Abstract
CC chemokines, a subfamily of 27 chemotactic cytokines, are a component of intercellular communication, which is crucial for the functioning of the tumor microenvironment. Although many individual chemokines have been well researched, there has been no comprehensive review presenting the role of all known human CC chemokines in the hallmarks of cancer, and this paper aims at filling this gap. The first part of this review discusses the importance of CCL1, CCL3, CCL4, CCL5, CCL18, CCL19, CCL20, CCL21, CCL25, CCL27, and CCL28 in cancer. Here, we discuss the significance of CCL2 (MCP-1), CCL7, CCL8, CCL11, CCL13, CCL14, CCL15, CCL16, CCL17, CCL22, CCL23, CCL24, and CCL26. The presentation of each chemokine includes its physiological function and then the role in tumor, including proliferation, drug resistance, migration, invasion, and organ-specific metastasis of tumor cells, as well as the effects on angiogenesis and lymphangiogenesis. We also discuss the effects of each CC chemokine on the recruitment of cancer-associated cells to the tumor niche (eosinophils, myeloid-derived suppressor cells (MDSC), tumor-associated macrophages (TAM), tumor-associated neutrophils (TAN), regulatory T cells (Treg)). On the other hand, we also present the anti-cancer properties of CC chemokines, consisting in the recruitment of tumor-infiltrating lymphocytes (TIL).
Keywords: chemokine, CC chemokine, cancer, tumor, organ-specific metastasis, angiogenesis, lymphangiogenesis, tumor microenvironment, anti-cancer therapy, MCP-1
1. Introduction
A high percentage of deaths in cancer treatment is partly due to inadequate treatment methods as a result of our incomplete understanding of cancer mechanisms. However, cancer models and treatment methods are improving and increasing their efficacy. Over the last 20 years, the perception of cancer cells in tumors has changed significantly [1,2]. Previously, research focused on the inside of a cancer cell, but, nowadays, more attention is paid to the tumor niche and tumor microenvironment, with a special focus on non-cancer cells and intercellular communication [3,4,5]. It is now known that tumor growth, and, thus, the progression of cancer, requires communication between cancer and non-cancer cells, involving chemokines among other things.
Chemokines are a group of almost 50 chemoattractant cytokines and are divided into sub-families according to the domain found at the N-terminus [6,7]. One such sub-family are twenty-seven CC chemokines with an N-terminal CC domain, four of which (chemokine (C-C motif) ligand 6 (CCL6), CCL9/CCL10, and CCL12) are murine chemokines [6,7]. Although CC chemokines are presented using 28 symbols (CCL1 to CCL28), their actual number is 27, as chemokines CCL9 and CCL10 are actually the same chemokine. CC chemokines are ligands for ten classical receptors and serve as an important component of the tumor microenvironment and of cell relationships in the tumor niche, evidenced by their receptors association with the prognosis of patients with a particular type of tumor (Table 1 and Table 2 based on “The Human Protein Atlas” (https://www.proteinatlas.org/) [8,9]. There are no single CC chemokines that give the same prognosis in all types of tumors, which is due to the differences between cancers and the fact that any given chemokine has both pro-cancer and anti-cancer properties. A chemokine may cause tumor infiltration of the tumor by tumor-infiltrating lymphocytes (TIL), the cells that destroy cancer cells [10,11], whilst also recruiting tumor-associated cells that cooperate with cancer cells in tumor development [12,13].
Table 1.
Influence of increased expression of individual CC chemokines discussed in this review on the prognosis of patients with various cancers according to “The Human Protein Atlas” (https://www.proteinatlas.org/) [8,9].
| Type of Cancer | Chemokine | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCL2 | CCL7 | CCL8 | CCL11 | CCL13 | CCL14 | CCL15 | CCL16 | CCL17 | CCL22 | CCL23 | CCL24 | CCL26 | |
| Glioma | ↓ | ↓ | ↓p = 0.095 | N/A | ↓p = 0.054 | -- | N/A | N/A | ↑ | -- | ↓ | ↓ | -- |
| Thyroid cancer | -- | -- | ↑p = 0.057 | -- | -- | -- | ↑ | -- | ↓ | ↓ | ↓ | -- | -- |
| Lung cancer | ↓p = 0.075 | ↓ | -- | ↓p = 0.081 | ↑p = 0.085 | -- | -- | -- | ↑ | ↑p = 0.089 | -- | -- | ↓p = 0.062 |
| Colorectal cancer | ↓ | ↓p = 0.085 | ↓ | ↑ | ↑ | ↑ | ↑ | -- | ↑p = 0.058 | ↑ | ↓p = 0.10 | ↑ | -- |
| Head and neck cancer | ↓p = 0.086 | -- | ↑ | ↑p = 0.058 | -- | ↑ | N/A | -- | ↑ | ↑ | -- | ↑p = 0.056 | ↓ |
| Stomach cancer | -- | -- | -- | ↓ | -- | ↓ | -- | ↓ | -- | ↑p = 0.077 | -- | ↑ | ↑ |
| Liver cancer | ↑ | N/A | -- | -- | ↓p = 0.076 | ↑ | ↓p = 0.089 | ↑ | -- | -- | ↑ | -- | ↓ |
| Pancreatic cancer | -- | ↓ | -- | ↓ | ↓ | ↑ | -- | ↑ | -- | ↑ | ↑p = 0.071 | -- | ↑p = 0.079 |
| Renal cancer | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓p = 0.060 | ↑ | ↓ | -- | ↓ |
| Urothelial cancer | ↓ | ↓ | ↓ | ↓ | ↑ | -- | ↑ | ↑ | ↑ | ↑ | -- | ↓ | ↓ |
| Prostate cancer | -- | ↑p = 0.070 | -- | -- | ↓p = 0.060 | ↑ | ↑ | -- | ↓ | -- | ↑p = 0.069 | -- | -- |
| Testicular cancer | ↓ | ↓p = 0.087 | ↓ | ↓ | -- | -- | -- | -- | ↓ | ↓ | -- | -- | -- |
| Breast cancer | ↑p = 0.064 | ↑p = 0.077 | ↓p = 0.075 | ↑ | ↑ | ↑ | -- | -- | ↑ | ↑ | ↑ | ↑ | -- |
| Cervical cancer | ↓ | ↓ | -- | -- | ↑ | ↑p = 0.058 | -- | -- | ↑ | ↑ | ↑ | -- | -- |
| Endometrial cancer | ↑p = 0.056 | ↓ | ↓ | ↓p = 0.082 | ↑ | ↓ | -- | ↓ | ↑ | ↑ | ↓p = 0.095 | ↑ | -- |
| Ovarian cancer | -- | ↑ | ↑ | -- | ↑ | ↓p = 0.081 | N/A | -- | ↑ | ↑ | ↑ | -- | ↑p = 0.087 |
| Melanoma | -- | -- | -- | -- | -- | -- | -- | -- | ↑p = 0.064 | -- | ↑p = 0.065 | ↓ | -- |
↑ blue background—better prognosis with higher expression of a given chemokine in a tumor; ↓ red background—worse prognosis with higher expression of a given chemokine in a tumor; -- means no correlation with higher expression of a given chemokine in a tumor.
Table 2.
Effects of increased expression of individual CC chemokine receptors discussed in this review on the prognosis of patients with various cancers according to “The Human Protein Atlas” (https://www.proteinatlas.org/) [8,9].
| Type of Cancer | Receptor | |||
|---|---|---|---|---|
| CCR1 | CCR2 | CCR3 | CCR4 | |
| Glioma | ↓p = 0.095 | ↓ | -- | -- |
| Thyroid cancer | -- | ↑ | -- | -- |
| Lung cancer | ↓p = 0.088 | ↑ | ↓ | ↑ |
| Colorectal cancer | -- | ↑ | -- | ↑ |
| Head and neck cancer | ↑p = 0.067 | ↑ | ↓ | ↑ |
| Stomach cancer | -- | -- | ↓ | -- |
| Liver cancer | -- | ↑p = 0.081 | ↓ | -- |
| Pancreatic cancer | -- | -- | ↓ | -- |
| Renal cancer | ↓ | ↓ | ↓ | ↓ |
| Urothelial cancer | -- | -- | ↓p = 0.098 | -- |
| Prostate cancer | -- | -- | ↑ | -- |
| Testicular cancer | ↓ | ↓ | ↓ | ↓ |
| Breast cancer | -- | ↑ | ↓ | ↑ |
| Cervical cancer | -- | ↑ | ↓ | ↑ |
| Endometrial cancer | -- | ↑ | ↓ | ↑ |
| Ovarian cancer | ↑ | ↑ | ↑ | ↑ |
| Melanoma | ↑ | ↑p = 0.063 | ↓ | ↑ |
↑ blue background—better prognosis with higher expression of a given chemokine in a tumor; ↓ red background—worse prognosis with higher expression of a given chemokine in a tumor; -- means no correlation with higher expression of a given chemokine in a tumor.
As there is no comprehensive and up-to-date compendium that discusses the role of all human CC chemokines in cancer, the aim of this review was to collect all information about the involvement of each human CC chemokine in the hallmarks of cancer. Due to the large amount of data, we decided to divide the paper into two parts. In the first part we discuss CC chemokine receptors CCR1, CCR2, CCR3, and CCR4 together with their main ligands (CCL2, CCL7, CCL8, CCL11, CCL13, CCL14, CCL15, CCL16, CCL17, CCL22, CCL23, CCL24, and CCL26) (Table 3 and Table 4) [6,7]. In the second part, we present receptors CCR5, CCR6, CCR7, CCR8, CCR9 and CCR10 and their ligands (CCL1, CCL3, CCL4, CCL5, CCL18, CCL19, CCL20, CCL21, CCL25, CCL27, and CCL28) [14]. The described chemokines are presented according to the receptor that they activate. However, many CC chemokines are ligands for more than one receptor, and for this reason, we also discuss them according to their shared properties. In order to better understand the role of individual CC chemokines in cancer, special attention has been paid to their physiological functions.
Table 3.
CC chemokines discussed in this part of the article, including cells recruited to the tumor niche.
| Name | Receptor | Effect on the Recruitment of Non-Cancer Cells into the Tumor | Induction of Angiogenesis or Lymphangiogenesis | Organ-Specific Metastasis |
|---|---|---|---|---|
| CCL2 | CCR1 (low-affinity binding), CCR2, CCR3 (antagonist), CCR4, CCR5 | TIL, MDSC, MSC, TAM, Treg, Th17, neural progenitor cells, microglia, hepatic stellate cells | Angiogenesis | Bone, perineural invasion |
| CCL7 | CCR1, CCR2, CCR3, CCR5 (antagonist) | TIL, TAM | ||
| CCL8 | CCR1, CCR2, CCR3, CCR5 | TAM, Treg | ||
| CCL11 | CCR2 (antagonist), CCR3, CCR5 | Eosinophils | Angiogenesis | |
| CCL13 | CCR2, CCR3, CCR5 | |||
| CCL14 | CCR1, CCR3 (low-affinity binding), CCR5 |
TAM | Angiogenesis | |
| CCL15 | CCR1, CCR3 | MDSC, MSC, TAM, TAN, osteoclast precursors, osteoclasts | Angiogenesis | |
| CCL16 | CCR1, CCR2, CCR5, CCR8, histamine H4 receptor | Angiogenesis | ||
| CCL17 | CCR4 | TIL, Treg, Th17, eosinophils | ||
| CCL22 | CCR4 | TIL, Treg, Th17, eosinophils | Bone | |
| CCL23 | CCR1 | Angiogenesis | ||
| CCL24 | CCR2 (antagonist), CCR3, | Eosinophils | Angiogenesis | |
| CCL26 | CCR1 (antagonist), CCR2 (antagonist), CCR3, CCR5 (antagonist), CX3CR1 | Eosinophils, MDSC, TAM | Angiogenesis |
MDSC—myeloid-derived suppressor cells; MSC—mesenchymal stem cells; TAM—tumor-associated macrophages; TAN—tumor-associated neutrophils; Th17—T helper 17; TIL—anti-cancer tumor-infiltrating lymphocytes; Treg—regulatory T cells.
Table 4.
Receptors of CC chemokines discussed in this part of the paper, with their ligands and functions in a tumor.
| Receptor | Ligand | Influence on the Recruitment of Cells into the Tumor Niche | Effects on Tumor Vascularization | Organ-Specific Metastasis |
|---|---|---|---|---|
| CCR1 | CCL2, CCL3, CCL4, CCL5, CCL7, CCL8, CCL14, CCL15, CCL16, CCL23 | MDSC, MSC, TAM, TAN, osteoclast precursors, osteoclasts | Increase in VEGF expression which leads to angiogenesis | Liver |
| CCR2 | CCL2, CCL7, CCL8, CCL13, CCL16 | MDSC, MSC, TAM, Treg | TAM-dependent angiogenesis | Bone, perineural invasion |
| CCR3 | CCL5, CCL7, CCL8, CCL11, CCL13, CCL14, CCL15, CCL24, CCL26, CCL28 |
Eosinophils, TAM | Angiogenesis | |
| CCR4 | CCL2, CCL17, CCL22 | TIL, Th17, Treg, | Lymph node, bone |
MDSC—myeloid-derived suppressor cells; MSC—mesenchymal stem cells; TAM—tumor-associated macrophages; TAN—tumor-associated neutrophils; Th17—T helper 17; TIL—anti-cancer tumor-infiltrating lymphocytes; Treg—regulatory T cells; VEGF—vascular endothelial growth factor.
2. CCR1
2.1. CCL14
CCL14 (also known as hemofiltrate CC chemokine (HCC)-1), occurs in high concentrations in plasma [15]. The expression of this chemokine has also been found in organs such as spleen, colon, small intestine, liver, muscle, and bone marrow but not in the brain and kidney [15]. CCL14 is a ligand of CCR1 [16,17], CCR5 [17,18], and a weak agonist of CCR3 [17]. For this reason, it serves as a chemotactic agent for monocytes but not for T lymphocytes, neutrophils, and eosinophils [15], although a later study showed that CCL14 may be a neutrophil chemoattractant [19].
CCL14 is secreted as an inactive proform. Following the cleavage of the 8-amino acid fragment by the urokinase-type plasminogen activator (uPA), plasmin or kallikrein-related peptidases, it is converted to an active form [20,21,22,23], which may be further processed by the cleavage of two amino acids by dipeptidyl peptidase IV (DPPIV)/CD26, which inactivates the active form of CCL14 [24]. The active form of CCL14 can also be broken down by atypical chemokine receptor 2 (ACKR2)/D6 [24]; however, the expression of this receptor decreases as the tumor develops [25,26,27].
The physiological function of CCL14 is poorly understood. It is important in the functioning of bone marrow by stimulating the proliferation of CD34+ human bone marrow cells [15]. CCL14 causes changes in extracellular matrix in the trophoblast, which induces trophoblast cell migration and embryo implantation [28].
CCL14 has anti- and pro-cancer properties. The expression of this chemokine is reduced in many solid tumors including in liver, breast, lung, and prostate cancer [29,30]. On the other hand, it is elevated in brain and esophageal cancer [30]. Since CCL14 is present in high concentrations in plasma and is activated by appropriate proteinases [15,20,21], it can act locally at the site of proteinase activity, e.g., uPA and plasmin.
CCL14 reduces the activation of the Wnt/β-catenin pathway in hepatocellular carcinoma cells, which inhibits their proliferation and causes their apoptosis [29]. CCL14 also has pro-cancer properties—it induces the migration of cancer cells, as shown by an experiment on breast cancer cells [31], and also causes angiogenesis [31]. It is also postulated that the CCL14→CCR1 axis is crucial in liver metastasis [15,32]. In addition, CCL14 participates in the recruitment of monocytes into the tumor niche, especially to bone marrow, as shown on a multiple myeloma model [33]. The monocytes are then converted into tumor-associated macrophages (TAM) by the tumor microenvironment. Subsequently, CCL14 increases the proliferation of TAM, which increases the number of these cells in the tumor niche [33]. Although CCL14 can participate in the infiltration of the tumor by anti-cancer TIL [30], a study on hepatocellular carcinoma has shown a negative correlation between the concentration of CCL14 and such infiltration [30], and so the effect of CCL14 on the response of the immune system to cancer requires further study.
2.2. CCL15
CCL15 (also known as HCC-2, macrophage inflammatory protein (MIP)-1δ, MIP-5, leukotactin-1) activates two receptors: CCR1 [34,35,36] and CCR3 [34,35], with the N-terminally truncated form of this chemokine showing a strong affinity for CCR1 [22,37,38]. CCL15 is a chemotactic agent for monocytes, eosinophils, and neutrophils [35,36]. It has the greatest expression in the gut and liver [36] and so is crucial for maintaining immune balance in these organs.
CCL15 is a serum biomarker and independent predictor of survival in hepatocellular carcinoma [39,40]. The higher the concentration of CCL15, the worse the prognosis—an effect associated with the migration and invasion of these cells, induced by CCL15 via CCR1 [40]. In head and neck squamous cell carcinoma, activation of CCR1 by the described chemokine induces apoptosis resistance and drug resistance by activating nuclear factor κB (NF-κB) [41]. CCL15 is crucial for the metastasis of renal cell carcinoma. If the cancer cell stops in the bone, CCL15, produced by the cancer cell, acts chemotactically on osteoclast precursors and osteoclasts, probably via CCR1 and CCR3 [42]. In this case, CCL15 causes osteoclastogenesis, which is followed by bone remodeling around the tumor cell and the formation of a metastatic niche [43]. The CCL15→CCR1 axis is also postulated to play an important role in liver metastasis [32,36].
CCL15 also acts on non-cancer cells in the tumor, for example causing angiogenesis mediated by CCR1 and CCR3 on vascular endothelial cells [44]. In addition, in hepatocellular carcinoma and colorectal cancer, CCL15 is responsible for recruiting TAM and myeloid-derived suppressor cells (MDSC) [45,46] and for recruiting mesenchymal stem cells (MSC) into the tumor niche [47]. Colorectal cancer models have shown that CCL15 causes tumor-associated neutrophils (TAN) recruitment to the tumor niche via CCR1 on these cells [48].
