Abstract
Background:
While fibroblast growth factor 23 (FGF23) is associated with heart failure (HF) and atrial fibrillation (AF), the mechanisms driving these associations are unclear. Sensitive measures of cardiovascular structure and function may provide mechanistic insight behind the associations of FGF23 and various cardiovascular diseases.
Methods:
In the Multi-Ethnic Study of Atherosclerosis (MESA), we evaluated the associations of baseline serum FGF23 (2000–2002) with measures of left ventricular (LV) and left atrial (LA) mechanical function on cardiac magnetic resonance (CMR) at 10-year follow up (2010–2012).
Results:
Of 2,276 participants with available FGF23 and CMR at 10-year follow up, participants with higher FGF23 levels were more likely white race, taking anti-hypertensive medications, and had lower kidney function. After covariate adjustment, FGF23 was associated with higher LV mass (β coefficient per 1 SD higher: 1.14, 95% CI:0.16, 2.12, P=0.02), worse LV global circumferential strain (β coefficient per 1 SD higher: 0.15, 95% CI: 0.05, 0.25, P= 0.003), worse LV mid-wall circumferential strain (β coefficient per 1 SD higher: 0.20, 95% CI: 0.08, 0.31, P= 0.001), and lower LA total emptying fraction (β coefficient per 1 SD higher: −0.52, 95% CI: −1.02, −0.02, P= 0.04). These associations were consistent across racial/ethnic groups and the spectrum of GFR. FGF23 was not associated with the presence of myocardial scar (OR per 1 SD higher: 1.12, 95% CI: 0.86–1.45, P= 0.42).
Conclusions:
In a multi-ethnic, community-based cohort, baseline FGF23 levels were independently associated with higher LV mass, lower LV systolic function, and reduced LA function over long-term follow up. These findings provide potential mechanistic insight into associations of FGF23 with incident HF and AF.
Keywords: heart failure, primary prevention, biomarkers, fibroblast growth factor 23, subclinical, cardiac function, atrial fibrillation
Fibroblast growth factor 23 (FGF23), a hormone predominantly secreted by osteocytes and osteoblasts, belongs to an overarching protein family that regulates cell proliferation.1 Specifically, FGF23 regulates phosphorus homeostasis through a reduction in phosphate reabsorption by the kidneys, modulation of parathyroid hormone production, and downregulation of activated vitamin D synthesis.2, 3 Circulating FGF23 is elevated in chronic kidney disease (CKD) and has primary target effects on the kidney tubules and parathyroid glands.4 In addition to its role in phosphate regulation, FGF23 has also demonstrated direct, adverse effects upon myocardial structure. Specifically, the administration of recombinant FGF23 induces cardiomyocyte hypertrophy along with upregulation of natriuretic peptides in murine experimental studies.5
Epidemiologic studies have further suggested that FGF23 may play a role in the development of cardiac structural abnormalities and overt cardiovascular disease. Indeed, higher FGF23 is associated with greater left ventricular (LV) mass,6–8 incident heart failure (HF),8–10 and incident atrial fibrillation (AF)11–13 in populations with and without CKD after adjustment for traditional risk factors. Importantly, FGF23 may be more strongly associated with HF with preserved ejection fraction (HFpEF) than HF with reduced ejection fraction (HFrEF).14 However, the specific mechanisms by which FGF23 may contribute to HF and AF are currently unclear. Sensitive measures of cardiovascular function may provide mechanistic insight behind the associations of FGF23 and specific clinical cardiovascular conditions, including HF and AF. Therefore, we evaluated the associations of FGF23 at baseline with sensitive indices of LV and left atrial (LA) function in later life among participants in the Multi-Ethnic Study of Atherosclerosis (MESA), a cohort free of prevalent cardiovascular disease at time of recruitment.
Methods
Study Population
The MESA study is a prospective cohort that initially recruited 6,814 community-dwelling adults aged 45–84 years, who identified themselves as white, black, Hispanic, or Chinese. Participants were recruited from 6 study sites between 2000 and 2002 (Baltimore, MD; Chicago, IL; St Paul, MN; Forsyth County, NC; New York, NY; and Los Angeles, CA). By design, the final study population was 38% white, 28% black, 22% Hispanic, and 12% Chinese. Full information regarding the recruitment and study design of MESA has been previously published.15 At recruitment, participants had no history of cardiovascular disease, defined as myocardial infarction, angina, stroke, transient ischemic attack, HF, AF, nitroglycerin use, angioplasty, pacemaker or defibrillator, or cardiac surgery. Following recruitment and a baseline in-person examination (Exam 1), 5 additional follow-up in-person examinations were completed at approximately 2- to 5-year intervals. For this analysis, we evaluated participants with available serum FGF23 concentrations at Exam 1 (conducted between 2000–2002) and who had cardiac magnetic resonance (CMR) performed at 10-year follow up (Exam 5; conducted between April 2010 to February 2012). Incident AF was identified from ECGs at Exam 5 visit, ICD-9 discharge diagnoses, and Medicare claims data, as previously described.11 Incident HF was adjudicated by 2 study physicians blinded to other study data through previously described medical record review.14 The study protocol was approved by the institutional review board of each study site and all participants provided informed consent. The data that support the findings of this study are available from the corresponding author upon reasonable request.
FGF23 Measurement
At Exam 1 (2000–2002), blood and urine samples were collected by MESA study personnel after overnight fasting.8 Blood samples were stored at the University of Vermont Laboratory for Clinical Biochemistry (Burlington, VT) using established methods.16 Subsequently, blood samples were shipped on dry ice to the University of Washington (Seattle, WA) where investigators measured serum FGF23 using the Kainos immunoassay.17 This assay detects the biologically intact, full-length FGF23 molecule through mid-molecule and distal epitopes on either side of the RXXR cleavage site. Quality control was assessed using standardized high and low value FGF23 controls. The coefficients of variation for high and low control samples were 6.7% and 12.4%, respectively.
