Synopsis:
Worldwide, over four million deaths annually are attributed to indoor air pollution. This largely preventable exposure represents a key target for reducing morbidity and mortality worldwide. Across settings, significant respiratory health effects are observed, ranging from attenuated lung growth and development in childhood to accelerated lung function decline and chronic obstructive pulmonary disease later in life. Numerous factors influence personal exposure to household air pollutants, including household characteristics, combustion of solid fuels, cooking practices, and allergens from household pests. This review outlines important sources of indoor air pollution, their respiratory health effects, and strategies to reduce household pollution and improve lung health across the globe.
Keywords: Asthma, Chronic Obstructive Pulmonary Disease, Indoor Air Pollution, Household Air Pollution, Respiratory Health Effects, Respiratory Tract Infections, Lung Function, Lung Development
Introduction: Why Indoor Air Pollution is important
The World Health Organization estimates that Household air pollution (HAP) accounts for an estimated 4.3 million premature deaths annually and 110 million disability-adjusted life years lost1. This largely preventable exposure has been listed by the Comparative Risk Assessment for the 2010 Global Burden of Diseases the third leading risk factor for morbidity and mortality worldwide, representing about 4.5% of global burden of disease2. Even in industrialized settings the home represents a critical source of pollutant exposure, with individuals spending approximately 90% of their time indoors, the majority of that being in the home3–5. While a significant source of morbidity related to household air pollution is from cardiovascular disease, HAP has a wide range of respiratory sequela across the lifespan, adversely affecting lung development early in life to potentially increasing the risk of chronic obstructive pulmonary disease (COPD) in adulthood. The impact on respiratory health has been observed across a variety of settings and disease processes, in both low-middle income and high-income countries. Today, organizations including the WHO, United Nations, American Thoracic Society, and European Respiratory Society have begun to invest in developing strategies to reduce the global respiratory health effects of HAP6,7. This article outlines sources of indoor air pollution, the respiratory effects of indoor air pollution in low-middle income and high-income countries, as well as strategies to reduce pollutant exposure in both settings (Figure 1).
Fig 1.
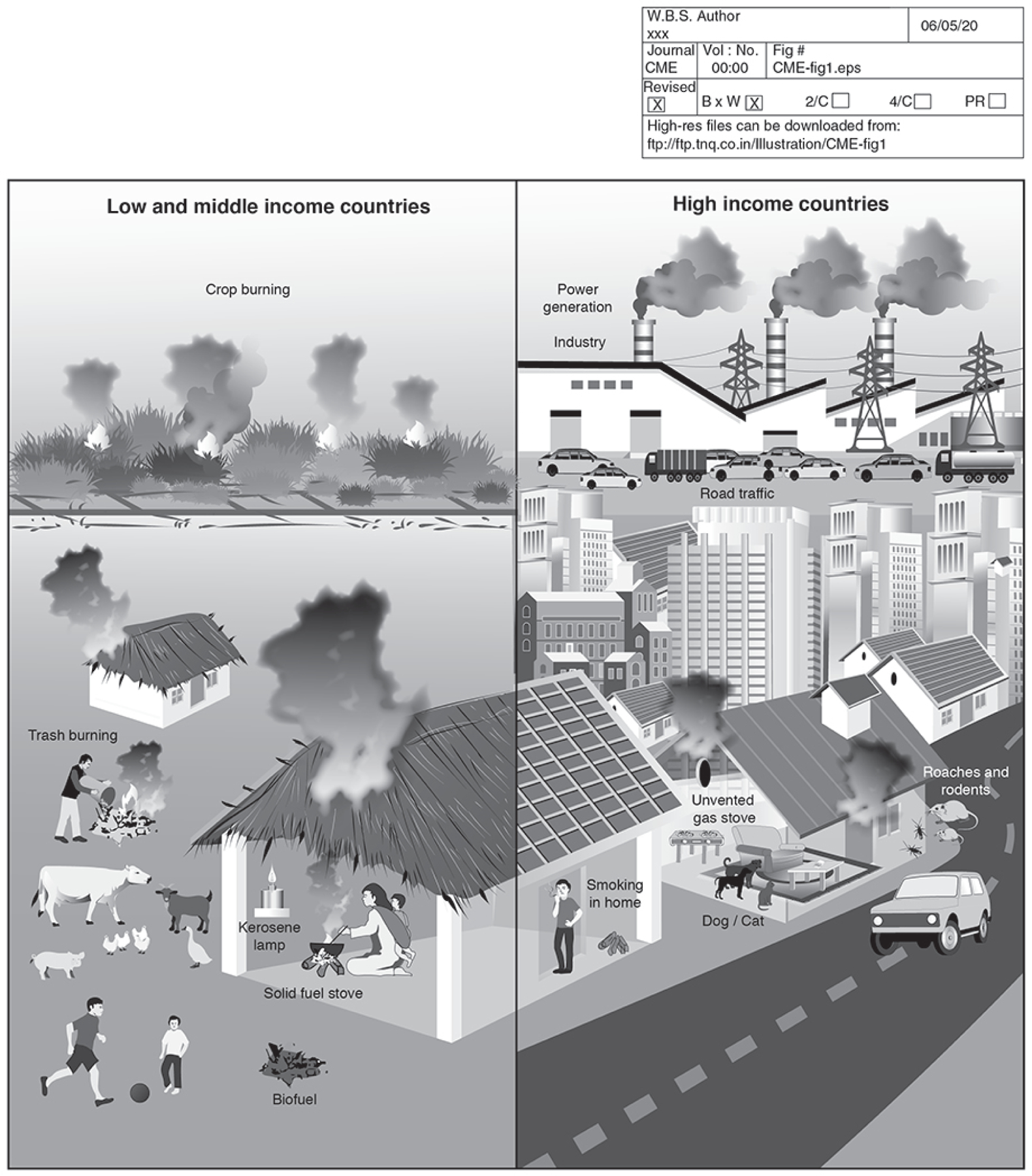
Indoor air pollution: the respiratory effects in low-middle income and high-income countries.
Indoor air pollution in Low-Middle Income Countries