2.3. CCL16
Chemokine CCL16 (other names: HCC-4, liver expressed chemokine (LEC), liver-specific CC chemokine-1 (LCC-1)) is constitutively expressed in the liver and by hepatoma cells [6,49], as well as by monocytes treated with interleukin (IL)-10 [50]. High concentrations of CCL16 can be found in the blood [51]. It is a ligand for CCR1 [51,52,53,54], CCR2 [51], CCR5 [51] and CCR8 [52]. CCL16 is a ligand of the histamine H4 receptor and therefore may act on eosinophils [55].
Receptor CCR1 is important in liver metastasis [32] and so it can be postulated that CCL14 [15], CCL15 [36] and CCL16 [49], i.e., chemokines with a high expression in the liver and at the same time, ligands for CCR1, may cause liver metastasis. Another pro-cancer property of CCL16 is the induction of angiogenesis, related to the expression of its receptor CCR1 on vascular endothelial cells [53]. CCL16 also causes the migration of cancer cells if they exhibit CCR1 expression [54].
On the other hand, CCL16 enhances the anti-cancer effects of cytotoxic T and dendritic cells (DC) lymphocytes [56,57] and some consider the possibility of using of this chemokine for enhancing the anticancer response in cancer immunotherapy [58,59,60,61].
2.4. CCL23
CCL23 (also known as CKβ8, MIP-3, and myeloid progenitor inhibitory factor-1 (MPIF-1)) is produced by eosinophils [62], monocyte-derived dendritic cells [63] and monocytes activated by interleukin (IL)-1β [64]. It serves as a chemoattractant for dendritic cells, resting T lymphocytes and monocytes, but not in T cells and eosinophils [63,64,65,66,67]. CCL23 is a ligand for CCR1 [67,68], and the N-terminally truncated form of this chemokine has a strong affinity for CCR1 [37,38]. Due to alternative splicing, CCL23 occurs in two forms, shorter CCL23α/CKβ8 and longer CCL23β/CKβ8-1 [66,67]. The shorter form activates CCR1 more strongly [66]. However, CCL23β can be cleaved at the C-terminus [69], which results in the release of SHAAGtide, a peptide which is a ligand for formyl peptide receptor-like 1 (FPRL1)—the activation of this receptor causes the migration of monocytes and neutrophils. CCL23 suppresses differentiation of myeloid progenitor cells [70]. For this reason, increased expression of CCL23 in acute myeloid leukemia cells leads to the suppression of hematopoiesis [71].
There are few studies indicating the involvement of CCL23 in cancer. However, it seems that this chemokine has both pro- and anti-cancer properties. It stimulates the proliferation of cells with CCR1 expression [72]. For this reason, it can be assumed that CCL23 increases the proliferation of cancer cells. CCL23 is secreted by macrophages in omentum and so the CCL23→CCR1 axis is crucial for omentum metastasis in ovarian cancer [73]. CCL23 is also a chemotactic agent for osteoclast precursors via CCR1 on these cells [74] and so it may play some role in bone remodeling and the formation of a metastatic niche in bones. However, the most important pro-cancer function of CCL23 may be the induction of angiogenesis by activating CCR1 on vascular endothelial cells [75,76], a process associated with increased expression and secretion of matrix metalloproteinase (MMP)-2 from these cells. CCL23 also induces an increase in the expression of the kinase insert domain-containing receptor (KDR)/fms-like tyrosine kinase 1 (Flk-1) on vascular endothelial cells [77], which enhances the effect of vascular endothelial growth factor (VEGF) on these cells. However, there are no studies on the involvement of CCL23 in angiogenesis inside the tumor.
CCL23 can also influence cancer-associated cells. For example, this chemokine may contribute to the function of eosinophils in a tumor [62]. It is also a chemoattractant for various cells of the immune system and therefore may cause the recruitment of some cells into the tumor niche [64,65,66,67,68]. It is yet to be established whether it causes tumor infiltration by anti-cancer TIL or participates in the recruitment of cancer-related cells, in particular MDSC, TAM, TAN and regulatory T cells (Treg).
3. CCR2 and Its Ligands: CCL2, CCL7, CCL8, and CCL13
CCL2 (also known as monocyte chemoattractant protein (MCP)-1) [78], CCL7 (also known as MCP-3) [79,80], CCL8 (also known as MCP-2) [81] and CCL13 (also known as MCP-4) [80,82,83] are ligands for CCR2. However, CCL2 can also activate CCR4 [84], CCR5 [85] and—at high concentrations—CCR1 [86]. CCL2 is also an antagonist of CCR3 [87]. CCL7 can also activate CCR1 [79,88] and CCR3 [89] and is an antagonist of CCR5 [85]. CCL8 can also activate CCR1 [81], CCR3 [89] and CCR5 [85,90,91]. CCL13 is also a ligand for CCR3 [82,83,89] and CCR5 [85]. CCL2, CCL7, and CCL8 are proteolytically spliced at the C-terminus, which is necessary for the acquisition of chemotactic properties by these chemokines [22]. However, cleavage at the N-terminus by MMPs makes them antagonists of their own receptors [22,92]. Another mechanism for reducing the activity of the described subgroup of chemokines is the chemokine decoy receptor ACKR2/D6 which reduces the level of this and many other CC chemokines [25,26,27]. However, the expression of this receptor in tumors is gradually reduced along with the progress of tumor growth.
One of the most important functions of the discussed subgroup of chemokines is the recruitment of monocytes to inflammatory reaction sites [93,94,95,96]. However, the discussed chemokines are involved in the recruitment of basophils, T cells, and NK (natural killer) cells [93,97]. In addition, CCL7 and CCL8 are chemoattractants for eosinophils [93]. Due to the activation of CCR3 by CCL13, this chemokine is important in the pathogenesis of allergic inflammation and asthma because it shows chemotactic activity against monocytes and eosinophils [82,98,99]. Due to the recruitment of monocytes, CCL2, CCL7, CCL8, and CCL13 are important in the pathogenesis of many diseases where an important role is played by monocytes and macrophages, in particular atherosclerosis, inflammatory bowel disease, and cancer.
The expression of chemokines from this subgroup increases in many cancers. Breast cancer is associated with increased CCL7 expression [100], while glioblastoma multiforme is accompanied by increased concentrations of CCL2 and CCL7 [101]. On the other hand, in some cancers, e.g., in ovarian adenocarcinoma, the expression of CCL2 decreases [102].
In a tumor, CCL2 is produced by cancer cells (Figure 1) [103,104,105,106,107,108,109]. The expression of this chemokine may be increased by factors such as growth factors [110], radiotherapy [107], cycling hypoxia [111] and anti-cancer drugs [112], or interactions with other cells, including cancer-associated fibroblasts (CAF) [113]. However, the expression of this chemokine in a tumor also occurs in MDSC [114], MSC [115], TAM [104,116,117,118], TAN [119], and CAF [120,121,122]. CCL7 and CCL8 are also expressed in CAF [123,124,125] and TAM [126]. The expression of CCL2, CCL7, and CCL8 in CAF is very important in cancer. Their expression is increased in CAF under the influence of interaction with a cancer cell, an important effect in the initial stages of metastatic niche formation and the functioning of a tumor [123,124,127,128,129,130].
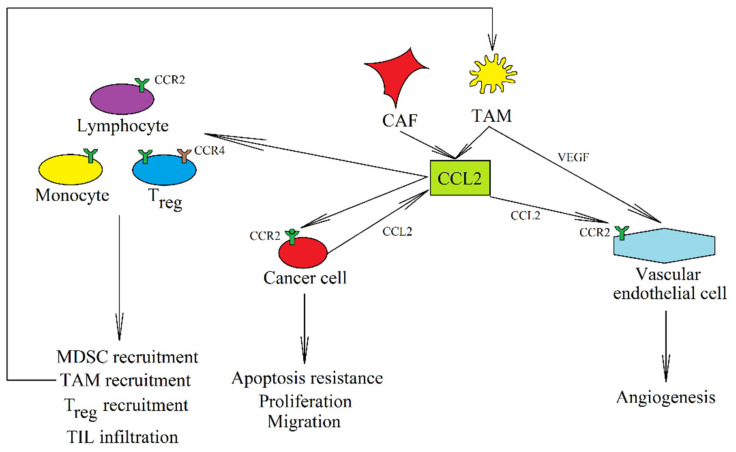
Figure 1.
The role of CCL2 in caner. In a tumor, CCL2 is produced by tumor cells and by CAF and TAM. It activates its receptor, CCR2, on a tumor cell, which stimulates the proliferation of cancer cells and causes their migration and resistance to apoptosis. CCL2 also acts on non-cancer cells, e.g., activating CCR2 on vascular endothelial cells which results in angiogenesis. CCL2 causes the recruitment of MDSC, TAM, and Treg into the tumor niche but also induces the infiltration of the tumor by anti-cancer TIL.
Increased expression of CCL2 in a tumor is associated with a worse prognosis for patients with solid tumors [131]. This is associated with the multiple pro-cancer properties of this chemokine. The most important function of CCL2 is the CCR2-mediated recruitment of TAM [12,13,103,132,133,134,135] and MDSC [13,122] into the tumor niche. CCL2 is also one of the factors contributing to M2-type macrophage polarization [136]. It can also recruit one of the Treg subsets that shows CCR2 expression [137,138]. The recruitment of other Treg subsets by CCL2 may also depend on the activation of CCR4 by this chemokine [84]. CCL2 can also recruit T helper type 17 (Th17) [139] and MSC [140] into the tumor niche. In brain tumors, CCL2 participates in the recruitment of neural progenitor cells [141] and microglia [142]. In liver cancer, CCL2 additionally causes the recruitment of hepatic stellate cells [143]. TAM recruitment can also be induced by CCL7 [144] and CCL8 [145], while Treg are recruited into the tumor niche by CCL8 [91] into the tumor niche.
CCL2 [10,11,146,147,148] and CCL7 [149,150] may also cause infiltration of the tumor by TIL, which has an anti-cancer effect. However, it seems that CCL2 in a tumor interferes with the function of anti-cancer T lymphocytes [151] and dendritic cells [152]. The discussed group of CC chemokines (CCL2, CCL7, CCL8, and CCL13) have numerous pro-cancer functions, which outweigh their anti-cancer properties. For example, the prognosis for patients is worse when the expression of CCL2 in the tumor is elevated [131].
CCL2 and CCL8 increase cancer cell proliferation [108,153,154]. CCL2 and CCL8 also cause enhanced tumor cell stemness and cancer stem cells self-renewal [127,155]. CCL2 also increases apoptosis resistance and drug resistance by activating the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)→ Akt/protein kinase B (PKB)→ mammalian target of rapamycin (mTOR) pathway [156,157,158]. This is of great importance as the expression of this chemokine can be increased by radiotherapy [107] and anti-cancer drugs [112]. For this reason, it is postulated to administer additional drugs to disrupt the function of CCL2 during cancer therapy.
CCL2 [86,156,159,160], CCL7 [123,161], and CCL8 [124,125,159] cause cancer cell migration. CCL2 also causes epithelial-to-mesenchymal transition (EMT) by activating the extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK)→ glycogen synthase kinase-3β (GSK-3β)→ Snail and PI3K→ Akt/PKB pathways [162,163]. CCL7 [164] and CCL8 [155,165] also cause EMT. CCL2 is crucial for the subsequent stages of metastasis. After EMT, a cancer cell increases the expression of CCL2 [166]. This allows it to effectively recruit macrophages, which participate in the early stage of the development of a metastatic niche [134,167]. However, at this stage CCL2 can also recruit neutrophils, which have a destructive effect on cancer cells [168].
In further stages of metastasis, CCL2 participates in the formation of a metastatic niche. As CCL2 is secreted by bone marrow endothelial cells [169], it causes the diapedesis of cancer cells into the bone tissue. Bone metastasis in prostate cancer is closely related to osteoblasts because prostate cancer cells secrete parathyroid hormone related proteins (PTHrP) that cause an increase in CCL2 expression in osteoblasts [170,171]. CCL2 can also be directly produced by other cancer cells [106,172,173]. Then the described chemokine causes the differentiation of osteoclasts, which are involved in bone remodeling and the formation of a metastatic niche [174]. CCL2 is also important in the perineural invasion of cervical cancer and prostate cancer [175,176], due to the expression of CCL2 in the nervous tissue. In particular, this chemokine is secreted by Schwann cells and supports the formation of metastasis by acting on cancer cells.
CCL2 can also directly cause angiogenesis, by acting on vascular endothelial cells on which it is expressed CCR2 [104,177]. However, some studies have shown that vascular endothelial cells in tumors do not express CCR2 [103,133], and so it seems that CCL2 may indirectly cause angiogenesis by recruiting TAM and increasing VEGF-A expression in these cells [132,133,135,178,179]. Angiogenesis may also be indirectly caused by the CCL2-mediated increase in VEGF expression in a cancer cell [108,156]. In comparison, there are no published studies showing the direct effects of CCL2, CCL7, CCL8, and CCL13 on lymphangiogenesis. It is possible that these CC chemokines have an indirect impact on this process via tumor-recruited TAM [180].
There are no studies showing the importance of CCL13 in cancer. However, it has been proven that this chemokine may increase apoptosis resistance [181]. In a tumor, it might cause cancer cells to become drug resistant. As this chemokine activates CCR2 and CCR3, it should have the same properties as other ligands for these receptors: CCL2 and eotaxins, but this needs to be confirmed by further research.
4. CCR3 and Eotaxins: CCL11 (Eotaxin-1), CCL24 (Eotaxin-2), and CCL26 (Eotaxin-3)
Eotaxins are three CC chemokines: CCL11 (eotaxin-1), CCL24 (eotaxin-2, also known as CK6 and MPIF-2) and CCL26 (eotaxin-3). Their most important receptor is CCR3 [182,183,184,185]. CCL11 is also a ligand of CCR5 [85,186] but also an antagonist of CCR2 [186]. CCL24 is an antagonist of CCR2 [87]. CCL26 is a ligand of CX3C motif chemokine receptor 1 (CX3CR1) [187,188] but also an antagonist of CCR1, CCR2, and CCR5 [189,190]. Eotaxins are the main chemotactic factors for eosinophils and to a lesser extent for basophils [7,183,191]. For this reason, eotaxins play a significant role in the pathophysiology of allergic reactions [184,192,193,194].
An elevated expression of eotaxins also occurs in tumors such as breast cancer [100], colorectal cancer [195] and oral squamous cell carcinomas [196], where it is associated with the recruitment of eosinophils into the tumor niche (Figure 2). However, the role of eosinophils in tumor is not clear [197,198,199], as they show both pro- and anti-cancer characteristics depending on the type of tumor. In addition to the recruitment of eosinophils, CCL26 has also been shown to cause TAM recruitment depending on CCR3 [200] and CX3CR1-dependent recruitment of MDSC into the tumor niche [188].
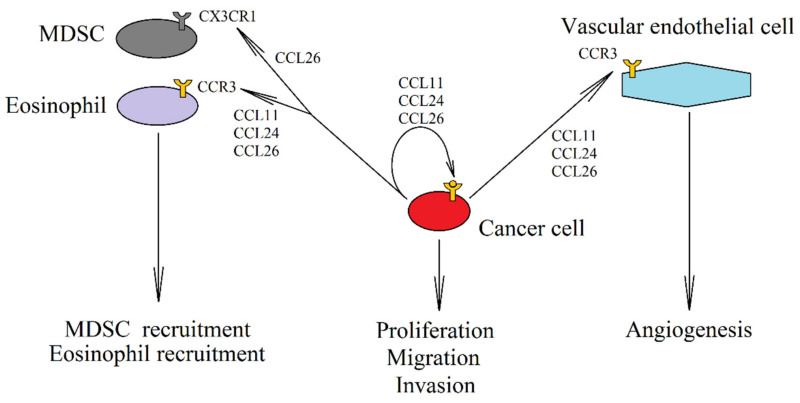
Figure 2.
The role of eotaxins in cancer. In a neoplastic cell there is an expression of CCL11 (eotaxin-1), CCL24 (eotaxin-2) and CCL26 (eotaxin-3). This increases the autocrine proliferation and causes the migration of cancer cells with CCR3 expression. Eotaxins also activate the CCR3 receptor on endothelial cells, which results in angiogenesis. Another effect of an increased expression of eotaxins in the tumor is the recruitment of cells into the tumor niche, in particular eosinophils, by all three eotaxins through the CCR3 receptor. MDSC are recruited into the tumor by CCL26 via CX3CR1.
Eotaxins do not only influence the composition of cells in the tumor niche. Increased expression of CCR3 has been reported in tumors such as renal cell carcinoma [201] or glioma [101]. Activation of this receptor on a cancer cell increases proliferation and migration [201,202,203]. CCL11 causes cancer cell apoptosis resistance by activating ERK MAPK [204]. Eotaxins also increase tumor vascularization. Due to the fact that CCR3 is expressed on vascular endothelial cells, eotaxins—especially CCL11—cause angiogenesis [205,206]. CCL11 may also indirectly affect angiogenesis, as the activation of the CCR3 receptor by CCL11 increases VEGF expression in the hepatocellular carcinoma cells and thus promotes angiogenesis [207]. On the other hand, angiogenesis is inhibited by eosinophils recruited by eotaxins, which leads to the necrosis of some areas in a tumor [199].