Cardiac Magnetic Resonance Protocol
At Exam 5 (2010–2012), MESA participants without contraindications underwent CMR using 1.5T scanners (Avanto and Espree, Siemens Medical Systems; Signa LX, GE Healthcare). The MESA CMR protocol has been described previously and was uniform across all study sites.18 Briefly, LV volumes, myocardial mass, and functional measures were assessed by a cine steady‐state free precession sequence. Twelve short axis slices, one 4‐chamber view and one 2‐ chamber view, were acquired as described previously. Additionally, 3 tagged short-axis slices (base, mid, apex) were additionally obtained. The protocol for tagged CMR has been previously described.19 Finally, a subset (n=1,342) of consenting participants with estimated glomerular filtration rates (eGFR) >60 mL/min/1.73m2 (Northwestern study site) or >45 mL/min/1.73m2 (remaining study sites) underwent late gadolinium enhanced (LGE) CMR 15 minutes after administration of 0.15 mmol/kg dose of gadolinium based contrast agent (Magnevist, Bayer Healthcare Pharmaceuticals, Montville, NJ). All MESA CMR images were analyzed for structure and function in a core laboratory and at a single image analysis center (Johns Hopkins Medical Center, Baltimore, MD).
Left Ventricular Structure and Function
LV structural parameters (LV mass and volumes) and LV ejection fraction were measured using commercially available software (CIM v6.2, Auckland, New Zealand). LV endocardial and epicardial borders were traced semi-automatically on short axis cine images. LV mass was calculated at end diastole as the sum of the myocardial area (difference between endocardial and epicardial contour) times the section thickness plus the intersection gap multiplied by the specific gravity of myocardium (1.05 g/mL). LV end-diastolic and end-systolic volumes were calculated using the Simpson’s biplane method. Stroke volume was calculated as the difference between LV end-diastolic volume and end-systolic volume. LV ejection fraction was calculated as the stroke volume divided by LV end-diastolic volume. Myocardial scar was defined as LGE either in 2 adjacent short axis slices or in 1 short axis and a corresponding long axis slice (Qmass v7.2; Medis, Leiden, the Netherlands).20
LV short-axis tagged slices were analyzed using the HARP method MATLAB software or HARP1.15, Diagnosoft, Palo Alto, CA, USA) to calculate circumferential myocardial strain. Using standardized protocols, circumferential strain (CS) was determined in 4 wall segments (anterior, lateral, posterior, and septal) in each of the 3 short-axis slices (apical, mid, and base), resulting in 12 total segments with strain curves. Global CS (GCS) was defined as the average of the 12 total segments. Mid-wall CS was defined as the average of the 4 mid-wall strain segments. As previously noted, the intraclass correlation coefficients for inter-observer and intra-observer agreement mid-wall CS were 0.80 and 0.84 in studies with good tag persistence.21
Left Atrial Structure and Function
Multimodality tissue tracking software (MTT; version 5.0, Toshiba, Japan) was used to quantify LA volumes from 4- and 2-chamber cine images.22 Endocardial and epicardial LA borders at end systole were identified by a trained investigator, and MTT software tracks these borders during the cardiac cycle to create volume curves. Maximal and minimal LA volumes were calculated from these volume curves using the area length method at end systole and end diastole, respectively. LA total emptying fraction was calculated as: (LA Volumemax-LA Volume min)/LA Volumemax. MTT also generates longitudinal strain curves of all LA segments in 2- and 4-chamber views during each cardiac cycle. Global longitudinal strain curves for the 2- and 4-chamber views were derived as the average of all longitudinal strain curves, which were subsequently averaged into to calculate LA peak longitudinal strain.
Statistical Analysis
Clinical characteristics at baseline examination (2000–2002) by quartile of FGF23 were compared using χ2 tests or Fisher’s exact tests for categorical variables and univariate general linear models for continuous variables, thus assessing overall trends across FGF23 quartiles. Penalized B splines were used to evaluate the linearity of relationships between FGF23 and measures of LV structure/function (LV mass, global CS, mid-wall CS, ejection fraction, stroke volume) or LA function (LA total emptying fraction, peak longitudinal strain) on CMR. Given the absence of non-linearity, multivariable general linear models were used to evaluate the associations of baseline FGF23 levels (continuous variable) with measures of cardiac function on CMR at Exam 5 (2010–2012). Covariates for adjustment were chosen a priori based on previous associations and known biology. Sex (male, female) and race (white, black, Hispanic, Chinese) were treated as dummy variables in all models. Model 1 adjusted for the following covariates obtained at baseline: age, sex, race, and income. Model 2 further adjusted for the following baseline covariates: smoking status, diabetes, systolic blood pressure, anti-hypertensive medication use, body mass index, eGFR by the CKD-EPI equation, low density lipoprotein cholesterol, urine albumin-to-creatinine ratio (UACR), 25- hydroxyvitamin D, calcium, urine phosphate, and parathyroid hormone. We also evaluated the association of FGF23 with presence of myocardial scar using multivariable logistic regression models. These models used the same covariates as previously outlined. We performed multiple sensitivity analyses to evaluate the consistency of our findings in models evaluating associations of FGF23 with LV GCS, mid-wall CS, and LA total emptying fraction. First, we performed sensitivity analyses adjusting for 1) C-reactive protein (CRP) or 2) vitamin D and iron supplementation in addition to Model 2 covariates. Second, we further adjusted for LV mass at Exam 5 in addition to Model 2 covariates, as LV mass may mediate the association between FGF23 and LV/LA function. Third, we further adjusted for Exam 1 (baseline) LV mass, LV ejection fraction, and LV end-diastolic volume on CMR in addition to Model 2 covariates. Finally, we assessed for interaction of incident HF and AF by Exam 5 on the association of FGF23 and GCS using separate interaction terms for HF and AF.
Given the known association between FGF23 with LV mass and the known contribution of LV hypertrophy/stiffness to LA dysfunction, we performed a formal mediation analysis of LV mass on the association between FGF23 and LA total emptying fraction using the mediation package in R (v4.5.0, R Foundation for Statistical Computing). First, regression models were performed to determine the independent associations between 1) FGF23 and LVH and 2) LVH and LA total emptying fraction. Multivariable-adjusted direct and indirect effects (i.e. mediation effect) were reported, with calculation of 95% confidence intervals (CIs) using bootstrapping with 1000 resamples. Statistically significant mediation was determined if the indirect effect was significantly different from zero.