In Low-Middle Income Countries (LMICS), nearly 3 billion people rely on biomass fuels for cooking and heating daily8. Biomass fuels, often referred to as solid fuels, include wood, dung, agricultural crop waste, and coal. Biomass is the main domestic energy source for ~40% of all households and ~90% of rural households in LMICs. Solid fuels are often burned in inefficient and poorly vented combustion devices (i.e., open fires, traditional stoves). Multiple respiratory health outcomes have been associated with HAP in LMICs, including preterm birth; low birth weight and attenuated lung function; childhood respiratory infection; as well as increased risk for development of asthma, chronic obstructive pulmonary disease (COPD), lung cancer and exacerbations of existing diseases9,10.
Indoor air pollution in High Income Countries
While respiratory health effects of pollution are most prominent in low-middle income countries, significant mortality has been observed at lower pollutant concentrations typical of high-income countries, including the US and Canada. In 2016, the WHO estimated that nearly 400,000 deaths in high-income countries were attributable to HAP, due to ongoing use of solid fuels, as well as other sources of pollution relevant to high-income countries. Currently 6.5 million people in the US still rely on solid fuels for heating, particularly those living in rural areas. Moreover, urbanization and the expanding built environment predispose individuals in high-income settings to other pollutants indoors, including indoor particulate matter (PM), environmental tobacco smoke, gases such as nitrogen dioxide (NO2) from cooking and heating, allergens from indoor pests, toxic cleaning chemicals, and molds related to increased indoor humidity and dampness. These important sources of Indoor air pollution are outlined in the following section.
Sources of Indoor Air Pollution
Indoor air includes pollutants that penetrate from the outdoors, as well as sources that are unique to the indoor environment. Thus, indoor pollution differs from outdoor pollution in source, composition, and concentration, and as a result the effects of indoor air pollution cannot be easily extrapolated from studies of outdoor air pollution11. The burning of solid fuels represents a significant source of household pollution across the globe, while numerous household activities more common in urban environments have also been associated with the generation of pollutants12,13. Environmental tobacco smoke (ETS) from primary and secondhand smoke remains an important source of household air pollution. In this review we primarily discuss non-tobacco sources of pollution that are often overlooked. However, initiatives to reduce smoking and environment tobacco smoke remain imperative and a continued high priority. We will focus on household behaviors and practices associated with increased production of pollutants (solid fuel combustion, cooking and heating practice, cleaning, and indoor smoking) in both LMICs and High-Income countries. Other sources of interest include general housing characteristics which directly influence (dampness, humidity, pests and allergens) and modify exposures (ventilation).
