Abstract
Tendon mechanical properties have been proposed as a biomarker of tendon health to track response to injury and treatment. Prior to utilizing these properties in an injured population, it is critical to understand how these are influenced by age and sex in an uninjured population. A retrospective analysis was conducted of 118 uninjured Achilles tendons to evaluate the relationship between tendon mechanical properties, age and sex. Mechanical properties (shear modulus and viscosity) were assessed using continuous shear wave elastography. A moderator regression analysis was completed to examine the relationship between tendon mechanical properties, age and sex, after adjusting for body mass index and physical activity level. There was an interaction between age and sex for shear modulus (p=0.049, R2 change=0.034). Females had a negative relationship between age and shear modulus (p=0.030, β=−0.350) but no relationship was observed for males (p=0.78, β=0.031). A positive relationship was found between age and viscosity (p=0.034, β=0.214). Increased viscosity was related to increased age with no difference between sexes. The effect of aging on shear modulus differed between men and women and may help explain sex specific injury risks and their differing response to mechanical load.
Keywords: tendinopathy, viscoelastic properties, shear wave elastography, human, ageing
Introduction
The Achilles tendon is a collagenous connective tissue running between the gastrocnemius and calcaneal insertion. This structure is critical to activities of daily living and sports performance since it is responsible for transferring force from the triceps surae muscles to the foot and storing and releasing energy during repetitive, dynamic tasks. The Achilles tendon is also a common site of injury, with Achilles tendinopathy occurring most frequently.1 Achilles tendinopathy is a clinical diagnosis of pain, swelling and impaired function of the Achilles tendon.2 It is one of the most commonly reported foot and ankle injuries in athletic populations but also occurs frequently in non-athletic populations.3,4 In a cross-sectional study of patients reporting to Dutch general practice physicians, the incidence rate for midportion Achilles tendinopathy was 1.85 per 1,000 patients but only 35% of the cases were related to sports activity.4
The incidence rate of Achilles tendinopathy increases with age until the middle decades of life, with the highest incidence occurring between ages 41–60.4 It is hypothesized that age-related changes in tendon mechanical properties may contribute to the increasing incidence of tendinopathy with age. However, the relationship between age and mechanical properties remains unclear. Animal studies have shown decreased,5,6 increased,7,8 or unchanged tendon compliance or mechanical properties in response to aging.9,10 In vitro and in vivo human studies are similarly inconclusive, as they have found either decreased or unchanged mechanical properties with age.11–16 The relationship between aging and tendon mechanical properties may also be influenced by sex, since female sex hormones appear to blunt the collagen synthesis response to loading.17 Furthermore, in human studies, it has been difficulty to separate the influence of aging from age-related declines in physical activity and increases in body mass index (BMI).18 Thus, the extent to which aging influences mechanical properties of tendon tissue remains elusive.
Recently, there has been increased interest in using tendon mechanical properties as a biomarker of tendon health and the response to injury. Our research group has developed a non-invasive form of ultrasound imaging called continuous shear wave elastography (cSWE), which has the ability to quantify mechanical properties in vivo.19 cSWE has the advantage of not relying on muscle contraction and can provide quantitative values for viscoelastic properties (shear modulus and viscosity).19 The ability to evaluate these properties may provide new insights into the aging process in tendon, the impact of injury, and help explain sex differences in the response to loading and treatment. However, prior to using tendon mechanical properties as a biomarker, it is crucial to understand how these properties are influenced by age and sex in an uninjured population. Therefore, the purpose of this study is to determine the relationship between viscoelastic properties of the human Achilles tendon, age, and sex, after adjusting for physical activity and BMI.
Materials and Methods
Study Design
This study is a retrospective analysis of uninjured Achilles tendons from studies performed in the Delaware Tendon Research Lab from November 2013 to December 2018.
Participants
Participants were either healthy or had unilateral Achilles tendinopathy or Achilles tendon rupture. For healthy participants, the right limb was selected for analysis. For participants with unilateral injuries, the contralateral, uninjured limb was used for analysis. Each subject signed an informed consent approved by the University of Delaware institutional review board. A licensed physical therapist assessed and screened each subject for tendon health through palpation and B-mode ultrasound imaging. Participants were excluded if they had pain on palpation or reported pain during loading of the Achilles tendon, or if they had hypoechoic regions or a tendon thickness greater than 7mm on B-mode ultrasound imaging. Additionally, only participants that had completed all measures described below were included for analysis.