Because of known associations of 1) FGF23 with renal function23 and 2) black race with HF risk,24 we assessed for interaction of renal function and race on the association of FGF23 and indices of LV and LA function using separate interaction terms for eGFR (continuous) and race. We also assessed for interaction of change in renal function on the association of FGF23 and indices of LV and LA function using an interaction term for change in eGFR from Exam 1 to Exam 5. Analyses were carried out using SAS version 9.4 (Cary, NC) and R version 3.5.1 (R Foundation for Statistical Computing). Two-tailed p-values <0.05 were considering statistically significant.
Results
Participant Characteristics
Of the 6,814 participants who were recruited into MESA at baseline examination, 2,098 did not attend Exam 5 (2010–2012), 2,360 did not have CMR performed, and 80 did not have available FGF23 levels at baseline. Therefore, the final analytic cohort for this analysis was 2,276 participants (Supplemental Figure 1). Baseline characteristics of the final analytic cohort by quartile of FGF23 are shown in Table 1. Across the analytic cohort, mean FGF23 was 39.8±14.4 pg/mL. Participants with higher FGF23 levels tended to have higher BMI, higher serum calcium, urinary phosphate, and 25-hydroxyvitamin D concentrations, and lower eGFR. There were relatively few participants who developed incident HF (n=28, 1.2%) or AF (n=102, 4.5%) by Exam 5 CMR. Compared with participants in the final analytic cohort, MESA participants who were excluded from this analysis were older, had higher prevalence of diabetes, and were more likely black race at baseline examination. In addition, excluded participants had lower eGFR, higher SBP, and higher fasting glucose at baseline compared to participants included in this analysis (Supplemental Table 1).
Table 1.
Baseline Characteristics by Quartile of FGF23.
| Characteristic | FGF23 Quartile 1 (n=568) |
FGF23 Quartile 2 (n=570) |
FGF23 Quartile 3 (n=569) |
FGF23 Quartile 4 (n=569) |
P value (linear trend) |
|---|---|---|---|---|---|
| Age, y, mean±SD | 58.7±9.2 | 59.4±9.1 | 59.0±9.4 | 60.1±9.5 | 0.06 |
| Female, n (%) | 329 (57.9) | 300 (52.6) | 295 (51.9) | 308 (54.1) | 0.17 |
| Race, n (%) | 0.04 | ||||
| Black | 139 (24.5) | 137 (24.0) | 133 (23.4) | 119 (20.9) | |
| Chinese | 78 (13.7) | 83 (14.6) | 69 (12.1) | 70 (12.3) | |
| Hispanic | 135 (23.8) | 115 (20.2) | 117 (20.6) | 100 (17.6) | |
| White | 216 (38.0) | 235 (41.2) | 250 (43.9) | 280 (49.2) | |
| Current Smoker, n (%) | 86 (15.2) | 48 (8.4) | 55 (9.7) | 45 (7.9) | 0.001 |
| Body mass index, kg/m2, mean ± SD | 27.0±4.8 | 27.4±4.9 | 27.5±4.8 | 28.4±5.2 | <0.001 |
| Systolic blood pressure, mmHg, mean ± SD | 122.2±21.3 | 121.8±19.7 | 121.9±19.4 | 123.7±19.2 | 0.37 |
| Anti-hypertensive medication, n (%) | 129 (22.7) | 142 (24.9) | 144 (25.3) | 194 (34.1) | <0.001 |
| Diabetes mellitus, n (%) | 39 (6.9) | 53 (9.3) | 48 (8.5) | 59 (10.4) | 0.20 |
| Total cholesterol, mg/dL, mean ± SD | 194.6±35.4 | 194.4±33.2 | 195.7±36.6 | 195.3±36.1 | 0.93 |
| LDL cholesterol, mg/dL, mean ± SD | 116.8±31.9 | 118.1±31.1 | 118.7±31.6 | 117.8±31.3 | 0.78 |
| Glucose, mg/dL, median (IQR) | 87 (81–96) | 88 (82–95) | 87 (82–96) | 88 (83–96) | 0.95 |
| eGFR mL/min/1.73 m2, mean ± SD | 83.8±14.4 | 80.4±13.8 | 79.8±14.6 | 74.6±15.6 | <0.001 |
| Calcium, mg/dL, mean ± SD | 9.6±0.4 | 9.6±0.4 | 9.7±0.4 | 9.7±0.4 | 0.003 |
| 25- hydroxyvitamin D, ng/mL, mean ± SD | 24.9±12.5 | 25.7±11.2 | 26.6±11.3 | 27.6±11.2 | <0.001 |
| Phosphate, urine, mg/dL, mean ± SD | 47.3±31.8 | 48.5±31.1 | 51.2±37.1 | 54.9±41.7 | 0.002 |
| Parathyroid hormone, pg/mL, mean ±SD | 42.1±18.2 | 41.8±17.1 | 42.4±18.1 | 42.7±18.1 | 0.86 |
| Angiotensin converting enzyme inhibitor, n (%) | 50 (8.8) | 53 (9.3) | 51 (9.0) | 66 (11.6) | 0.34 |
| Angiotensin receptor blocker, n(%) | 15 (2.7) | 23 (4.1) | 18 (3.2) | 39 (6.9) | 0.002 |
FGF23 = fibroblast growth factor 23; eGFR = estimated glomerular filtration rate; LDL = low-density lipoprotein
Unadjusted Associations of FGF23 with Cardiac Structure and Function
Comprehensive measures of cardiac structure and function on CMR at Exam 5 (2010–2012) by quartile of baseline FGF23 are shown in Table 2. Participants in higher quartile groups of FGF23 had higher LV mass and larger LV end-diastolic and end-systolic volumes. There were no differences in LV ejection fraction, stroke volume or prevalence of myocardial scar by quartile of FGF23. GCS and mid-wall CS were worse among participants with higher FGF23 levels. Participants with higher FGF23 levels had larger LA volumes and lower LA total emptying fraction. There was no difference in LA peak longitudinal strain by FGF23 quartile.
Table 2.