Household Behaviors and Practices: Smoking, Solid Fuel Combustion, Cooking & Heating, Cleaning,
Smoking.
Tobacco smoke is a major source of indoor particulate matter, accounting for 50–90% of indoor PM concentrations in high income countries14,15. Indoor tobacco smoke is a critical driver of environmental health disparities with as many as half of all children and up to 70% of African American children exposed to second hand smoke in the United States16.
Solid Fuel Combustion.
A significant body of evidence related to HAP and respiratory health has been generated from studies concerning the combustion of solid fuels, used for cooking and heating. The combustion products include gases (i.e NO2 and Carbon Monoxide (CO)) and particulate matter, including particles with median diameter <10um (PM10) and those with median diameter <2.5um (PM2.5). Additionally, formaldehyde, airborne endotoxins, and a number of toxic organic compounds (eg, benzene; 1,3 butadiene; benzo[a] pyrene, and other polycyclic aromatic hydrocarbons) are present in biomass smoke. While household pollution from the burning of biomass and solid fuels has been thought to mostly affect individuals from low-middle income countries, data from the US Census Bureau’s American Community Survey has indicated that 6.5 million people in the US live in homes heated primarily by wood or coal17. The burden of household pollution from solid fuels in the US has been shown to disproportionately affect those living in poor communities, with 53–65% of homes that primarily heat with wood or coal also facing household PM2.5 concentrations that exceed the WHO 24-hour particulate matter guidelines17–20.
Cooking and Heating.
Even in homes that do not use solid fuels, other common heating and cooking practices can increase household pollutant concentrations. Combustion of fuels used for cooking and heating can produce NO2, Volatile Organic Compounds (VOCs), Sulfur Dioxide (SO2), CO2, CO, and particulate matter. Exposure concentrations are driven by factors that include ventilation, duration of use of the cooking or heating equipment, and individual proximity to the source of pollutants. NO2 is of particular interest for homes that use gas stoves for cooking13. One study indicated that each hour of gas stove or furnace use was associated with an 18 ppb increase in 24-hour NO2 concentrations21. NO2 levels are even greater in poorly ventilated homes using unvented gas stoves. In addition to NO2, other studies have demonstrated that the frequency of stove use is also associated with significant increases in household PM2.5 concentrations12.
Cleaning represents a common household activity that can lead to the mobilization of particles and allergens. One study demonstrated indoor sweeping, which resuspends settled dust, was associated with increased household PM2.5 and PM1012. In addition, cleaning products and pesticides have direct toxic health effects that are discussed in more detail in a separate chapter of this issue.
Housing Characteristics: Ventilation, Humidity, Dampness, and Allergens from Pests
Ventilation.
One global factor that impacts household pollution concentrations is ventilation, as diminished indoor-outdoor air exchange rates can result in the accumulation of indoor pollutants. As insulation has improved, household air exchange rates have diminished22. The relationship between sources of pollution, air exchange rates, and health outcomes is an important consideration in understanding the respiratory health effects of indoor air pollution. In LMICs, poor ventilation, particularly around biomass-stoves, results in increased levels of indoor air pollution23,24. Ventilation in LMIC settings, primarily in tropical and subtropical regions of the world, often includes direct openings to the outdoors, and is typically greater compared to high income areas25. Households which utilize enclosed metal heating or cooking stoves with chimneys have significantly lower indoor pollutant concentrations than those that use open fires26,27. A number of studies have additionally demonstrated associations between ventilation and respiratory outcomes. Qian et al. demonstrated higher household ventilation was associated with lower reporting of persistent cough, phlegm, bronchitis, and wheeze among school children in four Chinese cities24. Household ventilation, as well as more efficient ventilated stoves has been outlined by the WHO as an important health intervention in LMICs.