Physical Activity Level
To describe participant activity level, all participants completed the Physical Activity Scale (PAS), which is a 6-point scale with higher scores indicating greater levels of physical activity.20
Mechanical Properties
Tendon mechanical properties were quantified using cSWE, as described in detail by Corrigan et al. and Cortes et al., using a SonixMDP Q+ ultrasound scanner (Ultrasonix, Vancouver, Canada) with a L14–5/38 transducer and a 128-channel external data acquisition unit.19,21 For this technique, the participant was positioned in prone with their feet secured against a footplate set at 10° dorsiflexion to remove the slack from the tendon. An external actuator (mini-shaker type 4810, Bruel & Kjaer, Naerum, Denmark) was used to propagate a shear wave along the length of the Achilles tendon at eleven known frequencies. The ultrasound probe was used to record raw radiofrequency data. For the uninjured subjects, the ultrasound probe was placed on the free portion of the right Achilles tendon, just distal to soleus junction. For the subjects with contralateral injury, the probe was placed on the uninjured tendon at the site equivalent to their pathological location on the injured tendon. Care was taken to ensure that the measures were obtained from the free tendon and not over the tendon-bone or tendon-muscle interface. A custom MatLab code (Mathworks, MA, USA) was used to quantify linear displacement of the tendon and shear wave speed. Based on wave speed, tendon shear modulus and viscosity were estimated using the Voigt model.19
Statistical Analysis
Statistical analysis was performed using IBM SPSS v.25 (Chicago, IL). Descriptive statistics were calculated for participant characteristics and mechanical properties for the complete sample, and by sex. Independent t-tests were used to determine if males and females differed for continuous, normally distributed variables. Mann-Whitney U tests were used for ordinal or non-normally distributed variables. Two Sequential Regression Models were performed, one for each mechanical property, shear modulus and viscosity, with four predictors (BMI, PAS, age and sex) and the age by sex interaction.22 In the first block the covariates to adjust for, BMI and PAS, were entered. Next the main effects of sex and age were added, and in the third block their interaction was included. For each model, the change in R2 was tested for significance to see if it significantly improved model fit. Assumptions were examined using visual inspection and Shapiro-Wilk’s test for normality. Additionally, data was screened for outliers and extreme cases. Alpha level was set at 0.05 for all analysis.
Results
One hundred and eighteen uninjured limbs were included in the analysis. Descriptive statistics for the complete sample, males, and females are presented in Table 1. Males and females differed in BMI (p<0.001) but there were no differences in age, physical activity level, shear modulus or viscosity (p>0.05).
Table 1.
Descriptive statistics of participant demographics and mechanical properties.
| Overall (n = 118) | Male (n=70) | Female (n=48) | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Median (Min-Max) | Median (Min-Max) | Median (Min-Max) | |
| Age (yrs) | 37.7 (14.7) | 39.1 (14.6) | 35.6 (14.8) |
| 32.0 (18.0–76.0) | 35.0 (18.0–76.0) | 30.0 (19.0–75.0) | |
| BMI (kg/m2) | 26.9 (5.8) | 28.0 (4.7) | 25.4 (6.8) |
| 26.2 (19.5–51.5) | 27.3 (20.1–48.0) | 23.1 (19.5–51.5)* | |
| PAS (AU) | 4.5 (1.3) | 4.6 (1.2) | 4.3 (1.4) |
| 5.0 (1.0–6.0) | 5.0 (1.0–6.0) | 4.5 (2.0–6.0) | |
| Shear Modulus (kPa) | 95.9 (14.9) | 95.9 (15.3) | 95.8 (14.4) |
| 93.8 (64.1–145.0) | 93.0 (64.1–145.0) | 95.6 (71.9–130.8) | |
| Viscosity (Pa*s) | 56.8 (11.6) | 58.0 (11.7) | 55.1 (11.3) |
| 57.2 (25.0–79.7) | 58.3 (30.6–79.2) | 55.1 (25.0–79.7) |
AU = arbitrary Units, SD = standard deviation,
p≤0.05
Regression Results
In the initial analysis, one outlier was identified for shear modulus. After removing the outlier, all assumptions were met for both outcomes. There was a significant interaction for shear after adjusting for physical activity level and BMI (p=0.049, R2 change = 0.034) (Table 2). Using a simple slopes approach, it was found that there was a significant negative relationship between age and shear modulus in females (p = 0.030, β = −0.350) and no relationship in males (p=0.78, β = 0.031) (Figure 1)23. For viscosity, after adjusting for physical activity level and BMI, the interaction of age and sex was not significant, therefore the main effects model is reported (p=0.728, R2 change = 0.001) (Table 3). The main effect of age was the only significant predictor (p = 0.034, β = 0.214) (Figure 2).