Cardiac Structure and Function on Cardiac Magnetic Resonance by Quartile of FGF23.
| CMR Measure | FGF23 Quartile 1 | FGF23 Quartile 2 | FGF23 Quartile 3 | FGF23 Quartile 4 | P value (linear trend) |
|---|---|---|---|---|---|
| LV Structure | |||||
| Mass, g, mean±SD | 117.4±33.3 | 121.5±32.6 | 122.7±33.0 | 124.4±32.7 | 0.003 |
| End diastolic volume, mL, mean±SD | 117.1±30.5 | 120.7±30.1 | 122.3±31.4 | 120.8±31.7 | 0.03 |
| End systolic volume, mL, mean±SD | 44.7±16.3 | 46.2±16.5 | 47.8±18.6 | 46.2±18.4 | 0.03 |
| Myocardial scar, n (%) | 20 (3.5) | 18 (3.2) | 33 (5.8) | 29 (5.1) | 0.14 |
| LV Systolic Function | |||||
| Ejection fraction, %, mean±SD | 62.3±6.8 | 62.2±6.9 | 61.5±7.6 | 62.3±7.4 | 0.16 |
| Stroke volume, mL, mean±SD | 72.4±18.1 | 74.5±18.0 | 74.5±18.6 | 75.6±18.8 | 0.12 |
| Global circumferential strain, %, mean±SD | −18.5±2.3 | −18.3±2.3 | −18.2±2.4 | −18.0±2.3 | 0.006 |
| Mid-wall circumferential strain, %, mean±SD | −18.7±2.7 | −18.4±2.7 | −18.2±2.8 | −18.1±2.8 | 0.003 |
| LA Structure | |||||
| LA maximal volume, mL, mean±SD | 62.7±22.2 | 66.7±24.0 | 65.1±23.1 | 66.2±22.1 | 0.02 |
| LA minimal volume, mL, mean±SD | 28.6±16.2 | 31.3±17.5 | 30.6±18.0 | 31.5±16.3 | 0.01 |
| LA Function | |||||
| Total emptying fraction, %, mean±SD | 56.6±11.4 | 55.1±11.3 | 55.2±12.4 | 54.5±11.7 | 0.03 |
| Peak longitudinal strain, %, mean±SD | 33.1±14.2 | 32.0±14.0 | 32.5±14.4 | 31.6±14.9 | 0.34 |
FGF23 = fibroblast growth factor 23; LA = left atrial; LV = left ventricular
Adjusted associations of FGF23 with Cardiac Structure and Function
The multivariable-adjusted associations of baseline FGF23 with measures of cardiac function in later life are shown in Table 3. After adjustment for demographic variables, higher baseline FGF23 was independently associated with worse LV GCS and LV mid-wall CS in later life. In final models further adjusting for clinical characteristics and laboratory covariates, baseline FGF23 remained significantly associated with worse GCS and mid-wall CS in later life, without considerable change in effect size (β coefficient). Similarly, higher baseline FGF23 levels were associated with lower LA total emptying fraction after full multivariable adjustment. There was no association of FGF23 with LA peak longitudinal strain. While FGF23 was associated with higher LV mass after multivariable adjustment (Table 3), there were no significant associations of FGF23 with presence of myocardial scar in the CMR subset (n=1,342) that underwent LGE imaging (OR per 1 SD higher FGF23: 1.12, 95% CI: 0.86–1.45, P= 0.42) (Supplemental Table 2). Similarly, FGF23 was not associated with traditional measures of LV function (LV ejection fraction or stroke volume). On sensitivity analyses, the associations between FGF23 with LV GCS, mid-wall CS, and LA total emptying fraction were consistent after further adjustment for CRP (Supplemental Table 3) or vitamin D and iron supplementation (Supplemental Table 4). While associations of FGF23 with LV GCS and mid-wall CS were consistent after further adjustment for LV mass, the association between FGF23 and LA total emptying fraction was slightly attenuated (Supplemental Table 5). Similarly, while the associations of FGF23 with LV GCS, and mid-wall CS were consistent after further adjustment for Exam 1 CMR variables, the association of FGF23 and LA total emptying fraction was slightly attenuated after Exam 1 LV mass adjustment (Supplemental Table 6).
Table 3.
Association of FGF23 with Cardiac Function.
| Measure of Cardiac Function | β coefficient per SD increase in FGF23 (95% CI) | P value |
|---|---|---|
| LV structure | ||
| LV mass | ||
| Model 1* | 2.02 (1.04, 3.01) | <0.001 |
| Model 2† | 1.14 (0.16, 2.12) | 0.02 |
| LV function | ||
| Global circumferential strain | ||
| Model 1* | 0.18 (0.08, 0.27) | <0.001 |
| Model 2† | 0.15 (0.05, 0.25) | 0.003 |
| Mid-wall circumferential strain | ||
| Model 1* | 0.22 (0.11, 0.33) | <0.001 |
| Model 2† | 0.20 (0.08, 0.31) | 0.001 |
| Ejection fraction | ||
| Model 1* | −0.10 (−0.38, 0.19) | 0.51 |
| Model 2† | −0.20 (−0.51, 0.11) | 0.21 |
| Stroke Volume | ||
| Model 1* | 0.35 (−0.27, 0.97) | 0.27 |
| Model 2† | 0.37 (−0.30, 1.05) | 0.28 |
| LA Function | ||
| Total Emptying Fraction | ||
| Model 1* | −0.51 (−0.97, −0.05) | 0.03 |
| Model 2† | −0.52 (−1.02, −0.02) | 0.04 |
| Peak longitudinal strain | ||
| Model 1* | −0.41 (−0.98, 0.16) | 0.16 |
| Model 2† | −0.27 (−0.89, 0.36) | 0.40 |
Adjusted for age, sex, race, and income at Exam 1
Adjusted for Model 1 covariates plus: smoking status, diabetes, systolic blood pressure, anti-hypertensive medication use, BMI, eGFR, LDL cholesterol, UACR, 25-hydroxyvitamin D, calcium, urine phosphate, and PTH at Exam 1
BMI = body mass index; eGFR = estimated glomerular filtration rate; FGF23 = fibroblast growth factor 23; LA = left atrial; LDL = low density lipoprotein; LV = left ventricular; PTH = parathyroid hormone; UACR = urinary albumin-to-creatinine ratio
There was no interaction by eGFR on the associations of FGF23 with 1) GCS (Pinteraction: 0.32; Figure 1), 2) mid-wall CS (Pinteraction: 0.92), 3) LA total emptying fraction (Pinteraction: 0.10; Figure 2) or 4) LV mass (Pinteraction: 0.73; Supplemental Figure 2). There was no interaction by race on the associations of FGF23 and 1) GCS (Pinteraction: 0.37), 2) mid-wall CS (Pinteraction: 0.54), or 3) LA total emptying fraction (Pinteraction: 0.36). There was no interaction by change in eGFR on the associations of GCS (Pinteraction: 0.72), mid wall CS (Pinteraction: 0.87), or LA total emptying fraction (Pinteraction: 0.36). Finally, there was no interaction by HF status (Pinteraction: 0.70) or AF status (Pinteraction: 0.20) at Exam 5 on the association of FGF23 with GCS.