5. CCR4 and Its Ligands CCL17 and CCL22
CCL17 (also known as thymus and activation regulated chemokine (TARC)) [208] and CCL22 (also known as macrophage-derived chemokine (MDC)) [209] are the ligands for CCR4. These chemokines are the chemotactic factor for Th2 and Treg due to the expression of the CCR4 on these cells [210] and for this reason they are important in the pathogenesis of asthma and allergy. CCL17 and CCL22 are also crucial in the homing of lymphocytes to the skin [210]. They exert an anti-cancer effect by causing the infiltration of TIL into the tumor [211,212], a process dependent on CCR4 on these cells.
On the other hand, the expression of CCL17 and CCL22 is elevated in a breast cancer tumor [100], CCL22 expression is increased in colorectal adenocarcinomas [213], while CCL17 expression is increased in glioblastoma multiforme [101]. CCL17 and CCL22 are both produced by cancer cells in a tumor (Figure 3) [214,215] and by TAM [118,214,216,217,218,219]. CCL17 is also produced in TAN [119,220] and CAF [221].
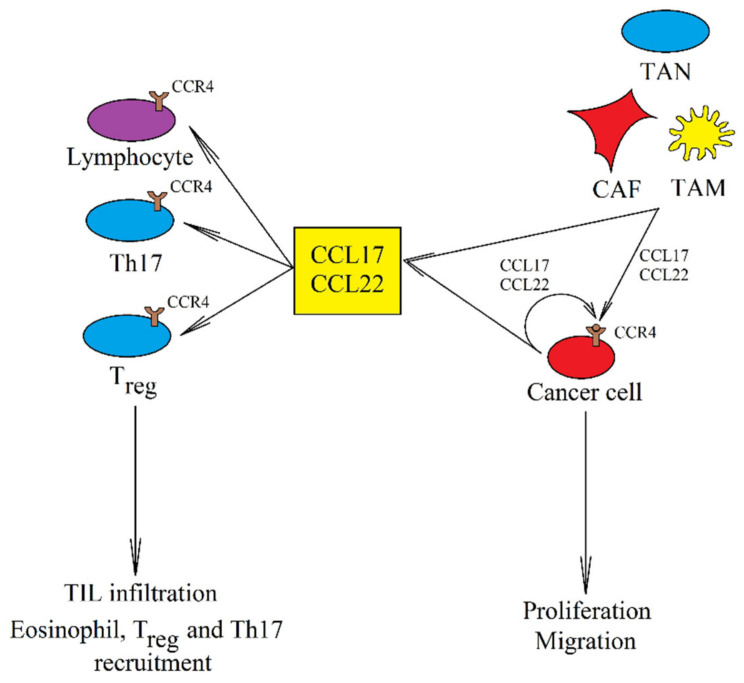
Figure 3.
The role of CCL17 and CCL22 in cancer. These two chemokines are produced by cancer cells, CAF, TAM, and TAN. They increase the proliferation of cancer cells and their migration and invasion. They also cause the recruitment of Treg and Th17. However, they can also cause infiltration of the tumor by TIL, which has an anti-cancer effect.
CCL17 and CCL22 are responsible for CCR4-dependent recruitment of Treg into the tumor niche, which enhances cancer immune evasion [220,222,223,224,225]. They can also recruit eosinophils [226] and Th17 [227] into the tumor niche. At the same time, these chemokines may also cause infiltration of the tumor by anti-cancer TIL [211,212]. Due to the recruitment of cancer-related cells in the squamous cell carcinoma of the tongue into the tumor niche, increased expression of CCL22 is associated with a poorer prognosis [219]. The same applies to the increased expression of CCL17 in breast cancer [228]. In contrast, an increased expression of CCL22 in the tumor improves prognosis in lung cancer patients [229] and elevated CCL17 blood levels improve prognosis in melanoma patients [230].
Although are no studies showing the direct effects of CCL17 and CCL22 on angiogenesis, Treg recruited by CCL17 and CCL22 do have pro-angiogenic properties via the secretion of VEGF [231].
CCL17 and CCL22 promote stemness of cancer cells with CCR4 expression [218], drug resistance [232], stimulate the proliferation of cancer cells [233], and cause cancer cell migration and EMT, as shown on many types of cancers [214,218,234]. Cancer cell migration is associated with metastasis. CCR4 expression has been linked to lymph node metastasis [214] and omental milky spots metastasis [235]. The CCL22→CCR4 axis also participates in bone metastasis due to the high expression of CCL22 in bones [236]. Brain [237] and lung [238] metastasis have also been associated with CCR4 expression. However, in these organs the expression of CCR4 ligands is low [239] and for this reason, the described axis may participate in metastasis only at the stage of induction where there is cell migration from the parent tumor.
6. CC Chemokines in Therapy
Many of the aforementioned CC chemokines simultaneously cause infiltration of a tumor by anti-cancer TIL while also recruiting cells which support the growth of the tumor. As the action of these chemokines depends on the tumor microenvironment [240], this a key area that should be studied in order to develop more effective therapeutic approaches. Importantly, a balance between pro- and anti-cancer mechanisms in the tumor microenvironment differs depending on the type of cancer, which means that an increased expression of a given CC chemokine may either improve or worsen a prognosis, as shown in Table 1 above [8,9]. Despite this, cancer therapies which target CC chemokines hold a lot of promise, as shown by in vivo and clinical trials, especially in combination with immunotherapy [241,242,243,244].
To date, few studies have centered on the significance of the ligands of CCR1 as therapeutic targets in cancer therapies. The most researched ligand has been CCL16 whose increased expression has shown an anti-cancer effect in mouse breast cancer [60,245], and colon carcinoma [245], and prostate cancer [246] due to an increase in the infiltration of the tumor by CD4+ T cells, CD8+ T cells, and DC. This effect may be enhanced by the use of factors that intensify the immune response, such as CpG (Toll-like receptor 9 ligand) and anti-IL-10 receptor antibody [245,246]. Another therapeutic approach includes the use of a CCR1 antagonist, for example BL5923, which reduces the recruitment of immature myeloid cells into the tumor and inhibits liver metastasis in mice with colon cancer [247].
CCL2 and CLL7 have also been well researched as therapeutic targets in cancer therapy due to their significant function in tumors. An increased expression of these ligands is often associated with a worse prognosis in various cancers [8,9,131]. Therefore, the use of CCL2 siRNA, CCR2 siRNA, CCL2-neutralizing antibodies, CCL2 inhibitors or CCR2 antagonists has given promising results in the therapy of cancers such as breast cancer [248,249], glioma [250,251,252], and hepatocellular cancer [253] in laboratory animals inoculated with cancer cells. CCR2-targeted drugs have also been tested, for example, CCR2-targeted apoptotic peptide was used in the therapy of melanoma in laboratory animals [254]. Anti-cancer properties have also been shown in the clinical trials of carlumab (CNTO888), a monoclonal antibody against CCL2, in patients with solid tumors [255].
Another therapeutic approach is to block the CCL2→CCR2 axis in chemotherapy or radiotherapy [256]. Chemotherapy induces an increase in CCL2 expression which results in apoptosis resistance [257]. As CCL2 causes a resistance to drugs such as cabazitaxel [157], docetaxel [257], and tamoxifen [158], blocking this chemokine increases the effectiveness of chemotherapy. CCL2 expression is also elevated by radiotherapy in breast cancer [107], colon cancer [258], head and neck squamous cell carcinoma [138], and pancreatic ductal adenocarcinoma [259], which leads to the resistance of these cells to radiotherapy [138,258,259,260] and causes their migration and then metastasis [261]. In addition, a radiotherapy-induced increase in CCL2 expression leads to neuroinflammation [262,263] and vascular dysfunction which leads to impaired lung function [264]. This indicates that the effectiveness of radiotherapy may be enhanced by the use of CCL2-neutralizing antibodies or CCR2 antagonists.
On the other hand, an increased expression of CCL2 and CCL7 has shown anti-tumor effects in cervical carcinoma [150], glioma [146] and mastocytoma [149] induced by gene therapy in laboratory animals. These effects included tumor infiltration by CD4+ and CD8+ cells [148,149], and NK cells [146,148,150]. CCL2 also causes tumor infiltration by type 1 cytotoxic γδ T lymphocytes in melanoma [11] and by cytotoxic T lymphocytes in colon cancer [147]. As all the aforementioned cells have anti-cancer properties, in some tumors, gene therapy can be used to increase the expression of CCL2, which in turn will enhance the accumulation of anti-cancer immune cells in the tumor. In contrast, in some tumors, such as in non-small cell lung cancer, CCL2 participates in tumor immune evasion [265,266]. In this case, the effectiveness of immunotherapy can be increased by the use of CCL2 neutralizing antibodies.
Compared to other chemokines, there are few studies exploring the role of eotaxins (CCL11, CCL24, and CCL26) in tumors and little has been published on therapies directed against these chemokines. However, some tumors, including colorectal cancer, have been reported to show an increased expression of eotaxins [267], which indicates the potential benefits of immunotherapy in which anti-tumor cells will have an increased expression of CCR3, a receptor for eotaxins [267]. Such cells will accumulate in tumors exhibiting an increased expression of ligands for this receptor when compared to normal tissue.
Animal studies on inoculated tumors, such as bladder cancer [268] and renal cell carcinoma [269], showed the anti-tumor effects of mogamulizumab or Affi-5 (monoclonal antibodies targeting CCR4) which reduced the number of Treg and increased the number of NK cells and CD4+ T cells in the tumor. Another therapeutic approach is the transduction of CCR4 to cytotoxic T cells [270], lymphocytes which accumulate in a tumor with a high expression of CCR4 ligands, for example, in pancreatic cancer [270], where they destroy cancer cells. In some cancers, an increased expression of CCR4 ligands (CCL17 and CCL22) in a tumor may play an anti-tumor role in colon carcinoma [212], lung cancer [271], ovarian cancer [272] and melanoma [211,272], resulting in an increase in CD4+ T cells and CD8+ T cells. An increased expression of CCL17 and CCL22 is consistent with the prognosis for patients with colon, lung, and ovarian cancers—the higher the expression of these chemokines in these tumors, the better the prognosis [8,9,229].
Clinical trials were also carried out in patients with various solid tumors who were administered mogamulizumab (KW-0761) [273,274], a defucosylated humanized monoclonal antibody targeting CCR4. This therapy led to a decrease in the number of Treg in the blood and caused changes in the cancer microenvironment which facilitated the effectiveness of immunotherapy. Mogamulizumab has also shown promising results in clinical trials involving patients with adult T-cell leukemia/lymphoma [275,276], cutaneous T-cell lymphoma [277,278] and peripheral T-cell lymphoma [275,277]. The use of mogamulizumab is also postulated in the treatment of the Epstein-Barr virus (EBV)-associated T/NK-cell lymphoproliferative diseases due to the expression of CCR4 in cells infected with EBV [279].
7. Conclusions
CC chemokines are an important component of the tumor microenvironment. Produced by tumor cells and tumor-associated cells such as CAF, TAM, and TAN, CC chemokines increase the proliferation, migration, and invasion of cancer cells, and induce their drug resistance. If a specific CC chemokine receptor is expressed on a circulating cancer cell, it will migrate to organs showing a high expression of the ligand of that receptor. In a similar way, CC chemokines recruit tumor-associated cells into the tumor niche—this mode of action of CC chemokines is local and depends on other factors in the tumor microenvironment. Yet although the recruited tumor-associated cells can enhance the growth of a tumor, some of them (namely, TIL), do have anti-cancer properties. This shows the importance of adjusting the therapeutic approach to the specific context in which a given CC chemokine operates. Therapies should try to use the anti-cancer properties of a given chemokine or suppress its pro-cancer properties. For example, they may increase the expression of a given chemokine in a tumor and then apply immunotherapy, in which anti-cancer immune cells accumulate in the tumor via this chemokine. On the other hand, they may also concentrate on blocking chemokines responsible for tumor immune evasion, and only then use immunotherapy.
Abbreviations
| CAF | cancer-associated fibroblasts |
| CCL | CC motif chemokine ligand |
| CCR | CC motif chemokine receptor |
| EMT | epithelial-to-mesenchymal transition |
| IL | interleukin |
| MDSC | myeloid-derived suppressor cells |
| MMP | matrix metalloproteinase |
| MSC | mesenchymal stem cells |
| NF-κB | nuclear factor κB |
| TAM | tumor-associated macrophages |
| TAN | tumor-associated neutrophils |
| Th17 | T helper 17 |
| TIL | tumor-infiltrating lymphocytes |
| Treg | regulatory T cells |
| VEGF | vascular endothelial growth factor |
Author Contributions
J.K.: writing—original draft preparation; K.K.: investigation; D.S.: investigation; R.B.: investigation; I.G.: investigation; D.C.: funding acquisition; I.B.-B.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the statutory budget of the Department of Biochemistry and Medical Chemistry Pomeranian Medical University in Szczecin, Poland.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Liu Q., Yang C., Wang S., Shi D., Wei C., Song J., Lin X., Dou R., Bai J., Xiang Z., et al. Wnt5a-induced M2 polarization of tumor-associated macrophages via IL-10 promotes colorectal cancer progression. Cell Commun. Signal. 2020;18:51. doi: 10.1186/s12964-020-00557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z., Yang Y., Cui Y., Wang C., Lai Z., Li Y., Zhang W., Mustonen H., Puolakkainen P., Ye Y., et al. Tumor-associated macrophages regulate gastric cancer cell invasion and metastasis through TGFβ2/NF-κB/Kindlin-2 axis. Chin. J. Cancer Res. 2020;32:72–88. doi: 10.21147/j.issn.1000-9604.2020.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu H., Zhang X., Han D., Cao J., Tian J. Tumour-associated macrophages mediate the invasion and metastasis of bladder cancer cells through CXCL8. PeerJ. 2020;8:e8721. doi: 10.7717/peerj.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zlotnik A., Yoshie O. Chemokines: A new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/S1074-7613(00)80165-X. [DOI] [PubMed] [Google Scholar]
- 7.Hughes C.E., Nibbs R.J.B. A guide to chemokines and their receptors. FEBS J. 2018;285:2944–2971. doi: 10.1111/febs.14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A., et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 9.Uhlen M., Zhang C., Lee S., Sjöstedt E., Fagerberg L., Bidkhori G., Benfeitas R., Arif M., Liu Z., Edfors F., et al. A pathology atlas of the human cancer transcriptome. Science. 2017;357:eaan2507. doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- 10.Iannello A., Thompson T.W., Ardolino M., Lowe S.W., Raulet D.H. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J. Exp. Med. 2013;210:2057–2069. doi: 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lança T., Costa M.F., Gonçalves-Sousa N., Rei M., Grosso A.R., Penido C., Silva-Santos B. Protective role of the inflammatory CCR2/CCL2 chemokine pathway through recruitment of type 1 cytotoxic γδ T lymphocytes to tumor beds. J. Immunol. 2013;190:6673–6680. doi: 10.4049/jimmunol.1300434. [DOI] [PubMed] [Google Scholar]
- 12.Bartneck M., Schrammen P.L., Möckel D., Govaere O., Liepelt A., Krenkel O., Ergen C., McCain M.V., Eulberg D., Luedde T., et al. The CCR2+ Macrophage Subset Promotes Pathogenic Angiogenesis for Tumor Vascularization in Fibrotic Livers. Cell Mol. Gastroenterol. Hepatol. 2019;7:371–390. doi: 10.1016/j.jcmgh.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F., Kitajima S., Kohno S., Yoshida A., Tange S., Sasaki S., Okada N., Nishimoto Y., Muranaka H., Nagatani N., et al. Retinoblastoma Inactivation Induces a Protumoral Microenvironment via Enhanced CCL2 Secretion. Cancer Res. 2019;79:3903–3915. doi: 10.1158/0008-5472.CAN-18-3604. [DOI] [PubMed] [Google Scholar]
