures have decreased during the same period. Due to the effects of urbanization, differences in motor vehicle fleets, fuel usage patterns and other factors, the global exposure picture is troubling. A 2018 assessment showed that 95% of the world’s population lived in areas where ambient PM2.5 levels exceeded the World Health Organization 10 μg/m3 (annual average) guideline11.
It has long been known that the size and composition of airborne particles can strongly influence exposure and the observed patterns of health effects12. Another fraction — ultrafine particles (UFP), defined as particles smaller than 0.1 microns in diameter — have been suggested as an important component of airborne particles that may be mechanistically responsible for some health effects13. While ultrafine particles are not regulated (like PM2.5 and other particulate matter fractions), the health effects of these exposures remain an active and important area of investigation.
Ozone –
Ozone, a key component of smog, is not emitted directly, but rather is formed in the atmosphere through complex series of photochemical reactions involving two other classes of pollutants: oxides of nitrogen (NOx) and volatile organic compounds (VOCs). In addition to its health effects, ozone is known to affect agricultural output and damage materials. Due to the photochemical origins of ozone, large urban areas located in warmer climates are more likely to experience high ozone levels. Ozone hotspots are frequently located downwind of emission sources, due to the timescales of the underlying chemical reactions.
NO2 –
Nitrogen dioxide (NO2) is formed during high temperature combustion and is both relevant for its direct effects on health as well as its role as a precursor of ozone. In urban areas, the most significant contributor to the levels and patterns of nitrogen dioxide exposure is traffic. Power plants and other industrial sources that burn fossil fuels are also major emitters of NO2 (and other oxides of nitrogen). Personal exposures to nitrogen dioxide are influenced by these ambient sources, as well as indoor combustion sources, such as emissions from gas stoves.
SO2 –
When burned, sulfur-containing fuels, such as coal, can release significant quantities of sulfur dioxide. While SO2 can be released naturally (e.g. volcanoes), most emissions are anthropogenic, from power plants, pulp and paper production, smelter and steel mill operations, and other industrial operations14. Historically, the focus on controlling SO2 emissions was based on its presumed contributions to acid rain, and not health effects.
TRAP/diesel –
Given the range of pollutants released from gasoline- and diesel-powered vehicles and the potential range of health effects, it has been convenient to refer to this pollutant mix as traffic-related air pollution, or TRAP. This designation is aligned with the large body of evidence that shows a consistent pattern of health effects associated with being exposed to high traffic density or living close to major roadways. Numerous exposure-focused studies have documented the influence of roadways on the concentration gradients for several air pollutants near these sources15.
The ability to estimate human exposure to ambient pollutants has evolved substantially over the past several decades. The primary measurement direct measurement devices for population-based pollution research have been central monitoring sites that are able to measure real-time and filter-based particle and gas composition in the local environment. However, sophisticated modeling that incorporates land use, green space, and other factors that affect the local exposure profiles have greatly enhanced the precision of these measures and ability to extrapolate from central monitors to discrete nearby locations. The use of satellite imagery to further enhance predictive models has led to accurate ground estimates as small as 1km across large portions of the U.S. More recently, the availability of low-cost sensors allows for more fine-scale or personal assessment of exposure to pollutants released by traffic and other urban sources. For example, recent studies reveal the small-scale variation of air pollution at the neighborhood level, and the potential associations with health16,17.
Health effects
Population health
Air pollution health effects are seen on a population scale in both healthy and vulnerable subjects. The World Health Organization estimates that ambient pollution is responsible for 4.2 million premature deaths annually18. Several studies in the developed and developing world have consistently demonstrated a higher risk of all-cause mortality in people exposed to high levels of pollutants. Liu and colleagues from the Multi-City Multi-Country (MCC) Collaborative Research Network recently reported on the effects of daily particulate exposure with daily mortality among 652 cities from 24 countries or regions19. Daily mortality increased 0.68% for each 10 μg/m3 increase in PM2.5. A recent study incorporating data from counties across the US from 1999 to 2015 observed excess PM2.5 was responsible for approximately 30,000 premature deaths, lowering the estimated national life expectancy by 0.15 years20. In counties where PM2.5 was reduced over the 15-year period, so did mortality. Highlighting these health effects, changes in emissions as a result of the U.S. Clean Air Act and its amendments over a 20 year period from 1980 – 2000 revealed that a 10 μg /m3 improvement in PM2.5 resulted in mean increased life expectancy of greater than 7 months across 51 US cities, which accounted for 15% of improved longevity overall5,21. These findings are consistent with other cohort studies over the same time period22,23. In 2011, the U.S. Environmental Protection Agency (EPA) estimated that improved PM exposure would result in 230,000 adult lives saved by 202024. Most notably, the dose-response of the relationship between particulate exposure and mortality is fairly consistent across concentration ranges, even at ambient levels below national and international thresholds19,20.
Respiratory specific mortality is an even higher individual risk. In the MCC data investigated by Liu et al, the corresponding increase in daily mortality from respiratory events was 0.74% for each 10 μg /m3 increase in PM2.5, higher than both all-cause and cardiovascular mortality attribution19. Respiratory mortality is driven by chronic obstructive pulmonary disease (COPD) and lung cancer, for which the estimates of respiratory mortality from the Global Burden of Disease study are >800,0000 persons and 280,000 in 2015, respectively2. However, in contrast to these provocative mortality data, a recent prospective study with individual level data within the multicenter European Study of Cohorts for Air Pollution Effects (ESCAPE) found no significant association of air pollution exposure with non-malignant respiratory mortality among 1559 deaths from 307,553 subjects across 16 cohorts25.
Pollutant transport to the respiratory tree
The size of the particulate and the solubility of the pollutant dictate the type of symptoms that are induced by pollutant exposure. Generally speaking, large particles are filtered by impaction in the upper airway and natural cleaning mechanisms in the nose (nasal hair and turbulent flow) and upper airway. Particle sizes 10 microns and above tend to be deposited in the upper airway and may cause nasal congestion and/or mucous membrane irritation of the eye. PM2.5 is small enough to reach the small airways and deposit as deep as the alveolar space. For this reason, PM2.5 is most highly associated with lower respiratory tract diseases, including asthma and airway infections.
Ultrafine particles freely cross the alveolar capillary membranes and can be transported to all organ systems. For gaseous pollutants, tissue specificity depends more on solubility profile. For example, water soluble pollutants, such as sulfur dioxide, cause eye and mucous membrane irritation in the upper airway. NO2 and O3 are less soluble and therefore penetrate deeper into the lower respiratory tract and lung1. However, it is important to recognize that real-world encounters with ambient pollutants are largely with pollutant mixtures that may contain a variety of particle sizes and gaseous components. Many studies have attempted to disentangle the effects of different pollutants in multipollutant models, but this is complicated by the number of contaminants and the variety of elemental components26,27 that contribute to particle mass at different levels.
Mechanism of biological effects
Mechanistic studies to determine how air pollution affects human health have uncovered several plausible pathways28, including oxidative stress and damage, innate29 and adaptive immunologic/inflammatory responses, allergic sensitization and Th2 airways inflammation, alteration of epigenetic loci that influences airways development, and direct effects on neuromuscular function of the airways.
Air pollution exposure may cause a variety of inflammatory changes in the airways which depend on the type of pollutant exposure, elemental composition of the particles and concurrent exposures. Additionally, host factors may play a significant role in the type of the inflammatory response. The inflammatory response to naturally occurring pollutant mixtures and co-exposure to other environmental factors are inherently difficult to systematically study due to the countless variables that would need to be accounted, so inferences are drawn from carefully designed single and co-exposure cell, animal and human studies. Perhaps the most ubiquitous response to air pollution exposure found in human studies has been the association with airways and lung injury via oxidative stress30,31 mechanisms which increase neutrophilic airway inflammation. Controlled human exposure studies have demonstrated increased levels of inflammatory cells, primarily neutrophils, but also mast cells and lymphocytes, along with increased inflammatory mediators, such as IL-8 and IL-13 in healthy volunteers32,33. Gene variants in the glutathione pathway have been shown to influence the effect of ambient pollutant exposure on lung function in children34,35, further supporting the role of oxidative stress as a mechanism in the adverse health effects of pollution exposure.
At-risk populations of patients with underlying asthma and atopy have found increased allergic responses, such as nasal and airway eosinophilia in response to exposure36. Moreover, studies of co-exposure of NO2 or PM with allergens has found these pollutants to have an adjuvant effect on allergic inflammatory response. Fine particles may be important conduits in which adherent allergens may reach the lower respiratory tract or cause epithelial disruption due to oxidative stress37.