Figure 1. Association of FGF23 with Global Circumferential Strain by eGFR Category.
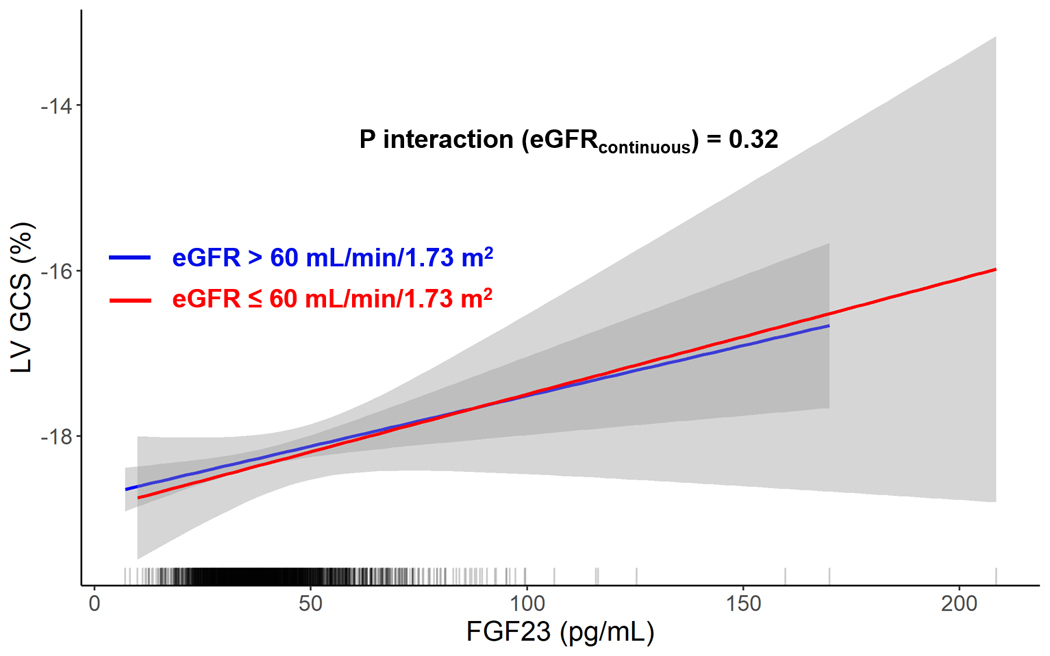
Shown are linear models displaying the association of FGF23 and global circumferential strain by eGFR strata. The rug plot shows the distribution of FGF23. FGF23 = fibroblast growth factor 23; eGFR = estimated glomerular filtration rate.
Figure 2. Association of FGF23 with Left Atrial Total Emptying Fraction by eGFR Category.
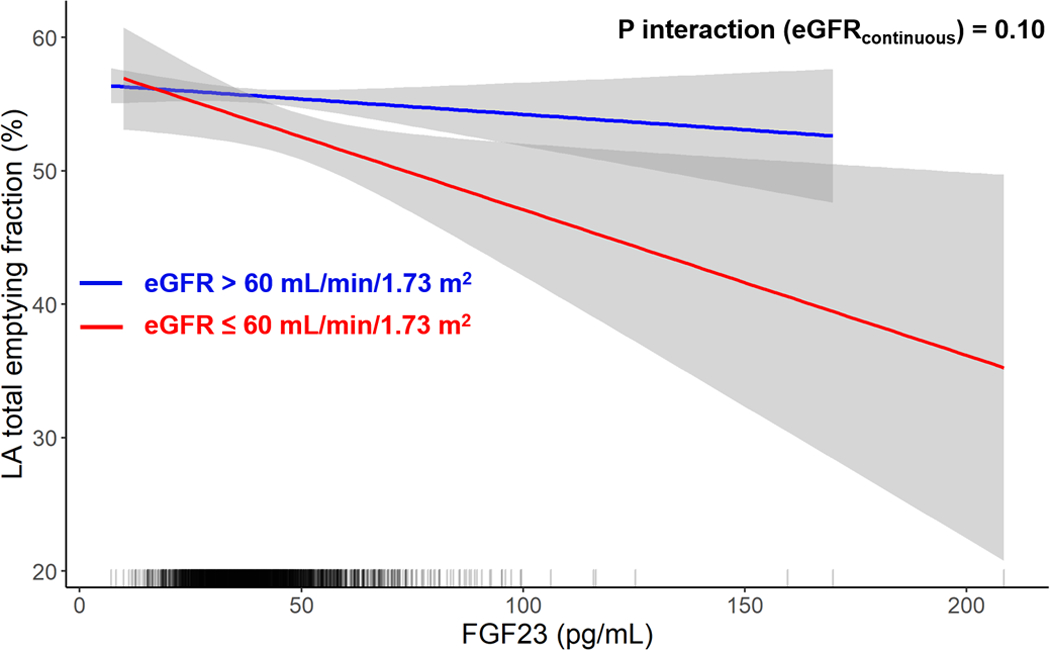
Shown are linear models displaying the association of FGF23 and LA total emptying fraction by eGFR strata. The rug plot shows the distribution of FGF23. FGF23 = fibroblast growth factor 23; eGFR = estimated glomerular filtration rate; LA = left atrial.
Results of mediation analyses of FGF23, LV mass at Exam 5, and LA total emptying fraction are displayed in Figure 3. LV mass was a partial mediator of the relationships between FGF23 and LA total emptying fraction. Specifically, LV mass appeared to mediate 16% of the association between FGF23 and LA total emptying fraction (P=0.04).