Table 2.
Regression analysis for shear modulus with outlier removed
| Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | p-value | B | SE B | β | p-value | B | SE B | β | p-value |
| Physical Activity Level | 1.130 | 1.100 | 0.103 | 0.306 | 1.007 | 1.148 | 0.091 | 0.382 | 0.974 | 1.133 | 0.088 | 0.392 |
| BMI | 0.094 | 0.246 | 0.038 | 0.704 | 0.204 | 0.268 | 0.083 | 0.450 | 0.330 | 0.272 | 0.134 | 0.229 |
| Age | −0.103 | 0.099 | −0.107 | 0.302 | −0.341 | .155 | −0.353 | 0.030 | ||||
| Sex | −1.003 | 2.832 | −0.035 | 0.724 | −15.08 | 7.612 | −0.524 | 0.050 | ||||
| Age x Sex | 0.374 | 0.188 | 0.586 | 0.049 | ||||||||
| R2 | 0.009 | 0.020 | 0.054 | |||||||||
| F for change in R2 | 0.528 | 0.631 | 3.953 | |||||||||
Note: Sex was coded with females as the reference group. Model 1: Physical activity level and BMI; Model 2: Physical activity level, BMI, age and sex; Model 3: Physical activity level, BMI, age, sex and age by sex interaction; SE = standard error; Bold indicates statistical significance.
Figure 1.
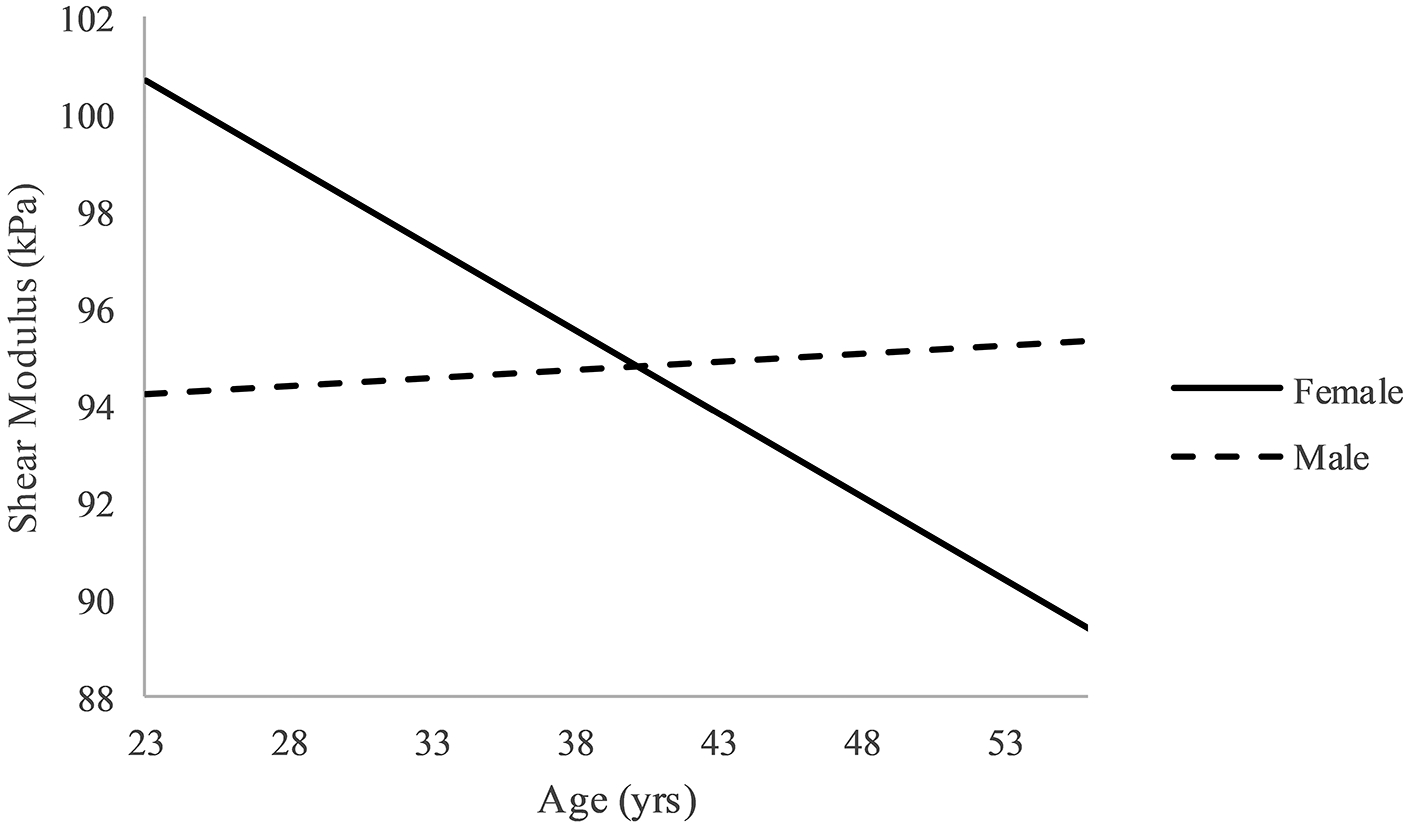
Interaction effect of age and sex for shear modulus. Note: X- and Y-axes truncated.