- 14.Korbecki J., Grochans S., Gutowska I., Barczak K., Baranowska-Bosiacka I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of Receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 Ligands. Int. J. Mol. Sci. 2020;21:7619. doi: 10.3390/ijms21207619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz-Knappe P., Mägert H.J., Dewald B., Meyer M., Cetin Y., Kubbies M., Tomeczkowski J., Kirchhoff K., Raida M., Adermann K., et al. HCC-1, a novel chemokine from human plasma. J. Exp. Med. 1996;183:295–299. doi: 10.1084/jem.183.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsou C.L., Gladue R.P., Carroll L.A., Paradis T., Boyd J.G., Nelson R.T., Neote K., Charo I.F. Identification of C-C chemokine receptor 1 (CCR1) as the monocyte hemofiltrate C-C chemokine (HCC)-1 receptor. J. Exp. Med. 1998;188:603–608. doi: 10.1084/jem.188.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Detheux M., Ständker L., Vakili J., Münch J., Forssmann U., Adermann K., Pöhlmann S., Vassart G., Kirchhoff F., Parmentier M., et al. Natural proteolytic processing of hemofiltrate CC chemokine 1 generates a potent CC chemokine receptor (CCR)1 and CCR5 agonist with anti-HIV properties. J. Exp. Med. 2000;192:1501–1508. doi: 10.1084/jem.192.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Münch J., Ständker L., Pöhlmann S., Baribaud F., Papkalla A., Rosorius O., Stauber R., Sass G., Heveker N., Adermann K., et al. Hemofiltrate CC chemokine 1[9–74] causes effective internalization of CCR5 and is a potent inhibitor of R5-tropic human immunodeficiency virus type 1 strains in primary T cells and macrophages. Antimicrob. Agents Chemother. 2002;46:982–990. doi: 10.1128/AAC.46.4.982-990.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Struyf S., Stoops G., Van Coillie E., Gouwy M., Schutyser E., Lenaerts J.P., Fiten P., Van Aelst I., Proost P., Opdenakker G., et al. Gene cloning of a new plasma CC chemokine, activating and attracting myeloid cells in synergy with other chemoattractants. Biochemistry. 2001;40:11715–11722. doi: 10.1021/bi010224+. [DOI] [PubMed] [Google Scholar]
- 20.Vakili J., Ständker L., Detheux M., Vassart G., Forssmann W.G., Parmentier M. Urokinase plasminogen activator and plasmin efficiently convert hemofiltrate CC chemokine 1 into its active. J. Immunol. 2001;167:3406–3413. doi: 10.4049/jimmunol.167.6.3406. [DOI] [PubMed] [Google Scholar]
- 21.Blain K.Y., Kwiatkowski W., Zhao Q., La Fleur D., Naik C., Chun T.W., Tsareva T., Kanakaraj P., Laird M.W., Shah R., et al. Structural and functional characterization of CC chemokine CCL14. Biochemistry. 2007;46:10008–10015. doi: 10.1021/bi700936w. [DOI] [PubMed] [Google Scholar]
- 22.Mortier A., Van Damme J., Proost P. Regulation of chemokine activity by posttranslational modification. Pharmacol. Ther. 2008;120:197–217. doi: 10.1016/j.pharmthera.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Grünberg M., Quandt D., Cynis H., Demuth H.U., Kindermann A., Magdolen V., Forssmann W.G., Seliger B., Mägert H.J. Kallikrein-related peptidases are activators of the CC chemokine CCL14. Eur. J. Immunol. 2018;48:1592–1594. doi: 10.1002/eji.201747452. [DOI] [PubMed] [Google Scholar]
- 24.Savino B., Borroni E.M., Torres N.M., Proost P., Struyf S., Mortier A., Mantovani A., Locati M., Bonecchi R. Recognition versus adaptive up-regulation and degradation of CC chemokines by the chemokine decoy receptor D6 are determined by their N-terminal sequence. J. Biol. Chem. 2009;284:26207–26215. doi: 10.1074/jbc.M109.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu F.Y., Ou Z.L., Feng L.Y., Luo J.M., Wang L.P., Shen Z.Z., Shao Z.M. Chemokine decoy receptor d6 plays a negative role in human breast cancer. Mol. Cancer Res. 2008;6:1276–1288. doi: 10.1158/1541-7786.MCR-07-2108. [DOI] [PubMed] [Google Scholar]
- 26.Langenes V., Svensson H., Börjesson L., Gustavsson B., Bemark M., Sjöling Å., Quiding-Järbrink M. Expression of the chemokine decoy receptor D6 is decreased in colon adenocarcinomas. Cancer Immunol. Immunother. 2013;62:1687–1695. doi: 10.1007/s00262-013-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massara M., Bonavita O., Mantovani A., Locati M., Bonecchi R. Atypical chemokine receptors in cancer: Friends or foes? J. Leukoc. Biol. 2016;99:927–933. doi: 10.1189/jlb.3MR0915-431RR. [DOI] [PubMed] [Google Scholar]
- 28.Hannan N.J., Salamonsen L.A. CX3CL1 and CCL14 regulate extracellular matrix and adhesion molecules in the trophoblast: Potential roles in human embryo implantation. Biol. Reprod. 2008;79:58–65. doi: 10.1095/biolreprod.107.066480. [DOI] [PubMed] [Google Scholar]
- 29.Zhu M., Xu W., Wei C., Huang J., Xu J., Zhang Y., Zhao Y., Chen J., Dong S., Liu B., et al. CCL14 serves as a novel prognostic factor and tumor suppressor of HCC by modulating cell cycle and promoting apoptosis. Cell Death Dis. 2019;10:796. doi: 10.1038/s41419-019-1966-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu Y., Li X., Bi Y., Zheng Y., Wang J., Li X., Huang Z., Chen L., Huang Y., Huang Y. CCL14 is a prognostic biomarker and correlates with immune infiltrates in hepatocellular carcinoma. Aging (Albany NY) 2020;12:784–807. doi: 10.18632/aging.102656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q., Shi L., Gui B., Yu W., Wang J., Zhang D., Han X., Yao Z., Shang Y. Binding of the JmjC demethylase JARID1B to LSD1/NuRD suppresses angiogenesis and metastasis in breast cancer cells by repressing chemokine CCL14. Cancer Res. 2011;71:6899–6908. doi: 10.1158/0008-5472.CAN-11-1523. [DOI] [PubMed] [Google Scholar]
- 32.Akram I.G., Georges R., Hielscher T., Adwan H., Berger M.R. The chemokines CCR1 and CCRL2 have a role in colorectal cancer liver metastasis. Tumour. Biol. 2016;37:2461–2471. doi: 10.1007/s13277-015-4089-4. [DOI] [PubMed] [Google Scholar]
- 33.Li Y., Zheng Y., Li T., Wang Q., Qian J., Lu Y., Zhang M., Bi E., Yang M., Reu F., et al. Chemokines CCL2, 3, 14 stimulate macrophage bone marrow homing, proliferation, and polarization in multiple myeloma. Oncotarget. 2015;6:24218–24229. doi: 10.18632/oncotarget.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coulin F., Power C.A., Alouani S., Peitsch M.C., Schroeder J.M., Moshizuki M., Clark-Lewis I., Wells T.N. Characterisation of macrophage inflammatory protein-5/human CC cytokine-2, a member of the macrophage-inflammatory-protein family of chemokines. Eur. J. Biochem. 1997;248:507–515. doi: 10.1111/j.1432-1033.1997.00507.x. [DOI] [PubMed] [Google Scholar]
- 35.Youn B.S., Zhang S.M., Lee E.K., Park D.H., Broxmeyer H.E., Murphy P.M., Locati M., Pease J.E., Kim K.K., Antol K., et al. Molecular cloning of leukotactin-1: A novel human beta-chemokine, a chemoattractant for neutrophils, monocytes, and lymphocytes, and a potent agonist at CC chemokine receptors 1 and 3. J. Immunol. 1997;159:5201–5205. [PubMed] [Google Scholar]
- 36.Pardigol A., Forssmann U., Zucht H.D., Loetscher P., Schulz-Knappe P., Baggiolini M., Forssmann W.G., Mägert H.J. HCC-2, a human chemokine: Gene structure, expression pattern, and biological activity. Proc. Natl. Acad. Sci. USA. 1998;95:6308–6313. doi: 10.1073/pnas.95.11.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berahovich R.D., Miao Z., Wang Y., Premack B., Howard M.C., Schall T.J. Proteolytic activation of alternative CCR1 ligands in inflammation. J. Immunol. 2005;174:7341–7351. doi: 10.4049/jimmunol.174.11.7341. [DOI] [PubMed] [Google Scholar]
- 38.Starr A.E., Dufour A., Maier J., Overall C.M. Biochemical analysis of matrix metalloproteinase activation of chemokines CCL15 and CCL23 and increased glycosaminoglycan binding of CCL16. J. Biol. Chem. 2012;287:5848–5860. doi: 10.1074/jbc.M111.314609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y., Wu J., Zhang W., Zhang N., Guo H. Identification of serum CCL15 in hepatocellular carcinoma. Br. J. Cancer. 2013;108:99–106. doi: 10.1038/bjc.2012.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y., Yu H.P., Zhang P. CCL15 overexpression predicts poor prognosis for hepatocellular carcinoma. Hepatol. Int. 2016;10:488–492. doi: 10.1007/s12072-015-9683-4. [DOI] [PubMed] [Google Scholar]
- 41.Yin X., Han S., Song C., Zou H., Wei Z., Xu W., Ran J., Tang C., Wang Y., Cai Y., et al. Metformin enhances gefitinib efficacy by interfering with interactions between tumor-associated macrophages and head and neck squamous cell carcinoma cells. Cell Oncol. (Dordr.) 2019;42:459–475. doi: 10.1007/s13402-019-00446-y. [DOI] [PubMed] [Google Scholar]
- 42.Kominsky S.L., Abdelmagid S.M., Doucet M., Brady K., Weber K.L. Macrophage inflammatory protein-1 delta: A novel osteoclast stimulating factor secreted by renal cell carcinoma bone metastasis. Cancer Res. 2008;68:1261–1266. doi: 10.1158/0008-5472.CAN-07-6122. [DOI] [PubMed] [Google Scholar]
- 43.Weber K.L., Doucet M., Shaner A., Hsu N., Huang D., Fogel J., Kominsky S.L. MIP-1δ activates NFATc1 and enhances osteoclastogenesis: Involvement of both PLCγ2 and NFκB signaling. PLoS ONE. 2012;7:e40799. doi: 10.1371/journal.pone.0040799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hwang J., Kim C.W., Son K.N., Han K.Y., Lee K.H., Kleinman H.K., Ko J., Na D.S., Kwon B.S., Gho Y.S., et al. Angiogenic activity of human CC chemokine CCL15 in vitro and in vivo. FEBS Lett. 2004;570:47–51. doi: 10.1016/j.febslet.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 45.Inamoto S., Itatani Y., Yamamoto T., Minamiguchi S., Hirai H., Iwamoto M., Hasegawa S., Taketo M.M., Sakai Y., Kawada K. Loss of SMAD4 Promotes Colorectal Cancer Progression by Accumulation of Myeloid-Derived Suppressor Cells through the CCL15-CCR1 Chemokine Axis. Clin. Cancer Res. 2016;22:492–501. doi: 10.1158/1078-0432.CCR-15-0726. [DOI] [PubMed] [Google Scholar]
- 46.Liu L.Z., Zhang Z., Zheng B.H., Shi Y., Duan M., Ma L.J., Wang Z.C., Dong L.Q., Dong P.P., Shi J.Y., et al. CCL15 Recruits Suppressive Monocytes to Facilitate Immune Escape and Disease Progression in Hepatocellular Carcinoma. Hepatology. 2019;69:143–159. doi: 10.1002/hep.30134. [DOI] [PubMed] [Google Scholar]
- 47.Gao Y., Zhou Z., Lu S., Huang X., Zhang C., Jiang R., Yao A., Sun B., Wang X. Chemokine CCL15 Mediates Migration of Human Bone Marrow-Derived Mesenchymal Stem Cells Toward Hepatocellular Carcinoma. Stem. Cells. 2016;34:1112–1122. doi: 10.1002/stem.2275. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto T., Kawada K., Itatani Y., Inamoto S., Okamura R., Iwamoto M., Miyamoto E., Chen-Yoshikawa T.F., Hirai H., Hasegawa S., et al. Loss of SMAD4 Promotes Lung Metastasis of Colorectal Cancer by Accumulation of CCR1+ Tumor-Associated Neutrophils through CCL15-CCR1 Axis. Clin. Cancer Res. 2017;23:833–844. doi: 10.1158/1078-0432.CCR-16-0520. [DOI] [PubMed] [Google Scholar]
- 49.Yang J.Y., Spanaus K.S., Widmer U. Cloning, characterization and genomic organization of LCC-1 (scya16), a novel human CC chemokine expressed in liver. Cytokine. 2000;12:101–109. doi: 10.1006/cyto.1999.0548. [DOI] [PubMed] [Google Scholar]
- 50.Musso T., Cappello P., Stornello S., Ravarino D., Caorsi C., Otero K., Novelli F., Badolato R., Giovarelli M. IL-10 enhances CCL2 release and chemotaxis induced by CCL16 in human monocytes. Int. J. Immunopathol. Pharmacol. 2005;18:339–349. doi: 10.1177/039463200501800216. [DOI] [PubMed] [Google Scholar]
- 51.Nomiyama H., Hieshima K., Nakayama T., Sakaguchi T., Fujisawa R., Tanase S., Nishiura H., Matsuno K., Takamori H., Tabira Y., et al. Human CC chemokine liver-expressed chemokine/CCL16 is a functional ligand for CCR1, CCR2 and CCR5, and constitutively expressed by hepatocytes. Int. Immunol. 2001;13:1021–1029. doi: 10.1093/intimm/13.8.1021. [DOI] [PubMed] [Google Scholar]
- 52.Howard O.M., Dong H.F., Shirakawa A.K., Oppenheim J.J. LEC induces chemotaxis and adhesion by interacting with CCR1 and CCR8. Blood. 2000;96:840–845. doi: 10.1182/blood.V96.3.840. [DOI] [PubMed] [Google Scholar]
- 53.Strasly M., Doronzo G., Cappello P., Valdembri D., Arese M., Mitola S., Moore P., Alessandri G., Giovarelli M., Bussolino F. CCL16 activates an angiogenic program in vascular endothelial cells. Blood. 2004;103:40–49. doi: 10.1182/blood-2003-05-1387. [DOI] [PubMed] [Google Scholar]
- 54.Kim I.S., Jang S.W., Sung H.J., Lee J.S., Ko J. Differential CCR1-mediated chemotaxis signaling induced by human CC chemokine HCC-4/CCL16 in HOS cells. FEBS Lett. 2005;579:6044–6048. doi: 10.1016/j.febslet.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 55.Nakayama T., Kato Y., Hieshima K., Nagakubo D., Kunori Y., Fujisawa T., Yoshie O. Liver-expressed chemokine/CC chemokine ligand 16 attracts eosinophils by interacting with histamine H4 receptor. J. Immunol. 2004;173:2078–2083. doi: 10.4049/jimmunol.173.3.2078. [DOI] [PubMed] [Google Scholar]
- 56.Cappello P., Caorsi C., Bosticardo M., De Angelis S., Novelli F., Forni G., Giovarelli M. CCL16/LEC powerfully triggers effector and antigen-presenting functions of macrophages and enhances T cell cytotoxicity. J. Leukoc. Biol. 2004;75:135–142. doi: 10.1189/jlb.0403146. [DOI] [PubMed] [Google Scholar]
- 57.Cappello P., Fraone T., Barberis L., Costa C., Hirsch E., Elia A.R., Caorsi C., Musso T., Novelli F., Giovarelli M. CC-chemokine ligand 16 induces a novel maturation program in human immature monocyte-derived dendritic cells. J. Immunol. 2006;177:6143–6151. doi: 10.4049/jimmunol.177.9.6143. [DOI] [PubMed] [Google Scholar]
- 58.Giovarelli M., Cappello P., Forni G., Salcedo T., Moore P.A., LeFleur D.W., Nardelli B., Di Carlo E., Lollini P.L., Ruben S., et al. Tumor rejection and immune memory elicited by locally released LEC chemokine are associated with an impressive recruitment of APCs, lymphocytes, and granulocytes. J. Immunol. 2000;164:3200–3206. doi: 10.4049/jimmunol.164.6.3200. [DOI] [PubMed] [Google Scholar]
- 59.Li J., Hu P., Khawli L.A., Epstein A.L. Complete regression of experimental solid tumors by combination LEC/chTNT-3 immunotherapy and CD25(+) T-cell depletion. Cancer Res. 2003;63:8384–8392. [PubMed] [Google Scholar]
- 60.Guiducci C., Di Carlo E., Parenza M., Hitt M., Giovarelli M., Musiani P., Colombo M.P. Intralesional injection of adenovirus encoding CC chemokine ligand 16 inhibits mammary tumor growth and prevents metastatic-induced death after surgical removal of the treated primary tumor. J. Immunol. 2004;172:4026–4036. doi: 10.4049/jimmunol.172.7.4026. [DOI] [PubMed] [Google Scholar]
- 61.Caorsi C., Cappello P., Ceruti P., Amici A., Marchini C., Novelli F., Forni G., Giovarelli M. CCL16 enhances the CD8+ and CD4+ T cell reactivity to human HER-2 elicited by dendritic cells loaded with rat ortholog HER-2. Int. J. Immunopathol. Pharmacol. 2008;21:867–877. doi: 10.1177/039463200802100411. [DOI] [PubMed] [Google Scholar]
- 62.Matsumoto K., Fukuda S., Hashimoto N., Saito H. Human eosinophils produce and release a novel chemokine, CCL23, in vitro. Int. Arch. Allergy Immunol. 2011;155(Suppl. 1):34–39. doi: 10.1159/000327263. [DOI] [PubMed] [Google Scholar]
- 63.Nardelli B., Morahan D.K., Bong G.W., Semenuk M.A., Kreider B.L., Garotta G. Dendritic cells and MPIF-1: Chemotactic activity and inhibition of endogenous chemokine production by IFN-gamma and CD40 ligation. J. Leukoc. Biol. 1999;65:822–828. doi: 10.1002/jlb.65.6.822. [DOI] [PubMed] [Google Scholar]
- 64.Forssmann U., Delgado M.B., Uguccioni M., Loetscher P., Garotta G., Baggiolini M. CKbeta8, a novel CC chemokine that predominantly acts on monocytes. FEBS Lett. 1997;408:211–216. doi: 10.1016/S0014-5793(97)00408-0. [DOI] [PubMed] [Google Scholar]
- 65.Patel V.P., Kreider B.L., Li Y., Li H., Leung K., Salcedo T., Nardelli B., Pippalla V., Gentz S., Thotakura R., et al. Molecular and functional characterization of two novel human C-C chemokines as inhibitors of two distinct classes of myeloid progenitors. J. Exp. Med. 1997;185:1163–1172. doi: 10.1084/jem.185.7.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Macphee C.H., Appelbaum E.R., Johanson K., Moores K.E., Imburgia C.S., Fornwald J., Berkhout T., Brawner M., Groot P.H., O’Donnell K., et al. Identification of a truncated form of the CC chemokine CK beta-8 demonstrating greatly enhanced biological activity. J. Immunol. 1998;161:6273–6279. [PubMed] [Google Scholar]