Additionally, diesel exhaust particles, a major component of urban pollution, have been shown to affect the vagal afferent nerves in in vitro and in vivo models by depolarization of the vagus nerve via airway c-fiber afferents, which could lead to respiratory symptoms by a direct neurostimulatory effect on airway smooth muscle38.
Respiratory symptoms
These biologic mechanisms affect respiratory symptoms in both general and vulnerable populations. In studies involving general populations of children and adults, exposure to particulate pollution has been associated with regular cough, wheezing and breathlessness27,39 which improve with reduction of pollutant levels40. A few case-control studies in children and adults have demonstrated higher rates of non-infectious conjunctivitis and conjunctival irritation associated with urban pollutant exposure41,42. Moreover, chronic exposure to air pollutants have been associated with dry eye disease across populations43,44.
Alteration of lung function
Chronic exposure to ambient pollution has been linked to impaired lung growth in children and progressive lung function decline in adult populations. Rice and colleagues evaluated chronic pollutant effects on early lung function development in a large birth cohort study and found that both proximity to roadway and home address estimates of black carbon (BC) and PM2.5 in the first seven years of life was associated with low forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) without significantly impacting the ratio of FEV1/FVC45. These data suggest that early life exposure to pollutants affects lung growth in a restrictive pattern rather than airway obstruction. In adolescence, the effects are similar. Perhaps the most demonstrative examples are from the California Health Study which demonstrated across three distinct time periods the association of ambient NO2, PM2.5 and BC with poor lung function in children46,47. While the most significant effects were reported for FEV1, the effect on FVC and maximal mid-expiratory flows were similarly reduced. Importantly, for children who moved from high exposure locations to lower exposures, there was a significant improvement in lung function48, highlighting that intervention on an individual level can alter long term lung growth. These findings extend to the prenatal period in which maternal exposure is associated with lower lung function in the child, as well as preterm birth49, which itself is associated with poor lung function outcomes50. These data suggest that perhaps alteration in DNA methylation is responsible for altered lung formation rather than direct effects of airway inflammation51. However, these associations have not been found in all cohorts52. The summation of the prenatal and pediatric findings are particularly salient as more and more data supports that long term lung function trajectories are determined in childhood53,54.
In adults, several longitudinal studies have demonstrated exposure to particulate pollutants are associated with lower lung function in cross-sectional and longitudinal analyses55–57. Moreover, across repeated measures, the Normative Aging Study found higher annual BC concentration was associated with accelerated rate of decline in FEV1 and FVC for elderly men living in Boston, MA USA55. Results from studies documenting the improvement in lung function in concert with decreased ambient PM add more convincing evidence of the causal effect of long term pollution on lung function outcomes58,59. The SAPALDIA study, a Swiss population-based study sampling over 9000 adults, demonstrated a 10 μg/m3 decrease in PM10 concentration was associated with a 9% decrease in annual FEV158.
Ambient pollution and respiratory disease
The effects of ambient pollutant exposure on respiratory symptoms and lung function in the general population are amplified in vulnerable populations of adults and children with existing lung disease. Moreover, for many chronic lung diseases, ambient exposure to particulates and gaseous pollutants have been implicated in the inception of the disease process. In the following sections, we detail the relationship of ambient pollution exposure to the inception and exacerbation of chronic lung diseases, focusing primarily on asthma and COPD, which represent the most common non-cancer lung diseases of childhood and adulthood. Figure 2 illustrates respiratory diseases affected by ambient air pollutants.
Figure 2.
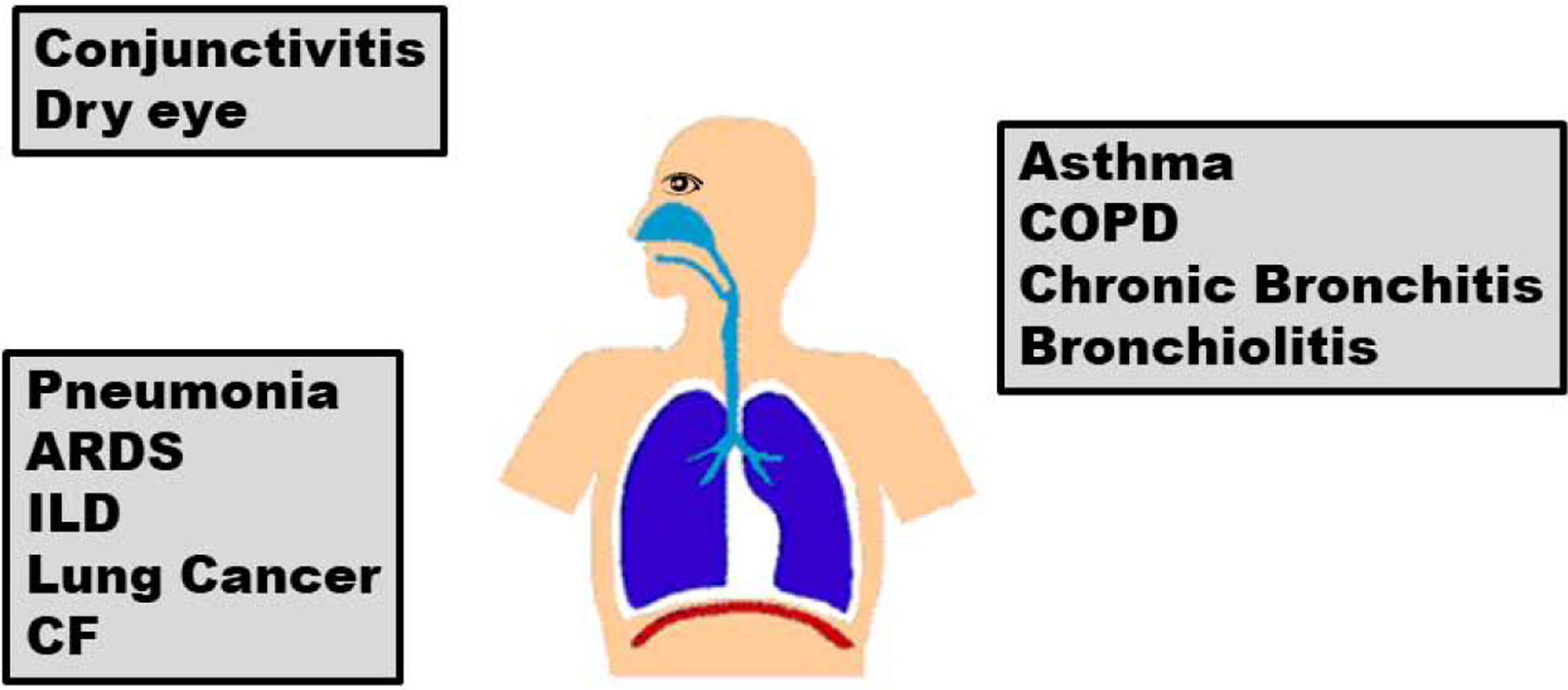
Respiratory diseases affected by ambient air pollutants. Schematic of respiratory diseases affected by ambient pollutant exposure. COPD: chronic obstructive pulmonary disease; ARDS, acute respiratory distress syndrome; ILD, interstitial lung disease; CF, cystic fibrosis.
Asthma
Asthma inception
The relationship between air pollution and the development of asthma has been found in population studies across all age groups. Prenatal exposure to ambient pollution may lead to epigenetic modifications that can be transmitted from mother to child in a nongenomic hereditary manner60. The Asthma Coalition on Community, Environment and Social Stress (ACCESS) project, an urban pregnancy cohort, found that exposure to BC and PM2.5, particularly in the second trimester were associated with infantile wheeze and childhood asthma, especially for boys61.