Humidity and dampness are also important factors that influence indoor air quality. A number of daily activities including showering, cooking, and humidifier use, can affect indoor moisture and humidity. Many homes additionally experience leaks and some degree of water damage over their lifetime. Molds, bacteria, insects, and mites thrive in humid and damp environments28. These pathogens in particular have been linked to a number of respiratory disorders including asthma, chronic rhino sinusitis, and hypersensitivity pneumonitis, along with increased respiratory symptoms (cough, wheeze, and exacerbations of underlying respiratory disease)29.
Allergens from Household Pests:
Exposure to household allergens from animals and pests represent significant but modifiable contributors to respiratory disease, particularly asthma and allergic rhinitis. In urban environments allergens from house dust mites, cockroaches, and mouse infestations are important to consider. House dust mites represent a significant source of allergens, with 13 different species found in household dust4. Allergens arise from mite bodies and feces. Dust mites are most commonly found on furniture, carpet, and bedding. House dust mite growth further increases in high humidity and temperature environments30. Similar to dust mites, allergens from cockroaches are increased in high temperature environments4. Mouse allergen can be found in small particle sizes between 0.5 and 10 µm and can remain airborne once resuspended from settled dust31. Mouse allergen has been detected in high concentration in settled dust from homes of children with asthma living in urban areas, as well as in air samples, where as many as a quarter of children had airborne mouse concentrations similar to those in mouse facilities32. Pest allergen, including cockroach and mouse represent important drivers of childhood asthma in urban environments.33,34 Other sources to consider, not included in this review, include allergens from pets and domestic animals that can also contribute to morbidity.
Other important housing factors to consider include residential crowding, along with proximity to roadways and high traffic corridors, which can lead to increases in indoor particulate matter and NO2 due to penetration from the outdoor environment.
Consequence of Indoor Air Pollution
The respiratory consequences of household air pollution are wide ranging, affecting lung development in childhood, incidence of lower respiratory tract infection, and both disease development and morbidity for COPD and asthma. The known impacts of household air pollution are described for each of these processes in both low-middle income countries and high-income countries.
Mechanisms
There have been a number of mechanisms proposed by which indoor air pollution results in pulmonary disease and alters host immune responses, leading to chronic respiratory conditions. Exposure to household air pollution results in pro-inflammatory states with associated increases in neutrophilic inflammation, proteolytic activity of matrix metalloproteinases, oxidative stress and apoptosis35–37 (Table 1). Loss of lung function from prolonged exposure to air pollution has been proposed as a leading cause of COPD globally38. Additionally, recurrent respiratory infections have been associated with increased levels of household air pollution, likely due to underlying immune dysregulation. Household air pollution has been demonstrated to impair macrophage phagocytosis and surface adherence, reduce bacterial and mucociliary clearance, and disrupt the alveolar-capillary barrier in the lungs39–41. A number of effects also relate to alterations in the airway microbiome as well as immune dysregulation and host response to commensals6. In particular Streptococcus Pneumoniae carriage, the most common cause of bacterial pneumonia, is thought to be increased in individuals with high HAP exposure. One study demonstrated that particulate matter exposure from woodsmoke decreased macrophage phagocytosis of S. Pneumonia and increased the duration and density of pneumococcal carriage42. Additionally immune responses to respiratory viruses may be impaired, with prior literature demonstrating that increased PM10 exposure can blunt alveolar macrophage responses to Respiratory syncytial virus (RSV)43. As a result, recent literature has focused on the impacts of HAP on both viral and bacterial ALRI. Air pollution additionally may result in genetic and epigenetic changes which alter inflammatory and oxidative stress responses even after the exposure is removed44.
Table 1.