Figure 3. Mediation Analysis of FGF23, LV Mass, and LA Total Emptying Fraction.
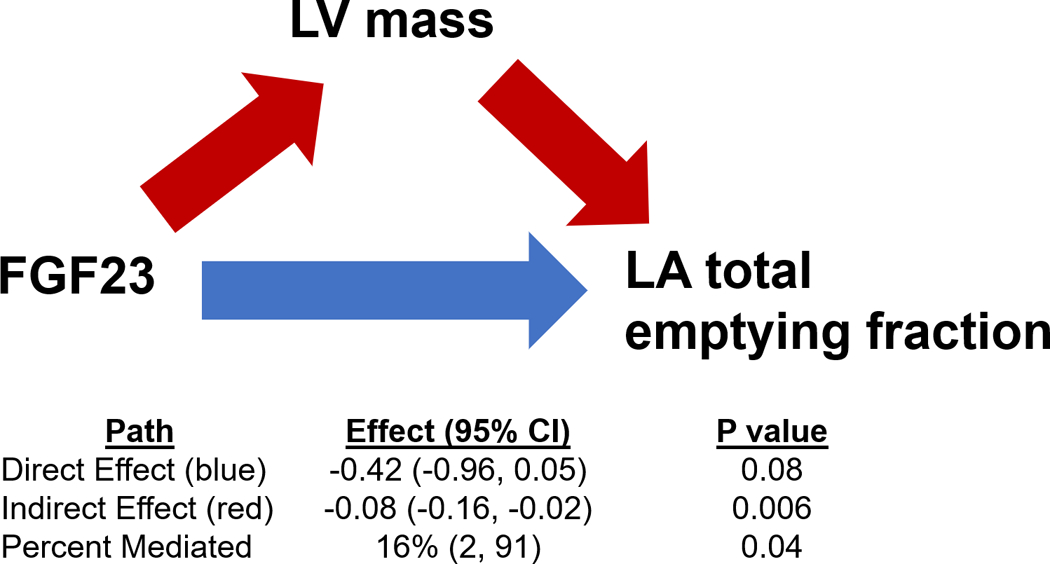
The path model and mediation analyses describing mediation of the relationship between FGF23, LV mass, and LA total emptying fraction are displayed. FGF23 = fibroblast growth factor 23. LA = left atrial; LV = left ventricular.
Discussion
In this multiethnic cohort of adults without cardiovascular disease at enrollment, we found associations of baseline FGF23 with worse LV systolic function, as measured by LV GCS and LV mid-wall CS, worse LA total emptying fraction, and higher LV mass at 10-year follow up after adjustment for baseline kidney function and other risk factors. Additionally, the associations of FGF23 with indices of LV systolic function and LA function were consistent across the spectra of renal function and race in the MESA study. Notably, FGF23 was not associated with traditional measures of LV function, including LV ejection fraction and stroke volume. While FGF23 was associated with higher LV mass, it was not associated with the presence of myocardial scar. Higher LV mass appeared to partially mediate the association between FGF23 and LA total emptying fraction. In total, these findings suggest a potential mechanistic role of FGF23 in driving the complex LA and LV myocardial substrate that may ultimately lead to the development of overt cardiovascular diseases, including HF and AF.
Experimental, epidemiologic, and post-hoc trial data have suggested specific associations of FGF23 with the development of HF. Indeed, FGF23 induces left ventricular hypertrophy in mice through signaling pathways that may be independent of Klotho, the traditional co-receptor to which the hormone binds.5 Additionally, FGF23 has been consistently associated with increased risk of HF in community-based cohorts, independent of kidney function.8–10 Among patients with stable ischemic heart disease or acute coronary syndromes, 2 particularly high-risk cohorts, baseline FGF23 levels have also been associated with higher risk of incident HF.25, 26 While FGF23 appears to be more strongly related to incident HFpEF as compared with HFrEF,14 the specific mechanisms by which FGF23 may contribute to HF pathogenesis remain unclear. Importantly, the associations of FGF23 with sensitive measures of cardiac function have not been described. Previously, FGF23 has been variably associated with traditional, crude indices of cardiac function. Among individuals with known coronary artery disease or those undergoing cardiac catheterization, FGF23 has been associated with lower LV ejection fraction.27–29 Importantly, such prior analyses have evaluated study populations with high prevalence of cardiovascular disease. Additionally, these previous investigations have measured traditional, gross measures of LV function (i.e., LV ejection fraction), which may lack sensitivity to detect subtle alterations in cardiac function that precede overt HF and offer limited insight regarding disease pathogenesis. In the current investigation, we provide a unique evaluation of the associations of baseline FGF23 levels with worse long-term cardiac function on CMR through indices of LV and LA strain among individuals free of cardiovascular disease at baseline, thus allowing for specific understanding of the substrate-specific alterations associated with higher FGF23.
FGF23 was independently associated with higher LV mass and subclinical alterations in LV systolic function as assessed by strain imaging, providing evidence for this hormone’s potential role in HF development, specifically with regard to HFpEF. HFpEF is a heterogeneous syndrome that may arise as a result of a variety of myocardial substrates. Indeed, HFpEF may arise secondary to chronic cardiovascular comorbidities, including hypertension, that lead to LV hypertrophy, reduced LV compliance, and subsequent elevation in LV filling pressures. Through high-fidelity CMR evaluation of LV mass, we confirm that FGF23 is independently associated with higher LV mass, a known risk factor for HFpEF.30 In addition, recent evidence suggests that HFpEF is indeed a syndrome of both diastolic and systolic dysfunction. In an analysis of the Prospective Comparison of ARNI with ARB on Management of Heart Failure with Preserved Ejection Fraction (PARAMOUNT) trial, HFpEF patients had worse LV GCS compared with a high-risk, community-based population without HFpEF.31 Additionally, reduced LV GCS was independently associated with a composite of incident HFpEF and HFrEF in the MESA study, and >90% of such incident HF cases were in fact HFpEF.21 In this analysis, we demonstrate long-term associations of FGF23 with subclinical measures of cardiac systolic dysfunction (i.e., LV GCS and mid-wall CS) that are known to precede the development of HFpEF and are also abnormal in prevalent HFpEF. Notably, the associations of FGF23 with derangement in LV GCS and mid-wall CS was independent of LV mass. Taken together, our findings suggest that FGF23 may exhibit multiple, distinct myocardial effects that drive HFpEF to exist: LV hypertrophy and LV systolic dysfunction. Previous experimental models support our findings that FGF23 may lead to cardiac systolic dysfunction. Namely, FGF23 administration to cardiomyocytes in vitro resulted in abnormalities in intracellular calcium handling.32 Thus, the independent associations of FGF23 with reduced systolic function in our study carry a strong molecular basis, as FGF23 elevation appears to influence intracellular calcium handing abnormalities in experimental studies. Finally, as FGF23 is strongly associated with subclinical LV systolic dysfunction and clinical HF, further investigation is required to understand if FGF23 mediates the associations of genetic risk with HF, which may further elucidate the hormone’s role in driving HF.