The Southern California Children’s Health Study identified home and school air pollution as a significant contributor to incident asthma in kindergarten and first grade students who were asthma and wheeze-free at the start of the study62. Similarly, a Swedish birth cohort found that TRAP and NO2 encountered in the first year of life had significant effects on lung function later in childhood and adolescence63,64. While a longitudinal European study incorporating multiple birth cohorts found no association between childhood air pollutant exposure and asthma prevalence65, the vast majority of studies from the developed world support the relationship66–71. Moreover, a population-based study including over 14,000 children from prospective birth cohorts in Germany, Sweden and the Netherlands extended the findings from childhood asthma measured primarily during early childhood to adolescents. They found a significant association of NO2 and BC (measured as PM2.5 absorbance) at the birth address with higher odds of incident asthma up to age 14–16 years72. Overall, these findings 1) demonstrate that long term associations persist between early life pollutant exposure and asthma, and 2) add confidence to the association at an age when asthma is more reliably diagnosed. The breadth of pediatric data strongly supports the relationship between ambient pollution and the inception of asthma in childhood across regions of the world and racial/ethnic differences, with a suggestion that early life exposures are the most important with different studies identifying differential effects of gender, allergic sensitization and family history of atopy that may modify the relationship.
Fewer data are available for adult onset asthma73,74. A study by Bowatte et al. identified that in the Tasmanian Longitudinal Health Study there was a significant association between home NO2 exposure or living within 200m from a major road and current wheeze and asthma74. The highest risk group had the null genotype of GSTT1, suggesting that lack of antioxidant activity may be a susceptibility factor for developing airways disease and thereby highlighting the role of an oxidative stress mechanism in explaining harm from pollutants. Aside from the association with the inception of adult asthma, exposure to ambient pollution may influence the development of asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS), which is noted to have higher morbidity and lung function decline than either condition alone. The prospective Canadian Community Healthy Survey study identified an almost 3-fold higher incidence of developing ACOS in those residing in locations with higher cumulative PM2.5 and 31% higher in areas of higher O375.
Asthma morbidity
Human controlled exposure studies in subjects with allergic asthma have shown both increased airway hyperresponsiveness to subsequent allergen challenge76 and reduced lung function after exposure to ambient pollutants at levels encountered in routine urban life77. The lung function deficits were accompanied by elevated markers of neutrophilic airways inflammation and acidification.
Epidemiologic studies extend these findings to demonstrate consistent alterations in lung function, increased symptoms and emergency healthcare services use in relation to short term increases in ambient pollutant exposures, primarily those included in TRAP, ozone, NO2 and particulate matter78. Children with asthma enrolled in the Childhood Asthma Management Program (CAMP) were found to have decreased indices of lung function and heightened airways reactivity with higher ambient exposure to CO, O3 and NO2 without evidence of treatment effect from inhaled steroids79. We similarly found a direct relationship between elevated levels of NO2 and airflow obstruction on spirometry in a cohort of inner city school children exposed to NO2 in their school classroom80. Notably, these affects were not driven by atopy and there was no relationship with fractional exhalation of nitric oxide (FeNO), so they appear independent of typical allergic asthma triggers.
Emergency department (ED) visits for pediatric asthma have been highly associated with spikes in ambient pollution in urban environments81,82; this relationship is potentially stronger in asthmatic patients with premature birth or from low income and Latino populations83,84. Time series analyses confirm increased emergency room visits for children and young adults with asthma exacerbations in concert with elevations in ambient pollutants84. While it is difficult to differentiate individual pollutants from pollutant mixtures, ozone appears to have the most potent effect on asthma exacerbations85 and is consistently found to be associated with ED visits86,87. In a study assessing asthma exacerbations presenting to emergency departments across Seoul, Korea, multiple triggers were assessed in time series analysis, stratified by age (infant, preschool children, school-aged children, adults and elderly (60 years or older))88. The most provocative association for asthma morbidity was air pollutant exposure, measured as individual gaseous or particle pollutants, which exceeded the effect of viral infection or environmental allergens. Moreover, in each age stratification, 7-day O3 exposure had the highest relative risk of asthma exacerbation, greater than any allergen, weather or viral factor. In summary, pollutant exposures, including particles, gases (ozone and NO2, primarily) and TRAP are strongly and consistently associated with increased asthma symptoms, lung function decline, and exacerbations of asthma across all ages89.
COPD
COPD inception
COPD is the second most common chronic respiratory disease in adults worldwide, affecting more than 170 million people, but has a disproportionately high rate of associated morbidity and mortality90. Cigarette smoking is the predominant etiology for the development of COPD; the mechanisms responsible likely mirror those for pollutant toxicity, however the evidence for causality between ambient pollution and COPD development is limited. Exposure to higher levels of ambient pollution is associated with accelerated loss of lung function in population based studies55,56. In parts of China, extremely high chronic exposures to PM have been found to have similar odds of COPD prevalence as smoking91; however this association is observed at PM levels greater than 5-fold higher than the World Health Organization recommended average annual exposure92. Large epidemiologic studies have been less consistent in identifying the relationship between air pollution and incident COPD. Initial COPD hospital admission over a 10-year period in a prospective Danish cohort of 53,000 subjects was associated with 35-year mean NO2 level at home residence93. However, a subsequent large English94 study with over 800,000 subjects did not find an association and an analysis of European cohorts included in the ESCAPE (European Studies on Chronic Air Pollution Effects) project found positive but non-significant associations between COPD incidence and prevalence with air pollution measure95. When using the GOLD criteria to define COPD, there was a significant association with traffic intensity near the home and suggested that female and never-smoker subgroups may be more strongly affected95.
The association of ambient pollution with exacerbation of COPD is much more consistent. A recent meta-analysis by Li and colleagues report a 3.1% increase in COPD related hospitalization and 2.5% increase in COPD mortality for each 10 μg/m3 increase in daily PM2.5 exposure96. In Chile, COPD ED admissions increase by up to 19% in association with increased pollutants by an interquartile range97.
Several panel studies of patients with COPD reporting person-level outcomes have demonstrated variable associations with air pollution and COPD morbidity. Several studies have demonstrated increased symptoms and exacerbations98–100 while the effects on lung function parameters101,102 are less consistent98. A large East London cohort with COPD identified a significant increase in symptoms with pollutant exposure and non-significant trends for exacerbations associated with local ambient pollutant exposure in East London103. Even moderate exercise in high traffic areas may reduce the benefits of exercise in patients with COPD104.
Perhaps the most convincing evidence of the relationship of exposure with disease progression comes from a recent longitudinal cohort from the Multi-Ethnic Study of Atherosclreosis (MESA) and Lung Studies. In this study, investigators found significant associations between ambient pollutant exposure and increases in percent emphysema measured on chest computed tomography (CT) scans over a 10 year period105. Concentrations of O3, PM2.5, NOx and BC, while only O3 and NOx at follow-up, were significantly associated with the 10-year change in percent emphysema. O3 was also significantly associated with decrease in FEV1 over the 10-year time period.
Other respiratory disorders
While the majority of air pollution respiratory literature focuses on asthma and COPD, it is important to highlight a few respiratory conditions in which there is evidence suggesting the influence of ambient air pollution: lung cancer, acute respiratory infection, acute respiratory distress syndrome (ARDS), and interstitial lung disease (ILD). Cancer incidence and cancer mortality are also both associated with exposure to high levels of ambient air pollution. The International Agency for Research on Cancer estimates that between 3–5% of lung cancer cases are attributable to ambient air pollution. The independent effect of pollution on lung cancer mortality is estimated at greater than 62,000 per year2.
A recently emerging body of evidence links ambient pollution exposure to risk of ARDS after trauma, an association that suggests pollutant exposure confers respiratory vulnerability in otherwise healthy adults such that a non-respiratory event that stresses the respiratory system can be overwhelming. Reilly and colleagues found that among 996 critically ill patients with acute trauma, both short and long term exposures with ambient pollutants were highly associated with development of ARDS106. These exposure levels were largely within acceptable ranges per U.S. and European Union air quality standards. The findings were mirrored in a separate cohort of critically ill patients with a specific risk associated with ozone exposure at their home location107.
Several studies have documented the increased risk of respiratory infection associated with air pollution exposure. The odds of acute lower respiratory tract infections, particularly bronchiolitis, following short term elevations in particulate matter increase by 7–15%108,109 and even higher for infants born preterm. Short term increases in air pollution have been associated with acute respiratory infection emergency visits and hospitalization in children and adults110–112.
Globally, increased levels of indoor and ambient pollutants are risk factors for pneumonia across the ages113,114. For patients with cystic fibrosis, a genetic disease of dysfunction chloride transport which leads to airways inflammation and chronic and acute infection, exposure to ambient pollution is significantly associated with respiratory exacerbations115–117 and decline in FEV1115.