Mechanism by Which Household Pollution Can Impact Respiratory Health
| • Neutrophilic Inflammation |
| • Reduced Bacterial Clearance and Alterations in Microbiome |
| • Increased Matrix Metalloproteinase Activity and Gene Expression |
| • Greater Oxidative Stress |
| • Increased Apoptosis |
| • Pulmonary Surfactant Deactivation |
| • Reduced Mucociliary Clearance |
| • Epigenetic Changes - DNA Damage and Methylation |
| • Disruption of Lung Endothelial Cell Barrier |
Lung Development in Children
Lung development begins in utero and by the fourth week of development nascent bronchi form. By gestation, only a small fraction of primary alveoli has developed, a process, which increases linearly up to the age of 18, with 85–90% of alveoli formed within the first six months of life. Fetal and adolescent lung development is a critical period, which predicts future lung function. Low lung function in infancy and childhood is associated with respiratory health sequela including, wheeze, airway hyper reactivity, asthma and COPD in adulthood45,46. Though a comprehensive review of second-hand smoke (SHS) exposure is beyond the scope of this paper, there is a large body of evidence concerning SHS as a contributor to indoor air pollution and lung development. SHS has been identified as a risk factor for low lung function in several studies47,48. A study of 2,500 children followed over six decades demonstrated that parental smoking during childhood is a determinant of low lung function trajectories, low lung function in adulthood and eventual development of COPD47,49
Studies related to ambient air pollution have demonstrated associations between prenatal exposures, lower lung function at birth and the likelihood of respiratory disease in adolescence and adulthood. However, few studies related to in-utero exposures have evaluated HAP or assessed personal exposure monitoring50,51. In a LMIC setting, Lee and colleagues examined the association between maternal carbon monoxide exposure (CO) from cookstove sources (traditional three stone fires with wood as the dominant fuel) and infant lung function at 30 days, and found a dose response relationship between elevated maternal CO exposure and reduced peak tidal expiratory flow to expiratory time and higher minute ventilation 52. Furthermore, the authors found this increase in minute ventilation was associated with increased risk for physician-assessed pneumonia and severe pneumonia in the first year of life52. Further works needs to be done to confirm the contribution of early exposure to household pollutants, outside of secondhand smoke, to early lung development.
Respiratory Tract Infections
A number of studies have demonstrated a strong association between household air pollution and the risk of childhood acute lower respiratory tract infection (ALRI) globally. It has been hypothesized that the contribution of HAP to lung development, along with the development of COPD and chronic bronchitis, is related to repeated infections over time.
LMICs.
In LMIC settings, HAP results in an estimated half a million deaths among children under 5 annually, specifically from pneumonia. Dherani and colleagues demonstrated exposure to HAP from solid fuel use during childhood increased the risk of ALRIs by 78% (pooled odds ratio = 1.78 (95% CI 1.45 to 2.18) in a meta-analysis of 24 studies53. As a result of the high prevalence and associated mortality, pediatric pneumonia has been a primary clinical outcome for interventions aiming to mitigate pollutions exposure with mixed results. (see Cookstove Interventions). While a number of studies have examined the association between HAP and pediatric pneumonia, the relationship with ALRI in adults is less clear. A recent systematic review limited to adults found mixed results, though some evidence of an increased risk of adult ALRI from exposure to HAP.54
High-Income Countries.
Within high-income countries more research exists on the association between outdoor pollution and healthcare encounters for ALRI among children and infants. A study by Horne and Colleague explored the relationship between rises in outdoor air pollution in Utah between 1999 and 2016 and odds of healthcare utilization related to lower respiratory tract infections55. The study demonstrated that a 10ug/m3 increase in ambient PM2.5 was associated with increased healthcare utilization for ALRIs (OR 1.15; 95% CI 1.12–1.19). The majority of individuals included in this study were again young children (0–2 years), though effects were still observed to the age of 18. These results have been replicated in other areas of the United States, including among adults in New York State, where increases in outdoor PM2.5 were associated with higher rates of Influenza and culture-negative pneumonia56. In contrast to the literature on ambient pollution, studies surrounding the association between household pollution and ALRI in high-income countries are much less robust, limited primarily to survey data with inconsistent findings54. The association between HAP and ALRI in high income countries still requires further investigation.
Asthma
Prior meta-analyses conducted across continents have demonstrated that early secondhand smoke exposure is associated with the childhood asthma development and subsequent increased risk for adult asthma57. As a result, many have hypothesized that particulate from other sources of HAP and airway irritants may lead to asthma development and increased morbidity for children living with asthma.
LMICS.