Table 3.
Regression analysis for viscosity
| Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | p-value | B | SE B | β | p-value | B | SE B | β | p-value |
| Physical Activity Level | −0.382 | 0.888 | −0.042 | 0.668 | −0.208 | 0.908 | −0.023 | 0.820 | −0.212 | 0.912 | −0.024 | 0.816 |
| BMI | 0.252 | 0.198 | 0.125 | 0.206 | 0.064 | 0.210 | 0.032 | 0.761 | 0.082 | 0.217 | 0.041 | 0.707 |
| Age | 0.169 | 0.079 | 0.214 | 0.034 | 0.135 | 0.125 | 0.171 | 0.280 | ||||
| Sex | 2.183 | 2.234 | 0.093 | 0.331 | 0.188 | 6.139 | 0.008 | 0.976 | ||||
| Age x Sex | 0.053 | 0.152 | 0.102 | 0.728 | ||||||||
| R2 | 0.021 | 0.070 | 0.071 | |||||||||
| F for change in R2 | 1.250 | 2.966 | 0.122 | |||||||||
Note: Sex was coded with females as the reference group. Model 1: Physical activity level and BMI; Model 2: Physical activity level, BMI, age and sex; Model 3: Physical activity level, BMI, age, sex and age by sex interaction; SE = standard error. Bold indicates statistical significance.
Figure 2.
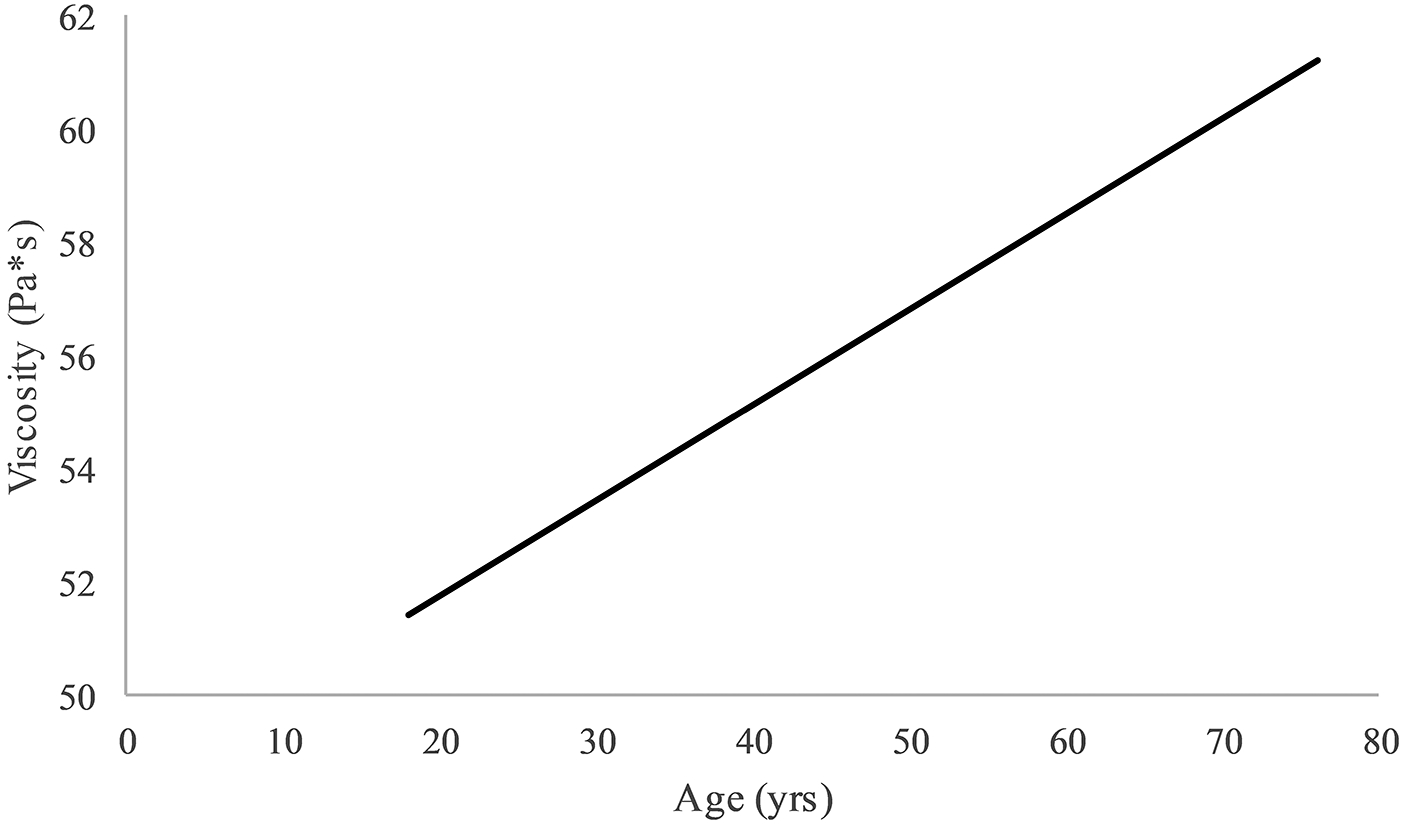
Relationship for age and viscosity after adjusting for sex, BMI and physical activity level. Note: Y-axis truncated.
Discussion
The purpose of this study was to investigate the relationship between viscoelastic properties of uninjured Achilles tendons, age and sex, after adjusting for BMI and physical activity level. We found that there was a significant interaction between age and sex for shear modulus, indicating that the relationship between age and shear modulus was dependent on sex. There was a negative relationship between age and shear modulus for females and no relationship for males. In the regression model for viscosity, there was no interaction between age and sex, and there was a positive relationship between age and viscosity.
In this study we chose to adjust for physical activity level and BMI, which have been shown to influence tendon structure and mechanical properties. Tendons are mechanosensitive, responding to loading with increased collagen synthesis and tendon remodeling.24 Previous studies in the Achilles and patellar tendons have demonstrated that strength training and habitual physical activity, such as running, results