While the full narrative on environmental and social determinants is unfolding, some early analyses suggest that COVID-related outcomes are related to ambient air pollution. Recently published studies from both China118 and Italy119 have demonstrated significant relationships between regions of high ambient particle and gaseous pollution and rates of COVID-19 infection. In a cross-sectional study using county-level U.S. data120, COVID-related mortality was highest in locations where long-term air pollution concentrations have been highest, adjusting for some key area-level risk factors, such as population size, age distribution, and population density. A similar study in the UK121 showed association between COVID mortality and ambient levels of nitrogen oxides and ozone.
The development of interstitial lung diseases (ILD) from exposure to occupational or intense environmental exposures have been well documented for conditions such as hypersensitivity pneumonitis, silicosis, asbestosis, and other pneumoconioses. More recently, outdoor air pollution has emerged as a plausible contributor to ILD122. Gaseous pollutants, O3 and NO2 have been associated with idiopathic pulmonary fibrosis (IPF) exacerbations123,124, while PM has been associated with decline in lung function125 and mortality126. In a longitudinal study by Johannson and colleagues, with weekly symptom and spirometry measures over 40 consecutive weeks, air pollution was associated with lower lung function across the study period, but not short-term variations in lung function127.
How clinicians can approach pollution and respiratory health
The summation of basic science, human exposure panel studies, cohort and large epidemiologic research overwhelmingly supports both the health risks to humans from exposure to ambient air pollution at levels encountered in industrialized and developing countries, alike. The risks are even higher for vulnerable populations with underlying chronic lung diseases, such as asthma and COPD. So how does the clinician coerce the breadth of research findings into daily practice? This, too, can be addressed on many levels. As the source apportionment of harmful environmental contaminants are derived largely from the burning of fossil fuels for industry, home and transportation uses, efforts to promote curtailing these practices should be encouraged. Supporting local, regional and national policies to encourage clean energy production and utilization and regulations to minimize emissions of pollutants may lead to improved air quality.
Alterations in personal use, such as minimizing gas and oil dependency in favor of renewable sources of energy for home (solar) and personal transportation (electric vehicles, public transportation, cycling and walking) can minimize the personal contribution to environmental pollution.
Healthcare providers have a unique relationship with patients with chronic disease. The office visit serves to assess and manage the individual’s physical disability but also offers a discrete opportunity to offer guidance on minimizing harmful exposures. One such publicly available tool providers can utilize for anticipatory guidance for patients is the air quality index (AQI). AirNow is a program by the U.S. EPA that is the national repository for real-time air quality data derived from federal ambient sampling sites for ozone and particle pollution with coverage of all 50 states, 6 Canadian provinces and 24 U.S. national parks128. The AQI is presented in a color-coded graphic to represent the air quality rating and levels of health concerns, specifically identifying conditions that may affect sensitive populations129 (Figure 3), and can be accessed by website, social media feed, and iPhone and Android apps. For individual patients, this daily information and forecasting of air quality can help provide advanced warning for adverse conditions for which the patient will want to avoid being outside and consider using an air cleaning device indoors.
Figure 3.
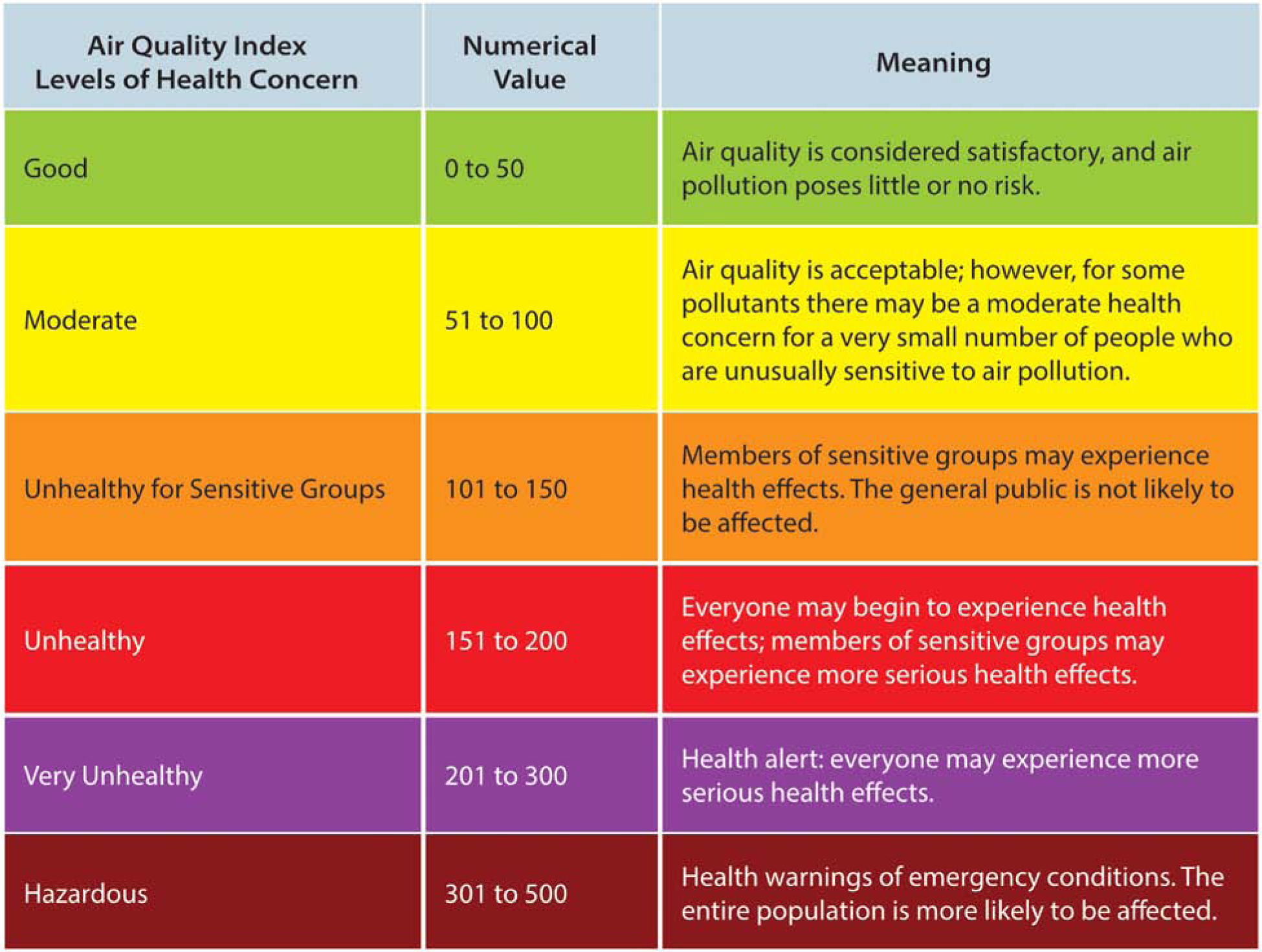
Schematic of Air Quality Index. From U.S. EPA’s AirNow Program. From U.S. EPA’s AirNow ProgramAir Quality Index (AQI) Basics. Available at: https://airnow.gov/index.cfm?action=aqibasics.aqi. Accessed February 11, 2020; with permission.
Conclusion
Air quality is an important influence on human health and disease. Our understanding of these relationships are limited by the complex interactions between exposure dynamics (e.g., pollutant mixtures, concentrations, the duration and intensity of exposure) and personal characteristics (e.g., age at exposure, underlying disease, genetic predisposition). While the adverse respiratory effects of air pollutants encountered in the outdoor environment presented in this article highlight the negative impact of pollutants on chronic disease and lung function growth and decline, it is important to note that human exposure to air pollution is not so neatly compartmentalized – sources of pollutants are ubiquitous in the indoor and outdoor environment and mixing of ambient and indoor air occurs constantly. Nevertheless, there are clear risks that poor ambient air quality presents to the development and exacerbation of chronic lung disease, especially COPD and asthma. Clinicians should be aware of the health effects of these environmental factors, as well as the publicly available resources to monitor them, in order to provide optimal guidance for their patients.
Synopsis:
Globally, exposure to ambient air pollutants is responsible for premature mortality and is implicated in the development and exacerbation of several acute and chronic lung disease across all ages. In this article, we discuss the source apportionment of ambient pollutants and the respiratory health effects in humans. We specifically discuss the evidence supporting ambient pollution in the development of asthma and COPD and acute exacerbations of each condition. Practical advice is given to healthcare providers in how to promote a healthy environment and advise patients with chronic conditions to avoid unsafe air quality.