Our study additionally provides insight into LA-specific myocardial derangements that may explain the associations of FGF23 with incident AF.11–13 While higher FGF23 has been associated with structural abnormalities (i.e., increased LA size) in cross-sectional analyses,11, 12 the longitudinal relationships between FGF23 and LA function has been incompletely understood. Additionally, it is unclear whether FGF23 is associated with reduced LA function independent of LV structure and function. In the current study, FGF23 was associated with lower LA total emptying fraction in later life. Indeed, LA total emptying fraction is a known predictor of future AF.22, 33 These findings suggest that FGF23 is more strongly associated with lower LA booster (i.e., contractile) function, as this parameter constitutes the ability of the LA to transfer blood into the LV and is captured by the aggregate metric of LA total emptying fraction. Notably, the association between FGF23 and LA total emptying fraction was partially mediated by higher LV mass, suggesting that LA dysfunction associated with FGF23 may be a downstream consequence of LV non-compliance and increased LA pressures. Further research is required to understand chamber-specific consequences of FGF23 elevation given the hormone’s association with both HF and AF.
Our study has strengths and limitations. Participants in the MESA study are ethnically diverse and have been closely followed for over a decade, allowing for unique understanding of the transition from risk factors to subclinical cardiac disease. Additionally, the biologically active form of FGF23 was measured in the current study, as opposed to the C-terminal fragment. Finally, CMR protocol was standardized across all sites and all interpretations were performed in a blinded fashion at a single, experienced reading center. Although our final analytic sample was large, a number of participants were excluded from the original cohort. Excluded participants tended to have higher prevalence of cardiovascular risk factors (and likely myocardial dysfunction), and thus our analytic cohort may indeed underestimate associations. While FGF23 was associated with reduced systolic function, there was no association between FGF23 and the presence of myocardial scar. Macroscopic cardiac fibrosis was infrequently noted in the current study, which may limit the power to detect an association, and we were unable to determine the extent of microscopic interstitial fibrosis through extracellular volume measurement in the current study. Diastolic function has not been comprehensively evaluated on MESA CMR at Exam 5, and we were thus unable to evaluate associations of FGF23 with LV early diastolic strain rate. Although we could not adjust for serum levels of hemoglobin and bicarbonate because they were not collected in the MESA cohort at Exam 1, the associations of FGF23 and cardiac function were consistent after multivariable adjustment.
In this investigation of a multi-ethnic cohort without known cardiovascular disease at baseline, FGF23 levels identified participants with hypertension and lower baseline kidney function. FGF23 at baseline was independently associated with higher LV mass, worse LV systolic function, as measured by LV GCS and LV mid-wall CS, and lower LA systolic function, as measured by LA total emptying fraction, in later life. These associations were independent of several clinical factors, including degree of renal dysfunction at baseline, and the association of FGF23 with lower systolic function was independent of LV mass. The associations of FGF23 and adverse LV and LA systolic mechanics were consistent across racial strata and the spectrum of eGFR. In total, these findings provide mechanistic support for the role of FGF23 in the development of overt HF, substantiate the strong association between FGF23 and HFpEF, and suggest that FGF23 may drive HFpEF through distinct effects upon the myocardium. Further investigation is required to understand if therapeutic reduction in FGF23 can alter derangements in cardiac function and prevent progression to overt cardiovascular disease.
Supplementary Material
Clinical Perspectives.
Fibroblast growth factor 23 (FGF23) is a hormone that increases in the setting of chronic kidney disease and has been associated with the development of multiple cardiovascular diseases, specifically heart failure (HF) and atrial fibrillation (AF). Additionally, FGF23 appears to be more strongly associated with HF with preserved ejection fraction (HFpEF). However, the mechanisms by which FGF23 leads to clinical HF and AF are unclear. We analyzed a community-based cohort that underwent cardiac magnetic resonance (CMR) imaging 10 years after FGF23 measurement. Baseline FGF23 was associated with lower LV systolic function, higher LV mass, and lower LA function at follow up over 10 years later. Through investigation of sensitive measures of cardiac structure and function on CMR, these findings provide insight into a potential multifaceted effect of FGF23 on cardiac function that may drive HF and AF. Further studies are required to understand if reduction in FGF23 may prevent progression to overt cardiovascular diseases.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI), by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS), and by grant R01HL096875 from the NHLBI. Dr. Ravi Patel is supported by the National Institutes of Health’s National Center for Advancing Translational Sciences (KL2TR001424).
Disclosures
Dr. Sanjiv Shah has received research grants from Actelion, AstraZeneca, Corvia, and Novartis, and consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Cardiora, Eisai, Ironwood, Merck, MyoKardia, Novartis, Sanofi, and United Therapeutics.
Footnotes
The remaining authors have nothing to disclose.