- 67.Youn B.S., Zhang S.M., Broxmeyer H.E., Cooper S., Antol K., Fraser M., Jr., Kwon B.S. Characterization of CKbeta8 and CKbeta8-1: Two alternatively spliced forms of human beta-chemokine, chemoattractants for neutrophils, monocytes, and lymphocytes, and potent agonists at CC chemokine receptor 1. Blood. 1998;91:3118–3126. doi: 10.1182/blood.V91.9.3118. [DOI] [PubMed] [Google Scholar]
- 68.Nardelli B., Tiffany H.L., Bong G.W., Yourey P.A., Morahan D.K., Li Y., Murphy P.M., Alderson R.F. Characterization of the signal transduction pathway activated in human monocytes and dendritic cells by MPIF-1, a specific ligand for CC chemokine receptor 1. J. Immunol. 1999;162:435–444. [PubMed] [Google Scholar]
- 69.Miao Z., Premack B.A., Wei Z., Wang Y., Gerard C., Showell H., Howard M., Schall T.J., Berahovich R. Proinflammatory proteases liberate a discrete high-affinity functional FPRL1 (CCR12) ligand from CCL23. J. Immunol. 2007;178:7395–7404. doi: 10.4049/jimmunol.178.11.7395. [DOI] [PubMed] [Google Scholar]
- 70.Han I.S., Ra J.S., Kim M.W., Lee E.A., Jun H.Y., Park S.K., Kwon B.S. Differentiation of CD34+ cells from human cord blood and murine bone marrow is suppressed by C6 beta-chemokines. Mol. Cells. 2003;15:176–180. [PubMed] [Google Scholar]
- 71.Çelik H., Lindblad K.E., Popescu B., Gui G., Goswami M., Valdez J., DeStefano C., Lai C., Thompson J., Ghannam J.Y., et al. Highly multiplexed proteomic assessment of human bone marrow in acute myeloid leukemia. Blood Adv. 2020;4:367–379. doi: 10.1182/bloodadvances.2019001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim J., Kim Y.S., Ko J. CKbeta8/CCL23 and its isoform CKbeta8-1 induce up-regulation of cyclins via the G(i)/G(o) protein/PLC/PKCdelta/ERK leading to cell-cycle progression. Cytokine. 2010;50:42–49. doi: 10.1016/j.cyto.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 73.Krishnan V., Tallapragada S., Schaar B., Kamat K., Chanana A.M., Zhang Y., Patel S., Parkash V., Rinker-Schaeffer C., Folkins A.K., et al. Omental macrophages secrete chemokine ligands that promote ovarian cancer colonization of the omentum via CCR1. Commun. Biol. 2020;3:524. doi: 10.1038/s42003-020-01246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Votta B.J., White J.R., Dodds R.A., James I.E., Connor J.R., Lee-Rykaczewski E., Eichman C.F., Kumar S., Lark M.W., Gowen M. CKbeta-8 [CCL23], a novel CC chemokine, is chemotactic for human osteoclast precursors and is expressed in bone tissues. J. Cell Physiol. 2000;183:196–207. doi: 10.1002/(SICI)1097-4652(200005)183:2<196::AID-JCP6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 75.Hwang J., Son K.N., Kim C.W., Ko J., Na D.S., Kwon B.S., Gho Y.S., Kim J. Human CC chemokine CCL23, a ligand for CCR1, induces endothelial cell migration and promotes angiogenesis. Cytokine. 2005;30:254–263. doi: 10.1016/j.cyto.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 76.Son K.N., Hwang J., Kwon B.S., Kim J. Human CC chemokine CCL23 enhances expression of matrix metalloproteinase-2 and invasion of vascular endothelial cells. Biochem. Biophys. Res. Commun. 2006;340:498–504. doi: 10.1016/j.bbrc.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 77.Han K.Y., Kim C.W., Lee T.H., Son Y., Kim J. CCL23 up-regulates expression of KDR/Flk-1 and potentiates VEGF-induced proliferation and migration of human endothelial cells. Biochem. Biophys. Res. Commun. 2009;382:124–128. doi: 10.1016/j.bbrc.2009.02.149. [DOI] [PubMed] [Google Scholar]
- 78.Monteclaro F.S., Charo I.F. The amino-terminal domain of CCR2 is both necessary and sufficient for high affinity binding of monocyte chemoattractant protein 1. Receptor activation by a pseudo-tethered ligand. J. Biol. Chem. 1997;272:23186–23190. doi: 10.1074/jbc.272.37.23186. [DOI] [PubMed] [Google Scholar]
- 79.Combadiere C., Ahuja S.K., Van Damme J., Tiffany H.L., Gao J.L., Murphy P.M. Monocyte chemoattractant protein-3 is a functional ligand for CC chemokine receptors 1 and 2B. J. Biol. Chem. 1995;270:29671–29675. doi: 10.1074/jbc.270.50.29671. [DOI] [PubMed] [Google Scholar]
- 80.Berkhout T.A., Sarau H.M., Moores K., White J.R., Elshourbagy N., Appelbaum E., Reape R.J., Brawner M., Makwana J., Foley J.J., et al. Cloning, in vitro expression, and functional characterization of a novel human CC chemokine of the monocyte chemotactic protein (MCP) family (MCP-4) that binds and signals through the CC chemokine receptor 2B. J. Biol. Chem. 1997;272:16404–16413. doi: 10.1074/jbc.272.26.16404. [DOI] [PubMed] [Google Scholar]
- 81.Gong X., Gong W., Kuhns D.B., Ben-Baruch A., Howard O.M., Wang J.M. Monocyte chemotactic protein-2 (MCP-2) uses CCR1 and CCR2B as its functional receptors. J. Biol. Chem. 1997;272:11682–11685. doi: 10.1074/jbc.272.18.11682. [DOI] [PubMed] [Google Scholar]
- 82.Garcia-Zepeda E.A., Combadiere C., Rothenberg M.E., Sarafi M.N., Lavigne F., Hamid Q., Murphy P.M., Luster A.D. Human monocyte chemoattractant protein (MCP)-4 is a novel CC chemokine with activities on monocytes, eosinophils, and basophils induced in allergic and nonallergic inflammation that signals through the CC chemokine receptors (CCR)-2 and -3. J. Immunol. 1996;157:5613–5626. [PubMed] [Google Scholar]
- 83.Stellato C., Collins P., Ponath P.D., Soler D., Newman W., La Rosa G., Li H., White J., Schwiebert L.M., Bickel C., et al. Production of the novel C-C chemokine MCP-4 by airway cells and comparison of its biological activity to other C-C chemokines. J. Clin. Invest. 1997;99:926–936. doi: 10.1172/JCI119257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun W., Li W.J., Wei F.Q., Wong T.S., Lei W.B., Zhu X.L., Li J., Wen W.P. Blockade of MCP-1/CCR4 signaling-induced recruitment of activated regulatory cells evokes an antitumor immune response in head and neck squamous cell carcinoma. Oncotarget. 2016;7:37714–37727. doi: 10.18632/oncotarget.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blanpain C., Migeotte I., Lee B., Vakili J., Doranz B.J., Govaerts C., Vassart G., Doms R.W., Parmentier M. CCR5 binds multiple CC-chemokines: MCP-3 acts as a natural antagonist. Blood. 1999;94:1899–1905. doi: 10.1182/blood.V94.6.1899. [DOI] [PubMed] [Google Scholar]
- 86.Dagouassat M., Suffee N., Hlawaty H., Haddad O., Charni F., Laguillier C., Vassy R., Martin L., Schischmanoff P.O., Gattegno L., et al. Monocyte chemoattractant protein-1 (MCP-1)/CCL2 secreted by hepatic myofibroblasts promotes migration and invasion of human hepatoma cells. Int. J. Cancer. 2010;126:1095–1108. doi: 10.1002/ijc.24800. [DOI] [PubMed] [Google Scholar]
- 87.Parody T.R., Stone M.J. High level expression, activation, and antagonism of CC chemokine receptors CCR2 and CCR3 in Chinese hamster ovary cells. Cytokine. 2004;27:38–46. doi: 10.1016/j.cyto.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 88.Liang M., Mallari C., Rosser M., Ng H.P., May K., Monahan S., Bauman J.G., Islam I., Ghannam A., Buckman B., et al. Identification and characterization of a potent, selective, and orally active antagonist of the CC chemokine receptor-1. J. Biol. Chem. 2000;275:19000–19008. doi: 10.1074/jbc.M001222200. [DOI] [PubMed] [Google Scholar]
- 89.Heath H., Qin S., Rao P., Wu L., LaRosa G., Kassam N., Ponath P.D., Mackay C.R. Chemokine receptor usage by human eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J. Clin. Invest. 1997;99:178–184. doi: 10.1172/JCI119145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruffing N., Sullivan N., Sharmeen L., Sodroski J., Wu L. CCR5 has an expanded ligand-binding repertoire and is the primary receptor used by MCP-2 on activated T cells. Cell Immunol. 1998;189:160–168. doi: 10.1006/cimm.1998.1379. [DOI] [PubMed] [Google Scholar]
- 91.Halvorsen E.C., Hamilton M.J., Young A., Wadsworth B.J., LePard N.E., Lee H.N., Firmino N., Collier J.L., Bennewith K.L. Maraviroc decreases CCL8-mediated migration of CCR5(+) regulatory T cells and reduces metastatic tumor growth in the lungs. Oncoimmunology. 2016;5:e1150398. doi: 10.1080/2162402X.2016.1150398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McQuibban G.A., Gong J.H., Wong J.P., Wallace J.L., Clark-Lewis I., Overall C.M. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–1167. doi: 10.1182/blood.V100.4.1160.h81602001160_1160_1167. [DOI] [PubMed] [Google Scholar]
- 93.Proost P., Wuyts A., Van Damme J. Human monocyte chemotactic proteins-2 and -3: Structural and functional comparison with MCP-1. J. Leukoc. Biol. 1996;59:67–74. doi: 10.1002/jlb.59.1.67. [DOI] [PubMed] [Google Scholar]
- 94.Boring L., Gosling J., Chensue S.W., Kunkel S.L., Farese R.V., Jr., Broxmeyer H.E., Charo I.F. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu B., Rutledge B.J., Gu L., Fiorillo J., Lukacs N.W., Kunkel S.L., North R., Gerard C., Rollins B.J. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deshmane S.L., Kremlev S., Amini S., Sawaya B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. Version 2. J. Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shou Q., Fu H., Huang X., Yang Y. PARP-1 controls NK cell recruitment to the site of viral infection. JCI Insight. 2019;4:e121291. doi: 10.1172/jci.insight.121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lamkhioued B., Garcia-Zepeda E.A., Abi-Younes S., Nakamura H., Jedrzkiewicz S., Wagner L., Renzi P.M., Allakhverdi Z., Lilly C., Hamid Q., et al. Monocyte chemoattractant protein (MCP)-4 expression in the airways of patients with asthma. Induction in epithelial cells and mononuclear cells by proinflammatory cytokines. Am. J. Respir. Crit. Care Med. 2000;162:723–732. doi: 10.1164/ajrccm.162.2.9901080. [DOI] [PubMed] [Google Scholar]
- 99.Kalayci O., Sonna L.A., Woodruff P.G., Camargo C.A., Jr., Luster A.D., Lilly C.M. Monocyte chemotactic protein-4 (MCP-4; CCL-13): A biomarker of asthma. J. Asthma. 2004;41:27–33. doi: 10.1081/JAS-120024590. [DOI] [PubMed] [Google Scholar]
- 100.Thomas J.K., Mir H., Kapur N., Bae S., Singh S. CC chemokines are differentially expressed in Breast Cancer and are associated with disparity in overall survival. Sci. Rep. 2019;9:4014. doi: 10.1038/s41598-019-40514-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharma I., Singh A., Sharma K.C., Saxena S. Gene Expression Profiling of Chemokines and Their Receptors in Low and High Grade Astrocytoma. Asian Pac. J. Cancer Prev. 2017;18:1307–1313. doi: 10.22034/APJCP.2017.18.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arnold J.M., Huggard P.R., Cummings M., Ramm G.A., Chenevix-Trench G. Reduced expression of chemokine (C-C motif) ligand-2 (CCL2) in ovarian adenocarcinoma. Br. J. Cancer. 2005;92:2024–2031. doi: 10.1038/sj.bjc.6602596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ohta M., Kitadai Y., Tanaka S., Yoshihara M., Yasui W., Mukaida N., Haruma K., Chayama K. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human esophageal squamous cell carcinomas. Int. J. Cancer. 2002;102:220–224. doi: 10.1002/ijc.10705. [DOI] [PubMed] [Google Scholar]
- 104.Koide N., Nishio A., Sato T., Sugiyama A., Miyagawa S. Significance of macrophage chemoattractant protein-1 expression and macrophage infiltration in squamous cell carcinoma of the esophagus. Am. J. Gastroenterol. 2004;99:1667–1674. doi: 10.1111/j.1572-0241.2004.30733.x. [DOI] [PubMed] [Google Scholar]
- 105.Desai S., Kumar A., Laskar S., Pandey B.N. Cytokine profile of conditioned medium from human tumor cell lines after acute and fractionated doses of gamma radiation and its effect on survival of bystander tumor cells. Cytokine. 2013;61:54–62. doi: 10.1016/j.cyto.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 106.Quan J., Morrison N.A., Johnson N.W., Gao J. MCP-1 as a potential target to inhibit the bone invasion by oral squamous cell carcinoma. J. Cell Biochem. 2014;115:1787–1798. doi: 10.1002/jcb.24849. [DOI] [PubMed] [Google Scholar]
- 107.Bravatà V., Minafra L., Forte G.I., Cammarata F.P., Russo G., Di Maggio F.M., Augello G., Lio D., Gilardi M.C. Cytokine profile of breast cell lines after different radiation doses. Int. J. Radiat. Biol. 2017;93:1217–1226. doi: 10.1080/09553002.2017.1362504. [DOI] [PubMed] [Google Scholar]
- 108.Lu B., Zhou Y., Su Z., Yan A., Ding P. Effect of CCL2 siRNA on proliferation and apoptosis in the U251 human glioma cell line. Mol. Med. Rep. 2017;16:3387–3394. doi: 10.3892/mmr.2017.6995. [DOI] [PubMed] [Google Scholar]
- 109.Dutta P., Sarkissyan M., Paico K., Wu Y., Vadgama J.V. MCP-1 is overexpressed in triple-negative breast cancers and drives cancer invasiveness and metastasis. Breast Cancer Res. Treat. 2018;170:477–486. doi: 10.1007/s10549-018-4760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ding M., He S.J., Yang J. MCP-1/CCL2 Mediated by Autocrine Loop of PDGF-BB Promotes Invasion of Lung Cancer Cell by Recruitment of Macrophages Via CCL2-CCR2 Axis. J. Interferon Cytokine Res. 2019;39:224–232. doi: 10.1089/jir.2018.0113. [DOI] [PubMed] [Google Scholar]
- 111.Kunz M., Bloss G., Gillitzer R., Gross G., Goebeler M., Rapp U.R., Ludwig S. Hypoxia/reoxygenation induction of monocyte chemoattractant protein-1 in melanoma cells: Involvement of nuclear factor-kappaB, stimulatory protein-1 transcription factors and mitogen-activated protein kinase pathways. Biochem. J. 2002;366:299–306. doi: 10.1042/bj20011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Geller M.A., Bui-Nguyen T.M., Rogers L.M., Ramakrishnan S. Chemotherapy induces macrophage chemoattractant protein-1 production in ovarian cancer. Int. J. Gynecol Cancer. 2010;20:918–925. doi: 10.1111/IGC.0b013e3181e5c442. [DOI] [PubMed] [Google Scholar]
- 113.Lin Z.Y., Chuang Y.H., Chuang W.L. Cancer-associated fibroblasts up-regulate CCL2, CCL26, IL6 and LOXL2 genes related to promotion of cancer progression in hepatocellular carcinoma cells. Biomed. Pharmacother. 2012;66:525–529. doi: 10.1016/j.biopha.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 114.Han E.C., Lee J., Ryu S.W., Choi C. Tumor-conditioned Gr-1(+)CD11b(+) myeloid cells induce angiogenesis through the synergistic action of CCL2 and CXCL16 in vitro. Biochem. Biophys. Res. Commun. 2014;443:1218–1225. doi: 10.1016/j.bbrc.2013.12.117. [DOI] [PubMed] [Google Scholar]
- 115.Jia X.H., Du Y., Mao D., Wang Z.L., He Z.Q., Qiu J.D., Ma X.B., Shang W.T., Ding D., Tian J. Zoledronic acid prevents the tumor-promoting effects of mesenchymal stem cells via MCP-1 dependent recruitment of macrophages. Oncotarget. 2015;6:26018–26028. doi: 10.18632/oncotarget.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mohamed M.M., El-Ghonaimy E.A., Nouh M.A., Schneider R.J., Sloane B.F., El-Shinawi M. Cytokines secreted by macrophages isolated from tumor microenvironment of inflammatory breast cancer patients possess chemotactic properties. Int. J. Biochem. Cell Biol. 2014;46:138–147. doi: 10.1016/j.biocel.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chang A.L., Miska J., Wainwright D.A., Dey M., Rivetta C.V., Yu D., Kanojia D., Pituch K.C., Qiao J., Pytel P., et al. CCL2 Produced by the Glioma Microenvironment Is Essential for the Recruitment of Regulatory T Cells and Myeloid-Derived Suppressor Cells. Cancer Res. 2016;76:5671–5682. doi: 10.1158/0008-5472.CAN-16-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gabrusiewicz K., Rodriguez B., Wei J., Hashimoto Y., Healy L.M., Maiti S.N., Thomas G., Zhou S., Wang Q., Elakkad A., et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight. 2016;1:e85841. doi: 10.1172/jci.insight.85841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou S.L., Zhou Z.J., Hu Z.Q., Huang X.W., Wang Z., Chen E.B., Fan J., Cao Y., Dai Z., Zhou J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology. 2016;150:1646–1658.e17. doi: 10.1053/j.gastro.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 120.Silzle T., Kreutz M., Dobler M.A., Brockhoff G., Knuechel R., Kunz-Schughart L.A. Tumor-associated fibroblasts recruit blood monocytes into tumor tissue. Eur. J. Immunol. 2003;33:1311–1320. doi: 10.1002/eji.200323057. [DOI] [PubMed] [Google Scholar]