in increased stiffness and Young’s modulus.25–27 Greater BMI may influence tendon mechanical properties in a couple ways. First, since these individuals are heavier, they subject their tendons to greater loads during daily activities, especially in weight-bearing tendons.28 Second, higher BMI is associated with greater adiposity and several systemic conditions, such as diabetes and high cholesterol, which may alter mechanical properties directly or through medications for their treatment.29,30 Therefore, it was critical to adjust for these factors to isolate the effects of sex and age on tendon mechanical properties.
Previous in-vitro and in-vivo studies of human tendon mechanical properties found no changes or reduced mechanical properties with aging.11–16 However, these studies have utilized measures of stiffness, Young’s modulus, or correlates, such as shear wave speed, and have not examined shear modulus or viscosity. Our results suggest that influence of aging on shear modulus differs for men and women, with shear modulus declining with age in females but remaining relatively unchanged in males. Estradiol, the primary female sex hormone, has an inhibitory effect on collagen synthesis in response to exercise, which may partially explain the sex specific response to aging.17 We theorize that as a result of this reduced collagen synthesis capacity, women may be less capable of maintaining tendon homeostasis, leading to a gradual decline in mechanical properties. There is some evidence to suggest that collagen synthesis capacity improves after menopause, once the inhibitory effect of estradiol is reduced.31 Magnusson et al. found that post-menopausal women have greater Achilles tendon cross-sectional area than pre-menopausal women, even though they load their tendons less.31 Menopause typically occurs during the 5th decade of life.32 Relatively few of the women included in this study were greater than 50 years old (11/48, 23%) and the number of women who had reached menopause is unknown. Therefore, we were not able to determine whether this relationship between age and shear modulus is maintained after menopause and this relationship requires further investigation.