Studies in LMICs to date have been inconsistent regarding HAP from solid fuel smoke and asthma among children and adults. Po and colleagues did not find a statistically significant association (children: OR = 0.50, 95% CI 0.12–1.98; adults: OR = 1.34, 95% CI 0.93–1.93) among 25 studies58,59. Chronic exposure to HAP is associated with increased levels of airway hyperresponsivness60. Additionally, HAP exposure can result in airway remodeling and inflammation with similar patterns of small airway disease seen in asthma. HAP from biomass contains known endotoxins and organic compounds, which increase the risk for asthma61. Air pollution from solid fuel combustion and existing allergens can become amalgamated resulting in increased serum IgE, eosinophils and neutrophils62. Nonetheless, the lack of consistent findings related to the association between HAP and asthma in LMICs require additional investigation.
High Income Settings.
Within high-income countries, studies of solid fuels and asthma development are limited to rural settings, where heating with wood and coal is relatively more common. One survey of adults in Southeastern Kentucky, conducted as part of the Burden of Lung Disease (BOLD) study, described an association between cooking with wood or coal and pediatric asthma (OR 2.3, 95% CI: 1.1–5.0). The study demonstrated no effect of heating with solid fuels on asthma development (OR 0.8, 95% CI: 0.4–1.8)63. Research from Ward and Noonan investigated the association between woodstove use for heating and asthma symptoms among children in Libby Montana, where woodstoves were the primary source of home heating in 33% of households. Their findings suggested that woodstove use was associated with greater odds of wheezing (OR 1.74, 95% CI 0.55–5.56) though findings were not statistically significant64.
There is more consistent evidence to suggest household pollution may increase respiratory morbidity among children with asthma in both urban and rural environments. Among populations with asthma, elevated levels of PM2.5 and PM10 are associated with higher rates of asthma attacks, medication use, and emergency department visits18,65,66. One study of household pollution in an urban inner-city environment, where the use of solid fuels is less common, demonstrated that increases in indoor PM2.5 were associated with greater asthma morbidity for children with both atopic and non-atopic forms of asthma67. In this study of 133 children ages 2–6, with repeated measured of household pollutants (PM2.5, PMcoarse), higher PMcoarse and PM2.5 concentrations were associated with greater risk for wheezing, rescue inhaler use, and nocturnal symptoms.66
In addition to PM, higher levels of elevated household NO2 may lead to greater asthma morbidity. Higher NO2 exposure has been tied to lower peak flow measurements in a nationwide analysis of urban asthmatic children in the National Cooperative Inner-City Asthma Study (NCICAS)68. A longitudinal study of asthmatic children in Baltimore demonstrated that each 20-ppb increase in NO2 exposure over a 72-hour period was associated with an increase in the number of days with limited speech (incidence rate ratio [IRR] = 1.15; [95% CI, 1.05–1.25]), cough (IRR = 1.10; 95% CI, 1.02–1.18), and nocturnal symptoms (IRR = 1.09; 95% CI, 1.02–1.16), after adjustment for potential confounders13. Paulin and colleagues demonstrated that daily changes in household NO2 exposure were associated with gas stove/oven use and led to worsened asthma symptoms and nighttime inhaler use among children with asthma21. Studies of school classrooms have also demonstrated that indoor NO2 is associated with increased airflow obstruction among children with asthma69.
There are still certain populations who are known to be more susceptible to the health effects of household pollution. Being overweight or obese may be risk factors that increases susceptibility to indoor PM2.5 and NO2 among children with asthma70. Studies have indicated that being overweight has been associated with a oxidative stress and inflammation, which may affect the ability to defend against oxidative and pro-inflammatory exposures, such as that from particulate and gaseous pollutants71,72. To investigate the hypothesis that obesity increases susceptibility to air pollution health effects, Lu and colleagues examined the effects of household PM2.5 and NO2 exposure 70. They observed that compared to normal weight children with asthma, overweight children experienced greater symptoms (cough, wheeze, and chest tightness) and rescue medication use, when faced with similar increases in household PM2.5 and NO2. Wu and colleagues similarly observed that overweight and obesity increased susceptibility to household secondhand smoke exposure among children with asthma73. These findings suggest that diet and weight loss, in addition to pollution reduction, may be important targets in efforts to reduce the respiratory health effects of indoor air pollution among children with asthma. Investigations of differential responses to air pollution by weight may provide insights as to pathways by which air pollutants elicit respiratory health effects. The majority of the studies of household pollution and asthma morbidity are conducted among children, and further research is needed to demonstrate whether these results can be extrapolated to adults.