- 121.Subramaniam K.S., Tham S.T., Mohamed Z., Woo Y.L., Mat Adenan N.A., Chung I. Cancer-associated fibroblasts promote proliferation of endometrial cancer cells. PLoS ONE. 2013;8:e68923. doi: 10.1371/journal.pone.0068923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang X., Lin Y., Shi Y., Li B., Liu W., Yin W., Dang Y., Chu Y., Fan J., He R. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res. 2016;76:4124–4135. doi: 10.1158/0008-5472.CAN-15-2973. [DOI] [PubMed] [Google Scholar]
- 123.Jung D.W., Che Z.M., Kim J., Kim K., Kim K.Y., Williams D., Kim J. Tumor-stromal crosstalk in invasion of oral squamous cell carcinoma: A pivotal role of CCL7. Int. J. Cancer. 2010;127:332–344. doi: 10.1002/ijc.25060. [DOI] [PubMed] [Google Scholar]
- 124.Barbai T., Fejős Z., Puskas L.G., Tímár J., Rásó E. The importance of microenvironment: The role of CCL8 in metastasis formation of melanoma. Oncotarget. 2015;6:29111–29128. doi: 10.18632/oncotarget.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Farmaki E., Chatzistamou I., Kaza V., Kiaris H. A CCL8 gradient drives breast cancer cell dissemination. Oncogene. 2016;35:6309–6318. doi: 10.1038/onc.2016.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yoshimura T., Imamichi T., Weiss J.M., Sato M., Li L., Matsukawa A., Wang J.M. Induction of Monocyte Chemoattractant Proteins in Macrophages via the Production of Granulocyte/Macrophage Colony-Stimulating Factor by Breast Cancer Cells. Front. Immunol. 2016;7:2. doi: 10.3389/fimmu.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tsuyada A., Chow A., Wu J., Somlo G., Chu P., Loera S., Luu T., Li A.X., Wu X., Ye W., et al. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res. 2012;72:2768–2779. doi: 10.1158/0008-5472.CAN-11-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Higashino N., Koma Y.I., Hosono M., Takase N., Okamoto M., Kodaira H., Nishio M., Shigeoka M., Kakeji Y., Yokozaki H. Fibroblast activation protein-positive fibroblasts promote tumor progression through secretion of CCL2 and interleukin-6 in esophageal squamous cell carcinoma. Lab. Invest. 2019;99:777–792. doi: 10.1038/s41374-018-0185-6. [DOI] [PubMed] [Google Scholar]
- 129.Pausch T.M., Aue E., Wirsik N.M., Freire Valls A., Shen Y., Radhakrishnan P., Hackert T., Schneider M., Schmidt T. Metastasis-associated fibroblasts promote angiogenesis in metastasized pancreatic cancer via the CXCL8 and the CCL2 axes. Sci. Rep. 2020;10:5420. doi: 10.1038/s41598-020-62416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vickman R.E., Broman M.M., Lanman N.A., Franco O.E., Sudyanti P.A.G., Ni Y., Ji Y., Helfand B.T., Petkewicz J., Paterakos M.C., et al. Heterogeneity of human prostate carcinoma-associated fibroblasts implicates a role for subpopulations in myeloid cell recruitment. Prostate. 2020;80:173–185. doi: 10.1002/pros.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang H., Zhang Q., Kong H., Zeng Y., Hao M., Yu T., Peng J., Xu Z., Chen J., Shi H. Monocyte chemotactic protein-1 expression as a prognosic biomarker in patients with solid tumor: A meta analysis. Int. J. Clin. Exp. Pathol. 2014;7:3876–3886. [PMC free article] [PubMed] [Google Scholar]
- 132.Goede V., Brogelli L., Ziche M., Augustin H.G. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int. J. Cancer. 1999;82:765–770. doi: 10.1002/(SICI)1097-0215(19990827)82:5<765::AID-IJC23>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 133.Kuroda T., Kitadai Y., Tanaka S., Yang X., Mukaida N., Yoshihara M., Chayama K. Monocyte chemoattractant protein-1 transfection induces angiogenesis and tumorigenesis of gastric carcinoma in nude mice via macrophage recruitment. Clin. Cancer Res. 2005;11:7629–7636. doi: 10.1158/1078-0432.CCR-05-0798. [DOI] [PubMed] [Google Scholar]
- 134.Qian B.Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L.R., Kaiser E.A., Snyder L.A., Pollard J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Low-Marchelli J.M., Ardi V.C., Vizcarra E.A., van Rooijen N., Quigley J.P., Yang J. Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Res. 2013;73:662–671. doi: 10.1158/0008-5472.CAN-12-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Roca H., Varsos Z.S., Sud S., Craig M.J., Ying C., Pienta K.J. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J. Biol. Chem. 2009;284:34342–34354. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Loyher P.L., Rochefort J., Baudesson de Chanville C., Hamon P., Lescaille G., Bertolus C., Guillot-Delost M., Krummel M.F., Lemoine F.M., Combadière C., et al. CCR2 Influences T Regulatory Cell Migration to Tumors and Serves as a Biomarker of Cyclophosphamide Sensitivity. Cancer Res. 2016;76:6483–6494. doi: 10.1158/0008-5472.CAN-16-0984. [DOI] [PubMed] [Google Scholar]
- 138.Mondini M., Loyher P.L., Hamon P., Gerbé de Thoré M., Laviron M., Berthelot K., Clémenson C., Salomon B.L., Combadière C., Deutsch E., et al. CCR2-Dependent Recruitment of Tregs and Monocytes Following Radiotherapy Is Associated with TNFα-Mediated Resistance. Cancer Immunol. Res. 2019;7:376–387. doi: 10.1158/2326-6066.CIR-18-0633. [DOI] [PubMed] [Google Scholar]
- 139.Su X., Ye J., Hsueh E.C., Zhang Y., Hoft D.F., Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J. Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 140.Xu F., Shi J., Yu B., Ni W., Wu X., Gu Z. Chemokines mediate mesenchymal stem cell migration toward gliomas in vitro. Oncol. Rep. 2010;23:1561–1567. doi: 10.3892/or_00000796. [DOI] [PubMed] [Google Scholar]
- 141.Magge S.N., Malik S.Z., Royo N.C., Chen H.I., Yu L., Snyder E.Y., O’Rourke D.M., Watson D.J. Role of monocyte chemoattractant protein-1 (MCP-1/CCL2) in migration of neural progenitor cells toward glial tumors. J. Neurosci. Res. 2009;87:1547–1555. doi: 10.1002/jnr.21983. [DOI] [PubMed] [Google Scholar]
- 142.Platten M., Kretz A., Naumann U., Aulwurm S., Egashira K., Isenmann S., Weller M. Monocyte chemoattractant protein-1 increases microglial infiltration and aggressiveness of gliomas. Ann. Neurol. 2003;54:388–392. doi: 10.1002/ana.10679. [DOI] [PubMed] [Google Scholar]
- 143.Yang X., Lu P., Ishida Y., Kuziel W.A., Fujii C., Mukaida N. Attenuated liver tumor formation in the absence of CCR2 with a concomitant reduction in the accumulation of hepatic stellate cells, macrophages and neovascularization. Int. J. Cancer. 2006;118:335–345. doi: 10.1002/ijc.21371. [DOI] [PubMed] [Google Scholar]
- 144.Ly L.V., Bronkhorst I.H., van Beelen E., Vrolijk J., Taylor A.W., Versluis M., Luyten G.P., Jager M.J. Inflammatory cytokines in eyes with uveal melanoma and relation with macrophage infiltration. Invest. Ophthalmol. Vis. Sci. 2010;51:5445–5451. doi: 10.1167/iovs.10-5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen X.J., Deng Y.R., Wang Z.C., Wei W.F., Zhou C.F., Zhang Y.M., Yan R.M., Liang L.J., Zhong M., Liang L., et al. Hypoxia-induced ZEB1 promotes cervical cancer progression via CCL8-dependent tumour-associated macrophage recruitment. Cell Death Dis. 2019;10:508. doi: 10.1038/s41419-019-1748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nagai M., Masuzawa T. Vaccination with MCP-1 cDNA transfectant on human malignant glioma in nude mice induces migration of monocytes and NK cells to the tumor. Int. Immunopharmacol. 2001;1:657–664. doi: 10.1016/S1567-5769(00)00050-3. [DOI] [PubMed] [Google Scholar]
- 147.Berencsi K., Rani P., Zhang T., Gross L., Mastrangelo M., Meropol N.J., Herlyn D., Somasundaram R. In vitro migration of cytotoxic T lymphocyte derived from a colon carcinoma patient is dependent on CCL2 and CCR2. J. Transl. Med. 2011;9:33. doi: 10.1186/1479-5876-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nakasone Y., Fujimoto M., Matsushita T., Hamaguchi Y., Huu D.L., Yanaba M., Sato S., Takehara K., Hasegawa M. Host-derived MCP-1 and MIP-1α regulate protective anti-tumor immunity to localized and metastatic B16 melanoma. Am. J. Pathol. 2012;180:365–374. doi: 10.1016/j.ajpath.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 149.Fioretti F., Fradelizi D., Stoppacciaro A., Ramponi S., Ruco L., Minty A., Sozzani S., Garlanda C., Vecchi A., Mantovani A. Reduced tumorigenicity and augmented leukocyte infiltration after monocyte chemotactic protein-3 (MCP-3) gene transfer: Perivascular accumulation of dendritic cells in peritumoral tissue and neutrophil recruitment within the tumor. J. Immunol. 1998;161:342–346. [PubMed] [Google Scholar]
- 150.Wetzel K., Menten P., Opdënakker G., Van Damme J., Gröne H.J., Giese N., Vecchi A., Sozzani S., Cornelis J.J., Rommelaere J., et al. Transduction of human MCP-3 by a parvoviral vector induces leukocyte infiltration and reduces growth of human cervical carcinoma cell xenografts. J. Gene Med. 2001;3:326–337. doi: 10.1002/jgm.191. [DOI] [PubMed] [Google Scholar]
- 151.Vitiello P.F., Shainheit M.G., Allison E.M., Adler E.P., Kurt R.A. Impact of tumor-derived CCL2 on T cell effector function. Immunol. Lett. 2004;91:239–245. doi: 10.1016/j.imlet.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 152.Michielsen A.J., Hogan A.E., Marry J., Tosetto M., Cox F., Hyland J.M., Sheahan K.D., O’Donoghue D.P., Mulcahy H.E., Ryan E.J., et al. Tumour tissue microenvironment can inhibit dendritic cell maturation in colorectal cancer. PLoS ONE. 2011;6:e27944. doi: 10.1371/journal.pone.0027944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mou T., Xie F., Zhong P., Hua H., Lai L., Yang Q., Wang J. MiR-345-5p functions as a tumor suppressor in pancreatic cancer by directly targeting CCL8. Biomed. Pharmacother. 2019;111:891–900. doi: 10.1016/j.biopha.2018.12.121. [DOI] [PubMed] [Google Scholar]
- 154.Yao M., Fang W., Smart C., Hu Q., Huang S., Alvarez N., Fields P., Cheng N. CCR2 Chemokine Receptors Enhance Growth and Cell-Cycle Progression of Breast Cancer Cells through SRC and PKC Activation. Mol. Cancer Res. 2019;17:604–617. doi: 10.1158/1541-7786.MCR-18-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang X., Chen L., Dang W.Q., Cao M.F., Xiao J.F., Lv S.Q., Jiang W.J., Yao X.H., Lu H.M., Miao J.Y., et al. CCL8 secreted by tumor-associated macrophages promotes invasion and stemness of glioblastoma cells via ERK1/2 signaling. Lab. Invest. 2020;100:619–629. doi: 10.1038/s41374-019-0345-3. [DOI] [PubMed] [Google Scholar]
- 156.Shi C.L., Yu C.H., Zhang Y., Zhao D., Chang X.H., Wang W.H. Monocyte chemoattractant protein-1 modulates invasion and apoptosis of PC-3M prostate cancer cells via regulating expression of VEGF, MMP9 and caspase-3. Asian Pac. J. Cancer Prev. 2011;12:555–559. [PubMed] [Google Scholar]
- 157.Natsagdorj A., Izumi K., Hiratsuka K., Machioka K., Iwamoto H., Naito R., Makino T., Kadomoto S., Shigehara K., Kadono Y., et al. CCL2 induces resistance to the antiproliferative effect of cabazitaxel in prostate cancer cells. Cancer Sci. 2019;110:279–288. doi: 10.1111/cas.13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li D., Ji H., Niu X., Yin L., Wang Y., Gu Y., Wang J., Zhou X., Zhang H., Zhang Q. Tumor-associated macrophages secrete CC-chemokine ligand 2 and induce tamoxifen resistance by activating PI3K/Akt/mTOR in breast cancer. Cancer Sci. 2020;111:47–58. doi: 10.1111/cas.14230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Torres S., Bartolomé R.A., Mendes M., Barderas R., Fernandez-Aceñero M.J., Peláez-García A., Peña C., Lopez-Lucendo M., Villar-Vázquez R., de Herreros A.G., et al. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin. Cancer Res. 2013;19:6006–6019. doi: 10.1158/1078-0432.CCR-13-1130. [DOI] [PubMed] [Google Scholar]
- 160.Grimm S., Jennek S., Singh R., Enkelmann A., Junker K., Rippaus N., Berndt A., Friedrich K. Malignancy of bladder cancer cells is enhanced by tumor-associated fibroblasts through a multifaceted cytokine-chemokine loop. Exp. Cell Res. 2015;335:1–11. doi: 10.1016/j.yexcr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 161.Liu J., Chen S., Wang W., Ning B.F., Chen F., Shen W., Ding J., Chen W., Xie W.F., Zhang X. Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-β pathways. Cancer Lett. 2016;379:49–59. doi: 10.1016/j.canlet.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 162.Lee C.C., Ho H.C., Su Y.C., Lee M.S., Hung S.K., Lin C.H. MCP1-Induced Epithelial-Mesenchymal Transition in Head and Neck Cancer by AKT Activation. Anticancer Res. 2015;35:3299–3306. [PubMed] [Google Scholar]
- 163.Li S., Lu J., Chen Y., Xiong N., Li L., Zhang J., Yang H., Wu C., Zeng H., Liu Y. MCP-1-induced ERK/GSK-3β/Snail signaling facilitates the epithelial-mesenchymal transition and promotes the migration of MCF-7 human breast carcinoma cells. Cell Mol. Immunol. 2017;14:621–630. doi: 10.1038/cmi.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee Y.S., Kim S.Y., Song S.J., Hong H.K., Lee Y., Oh B.Y., Lee W.Y., Cho Y.B. Crosstalk between CCL7 and CCR3 promotes metastasis of colon cancer cells via ERK-JNK signaling pathways. Oncotarget. 2016;7:36842–36853. doi: 10.18632/oncotarget.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhou J., Zheng S., Liu T., Liu Q., Chen Y., Tan D., Ma R., Lu X. MCP2 activates NF-κB signaling pathway promoting the migration and invasion of ESCC cells. Cell Biol. Int. 2018;42:365–372. doi: 10.1002/cbin.10909. [DOI] [PubMed] [Google Scholar]
- 166.Mestdagt M., Polette M., Buttice G., Noël A., Ueda A., Foidart J.M., Gilles C. Transactivation of MCP-1/CCL2 by beta-catenin/TCF-4 in human breast cancer cells. Int. J. Cancer. 2006;118:35–42. doi: 10.1002/ijc.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kitamura T., Qian B.Z., Soong D., Cassetta L., Noy R., Sugano G., Kato Y., Li J., Pollard J.W. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 2015;212:1043–1059. doi: 10.1084/jem.20141836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Granot Z., Henke E., Comen E.A., King T.A., Norton L., Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20:300–314. doi: 10.1016/j.ccr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Van Golen K.L., Ying C., Sequeira L., Dubyk C.W., Reisenberger T., Chinnaiyan A.M., Pienta K.J., Loberg R.D. CCL2 induces prostate cancer transendothelial cell migration via activation of the small GTPase Rac. J. Cell Biochem. 2008;104:1587–1597. doi: 10.1002/jcb.21652. [DOI] [PubMed] [Google Scholar]
- 170.Li X., Loberg R., Liao J., Ying C., Snyder L.A., Pienta K.J., McCauley L.K. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Res. 2009;69:1685–1692. doi: 10.1158/0008-5472.CAN-08-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang N., Docherty F.E., Brown H.K., Reeves K.J., Fowles A.C., Ottewell P.D., Dear T.N., Holen I., Croucher P.I., Eaton C.L. Prostate cancer cells preferentially home to osteoblast-rich areas in the early stages of bone metastasis: Evidence from in vivo models. J. Bone Miner. Res. 2014;29:2688–2696. doi: 10.1002/jbmr.2300. [DOI] [PubMed] [Google Scholar]
- 172.Cai Z., Chen Q., Chen J., Lu Y., Xiao G., Wu Z., Zhou Q., Zhang J. Monocyte chemotactic protein 1 promotes lung cancer-induced bone resorptive lesions in vivo. Neoplasia. 2009;11:228–236. doi: 10.1593/neo.81282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.He Z., He J., Liu Z., Xu J., Yi S.F., Liu H., Yang J. MAPK11 in breast cancer cells enhances osteoclastogenesis and bone resorption. Biochimie. 2014;106:24–32. doi: 10.1016/j.biochi.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lu Y., Xiao G., Galson D.L., Nishio Y., Mizokami A., Keller E.T., Yao Z., Zhang J. PTHrP-induced MCP-1 production by human bone marrow endothelial cells and osteoblasts promotes osteoclast differentiation and prostate cancer cell proliferation and invasion in vitro. Int. J. Cancer. 2007;121:724–733. doi: 10.1002/ijc.22704. [DOI] [PubMed] [Google Scholar]