References
- 1.Liu S and Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18:1637–47. [DOI] [PubMed] [Google Scholar]
- 2.Isakova T, Cai X, Lee J, Mehta R, Zhang X, Yang W, Nessel L, Anderson AH, Lo J, Porter A et al. Longitudinal Evolution of Markers of Mineral Metabolism in Patients With CKD: The Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2020;75:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vervloet M Renal and extrarenal effects of fibroblast growth factor 23. Nat Rev Nephrol. 2019;15:109–120. [DOI] [PubMed] [Google Scholar]
- 5.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jovanovich A, Ix JH, Gottdiener J, McFann K, Katz R, Kestenbaum B, de Boer IH, Sarnak M, Shlipak MG, Mukamal KJ et al. Fibroblast growth factor 23, left ventricular mass, and left ventricular hypertrophy in community-dwelling older adults. Atherosclerosis. 2013;231:114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kestenbaum B, Sachs MC, Hoofnagle AN, Siscovick DS, Ix JH, Robinson-Cohen C, Lima JA, Polak JF, Blondon M, Ruzinski J et al. Fibroblast growth factor-23 and cardiovascular disease in the general population: the Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2014;7:409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ and Shlipak MG. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol. 2012;60:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutsey PL, Alonso A, Selvin E, Pankow JS, Michos ED, Agarwal SK, Loehr LR, Eckfeldt JH and Coresh J. Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: the Atherosclerosis Risk in Communities study. J Am Heart Assoc. 2014;3:e000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathew JS, Sachs MC, Katz R, Patton KK, Heckbert SR, Hoofnagle AN, Alonso A, Chonchol M, Deo R, Ix JH et al. Fibroblast growth factor-23 and incident atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS). Circulation. 2014;130:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta R, Cai X, Lee J, Scialla JJ, Bansal N, Sondheimer JH, Chen J, Hamm LL, Ricardo AC, Navaneethan SD et al. Association of Fibroblast Growth Factor 23 With Atrial Fibrillation in Chronic Kidney Disease, From the Chronic Renal Insufficiency Cohort Study. JAMA Cardiol. 2016;1:548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso A, Misialek JR, Eckfeldt JH, Selvin E, Coresh J, Chen LY, Soliman EZ, Agarwal SK and Lutsey PL. Circulating fibroblast growth factor-23 and the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities study. J Am Heart Assoc. 2014;3:e001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almahmoud MF, Soliman EZ, Bertoni AG, Kestenbaum B, Katz R, Lima JAC, Ouyang P, Miller PE, Michos ED and Herrington DM. Fibroblast Growth Factor-23 and Heart Failure With Reduced Versus Preserved Ejection Fraction: MESA. J Am Heart Assoc. 2018;7:e008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 16.Lewis MR, Callas PW, Jenny NS and Tracy RP. Longitudinal stability of coagulation, fibrinolysis, and inflammation factors in stored plasma samples. Thromb Haemost. 2001;86:1495–500. [PubMed] [Google Scholar]
- 17.Imel EA, Peacock M, Pitukcheewanont P, Heller HJ, Ward LM, Shulman D, Kassem M, Rackoff P, Zimering M, Dalkin A et al. Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab. 2006;91:2055–61. [DOI] [PubMed] [Google Scholar]
- 18.Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, Liu Y, Hundley WG, Gomes AS, Liu S et al. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2013;62:1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castillo E, Osman NF, Rosen BD, El-Shehaby I, Pan L, Jerosch-Herold M, Lai S, Bluemke DA and Lima JA. Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase (HARP) MRI. J Cardiovasc Magn Reson. 2005;7:783–91. [DOI] [PubMed] [Google Scholar]
- 20.Turkbey EB, Nacif MS, Guo M, McClelland RL, Teixeira PB, Bild DE, Barr RG, Shea S, Post W, Burke G et al. Prevalence and Correlates of Myocardial Scar in a US Cohort. JAMA. 2015;314:1945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi EY, Rosen BD, Fernandes VR, Yan RT, Yoneyama K, Donekal S, Opdahl A, Almeida AL, Wu CO, Gomes AS et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2013;34:2354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habibi M, Samiei S, Ambale Venkatesh B, Opdahl A, Helle-Valle TM, Zareian M, Almeida AL, Choi EY, Wu C, Alonso A et al. Cardiac Magnetic Resonance-Measured Left Atrial Volume and Function and Incident Atrial Fibrillation: Results From MESA (Multi-Ethnic Study of Atherosclerosis). Circ Cardiovasc Imaging. 2016;9: 10.1161/CIRCIMAGING.115.004299 e004299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Group MS, Kuen E et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–8. [DOI] [PubMed] [Google Scholar]
- 24.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL and Lima JA. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergmark BA, Udell JA, Morrow DA, Cannon CP, Steen DL, Jarolim P, Budaj A, Hamm C, Guo J, Im K et al. Association of Fibroblast Growth Factor 23 With Recurrent Cardiovascular Events in Patients After an Acute Coronary Syndrome: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2018;3:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Udell JA, Morrow DA, Jarolim P, Sloan S, Hoffman EB, O’Donnell TF, Vora AN, Omland T, Solomon SD, Pfeffer MA et al. Fibroblast growth factor-23, cardiovascular prognosis, and benefit of angiotensin-converting enzyme inhibition in stable ischemic heart disease. J Am Coll Cardiol. 2014;63:2421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal I, Ide N, Ix JH, Kestenbaum B, Lanske B, Schiller NB, Whooley MA and Mukamal KJ. Fibroblast growth factor-23 and cardiac structure and function. J Am Heart Assoc. 2014;3:e000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seiler S, Cremers B, Rebling NM, Hornof F, Jeken J, Kersting S, Steimle C, Ege P, Fehrenz M, Rogacev KS et al. The phosphatonin fibroblast growth factor 23 links calcium-phosphate metabolism with left-ventricular dysfunction and atrial fibrillation. Eur Heart J. 2011;32:2688–96. [DOI] [PubMed] [Google Scholar]
- 29.Shibata K, Fujita S, Morita H, Okamoto Y, Sohmiya K, Hoshiga M and Ishizaka N. Association between circulating fibroblast growth factor 23, alpha-Klotho, and the left ventricular ejection fraction and left ventricular mass in cardiology inpatients. PLoS One. 2013;8:e73184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL and Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Touchberry CD, Green TM, Tchikrizov V, Mannix JE, Mao TF, Carney BW, Girgis M, Vincent RJ, Wetmore LA, Dawn B et al. FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am J Physiol Endocrinol Metab. 2013;304:E863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sardana M, Lessard D, Tsao CW, Parikh NI, Barton BA, Nah G, Thomas RC, Cheng S, Schiller NB, Aragam JR et al. Association of Left Atrial Function Index with Atrial Fibrillation and Cardiovascular Disease: The Framingham Offspring Study. J Am Heart Assoc. 2018;7:e008435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