To our knowledge, this is the first study to directly investigate the effect of aging on tendon viscosity. We found a positive relationship between age and viscosity, which was not dependent on sex, indicating that viscosity increases with age. The viscosity of the tendon is likely influenced by the water content in the tendon. A decrease in water content has been reported to occur with aging and could be a reason for the increase in viscosity with age seen in this study.33 Advanced glycolytic end-products (AGEs) accumulate with age, especially in tissues with slow collagen turnover, such as tendon.33 AGEs form cross-links between collagen fibers, which contribute to dehydration of collagen fibers and reduced water content.29,34 Furthermore, animal studies have demonstrated a decrease in proteoglycans within the tendon with age, which further contributes to reduced water content.33 AGEs can also form cross-links in the collagen of tendons proposed to increase the stiffness of the tendon and that might also account for the increased viscosity with age.29 The risk of tendon injuries increases with age however,35 in this study the relationship between age and viscosity is the opposite of what we have observed in injured Achilles tendons, where viscosity is reduced.19,36 Further study is needed to determine how viscosity can be used for evaluating injury severity and response to mechanical loading.
Studying the effects of aging on tendon mechanical properties has been challenging, since prior methods for assessing mechanical properties in-vivo have relied on muscle contraction.37 Since strength often decreases with age, alterations in mechanical properties in prior studies may reflect decreased force or torque output rather than changes within the tendon. This limitation can be negated to a certain extent by normalizing to the maximal common force or torque across participants. However, this method may underestimate mechanical properties.38 In this study, we utilized cSWE, which is not reliant on muscle contraction, thereby eliminating the confounding factor of muscle strength.19 Additionally, the method has the advantage of providing multiple measures of tendon mechanical properties, shear modulus and viscosity.19 Shear modulus is obtainable from commercial elastography systems but viscosity is not. The diverging relationships between these two properties and age suggests that they may be measuring different aspects of tendon changes, which may provide a more nuanced representation of tendon health and response to injury.
We acknowledge that there are several limitations to this study, which may limit the generalizability to a larger population. First, shear modulus and viscosity were calculated using the Voigt model that assumes a linear isotropic viscoelastic medium under no stress. In other words, the Voigt model assumes that the relationship between the applied shear stress and the values obtained for mechanical properties are linear and these values are the same, regardless of which direction along the material the measurement is made. Tendon is a nonlinear structure and the mechanical properties differ based on the direction of an applied load, due to its fibrous composition. However, since the shear deformation are very small, the nonlinearity of the tendon is expected to have a negligible effect. Additionally, there is a proportional relationship between the square of the speed and the shear modulus in anisotropic materials when the wave is traveling in one of the main anisotropy axes, which is similar to the relationship for isotropic materials. Finally, the wave speed is also affected by tendon thickness and tensile stress.39,40 Unfortunately, there is currently no formulation available that includes all those effects. However, in our recent publication, we demonstrated that the shear modulus measured using the proposed method is linearly proportional to the Young’s modulus at different values of ankle dorsiflexion.21 This linearity suggest that the shear modulus reported in this study is closely related to material properties of clinical relevance for the tendon. Secondly, many of the participants had contralateral injuries. Although the included tendons were screened to ensure that they were uninjured and did not display signs of structural changes on ultrasound imaging, these tendons may have had subclinical changes that were not detected that may influence mechanical properties. Third, the menstruation status of the female participants is unknown, so it is unclear what percentage of female participants were pre- or post-menopausal. This may influence these relationships due to a drop in circulating levels of estradiol, a collagen synthesis inhibitor, after menopause. Finally, the majority of participants were less than 50 years old, so it is possible that the relationships observed may be different in the later decades of life.
Perspectives
This study utilized cSWE to determine the influence of age and sex on Achilles tendon mechanical properties, after adjusting for BMI and physical activity level. Shear modulus was negatively related to age in women and was not related to age in men. Additionally, viscosity was positively related to age but the relationship did not differ between men and women. These results illustrate that the response to aging differs between men and women. Mechanical properties have been proposed as a biomarker of tendon health. Therefore, these results will help separate the alterations in these properties due to age and sex from the impact of injury.