- 175.He S., He S., Chen C.H., Deborde S., Bakst R.L., Chernichenko N., McNamara W.F., Lee S.Y., Barajas F., Yu Z., et al. The chemokine (CCL2-CCR2) signaling axis mediates perineural invasion. Mol. Cancer Res. 2015;13:380–390. doi: 10.1158/1541-7786.MCR-14-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huang T., Fan Q., Wang Y., Cui Y., Wang Z., Yang L., Sun X., Wang Y. Schwann Cell-Derived CCL2 Promotes the Perineural Invasion of Cervical Cancer. Front. Oncol. 2020;10:19. doi: 10.3389/fonc.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Salcedo R., Ponce M.L., Young H.A., Wasserman K., Ward J.M., Kleinman H.K., Oppenheim J.J., Murphy W.J. Human endothelial cells express CCR2 and respond to MCP-1: Direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. doi: 10.1182/blood.V96.1.34. [DOI] [PubMed] [Google Scholar]
- 178.Varney M.L., Olsen K.J., Mosley R.L., Singh R.K. Paracrine regulation of vascular endothelial growth factor--a expression during macrophage-melanoma cell interaction: Role of monocyte chemotactic protein-1 and macrophage colony-stimulating factor. J. Interferon Cytokine Res. 2005;25:674–683. doi: 10.1089/jir.2005.25.674. [DOI] [PubMed] [Google Scholar]
- 179.Jetten N., Verbruggen S., Gijbels M.J., Post M.J., De Winther M.P., Donners M.M. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17:109–118. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 180.Lee K.M., Danuser R., Stein J.V., Graham D., Nibbs R.J., Graham G.J. The chemokine receptors ACKR2 and CCR2 reciprocally regulate lymphatic vessel density. EMBO J. 2014;33:2564–2580. doi: 10.15252/embj.201488887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yamaguchi A., Nozawa K., Fujishiro M., Kawasaki M., Suzuki F., Takamori K., Ogawa H., Takasaki Y., Sekigawa I. CC motif chemokine ligand 13 is associated with rheumatoid arthritis pathogenesis. Mod. Rheumatol. 2013;23:856–863. doi: 10.3109/s10165-012-0752-4. [DOI] [PubMed] [Google Scholar]
- 182.Kitaura M., Nakajima T., Imai T., Harada S., Combadiere C., Tiffany H.L., Murphy P.M., Yoshie O. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J. Biol. Chem. 1996;271:7725–7730. doi: 10.1074/jbc.271.13.7725. [DOI] [PubMed] [Google Scholar]
- 183.Forssmann U., Uguccioni M., Loetscher P., Dahinden C.A., Langen H., Thelen M., Baggiolini M. Eotaxin-2, a novel CC chemokine that is selective for the chemokine receptor CCR3, and acts like eotaxin on human eosinophil and basophil leukocytes. J. Exp. Med. 1997;185:2171–2176. doi: 10.1084/jem.185.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.White J.R., Imburgia C., Dul E., Appelbaum E., O’Donnell K., O’Shannessy D.J., Brawner M., Fornwald J., Adamou J., Elshourbagy N.A., et al. Cloning and functional characterization of a novel human CC chemokine that binds to the CCR3 receptor and activates human eosinophils. J. Leukoc. Biol. 1997;62:667–675. doi: 10.1002/jlb.62.5.667. [DOI] [PubMed] [Google Scholar]
- 185.Kitaura M., Suzuki N., Imai T., Takagi S., Suzuki R., Nakajima T., Hirai K., Nomiyama H., Yoshie O. Molecular cloning of a novel human CC chemokine (Eotaxin-3) that is a functional ligand of CC chemokine receptor 3. J. Biol. Chem. 1999;274:27975–27980. doi: 10.1074/jbc.274.39.27975. [DOI] [PubMed] [Google Scholar]
- 186.Ogilvie P., Bardi G., Clark-Lewis I., Baggiolini M., Uguccioni M. Eotaxin is a natural antagonist for CCR2 and an agonist for CCR5. Blood. 2001;97:1920–1924. doi: 10.1182/blood.V97.7.1920. [DOI] [PubMed] [Google Scholar]
- 187.Nakayama T., Watanabe Y., Oiso N., Higuchi T., Shigeta A., Mizuguchi N., Katou F., Hashimoto K., Kawada A., Yoshie O. Eotaxin-3/CC chemokine ligand 26 is a functional ligand for CX3CR1. J. Immunol. 2010;185:6472–6479. doi: 10.4049/jimmunol.0904126. [DOI] [PubMed] [Google Scholar]
- 188.Chiu D.K., Xu I.M., Lai R.K., Tse A.P., Wei L.L., Koh H.Y., Li L.L., Lee D., Lo R.C., Wong C.M., et al. Hypoxia induces myeloid-derived suppressor cell recruitment to hepatocellular carcinoma through chemokine (C-C motif) ligand 26. Hepatology. 2016;64:797–813. doi: 10.1002/hep.28655. [DOI] [PubMed] [Google Scholar]
- 189.Ogilvie P., Paoletti S., Clark-Lewis I., Uguccioni M. Eotaxin-3 is a natural antagonist for CCR2 and exerts a repulsive effect on human monocytes. Blood. 2003;102:789–794. doi: 10.1182/blood-2002-09-2773. [DOI] [PubMed] [Google Scholar]
- 190.Petkovic V., Moghini C., Paoletti S., Uguccioni M., Gerber B. Eotaxin-3/CCL26 is a natural antagonist for CC chemokine receptors 1 and 5. A human chemokine with a regulatory role. J. Biol. Chem. 2004;279:23357–23363. doi: 10.1074/jbc.M309283200. [DOI] [PubMed] [Google Scholar]
- 191.Yamada H., Hirai K., Miyamasu M., Iikura M., Misaki Y., Shoji S., Takaishi T., Kasahara T., Morita Y., Ito K. Eotaxin is a potent chemotaxin for human basophils. Biochem. Biophys. Res. Commun. 1997;231:365–368. doi: 10.1006/bbrc.1997.6100. [DOI] [PubMed] [Google Scholar]
- 192.Ponath P.D., Qin S., Ringler D.J., Clark-Lewis I., Wang J., Kassam N., Smith H., Shi X., Gonzalo J.A., Newman W., et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J. Clin. Invest. 1996;97:604–612. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Menzies-Gow A., Ying S., Sabroe I., Stubbs V.L., Soler D., Williams T.J., Kay A.B. Eotaxin (CCL11) and eotaxin-2 (CCL24) induce recruitment of eosinophils, basophils, neutrophils, and macrophages as well as features of early- and late-phase allergic reactions following cutaneous injection in human atopic and nonatopic volunteers. J. Immunol. 2002;169:2712–2718. doi: 10.4049/jimmunol.169.5.2712. [DOI] [PubMed] [Google Scholar]
- 194.Provost V., Larose M.C., Langlois A., Rola-Pleszczynski M., Flamand N., Laviolette M. CCL26/eotaxin-3 is more effective to induce the migration of eosinophils of asthmatics than CCL11/eotaxin-1 and CCL24/eotaxin-2. J. Leukoc. Biol. 2013;94:213–222. doi: 10.1189/jlb.0212074. [DOI] [PubMed] [Google Scholar]
- 195.Cho H., Lim S.J., Won K.Y., Bae G.E., Kim G.Y., Min J.W., Noh B.J. Eosinophils in Colorectal Neoplasms Associated with Expression of CCL11 and CCL24. J. Pathol. Transl. Med. 2016;50:45–51. doi: 10.4132/jptm.2015.10.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lorena S.C., Oliveira D.T., Dorta R.G., Landman G., Kowalski L.P. Eotaxin expression in oral squamous cell carcinomas with and without tumour associated tissue eosinophilia. Oral. Dis. 2003;9:279–283. doi: 10.1034/j.1601-0825.2003.00958.x. [DOI] [PubMed] [Google Scholar]
- 197.Da Silva J.M., Queiroz-Junior C.M., Batista A.C., Rachid M.A., Teixeira M.M., da Silva T.A. Eosinophil depletion protects mice from tongue squamous cell carcinoma induced by 4-nitroquinoline-1-oxide. Histol. Histopathol. 2014;29:387–396. doi: 10.14670/HH-29.387. [DOI] [PubMed] [Google Scholar]
- 198.Sakkal S., Miller S., Apostolopoulos V., Nurgali K. Eosinophils in Cancer: Favourable or Unfavourable? Curr. Med. Chem. 2016;23:650–666. doi: 10.2174/0929867323666160119094313. [DOI] [PubMed] [Google Scholar]
- 199.Xing Y., Tian Y., Kurosawa T., Matsui S., Touma M., Yanai T., Wu Q., Sugimoto K. CCL11-induced eosinophils inhibit the formation of blood vessels and cause tumor necrosis. Genes Cells. 2016;21:624–638. doi: 10.1111/gtc.12371. [DOI] [PubMed] [Google Scholar]
- 200.Lan Q., Lai W., Zeng Y., Liu L., Li S., Jin S., Zhang Y., Luo X., Xu H., Lin X., et al. CCL26 Participates in the PRL-3-Induced Promotion of Colorectal Cancer Invasion by Stimulating Tumor-Associated Macrophage Infiltration. Mol. Cancer Ther. 2018;17:276–289. doi: 10.1158/1535-7163.MCT-17-0507. [DOI] [PubMed] [Google Scholar]
- 201.Jöhrer K., Zelle-Rieser C., Perathoner A., Moser P., Hager M., Ramoner R., Gander H., Höltl L., Bartsch G., Greil R., et al. Up-regulation of functional chemokine receptor CCR3 in human renal cell carcinoma. Clin. Cancer Res. 2005;11:2459–2465. doi: 10.1158/1078-0432.CCR-04-0405. [DOI] [PubMed] [Google Scholar]
- 202.Zhu F., Liu P., Li J., Zhang Y. Eotaxin-1 promotes prostate cancer cell invasion via activation of the CCR3-ERK pathway and upregulation of MMP-3 expression. Oncol. Rep. 2014;31:2049–2054. doi: 10.3892/or.2014.3060. [DOI] [PubMed] [Google Scholar]
- 203.Tian M., Chen L., Ma L., Wang D., Shao B., Wu J., Wu H., Jin Y. Expression and prognostic significance of CCL11/CCR3 in glioblastoma. Oncotarget. 2016;7:32617–32627. doi: 10.18632/oncotarget.8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Miyagaki T., Sugaya M., Murakami T., Asano Y., Tada Y., Kadono T., Okochi H., Tamaki K., Sato S. CCL11-CCR3 interactions promote survival of anaplastic large cell lymphoma cells via ERK1/2 activation. Cancer Res. 2011;71:2056–2065. doi: 10.1158/0008-5472.CAN-10-3764. [DOI] [PubMed] [Google Scholar]
- 205.Salcedo R., Young H.A., Ponce M.L., Ward J.M., Kleinman H.K., Murphy W.J., Oppenheim J.J. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J. Immunol. 2001;166:7571–7578. doi: 10.4049/jimmunol.166.12.7571. [DOI] [PubMed] [Google Scholar]
- 206.Park J.Y., Kang Y.W., Choi B.Y., Yang Y.C., Cho B.P., Cho W.G. CCL11 promotes angiogenic activity by activating the PI3K/Akt pathway in HUVECs. J. Recept. Signal. Transduct. Res. 2017;37:416–421. doi: 10.1080/10799893.2017.1298132. [DOI] [PubMed] [Google Scholar]
- 207.Jin L., Liu W.R., Tian M.X., Jiang X.F., Wang H., Zhou P.Y., Ding Z.B., Peng Y.F., Dai Z., Qiu S.J., et al. CCL24 contributes to HCC malignancy via RhoB- VEGFA-VEGFR2 angiogenesis pathway and indicates poor prognosis. Oncotarget. 2017;8:5135–5148. doi: 10.18632/oncotarget.14095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Imai T., Baba M., Nishimura M., Kakizaki M., Takagi S., Yoshie O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J. Biol. Chem. 1997;272:15036–15042. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- 209.Imai T., Chantry D., Raport C.J., Wood C.L., Nishimura M., Godiska R., Yoshie O., Gray P.W. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J. Biol. Chem. 1998;273:1764–1768. doi: 10.1074/jbc.273.3.1764. [DOI] [PubMed] [Google Scholar]
- 210.Yoshie O., Matsushima K. CCR4 and its ligands: From bench to bedside. Int. Immunol. 2015;27:11–20. doi: 10.1093/intimm/dxu079. [DOI] [PubMed] [Google Scholar]
- 211.Okada N., Sasaki A., Niwa M., Okada Y., Hatanaka Y., Tani Y., Mizuguchi H., Nakagawa S., Fujita T., Yamamoto A. Tumor suppressive efficacy through augmentation of tumor-infiltrating immune cells by intratumoral injection of chemokine-expressing adenoviral vector. Cancer Gene Ther. 2006;13:393–405. doi: 10.1038/sj.cgt.7700903. [DOI] [PubMed] [Google Scholar]
- 212.Kanagawa N., Niwa M., Hatanaka Y., Tani Y., Nakagawa S., Fujita T., Yamamoto A., Okada N. CC-chemokine ligand 17 gene therapy induces tumor regression through augmentation of tumor-infiltrating immune cells in a murine model of preexisting CT26 colon carcinoma. Int. J. Cancer. 2007;121:2013–2022. doi: 10.1002/ijc.22908. [DOI] [PubMed] [Google Scholar]
- 213.Wågsäter D., Dienus O., Löfgren S., Hugander A., Dimberg J. Quantification of the chemokines CCL17 and CCL22 in human colorectal adenocarcinomas. Mol. Med. Rep. 2008;1:211–217. [PubMed] [Google Scholar]
- 214.Tsujikawa T., Yaguchi T., Ohmura G., Ohta S., Kobayashi A., Kawamura N., Fujita T., Nakano H., Shimada T., Takahashi T., et al. Autocrine and paracrine loops between cancer cells and macrophages promote lymph node metastasis via CCR4/CCL22 in head and neck squamous cell carcinoma. Int. J. Cancer. 2013;132:2755–2766. doi: 10.1002/ijc.27966. [DOI] [PubMed] [Google Scholar]
- 215.Tripathi C., Tewari B.N., Kanchan R.K., Baghel K.S., Nautiyal N., Shrivastava R., Kaur H., Bhatt M.L., Bhadauria S. Macrophages are recruited to hypoxic tumor areas and acquire a pro-angiogenic M2-polarized phenotype via hypoxic cancer cell derived cytokines Oncostatin M and Eotaxin. Oncotarget. 2014;5:5350–5368. doi: 10.18632/oncotarget.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wertel I., Surówka J., Polak G., Barczyński B., Bednarek W., Jakubowicz-Gil J., Bojarska-Junak A., Kotarski J. Macrophage-derived chemokine CCL22 and regulatory T cells in ovarian cancer patients. Tumour. Biol. 2015;36:4811–4817. doi: 10.1007/s13277-015-3133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang W., Chen L., Ma K., Zhao Y., Liu X., Wang Y., Liu M., Liang S., Zhu H., Xu N. Polarization of macrophages in the tumor microenvironment is influenced by EGFR signaling within colon cancer cells. Oncotarget. 2016;7:75366–75378. doi: 10.18632/oncotarget.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhu F., Li X., Chen S., Zeng Q., Zhao Y., Luo F. Tumor-associated macrophage or chemokine ligand CCL17 positively regulates the tumorigenesis of hepatocellular carcinoma. Med. Oncol. 2016;33:17. doi: 10.1007/s12032-016-0729-9. [DOI] [PubMed] [Google Scholar]
- 219.Kimura S., Nanbu U., Noguchi H., Harada Y., Kumamoto K., Sasaguri Y., Nakayama T. Macrophage CCL22 expression in the tumor microenvironment and implications for survival in patients with squamous cell carcinoma of the tongue. J. Oral. Pathol. Med. 2019;48:677–685. doi: 10.1111/jop.12885. [DOI] [PubMed] [Google Scholar]
- 220.Mishalian I., Bayuh R., Eruslanov E., Michaeli J., Levy L., Zolotarov L., Singhal S., Albelda S.M., Granot Z., Fridlender Z.G. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17--a new mechanism of impaired antitumor immunity. Int. J. Cancer. 2014;135:1178–1186. doi: 10.1002/ijc.28770. [DOI] [PubMed] [Google Scholar]
- 221.Omland S.H., Wettergren E.E., Mollerup S., Asplund M., Mourier T., Hansen A.J., Gniadecki R. Cancer associated fibroblasts (CAFs) are activated in cutaneous basal cell carcinoma and in the peritumoural skin. BMC Cancer. 2017;17:675. doi: 10.1186/s12885-017-3663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mizukami Y., Kono K., Kawaguchi Y., Akaike H., Kamimura K., Sugai H., Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int. J. Cancer. 2008;122:2286–2293. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- 223.Qin X.J., Shi H.Z., Deng J.M., Liang Q.L., Jiang J., Ye Z.J. CCL22 recruits CD4-positive CD25-positive regulatory T cells into malignant pleural effusion. Clin. Cancer Res. 2009;15:2231–2237. doi: 10.1158/1078-0432.CCR-08-2641. [DOI] [PubMed] [Google Scholar]
- 224.Maruyama T., Kono K., Izawa S., Mizukami Y., Kawaguchi Y., Mimura K., Watanabe M., Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to infiltration of regulatory T cells in esophageal squamous cell carcinoma. Dis. Esophagus. 2010;23:422–429. doi: 10.1111/j.1442-2050.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 225.Liu W., Wei X., Li L., Wu X., Yan J., Yang H., Song F. CCR4 mediated chemotaxis of regulatory T cells suppress the activation of T cells and NK cells via TGF-β pathway in human non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2017;488:196–203. doi: 10.1016/j.bbrc.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 226.Xie F., Liu L.B., Shang W.Q., Chang K.K., Meng Y.H., Mei J., Yu J.J., Li D.J., Li M.Q. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 2015;364:106–117. doi: 10.1016/j.canlet.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 227.Chen D., Jiang R., Mao C., Shi L., Wang S., Yu L., Hu Q., Dai D., Xu H. Chemokine/chemokine receptor interactions contribute to the accumulation of Th17 cells in patients with esophageal squamous cell carcinoma. Hum. Immunol. 2012;73:1068–1072. doi: 10.1016/j.humimm.2012.07.333. [DOI] [PubMed] [Google Scholar]