Multiple studies have proposed a synergistic relationship between allergen and pollutant exposure but have focused on outdoor air pollution exposures. A prospective cohort study of children followed from infancy to 4 years demonstrated that exposure to traffic-related diesel exhaust was associated with increased risk of aeroallergen sensitization74. Controlled exposure studies in atopic volunteers have demonstrated that diesel exhaust increases markers of allergic and non-allergic inflammation and that allergen and diesel exhaust have exposure specific responses75,76. Previous studies have demonstrated that increased ambient PM2.5, NO2, and ozone exposure are associated with a significant increases in risk for asthma and allergic rhinitis among children with allergic sensitization77,78. Several studies of children living in urban settings have demonstrated that pest allergen, including mouse and cockroach, are associated with increased asthma morbidity34,79,80. Children are often co-exposed to high levels of indoor PM2.5, NO2, and dust allergens81. The combined effects of indoor air pollution and allergen exposure are not well understood and can provide insight as to priority targets for multi-faceted intervention studies.
COPD
LMICs.
There have been a number of studies that have examined the association between biomass and COPD in LMICs, with heterogeneity in exposure history and outcomes60,82. A meta-analysis of 23 papers by Kurmi and colleagues found an increased odds of COPD (OR = 2.80, 95% CI 1.85 – 4.0) and chronic bronchitis (OR =2.32, 95% CI 1.92 – 2.80)38. Similarly, Hu and colleagues demonstrated a similar association (OR = 2.44, 95% CI, 1.9–3.33) between HAP and COPD, relative to those not exposed to biomass smoke. While the vast majority of studies related to COPD pertain to tobacco exposure in high-income settings, less is known about how HAP can lead to COPD development in LMICs. Although evidence of direct causal relationship between HAP and COPD is not definitive, it is likely that HAP exposure over the course of the lifespan has direct and indirect effects (recurrent ALRI, low socioeconomic status) on lung function that predispose to COPD83.
Individuals with biomass-related COPD demonstrate a distinct pattern of lung injury with increased anthracosis, small airway thickening and peripheral fibrosis on lung biopsy compared to individuals with tobacco smoke mediated COPD6. Women with biomass-related COPD have lower rates of emphysema and higher rates of air trapping and bronchiectasis compared to those with tobacco-related disease84. In addition, individuals with biomass-related COPD have distinct patterns of airway disease, which may be related to the size of particles deposited in the airways during biomass exposure85. This phenotype is marked by increased cough, phlegm on respiratory symptom questionnaire, as well as higher rates of bronchodilator reversibility and hyper-responsiveness, signifying an elevated degree of airway inflammation60. Biomass-related COPD results in a different inflammatory profile, with higher circulating levels of CD4 inflammatory mediators (TH2, IL-4 and IL-10) than tobacco related disease86. Furthermore, those with biomass-related COPD have higher levels of malonylaldehide and superoxide dismutase, measures of oxidative stress that correlate inversely to FEV187.
High-Income Countries.