Supplementary Material
Acknowledgements:
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers R21AR067390, T32HD00749019, R01AR0720340, and Florence P. Kendall and Promotion of Doctoral Studies I scholarships from the Foundation for Physical Therapy.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
References:
- 1.Kvist M Achilles tendon injuries in athletes. Ann Chir Gynaecol 1991;80:188–201. [PubMed] [Google Scholar]
- 2.Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: Time to change a confusing terminology. Arthroscopy 1998;14:840–843. [DOI] [PubMed] [Google Scholar]
- 3.Sobhani S, Dekker R, Postema K, Dijkstra PU. Epidemiology of ankle and foot overuse injuries in sports: A systematic review. Scand J Med Sci Sport 2013;23:669–686. [DOI] [PubMed] [Google Scholar]
- 4.De Jonge S, Van Den Berg C, de Vos RJ, Van Der Heide HJLL, Weir A, Verhaar JANN, Bierma-Zeinstra SMAA, Tol JL. Incidence of midportion Achilles tendinopathy in the general population. Br J Sports Med 2011;45:1026–1028. [DOI] [PubMed] [Google Scholar]
- 5.Simonsen EB, Klitgaard H, Bojsen-Møller F. The influence of strength training, swim training and ageing on the Achilles tendon and m. soleus of the rat. J Sports Sci 1995;13:291–5. [DOI] [PubMed] [Google Scholar]
- 6.LaCroix AS, Duenwald-Kuehl SE, Brickson S, Akins TL, Diffee G, Aiken J, Vanderby R, Lakes RS. Effect of age and exercise on the viscoelastic properties of rat tail tendon. Ann Biomed Eng 2013;41:1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viidik A, Nielsen HM, Skalicky M. Influence of physical exercise on aging rats: II. Life-long exercise delays aging of tail tendon collagen. Mech Ageing Dev 1996;88:139–48. [DOI] [PubMed] [Google Scholar]
- 8.Wood LK, Arruda EM, Brooks S V. Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J Appl Physiol 2011;111:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa Y, Hayashi K, Yamamoto N, Nagashima K. Age-related changes in biomechanical properties of the Achilles tendon in rabbits. Eur J Appl Physiol Occup Physiol 1996;73:7–10. [DOI] [PubMed] [Google Scholar]
- 10.Haut RC. Age-dependent influence of strain rate on the tensile failure of rat-tail tendon. J Biomech Eng 1983;105:296–9. [DOI] [PubMed] [Google Scholar]
- 11.Hubbard RP, Soutas-Little RW. Mechanical properties of human tendon and their age dependence. J Biomech Eng 1984;106:144–150. [DOI] [PubMed] [Google Scholar]
- 12.Couppé C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol 2009;107:880–6. [DOI] [PubMed] [Google Scholar]
- 13.Kubo K, Kanehisa H, Miyatani M, Tachi M, Fukunaga T. Effect of low-load resistance training on the tendon properties in middle-aged and elderly women. Acta Physiol Scand 2003;178:25–32. [DOI] [PubMed] [Google Scholar]
- 14.Onambele GL, Narici MV., Maganaris CN. Calf muscle-tendon properties and postural balance in old age. J Appl Physiol 2006;100:2048–56. [DOI] [PubMed] [Google Scholar]
- 15.Slane LC, DeWall R, Martin J, Lee K, Thelen DG. Middle-aged adults exhibit altered spatial variations in Achilles tendon wave speed. Physiol Meas 2015;36:1485–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slane LC, Martin J, DeWall R, Thelen D, Lee K. Quantitative ultrasound mapping of regional variations in shear wave speeds of the aging Achilles tendon. Eur Radiol 2017;27:474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnusson SP, Hansen M, Langberg H, Miller B, Haraldsson B, Westh EK, Koskinen S, Aagaard P, Kjaer M. The adaptability of tendon to loading differs in men and women. Int J Exp Pathol 2007;88:237–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazarus NR, Harridge SDR. Declining performance of master athletes: silhouettes of the trajectory of healthy human ageing? J Physiol 2017;595:2941–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortes DH, Suydam SM, Silbernagel KG, Buchanan TS, Elliott DM. Continuous Shear Wave Elastography: A New Method to Measure Viscoelastic Properties of Tendons in Vivo. Ultrasound Med Biol 2015;41:1518–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimby G Physical activity and muscle training in the elderly. Acta Med Scand 1986;220:233–237. [DOI] [PubMed] [Google Scholar]