- 228.Li Y.Q., Liu F.F., Zhang X.M., Guo X.J., Ren M.J., Fu L. Tumor secretion of CCL22 activates intratumoral Treg infiltration and is independent prognostic predictor of breast cancer. PLoS ONE. 2013;8:e76379. doi: 10.1371/journal.pone.0076379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Nakanishi T., Imaizumi K., Hasegawa Y., Kawabe T., Hashimoto N., Okamoto M., Shimokata K. Expression of macrophage-derived chemokine (MDC)/CCL22 in human lung cancer. Cancer Immunol. Immunother. 2006;55:1320–1329. doi: 10.1007/s00262-006-0133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Weide B., Allgaier N., Hector A., Forschner A., Leiter U., Eigentler T.K., Garbe C., Hartl D. Increased CCL17 serum levels are associated with improved survival in advanced melanoma. Cancer Immunol. Immunother. 2015;64:1075–1082. doi: 10.1007/s00262-015-1714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Li X., Li D., Shi Q., Huang X., Ju X. Umbilical cord blood-derived Helios-positive regulatory T cells promote angiogenesis in acute lymphoblastic leukemia in mice via CCL22 and the VEGFA-VEGFR2 pathway. Mol. Med. Rep. 2019;19:4195–4204. doi: 10.3892/mmr.2019.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wei C., Yang C., Wang S., Shi D., Zhang C., Lin X., Xiong B. M2 macrophages confer resistance to 5-fluorouracil in colorectal cancer through the activation of CCL22/PI3K/AKT signaling. Oncol. Targets Ther. 2019;12:3051–3063. doi: 10.2147/OTT.S198126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Liu L.B., Xie F., Chang K.K., Shang W.Q., Meng Y.H., Yu J.J., Li H., Sun Q., Yuan M.M., Jin L.P., et al. Chemokine CCL17 induced by hypoxia promotes the proliferation of cervical cancer cell. Am. J. Cancer Res. 2015;5:3072–3084. [PMC free article] [PubMed] [Google Scholar]
- 234.Al-haidari A.A., Syk I., Jirström K., Thorlacius H. CCR4 mediates CCL17 (TARC)-induced migration of human colon cancer cells via RhoA/Rho-kinase signaling. Int. J. Colorectal Dis. 2013;28:1479–1487. doi: 10.1007/s00384-013-1712-y. [DOI] [PubMed] [Google Scholar]
- 235.Cao L., Hu X., Zhang J., Huang G., Zhang Y. The role of the CCL22-CCR4 axis in the metastasis of gastric cancer cells into omental milky spots. J. Transl. Med. 2014;12:267. doi: 10.1186/s12967-014-0267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Nakamura E.S., Koizumi K., Kobayashi M., Saitoh Y., Arita Y., Nakayama T., Sakurai H., Yoshie O., Saiki I. RANKL-induced CCL22/macrophage-derived chemokine produced from osteoclasts potentially promotes the bone metastasis of lung cancer expressing its receptor CCR4. Clin. Exp. Metastasis. 2006;23:9–18. doi: 10.1007/s10585-006-9006-1. [DOI] [PubMed] [Google Scholar]
- 237.Klein A., Sagi-Assif O., Meshel T., Telerman A., Izraely S., Ben-Menachem S., Bayry J., Marzese D.M., Ohe S., Hoon D.S.B., et al. CCR4 is a determinant of melanoma brain metastasis. Oncotarget. 2017;8:31079–31091. doi: 10.18632/oncotarget.16076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Olkhanud P.B., Baatar D., Bodogai M., Hakim F., Gress R., Anderson R.L., Deng J., Xu M., Briest S., Biragyn A. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Fagerberg L., Hallström B.M., Oksvold P., Kampf C., Djureinovic D., Odeberg J., Habuka M., Tahmasebpoor S., Danielsson A., Edlund K., et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteomics. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Arneth B. Tumor Microenvironment. Medicina (Kaunas) 2019;56:15. doi: 10.3390/medicina56010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lapteva N., Aldrich M., Weksberg D., Rollins L., Goltsova T., Chen S.Y., Huang X.F. Targeting the intratumoral dendritic cells by the oncolytic adenoviral vaccine expressing RANTES elicits potent antitumor immunity. J. Immunother. 2009;32:145–156. doi: 10.1097/CJI.0b013e318193d31e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Igoucheva O., Grazzini M., Pidich A., Kemp D.M., Larijani M., Farber M., Lorton J., Rodeck U., Alexeev V. Immunotargeting and eradication of orthotopic melanoma using a chemokine-enhanced DNA vaccine. Gene Ther. 2013;20:939–948. doi: 10.1038/gt.2013.17. [DOI] [PubMed] [Google Scholar]
- 243.Li J., Liu H., Li L., Wu H., Wang C., Yan Z., Wang Y., Su C., Jin H., Zhou F., et al. The combination of an oxygen-dependent degradation domain-regulated adenovirus expressing the chemokine RANTES/CCL5 and NK-92 cells exerts enhanced antitumor activity in hepatocellular carcinoma. Oncol. Rep. 2013;29:895–902. doi: 10.3892/or.2012.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lai J.Z., Zhu Y.Y., Ruan M., Chen L., Zhang Q.Y. Local Irradiation Sensitized Tumors to Adoptive T Cell Therapy via Enhancing the Cross-Priming, Homing, and Cytotoxicity of Antigen-Specific CD8 T Cells. Front. Immunol. 2019;10:2857. doi: 10.3389/fimmu.2019.02857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Guiducci C., Vicari A.P., Sangaletti S., Trinchieri G., Colombo M.P. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 246.Mauri G., Chiodoni C., Parenza M., Arioli I., Tripodo C., Colombo M.P. Ultrasound-guided intra-tumor injection of combined immunotherapy cures mice from orthotopic prostate cancer. Cancer Immunol. Immunother. 2013;62:1811–1819. doi: 10.1007/s00262-013-1486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kitamura T., Fujishita T., Loetscher P., Revesz L., Hashida H., Kizaka-Kondoh S., Aoki M., Taketo M.M. Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model. Proc. Natl. Acad. Sci. USA. 2010;107:13063–13068. doi: 10.1073/pnas.1002372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Fang W.B., Yao M., Brummer G., Acevedo D., Alhakamy N., Berkland C., Cheng N. Targeted gene silencing of CCL2 inhibits triple negative breast cancer progression by blocking cancer stem cell renewal and M2 macrophage recruitment. Oncotarget. 2016;7:49349–49367. doi: 10.18632/oncotarget.9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Shen S., Zhang Y., Chen K.G., Luo Y.L., Wang J. Cationic Polymeric Nanoparticle Delivering CCR2 siRNA to Inflammatory Monocytes for Tumor Microenvironment Modification and Cancer Therapy. Mol. Pharm. 2018;15:3642–3653. doi: 10.1021/acs.molpharmaceut.7b00997. [DOI] [PubMed] [Google Scholar]
- 250.Zhu X., Fujita M., Snyder L.A., Okada H. Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. J. Neurooncol. 2011;104:83–92. doi: 10.1007/s11060-010-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Cho H.R., Kumari N., Thi V.H., Kim H., Park C.K., Choi S.H. Increased Antiangiogenic Effect by Blocking CCL2-dependent Macrophages in a Rodent Glioblastoma Model: Correlation Study with Dynamic Susceptibility Contrast Perfusion MRI. Sci. Rep. 2019;9:11085. doi: 10.1038/s41598-019-47438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Flores-Toro J.A., Luo D., Gopinath A., Sarkisian M.R., Campbell J.J., Charo I.F., Singh R., Schall T.J., Datta M., Jain R.K., et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc. Natl. Acad. Sci. USA. 2020;117:1129–1138. doi: 10.1073/pnas.1910856117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Teng K.Y., Han J., Zhang X., Hsu S.H., He S., Wani N.A., Barajas J.M., Snyder L.A., Frankel W.L., Caligiuri M.A., et al. Blocking the CCL2-CCR2 Axis Using CCL2-Neutralizing Antibody Is an Effective Therapy for Hepatocellular Cancer in a Mouse Model. Mol. Cancer Ther. 2017;16:312–322. doi: 10.1158/1535-7163.MCT-16-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Trac N., Chen L.Y., Zhang A., Liao C.P., Poon C., Wang J., Ando Y., Joo J., Garri C., Shen K., et al. CCR2-targeted micelles for anti-cancer peptide delivery and immune stimulation. J. Control. Release. 2020 doi: 10.1016/j.jconrel.2020.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sandhu S.K., Papadopoulos K., Fong P.C., Patnaik A., Messiou C., Olmos D., Wang G., Tromp B.J., Puchalski T.A., Balkwill F., et al. A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemother. Pharmacol. 2013;71:1041–1050. doi: 10.1007/s00280-013-2099-8. [DOI] [PubMed] [Google Scholar]
- 256.Moisan F., Francisco E.B., Brozovic A., Duran G.E., Wang Y.C., Chaturvedi S., Seetharam S., Snyder L.A., Doshi P., Sikic B.I. Enhancement of paclitaxel and carboplatin therapies by CCL2 blockade in ovarian cancers. Mol. Oncol. 2014;8:1231–1239. doi: 10.1016/j.molonc.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Qian D.Z., Rademacher B.L., Pittsenbarger J., Huang C.Y., Myrthue A., Higano C.S., Garzotto M., Nelson P.S., Beer T.M. CCL2 is induced by chemotherapy and protects prostate cancer cells from docetaxel-induced cytotoxicity. Prostate. 2010;70:433–442. doi: 10.1002/pros.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Connolly K.A., Belt B.A., Figueroa N.M., Murthy A., Patel A., Kim M., Lord E.M., Linehan D.C., Gerber S.A. Increasing the efficacy of radiotherapy by modulating the CCR2/CCR5 chemokine axes. Oncotarget. 2016;7:86522–86535. doi: 10.18632/oncotarget.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kalbasi A., Komar C., Tooker G.M., Liu M., Lee J.W., Gladney W.L., Ben-Josef E., Beatty G.L. Tumor-Derived CCL2 Mediates Resistance to Radiotherapy in Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2017;23:137–148. doi: 10.1158/1078-0432.CCR-16-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Guo S.S., Liu R., Wen Y.F., Liu L.T., Yuan L., Li Y.X., Li Y., Hao W.W., Peng J.Y., Chen D.N., et al. Endogenous production of C-C motif chemokine ligand 2 by nasopharyngeal carcinoma cells drives radioresistance-associated metastasis. Cancer Lett. 2020;468:27–40. doi: 10.1016/j.canlet.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 261.Yan C., Luo L., Urata Y., Goto S., Li T.S. Nicaraven reduces cancer metastasis to irradiated lungs by decreasing CCL8 and macrophage recruitment. Cancer Lett. 2018;418:204–210. doi: 10.1016/j.canlet.2018.01.037. [DOI] [PubMed] [Google Scholar]
- 262.Belarbi K., Jopson T., Arellano C., Fike J.R., Rosi S. CCR2 deficiency prevents neuronal dysfunction and cognitive impairments induced by cranial irradiation. Cancer Res. 2013;73:1201–1210. doi: 10.1158/0008-5472.CAN-12-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lee S.W., Haditsch U., Cord B.J., Guzman R., Kim S.J., Boettcher C., Priller J., Ormerod B.K., Palmer T.D. Absence of CCL2 is sufficient to restore hippocampal neurogenesis following cranial irradiation. Brain Behav. Immun. 2013;30:33–44. doi: 10.1016/j.bbi.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wiesemann A., Ketteler J., Slama A., Wirsdörfer F., Hager T., Röck K., Engel D.R., Fischer J.W., Aigner C., Jendrossek V., et al. Inhibition of Radiation-Induced Ccl2 Signaling Protects Lungs from Vascular Dysfunction and Endothelial Cell Loss. Antioxid. Redox Signal. 2019;30:213–231. doi: 10.1089/ars.2017.7458. [DOI] [PubMed] [Google Scholar]
- 265.Fridlender Z.G., Buchlis G., Kapoor V., Cheng G., Sun J., Singhal S., Crisanti M.C., Wang L.C., Heitjan D., Snyder L.A., et al. CCL2 blockade augments cancer immunotherapy. Cancer Res. 2010;70:109–118. doi: 10.1158/0008-5472.CAN-09-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Fridlender Z.G., Kapoor V., Buchlis G., Cheng G., Sun J., Wang L.C., Singhal S., Snyder L.A., Albelda S.M. Monocyte chemoattractant protein-1 blockade inhibits lung cancer tumor growth by altering macrophage phenotype and activating CD8+ cells. Am. J. Respir. Cell Mol. Biol. 2011;44:230–237. doi: 10.1165/rcmb.2010-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Cheadle E.J., Riyad K., Subar D., Rothwell D.G., Ashton G., Batha H., Sherlock D.J., Hawkins R.E., Gilham D.E. Eotaxin-2 and colorectal cancer: A potential target for immune therapy. Clin. Cancer Res. 2007;13:5719–5728. doi: 10.1158/1078-0432.CCR-07-1145. [DOI] [PubMed] [Google Scholar]
- 268.Maeda S., Murakami K., Inoue A., Yonezawa T., Matsuki N. CCR4 Blockade Depletes Regulatory T Cells and Prolongs Survival in a Canine Model of Bladder Cancer. Cancer Immunol. Res. 2019;7:1175–1187. doi: 10.1158/2326-6066.CIR-18-0751. [DOI] [PubMed] [Google Scholar]
- 269.Berlato C., Khan M.N., Schioppa T., Thompson R., Maniati E., Montfort A., Jangani M., Canosa M., Kulbe H., Hagemann U.B., et al. A CCR4 antagonist reverses the tumor-promoting microenvironment of renal cancer. J. Clin. Invest. 2017;127:801–813. doi: 10.1172/JCI82976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rapp M., Grassmann S., Chaloupka M., Layritz P., Kruger S., Ormanns S., Rataj F., Janssen K.P., Endres S., Anz D., et al. C-C chemokine receptor type-4 transduction of T cells enhances interaction with dendritic cells, tumor infiltration and therapeutic efficacy of adoptive T cell transfer. Oncoimmunology. 2015;5:e1105428. doi: 10.1080/2162402X.2015.1105428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Guo J., Wang B., Zhang M., Chen T., Yu Y., Regulier E., Homann H.E., Qin Z., Ju D.W., Cao X. Macrophage-derived chemokine gene transfer results in tumor regression in murine lung carcinoma model through efficient induction of antitumor immunity. Gene Ther. 2002;9:793–803. doi: 10.1038/sj.gt.3301688. [DOI] [PubMed] [Google Scholar]
- 272.Okada N., Gao J.Q., Sasaki A., Niwa M., Okada Y., Nakayama T., Yoshie O., Mizuguchi H., Hayakawa T., Fujita T., et al. Anti-tumor activity of chemokine is affected by both kinds of tumors and the activation state of the host’s immune system: Implications for chemokine-based cancer immunotherapy. Biochem. Biophys. Res. Commun. 2004;317:68–76. doi: 10.1016/j.bbrc.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 273.Cohen E.E.W., Pishvaian M.J., Shepard D.R., Wang D., Weiss J., Johnson M.L., Chung C.H., Chen Y., Huang B., Davis C.B., et al. A phase Ib study of utomilumab (PF-05082566) in combination with mogamulizumab in patients with advanced solid tumors. J. Immunother. Cancer. 2019;7:342. doi: 10.1186/s40425-019-0815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Doi T., Muro K., Ishii H., Kato T., Tsushima T., Takenoyama M., Oizumi S., Gemmoto K., Suna H., Enokitani K., et al. A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors. Clin. Cancer Res. 2019;25:6614–6622. doi: 10.1158/1078-0432.CCR-19-1090. [DOI] [PubMed] [Google Scholar]
- 275.Yamamoto K., Utsunomiya A., Tobinai K., Tsukasaki K., Uike N., Uozumi K., Yamaguchi K., Yamada Y., Hanada S., Tamura K., et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J. Clin. Oncol. 2010;28:1591–1598. doi: 10.1200/JCO.2009.25.3575. [DOI] [PubMed] [Google Scholar]
- 276.Ishida T., Utsunomiya A., Jo T., Yamamoto K., Kato K., Yoshida S., Takemoto S., Suzushima H., Kobayashi Y., Imaizumi Y., et al. Mogamulizumab for relapsed adult T-cell leukemia-lymphoma: Updated follow-up analysis of phase I and II studies. Cancer Sci. 2017;108:2022–2029. doi: 10.1111/cas.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ogura M., Ishida T., Hatake K., Taniwaki M., Ando K., Tobinai K., Fujimoto K., Yamamoto K., Miyamoto T., Uike N., et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J. Clin. Oncol. 2014;32:1157–1163. doi: 10.1200/JCO.2013.52.0924. [DOI] [PubMed] [Google Scholar]
- 278.Duvic M., Pinter-Brown L.C., Foss F.M., Sokol L., Jorgensen J.L., Challagundla P., Dwyer K.M., Zhang X., Kurman M.R., Ballerini R., et al. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood. 2015;125:1883–1889. doi: 10.1182/blood-2014-09-600924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kanazawa T., Hiramatsu Y., Iwata S., Siddiquey M., Sato Y., Suzuki M., Ito Y., Goshima F., Murata T., Kimura H. Anti-CCR4 monoclonal antibody mogamulizumab for the treatment of EBV-associated T- and NK-cell lymphoproliferative diseases. Clin. Cancer Res. 2014;20:5075–5084. doi: 10.1158/1078-0432.CCR-14-0580. [DOI] [PubMed] [Google Scholar]