Key points:
Ambient air pollution is emitted primarily as a result of fossil fuel combustion and atmospheric chemical reactions
Ambient air pollution exposure is responsible for premature mortality due to its effect on multiple organ systems, particularly the respiratory and cardiovascular system
Outdoor pollutant exposure is highly associated with impaired lung development and accelerated lung function decline
Outdoor air pollution is a potent modifiable risk factor for exacerbations of asthma, COPD, and acute bronchopulmonary infections
Funding sources:
This manuscript is supported by NIH grants R01 ES 030100, K23AI106945 (PI, Dr. Gaffin),
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None of the authors have any commercial/financial conflicts of interest.
Contributor Information
Jahred Liddie, Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, MA 02115, USA..
Jonathan M. Gaffin, Division of Pulmonary Medicine, Boston Children’s Hospital, Harvard Medical School, Boston MA 02115, USA..
References
- 1.Schraufnagel DE, Balmes JR, Cowl CT, et al. Air Pollution and Noncommunicable Diseases: A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 1: The Damaging Effects of Air Pollution. Chest. 2019;155(2):417–426. doi: 10.1016/j.chest.2018.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen AJ, Brauer M, Burnett R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet (London, England). 2017;389(10082):1907–1918. doi: 10.1016/S0140-6736(17)30505-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525(7569):367–371. doi: 10.1038/nature15371 [DOI] [PubMed] [Google Scholar]
- 4.Landrigan PJ, Fuller R, Acosta NJR, et al. The Lancet Commission on pollution and health. Lancet. 2018. doi: 10.1016/S0140-6736(17)32345-0 [DOI] [PubMed] [Google Scholar]
- 5.Pope CA 3rd, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med. 2009;360(4):376–386. doi: 10.1056/NEJMsa0805646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajat A, Hsia C, O’Neill MS. Socioeconomic Disparities and Air Pollution Exposure: a Global Review. Curr Environ Heal reports. 2015. doi: 10.1007/s40572-015-0069-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karagulian F, Belis CA, Dora CFC, et al. Contributions to cities’ ambient particulate matter (PM): A systematic review of local source contributions at global level. Atmos Environ. 2015;120:475–483. doi: 10.1016/j.atmosenv.2015.08.087 [DOI] [Google Scholar]
- 8.Thurston GD, Ito K, Lall R. A source apportionment of U.S. fine particulate matter air pollution. Atmos Environ. 2011;45(24):3924–3936. doi: 10.1016/j.atmosenv.2011.04.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikati I, Benson AF, Luben TJ, Sacks JD, Richmond-Bryant J. Disparities in Distribution of Particulate Matter Emission Sources by Race and Poverty Status. Am J Public Health. 2018;108(4):480–485. doi: 10.2105/AJPH.2017.304297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosofsky A, Levy JI, Zanobetti A, Janulewicz P, Fabian MP. Temporal trends in air pollution exposure inequality in Massachusetts. Environ Res. 2018. doi: 10.1016/j.envres.2017.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaddick G, Thomas ML, Amini H, et al. Data Integration for the Assessment of Population Exposure to Ambient Air Pollution for Global Burden of Disease Assessment. Environ Sci Technol. 2018;52(16):9069–9078. doi: 10.1021/acs.est.8b02864 [DOI] [PubMed] [Google Scholar]
- 12.Kelly FJ, Fussell JC. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos Environ. 2012. doi: 10.1016/j.atmosenv.2012.06.039 [DOI] [Google Scholar]
- 13.Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ Health Perspect. 2005. doi: 10.1289/ehp.7938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US EPA O. Integrated Science Assessment (ISA) for Sulfur Oxides - Health Criteria.
- 15.Karner AA, Eisinger DS, Niemeier DA. Near-roadway air quality: Synthesizing the findings from real-world data. Environ Sci Technol. 2010. doi: 10.1021/es100008x [DOI] [PubMed] [Google Scholar]
- 16.Alexeeff SE, Roy A, Shan J, et al. High-resolution mapping of traffic related air pollution with Google street view cars and incidence of cardiovascular events within neighborhoods in Oakland, CA. Environ Heal A Glob Access Sci Source. 2018. doi: 10.1186/s12940-018-0382-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apte JS, Messier KP, Gani S, et al. High-Resolution Air Pollution Mapping with Google Street View Cars: Exploiting Big Data. Environ Sci Technol. 2017. doi: 10.1021/acs.est.7b00891 [DOI] [PubMed] [Google Scholar]
- 18.Ambient (outdoor) air pollution. https://www.who.int/en/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health. Accessed February 4, 2020.
- 19.Liu C, Chen R, Sera F, et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N Engl J Med. 2019;381(8):705–715. doi: 10.1056/NEJMoa1817364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett JE, Tamura-Wicks H, Parks RM, et al. Particulate matter air pollution and national and county life expectancy loss in the USA: A spatiotemporal analysis. PLoS Med. 2019;16(7):e1002856–e1002856. doi: 10.1371/journal.pmed.1002856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerrett M, Burnett RT, Pope CA 3rd, et al. Long-term ozone exposure and mortality. N Engl J Med. 2009;360(11):1085–1095. doi: 10.1056/NEJMoa0803894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart JE, Garshick E, Dockery DW, Smith TJ, Ryan L, Laden F. Long-term ambient multipollutant exposures and mortality. Am J Respir Crit Care Med. 2011;183(1):73–78. doi: 10.1164/rccm.200912-1903OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities Study. Am J Respir Crit Care Med. 2006. doi: 10.1164/rccm.200503-443OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epa U, of Policy Analysis O. The Benefits and Costs of the Clean Air Act from 1990 to 2020, Final Report, Revision A, April 2011.; 2011. [Google Scholar]
- 25.Dimakopoulou K, Samoli E, Beelen R, et al. Air pollution and nonmalignant respiratory mortality in 16 cohorts within the ESCAPE project. Am J Respir Crit Care Med. 2014;189(6):684–696. doi: 10.1164/rccm.201310-1777OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell ML, Ebisu K, Peng RD, Samet JM, Dominici F. Hospital admissions and chemical composition of fine particle air pollution. Am J Respir Crit Care Med. 2009;179(12):1115–1120. doi: 10.1164/rccm.200808-1240OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel MM, Hoepner L, Garfinkel R, et al. Ambient metals, elemental carbon, and wheeze and cough in New York City children through 24 months of age. Am J Respir Crit Care Med. 2009;180(11):1107–1113. doi: 10.1164/rccm.200901-0122OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gowers AM, Cullinan P, Ayres JG, et al. Does outdoor air pollution induce new cases of asthma? Biological plausibility and evidence; a review. Respirology. 2012;17(6):887–898. doi: 10.1111/j.1440-1843.2012.02195.x [DOI] [PubMed] [Google Scholar]
- 29.Bauer RN, Diaz-Sanchez D, Jaspers I. Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors. J Allergy Clin Immunol. 2012;129(1):14–16. doi: 10.1016/j.jaci.2011.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008;122(3):456–470. doi: 10.1016/j.jaci.2008.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang W, Wang G, Lu S-E, et al. Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. Am J Respir Crit Care Med. 2012;186(11):1150–1159. doi: 10.1164/rccm.201205-0850OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim CS, Alexis NE, Rappold AG, et al. Lung function and inflammatory responses in healthy young adults exposed to 0.06 ppm ozone for 6.6 hours. Am J Respir Crit Care Med. 2011;183(9):1215–1221. doi: 10.1164/rccm.201011-1813OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu W, Jin Y, Carlsten C. Inflammatory health effects of indoor and outdoor particulate matter. J Allergy Clin Immunol. 2018;141(3):833–844. doi: 10.1016/j.jaci.2017.12.981 [DOI] [PubMed] [Google Scholar]