- 21.Corrigan P, Zellers JA, Balascio P, Silbernagel KG, Cortes DH. Quantification of mechanical properties in healthy Achilles tendon using continous shear wave elastography: a reliability and validation study. Ultrasound Med Biol 2019;45:1574–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J, Cohen P. Applied Multiple Regression and Correlation Analysis for the Behavioral Sciences. Mahwah, N.J: L. Erlbaum Associates; 2003. [Google Scholar]
- 23.Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Newbury Park: SAGE Publications, Incorporated; 1991. [Google Scholar]
- 24.Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M, Magnusson SP. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol (Oxf) 2007;191:111–21. [DOI] [PubMed] [Google Scholar]
- 25.Kubo K, Kanehisa H, Ito M, Fukunaga T. Effects of isometric training on the elasticity of human tendon structures in vivo. J Appl Physiol 2001;91:26–32. [DOI] [PubMed] [Google Scholar]
- 26.Albracht K, Arampatzis A. Exercise-induced changes in triceps surae tendon stiffness and muscle strength affect running economy in humans. Eur J Appl Physiol 2013;113:1605–15. [DOI] [PubMed] [Google Scholar]
- 27.Couppé C, Kongsgaard M, Aagaard P, Hansen P, Bojsen-Moller J, Kjaer M, Magnusson SP. Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J Appl Physiol 2008;105:805–10. [DOI] [PubMed] [Google Scholar]
- 28.Gaida JE, Ashe MC, Bass SL, Cook JL. Is adiposity an under-recognized risk factor for tendinopathy? A systematic review. Arthritis Rheum 2009;61:840–9. [DOI] [PubMed] [Google Scholar]
- 29.Couppé C, Svensson RB, Kongsgaard M, Kovanen V, Grosset JF, Snorgaard O, Bencke J, Larsen JO, Bandholm T, Christensen TM, Boesen A, Helmark IC, Aagaard P, Kjaer M, Magnusson SP. Human Achilles tendon glycation and function in diabetes. J Appl Physiol 2016;120:130–7. [DOI] [PubMed] [Google Scholar]
- 30.de Oliveira LP, Vieira CP, Guerra FD, Almeida MS, Pimentel ER. Structural and biomechanical changes in the Achilles tendon after chronic treatment with statins. Food Chem Toxicol 2015;77:50–7. [DOI] [PubMed] [Google Scholar]
- 31.Magnusson SP, Beyer N, Abrahamsen H, Aagaard P, Neergaard K, Kjaer M. Increased cross-sectional area and reduced tensile stress of the Achilles tendon in elderly compared with young women. J Gerontol A Biol Sci Med Sci 2003;58:123–7. [DOI] [PubMed] [Google Scholar]
- 32.Hill K The demography of menopause. Maturitas 1996;23:113–127. [DOI] [PubMed] [Google Scholar]
- 33.Svensson RB, Heinemeier KM, Couppé C, Kjaer M, Magnusson SP. Effect of aging and exercise on the tendon. J Appl Physiol 2016;121:1237–1246. [DOI] [PubMed] [Google Scholar]
- 34.Miles CA, Avery NC, Rodin VV., Bailey AJ. The increase in denaturation temperature following cross-linking of collagen is caused by dehydration of the fibres. J Mol Biol 2005;346:551–556. [DOI] [PubMed] [Google Scholar]
- 35.Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med 2003;22:675–92. [DOI] [PubMed] [Google Scholar]
- 36.Zellers JA, Cortes DH, Corrigan P, Pontiggia L, Silbernagel KG. Side-to-side differences in Achilles tendon geometry and mechanical properties following achilles tendon rupture. Muscles Ligaments Tendons J 2017;7:541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maganaris CN, Paul JP. Tensile properties of the in vivo human gastrocnemius tendon. J Biomech 2002;35:1639–1646. [DOI] [PubMed] [Google Scholar]
- 38.Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol 1999;521 Pt 1:307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeWall RJ, Slane LC, Lee KS, Thelen DG. Spatial variations in Achilles tendon shear wave speed. J Biomech 2014;47:2685–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin JA, Brandon SCE, Keuler EM, Hermus JR, Ehlers AC, Segalman DJ, Allen MS, Thelen DG. Gauging force by tapping tendons. Nat Commun 2018;9:1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