- 34.Breton CV, Salam MT, Vora H, Gauderman WJ, Gilliland FD. Genetic variation in the glutathione synthesis pathway, air pollution, and children’s lung function growth. Am J Respir Crit Care Med. 2011;183(2):243–248. doi: 10.1164/rccm.201006-0849OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai X, Bowatte G, Lowe AJ, et al. Do Glutathione S-Transferase Genes Modify the Link between Indoor Air Pollution and Asthma, Allergies, and Lung Function? A Systematic Review. Curr Allergy Asthma Rep. 2018;18(3):20. doi: 10.1007/s11882-018-0771-0 [DOI] [PubMed] [Google Scholar]
- 36.Noah TL, Zhou H, Zhang H, et al. Diesel exhaust exposure and nasal response to attenuated influenza in normal and allergic volunteers. Am J Respir Crit Care Med. 2012;185(2):179–185. doi: 10.1164/rccm.201103-0465OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diaz-Sanchez D, Garcia MP, Wang M, Jyrala M, Saxon A. Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. J Allergy Clin Immunol. 1999. doi: 10.1016/S0091-6749(99)70011-4 [DOI] [PubMed] [Google Scholar]
- 38.Robinson RK, Birrell MA, Adcock JJ, et al. Mechanistic link between diesel exhaust particles and respiratory reflexes. J Allergy Clin Immunol. 2018;141(3):1074–1084.e9. doi: 10.1016/j.jaci.2017.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan PH, Bernstein DI, Lockey J, et al. Exposure to traffic-related particles and endotoxin during infancy is associated with wheezing at age 3 years. Am J Respir Crit Care Med. 2009;180(11):1068–1075. doi: 10.1164/rccm.200808-1307OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schindler C, Keidel D, Gerbase MW, et al. Improvements in PM10 exposure and reduced rates of respiratory symptoms in a cohort of Swiss adults (SAPALDIA). Am J Respir Crit Care Med. 2009;179(7):579–587. doi: 10.1164/rccm.200803-388OC [DOI] [PubMed] [Google Scholar]
- 41.A Gutiérrez M, Giuliani D, A Porta A, Andrinolo D. Relationship between Ocular Surface Alterations and Concentrations of Aerial Particulate Matter. J Ophthalmic Vis Res. 2019;14(4):419–427. doi: 10.18502/jovr.v14i4.5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nucci P, Sacchi M, Pichi F, et al. Pediatric Conjunctivitis and Air Pollution Exposure: A Prospective Observational Study. Semin Ophthalmol. 2017;32(4):407–411. doi: 10.3109/08820538.2015.1115088 [DOI] [PubMed] [Google Scholar]
- 43.Alves M, Novaes P, Morraye M de A, Reinach PS, Rocha EM. Is dry eye an environmental disease? Arq Bras Oftalmol. 2014;77(3):193–200. doi: 10.5935/0004-2749.20140050 [DOI] [PubMed] [Google Scholar]
- 44.Mo Z, Fu Q, Lyu D, et al. Impacts of air pollution on dry eye disease among residents in Hangzhou, China: A case-crossover study. Environ Pollut. 2019;246:183–189. doi: 10.1016/j.envpol.2018.11.109 [DOI] [PubMed] [Google Scholar]
- 45.Rice MB, Rifas-Shiman SL, Litonjua AA, et al. Lifetime Exposure to Ambient Pollution and Lung Function in Children. Am J Respir Crit Care Med. 2016;193(8):881–888. doi: 10.1164/rccm.201506-1058OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gauderman WJ, McConnell R, Gilliland F, et al. Association between air pollution and lung function growth in southern California children. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1383–1390. doi: 10.1164/ajrccm.162.4.9909096 [DOI] [PubMed] [Google Scholar]
- 47.Gauderman WJ, Urman R, Avol E, et al. Association of improved air quality with lung development in children. N Engl J Med. 2015;372(10):905–913. doi: 10.1056/NEJMoa1414123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avol EL, Gauderman WJ, Tan SM, London SJ, Peters JM. Respiratory effects of relocating to areas of differing air pollution levels. Am J Respir Crit Care Med. 2001;164(11):2067–2072. doi: 10.1164/ajrccm.164.11.2102005 [DOI] [PubMed] [Google Scholar]
- 49.Jun W, Cizao R, J. DR, Judith C, Michelle W, Beate R. Association between Local Traffic-Generated Air Pollution and Preeclampsia and Preterm Delivery in the South Coast Air Basin of California. Environ Health Perspect. 2009;117(11):1773–1779. doi: 10.1289/ehp.0800334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Korten I, Ramsey K, Latzin P. Air pollution during pregnancy and lung development in the child. Paediatr Respir Rev. 2017;21:38–46. doi: 10.1016/j.prrv.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 51.Janssen BG, Byun H-M, Gyselaers W, Lefebvre W, Baccarelli AA, Nawrot TS. Placental mitochondrial methylation and exposure to airborne particulate matter in the early life environment: An ENVIRONAGE birth cohort study. Epigenetics. 2015;10(6):536–544. doi: 10.1080/15592294.2015.1048412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuertes E, Bracher J, Flexeder C, et al. Long-term air pollution exposure and lung function in 15 year-old adolescents living in an urban and rural area in Germany: The GINIplus and LISAplus cohorts. Int J Hyg Environ Health. 2015;218(7):656–665. doi: 10.1016/j.ijheh.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 53.McGeachie MJ, Yates KP, Zhou X, et al. Patterns of Growth and Decline in Lung Function in Persistent Childhood Asthma. N Engl J Med. 2016;374(19):1842–1852. doi: 10.1056/NEJMoa1513737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez FD. Early-Life Origins of Chronic Obstructive Pulmonary Disease. N Engl J Med. 2016;375(9):871–878. doi: 10.1056/NEJMra1603287 [DOI] [PubMed] [Google Scholar]
- 55.Lepeule J, Litonjua AA, Coull B, et al. Long-term effects of traffic particles on lung function decline in the elderly. Am J Respir Crit Care Med. 2014;190(5):542–548. doi: 10.1164/rccm.201402-0350OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rice MB, Ljungman PL, Wilker EH, et al. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study. Am J Respir Crit Care Med. 2015;191(6):656–664. doi: 10.1164/rccm.201410-1875OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ackermann-Liebrich U, Leuenberger P, Schwartz J, et al. Lung function and long term exposure to air pollutants in Switzerland. Study on Air Pollution and Lung Diseases in Adults (SAPALDIA) Team. Am J Respir Crit Care Med. 1997;155(1):122–129. doi: 10.1164/ajrccm.155.1.9001300 [DOI] [PubMed] [Google Scholar]
- 58.Downs SH, Schindler C, Liu L-JS, et al. Reduced exposure to PM10 and attenuated age-related decline in lung function. N Engl J Med. 2007;357(23):2338–2347. doi: 10.1056/NEJMoa073625 [DOI] [PubMed] [Google Scholar]
- 59.Boogaard H, Fischer PH, Janssen NAH, et al. Respiratory effects of a reduction in outdoor air pollution concentrations. Epidemiology. 2013;24(5):753–761. doi: 10.1097/EDE.0b013e31829e1639 [DOI] [PubMed] [Google Scholar]
- 60.Burbank AJ, Sood AK, Kesic MJ, Peden DB, Hernandez ML, Hill C. Environmental determinants of allergy and asthma in early life. J Allergy Clin Immunol. 2017;140(1):1–12. doi: 10.1016/j.jaci.2017.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsu H-HL, Chiu Y-HM, Coull BA, et al. Prenatal Particulate Air Pollution and Asthma Onset in Urban Children. Identifying Sensitive Windows and Sex Differences. Am J Respir Crit Care Med. 2015;192(9):1052–1059. doi: 10.1164/rccm.201504-0658OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McConnell R, Islam T, Shankardass K, et al. Childhood incident asthma and traffic-related air pollution at home and school. Env Heal Perspect. 2010;118(7):1021–1026. doi: 10.1289/ehp.0901232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schultz ES, Hallberg J, Bellander T, et al. Early-Life Exposure to Traffic-related Air Pollution and Lung Function in Adolescence. Am J Respir Crit Care Med. 2016;193(2):171–177. doi: 10.1164/rccm.201505-0928OC [DOI] [PubMed] [Google Scholar]
- 64.Schultz ES, Gruzieva O, Bellander T, et al. Traffic-related air pollution and lung function in children at 8 years of age: a birth cohort study. Am J Respir Crit Care Med. 2012;186(12):1286–1291. doi: 10.1164/rccm.201206-1045OC [DOI] [PubMed] [Google Scholar]
- 65.Molter A, Simpson A, Berdel D, et al. A multicentre study of air pollution exposure and childhood asthma prevalence: the ESCAPE project. Eur Respir J. 2015;45(3):610–624. doi: 10.1183/09031936.00083614 [DOI] [PubMed] [Google Scholar]
- 66.Nishimura KK, Iwanaga K, Oh SS, et al. Early-life ozone exposure associated with asthma without sensitization in Latino children. J Allergy Clin Immunol. 2016;138(6):1703–1706.e1. doi: 10.1016/j.jaci.2016.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gehring U, Wijga AH, Brauer M, et al. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am J Respir Crit Care Med. 2010;181(6):596–603. doi: 10.1164/rccm.200906-0858OC [DOI] [PubMed] [Google Scholar]
- 68.Jerrett M, Shankardass K, Berhane K, et al. Traffic-related air pollution and asthma onset in children: a prospective cohort study with individual exposure measurement. Environ Health Perspect. 2008;116(10):1433–1438. doi: 10.1289/ehp.10968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nishimura KK, Galanter JM, Roth LA, et al. Early-life air pollution and asthma risk in minority children. The GALA II and SAGE II studies. Am J Respir Crit Care Med. 2013;188(3):309–318. doi: 10.1164/rccm.201302-0264OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brunst KJ, Ryan PH, Brokamp C, et al. Timing and Duration of Traffic-related Air Pollution Exposure and the Risk for Childhood Wheeze and Asthma. Am J Respir Crit Care Med. 2015;192(4):421–427. doi: 10.1164/rccm.201407-1314OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bowatte G, Lodge C, Lowe AJ, et al. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. 2015;70(3):245–256. doi: 10.1111/all.12561 [DOI] [PubMed] [Google Scholar]
- 72.Gehring U, Wijga AH, Hoek G, et al. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med. 2015;3(12):933–942. doi: 10.1016/s2213-2600(15)00426-9 [DOI] [PubMed] [Google Scholar]
- 73.Young MT, Sandler DP, DeRoo LA, Vedal S, Kaufman JD, London SJ. Ambient air pollution exposure and incident adult asthma in a nationwide cohort of U.S. women. Am J Respir Crit Care Med. 2014;190(8):914–921. doi: 10.1164/rccm.201403-0525OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bowatte G, Lodge CJ, Knibbs LD, et al. Traffic-related air pollution exposure is associated with allergic sensitization, asthma, and poor lung function in middle age. J Allergy Clin Immunol. 2017;139(1):122–129 e1. doi: 10.1016/j.jaci.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 75.To T, Zhu J, Larsen K, et al. Progression from Asthma to Chronic Obstructive Pulmonary Disease. Is Air Pollution a Risk Factor? Am J Respir Crit Care Med. 2016;194(4):429–438. doi: 10.1164/rccm.201510-1932OC [DOI] [PubMed] [Google Scholar]
- 76.Svartengren M, Strand V, Bylin G, Jarup L, Pershagen G. Short-term exposure to air pollution in a road tunnel enhances the asthmatic response to allergen. Eur Respir J. 2000;15(4):716–724. [DOI] [PubMed] [Google Scholar]
- 77.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007;357(23):2348–2358. doi:357/23/2348 [pii]10.1056/NEJMoa071535 [DOI] [PubMed] [Google Scholar]
- 78.Burbank AJ, Peden DB. Assessing the impact of air pollution on childhood asthma morbidity: how, when, and what to do. Curr Opin Allergy Clin Immunol. 2018;18(2):124–131. doi: 10.1097/ACI.0000000000000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ierodiakonou D, Zanobetti A, Coull BA, et al. Ambient air pollution, lung function, and airway responsiveness in asthmatic children. J Allergy Clin Immunol. 2016;137(2):390–399. doi: 10.1016/j.jaci.2015.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gaffin JM, Hauptman M, Petty CR, et al. Nitrogen dioxide exposure in school classrooms of inner-city children with asthma. J Allergy Clin Immunol. 2017. doi: 10.1016/j.jaci.2017.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Strickland MJ, Darrow LA, Klein M, et al. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med. 2010;182(3):307–316. doi: 10.1164/rccm.200908-1201OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng XY, Ding H, Jiang LN, et al. Association between Air Pollutants and Asthma Emergency Room Visits and Hospital Admissions in Time Series Studies: A Systematic Review and Meta-Analysis. PLoS One. 2015;10(9):e0138146. doi: 10.1371/journal.pone.0138146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strickland MJ, Klein M, Flanders WD, et al. Modification of the effect of ambient air pollution on pediatric asthma emergency visits: susceptible subpopulations. Epidemiology. 2014;25(6):843–850. doi: 10.1097/ede.0000000000000170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Byers N, Ritchey M, Vaidyanathan A, Brandt AJ, Yip F. Short-term effects of ambient air pollutants on asthma-related emergency department visits in Indianapolis, Indiana, 2007–2011. J Asthma. 2016;53(3):245–252. doi: 10.3109/02770903.2015.1091006 [DOI] [PubMed] [Google Scholar]
- 85.Gass K, Klein M, Sarnat SE, et al. Associations between ambient air pollutant mixtures and pediatric asthma emergency department visits in three cities: a classification and regression tree approach. Env Heal. 2015;14:58. doi: 10.1186/s12940-015-0044-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Noh J, Sohn J, Cho J, et al. Short-term Effects of Ambient Air Pollution on Emergency Department Visits for Asthma: An Assessment of Effect Modification by Prior Allergic Disease History. J Prev Med Public Heal. 2016;49(5):329–341. doi: 10.3961/jpmph.16.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tétreault L-F, Doucet M, Gamache P, et al. Severe and Moderate Asthma Exacerbations in Asthmatic Children and Exposure to Ambient Air Pollutants. Int J Environ Res Public Health. 2016;13(8):771. doi: 10.3390/ijerph13080771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee SW, Yon DK, James CC, et al. Short-term effects of multiple outdoor environmental factors on risk of asthma exacerbations: Age-stratified time-series analysis. J Allergy Clin Immunol. 2019. doi: 10.1016/j.jaci.2019.08.037 [DOI] [PubMed] [Google Scholar]
- 89.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383(9928):1581–1592. doi: 10.1016/S0140-6736(14)60617-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Collaborators GBD 2015 CRD. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. doi: 10.1016/S2213-2600(17)30293-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet (London, England). 2018;391(10131):1706–1717. doi: 10.1016/S0140-6736(18)30841-9 [DOI] [PubMed] [Google Scholar]
- 92.World Health Organization Regional Office for Europe.; 2006. www.euro.who.int. Accessed February 10, 2020.
- 93.Andersen ZJ, Hvidberg M, Jensen SS, et al. Chronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution: a cohort study. Am J Respir Crit Care Med. 2011;183(4):455–461. doi: 10.1164/rccm.201006-0937OC [DOI] [PubMed] [Google Scholar]
- 94.Atkinson RW, Carey IM, Kent AJ, van Staa TP, Anderson HR, Cook DG. Long-term exposure to outdoor air pollution and the incidence of chronic obstructive pulmonary disease in a national English cohort. Occup Environ Med. 2015;72(1):42–48. doi: 10.1136/oemed-2014-102266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schikowski T, Adam M, Marcon A, et al. Association of ambient air pollution with the prevalence and incidence of COPD. Eur Respir J. 2014;44(3):614–626. doi: 10.1183/09031936.00132213 [DOI] [PubMed] [Google Scholar]
- 96.Li M-H, Fan L-C, Mao B, et al. Short-term Exposure to Ambient Fine Particulate Matter Increases Hospitalizations and Mortality in COPD: A Systematic Review and Meta-analysis. Chest. 2016;149(2):447–458. doi: 10.1378/chest.15-0513 [DOI] [PubMed] [Google Scholar]
- 97.Arbex MA, de Souza Conceição GM, Cendon SP, et al. Urban air pollution and chronic obstructive pulmonary disease-related emergency department visits. J Epidemiol Community Health. 2009;63(10):777 LP – 783. doi: 10.1136/jech.2008.078360 [DOI] [PubMed] [Google Scholar]
- 98.Harré ESM, Price PD, Ayrey RB, Toop LJ, Martin IR, Town GI. Respiratory effects of air pollution in chronic obstructive pulmonary disease: A three month prospective study. Thorax. 1997. doi: 10.1136/thx.52.12.1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Desqueyroux H, Pujet JC, Prosper M, Momas I, Le Moullec Y. Effects of Air Pollution on Adults with Chronic Obstructive Pulmonary Disease. Arch Environ Health. 2002. doi: 10.1080/00039890209602088 [DOI] [PubMed] [Google Scholar]
- 100.Van Der Zee SC, Hoek G, Boezen MH, Schouten JP, Van Wijnen JH, Brunekreef B. Acute effects of air pollution on respiratory health of 50–70 yr old adults. Eur Respir J. 2000. doi: 10.1034/j.1399-3003.2000.15d13.x [DOI] [PubMed] [Google Scholar]
- 101.Lagorio S, Forastiere F, Pistelli R, et al. Air pollution and lung function among susceptible adult subjects: A panel study. Environ Heal A Glob Access Sci Source. 2006. doi: 10.1186/1476-069X-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trenga CA, Sullivan JH, Schildcrout JS, et al. Effect of particulate air pollution on lung function in adult and pediatric subjects in a Seattle panel study. Chest. 2006. doi: 10.1378/chest.129.6.1614 [DOI] [PubMed] [Google Scholar]
- 103.Peacock JL, Anderson HR, Bremner SA, et al. Outdoor air pollution and respiratory health in patients with COPD. Thorax. 2011;66(7):591–596. doi: 10.1136/thx.2010.155358 [DOI] [PubMed] [Google Scholar]
- 104.Sinharay R, Gong J, Barratt B, et al. Respiratory and cardiovascular responses to walking down a traffic-polluted road compared with walking in a traffic-free area in participants aged 60 years and older with chronic lung or heart disease and age-matched healthy controls: a randomised, cross. Lancet (London, England). 2018;391(10118):339–349. doi: 10.1016/S0140-6736(17)32643-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang M, Aaron CP, Madrigano J, et al. Association Between Long-term Exposure to Ambient Air Pollution and Change in Quantitatively Assessed Emphysema and Lung Function. JAMA. 2019;322(6):546–556. doi: 10.1001/jama.2019.10255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reilly JP, Zhao Z, Shashaty MGS, et al. Low to Moderate Air Pollutant Exposure and Acute Respiratory Distress Syndrome after Severe Trauma. Am J Respir Crit Care Med. 2019;199(1):62–70. doi: 10.1164/rccm.201803-0435OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ware LB, Zhao Z, Koyama T, et al. Long-Term Ozone Exposure Increases the Risk of Developing the Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2016;193(10):1143–1150. doi: 10.1164/rccm.201507-1418OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Horne BD, Joy EA, Hofmann MG, et al. Short-Term Elevation of Fine Particulate Matter Air Pollution and Acute Lower Respiratory Infection. Am J Respir Crit Care Med. 2018;198(6):759–766. doi: 10.1164/rccm.201709-1883OC [DOI] [PubMed] [Google Scholar]
- 109.Girguis MS, Strickland MJ, Hu X, et al. Exposure to acute air pollution and risk of bronchiolitis and otitis media for preterm and term infants. J Expo Sci Environ Epidemiol. 2018;28(4):348–357. doi: 10.1038/s41370-017-0006-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Darrow LA, Klein M, Flanders WD, Mulholland JA, Tolbert PE, Strickland MJ. Air pollution and acute respiratory infections among children 0–4 years of age: an 18-year time-series study. Am J Epidemiol. 2014;180(10):968–977. doi: 10.1093/aje/kwu234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barnett AG, Williams GM, Schwartz J, et al. Air pollution and child respiratory health: a case-crossover study in Australia and New Zealand. Am J Respir Crit Care Med. 2005;171(11):1272–1278. doi: 10.1164/rccm.200411-1586OC [DOI] [PubMed] [Google Scholar]
- 112.Cakmak S, Dales RE, Gultekin T, et al. Components of particulate air pollution and emergency department visits in Chile. Arch Environ Occup Health. 2009;64(3):148–155. doi: 10.1080/19338240903240228 [DOI] [PubMed] [Google Scholar]
- 113.Pirozzi CS, Jones BE, VanDerslice JA, Zhang Y, Paine R 3rd, Dean NC. Short-Term Air Pollution and Incident Pneumonia. A Case-Crossover Study. Ann Am Thorac Soc. 2018;15(4):449–459. doi: 10.1513/AnnalsATS.201706-495OC [DOI] [PubMed] [Google Scholar]
- 114.Nhung NTT, Amini H, Schindler C, et al. Short-term association between ambient air pollution and pneumonia in children: A systematic review and meta-analysis of time-series and case-crossover studies. Environ Pollut. 2017;230:1000–1008. doi: 10.1016/j.envpol.2017.07.063 [DOI] [PubMed] [Google Scholar]
- 115.Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD. Effect of Ambient Air Pollution on Pulmonary Exacerbations and Lung Function in Cystic Fibrosis. Am J Respir Crit Care Med. 2004;169(7):816–821. doi: 10.1164/rccm.200306-779OC [DOI] [PubMed] [Google Scholar]
- 116.Farhat SCL, Almeida MB, Silva-Filho LVRF, Farhat J, Rodrigues JC, Braga ALF. Ozone is associated with an increased risk of respiratory exacerbations in patients with cystic fibrosis. Chest. 2013;144(4):1186–1192. doi: 10.1378/chest.12-2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goeminne PC, Kiciński M, Vermeulen F, et al. Impact of Air Pollution on Cystic Fibrosis Pulmonary Exacerbations: A Case-Crossover Analysis. Chest. 2013;143(4):946–954. doi: 10.1378/chest.12-1005 [DOI] [PubMed] [Google Scholar]
- 118.Zhu Y, Xie J, Huang F, Cao L. Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China. Sci Total Environ. 2020;727:138704. doi: 10.1016/j.scitotenv.2020.138704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fattorini D, Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ Pollut. 2020;264:114732. doi: 10.1016/j.envpol.2020.114732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu X, Nethery RC, Sabath BM, Braun D, Dominici F. Exposure to air pollution and COVID-19 mortality in the United States: A nationwide cross-sectional study. medRxiv. April 2020:2020.04.05.20054502. doi: 10.1101/2020.04.05.20054502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Travaglio M, Popovic R, Yu Y, Leal N, Martins LM. Links between air pollution and COVID-19 in England. medRxiv. May 2020:2020.04.16.20067405. doi: 10.1101/2020.04.16.20067405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Johannson KA, Balmes JR, Collard HR. Air pollution exposure: a novel environmental risk factor for interstitial lung disease? Chest. 2015;147(4):1161–1167. doi: 10.1378/chest.14-1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sese L, Annesi-Maesano I, Nunes H. Impact of Particulate Matter on the Natural History of IPF: A Matter of Concentrations? Chest. 2018;154(3):726–727. doi: 10.1016/j.chest.2018.05.043 [DOI] [PubMed] [Google Scholar]
- 124.Johannson KA, Vittinghoff E, Lee K, et al. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur Respir J. 2014;43(4):1124 LP – 1131. doi: 10.1183/09031936.00122213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Winterbottom CJ, Shah RJ, Patterson KC, et al. Exposure to Ambient Particulate Matter Is Associated With Accelerated Functional Decline in Idiopathic Pulmonary Fibrosis. Chest. 2018;153(5):1221–1228. doi: 10.1016/J.CHEST.2017.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sesé L, Nunes H, Cottin V, et al. Role of atmospheric pollution on the natural history of idiopathic pulmonary fibrosis. Thorax. 2018;73(2):145 LP – 150. doi: 10.1136/thoraxjnl-2017-209967 [DOI] [PubMed] [Google Scholar]
- 127.Johannson KA, Vittinghoff E, Morisset J, et al. Air Pollution Exposure Is Associated With Lower Lung Function, but Not Changes in Lung Function, in Patients With Idiopathic Pulmonary Fibrosis. Chest. 2018;154(1):119–125. doi: 10.1016/j.chest.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.About AirNow. https://airnow.gov/index.cfm?action=topics.about_airnow. Accessed February 11, 2020.
- 129.Air Quality Index (AQI) Basics. https://airnow.gov/index.cfm?action=aqibasics.aqi. Accessed February 11, 2020.
- 130.Ground-level Ozone Basics | Ground-level Ozone Pollution | US EPA; https://www.epa.gov/ground-level-ozone-pollution/ground-level-ozone-basics. Accessed May 24, 2020. [Google Scholar]
- 131.Basic Information about NO2 | Nitrogen Dioxide (NO2) Pollution | US EPA; https://www.epa.gov/no2-pollution/basic-information-about-no2#WhatisNO2. Accessed May 24, 2020. [Google Scholar]
- 132.Sulfur Dioxide Basics | Sulfur Dioxide (SO2) Pollution | US EPA; https://www.epa.gov/so2-pollution/sulfur-dioxide-basics. Accessed May 24, 2020. [Google Scholar]
- 133.Sulfur Dioxide: Your Environment, Your Health | National Library of Medicine. https://toxtown.nlm.nih.gov/chemicals-and-contaminants/sulfur-dioxide. Accessed May 24, 2020.