Given lower utilization of solid fuels in high-income countries the relationship between household use of solid fuels and COPD development in high-income studies has not been as intensively investigated. However, a few studies have demonstrated a potential link between the utilization of solid fuels for heating and COPD development in the United States. A 2010 study by Sood and colleagues demonstrated that self-reported wood smoke exposure was associated with greater odds of airflow obstruction and chronic bronchitis (OR 1.96, 95% CI 1.52–2.54) and 1.64 (95% 1.36–2.06), respectively88. This study was limited to a cohort of primarily smokers in New Mexico. A subsequent, nationally representative study, which utilized the National Health Interview Survey for data on COPD prevalence and the US Census for information on community use of solid fuels (coal and wood) for heating, demonstrated that higher use of coal for heating, at the community level, was associated with greater odds of self-reported COPD (OR 1.09; P<0.001), among never-smokers89. A similar study which utilized data from the National Health and Nutrition Examination Survey demonstrated that increased use of wood for heating, at the community level, was associated with greater odd of COPD, defined by spirometry (OR 1.12; P<0.001), among never smokers. Both of these studies demonstrated that living in a rural region of the United States was associated with greater odds of COPD, even after taking account traditional risk factors such as smoking, community level poverty and individual socioeconomic status. Rural regions notably reported a higher percentage of homes using solid fuels (wood and coal) for heating, which may be an explanation for this disparity. All three of these studies were limited by a lack of information on individual level exposures, as they relied on the census level data, and did not contain direct measurement of pollutants. As a result, there remains a significant gap in our knowledge of the contribution of these household pollutants to COPD development in high income settings.
Among individuals living with COPD indoor air pollution has been associated with increased respiratory morbidity, even in communities that do not use solid fuels for heating or cooking. Hansel and colleagues demonstrated in an urban cohort of 84 former smokers with COPD that increases in PM2.5 and NO2 are associated with worsened COPD morbidity90. An increase of 10ug/m3 PM2.5 in the main living area was independently associated with increases in nocturnal symptoms, wheeze, worse respiratory status based on St. George Respiratory Questionnaire scores, and greater odds of severe exacerbation (OR 1.50; 95% CI 1.04 to 2.18). A 20ppb increase in NO2 in the main living area was associated with increased modified medical research council (mMRC) dyspnea scores while a similar increase in bedroom NO2 was associated with increased odds of severe exacerbations (OR 2.71; 95% CI 1.05 to 6.93). One notable thing about this study is that these effects were seen despite relatively low household pollutant concentrations (median [IQR] PM2.5 8.3 [4.9 to 14.4] ug/m3 and NO2 6.8 [4.2 to 14.5] ppb), which are typical of high-income countries. These studies did not show an effect of PM2.5 or NO2 on FEV1, though the follow-up period was limited to 6 months. In addition to PM2.5 and NO2, studies have highlighted the impact of household allergen sensitization on COPD morbidity. Data from an urban COPD cohort demonstrated that sensitization to allergens from mouse, cockroach, cat, dog, and dust mites increased respiratory morbidity91. In this study, the authors measured detectable IgE levels for the aforementioned allergens, as well as markers of allergen exposure and sensitization, among 77 participants with COPD. After adjusting for confounders, an increasing number of sensitizations was associated higher risk for cough, wheeze, and nocturnal dyspnea, though the effects were primarily observed among those with 3 or more sensitizations. Those with only 1–2 sensitization still had greater odds of reporting an ED visit (OR 10.0; 95% CI 1.6–60.7). This study was limited by a small sample size, with broad effect estimates, but still provides evidence to suggest that household allergen exposure and sensitization can lead to increased morbidity for those with COPD.
Certain factors can also increase susceptibility to the impact of household pollutants for those living with COPD. Similar to what has been observed among children with asthma, obesity may increase susceptibility to the effects of HAP among adults with COPD. One study of 84 participants with moderate to severe COPD demonstrated that obese individuals had exaggerated increases in nocturnal symptoms, dyspnea and rescue medication use compared to non-obese in response to PM2.5 and PMcoarse 92. Other studies have demonstrated that extremes of temperature – both hot and cold –may also influence the effects of indoor pollution on COPD morbidity93,94. One study in particular demonstrated that high heat increased the effect of PM2.5 and NO2 exposures on rescue inhaler use, cough, and sputum production95. We do note the majority of the data presented here regarding the effects of household pollution on COPD morbidity in high income countries are from urban environments. To date there are few studies, with direct measurements of household pollutants, in rural regions of the United States that have studied the effects of household pollution on COPD morbidity. While the literature is suggestive of this, we do not know if there is a difference in the impact of household air pollution between urban and rural environments in the United States remains an important area for future study. The present studies have also not shown that increases in household PM2.5 or NO2 exposure are associated with more accelerated lung function decline for those with COPD. This may ultimately be related to relatively short-term follow-up (<1-year followup) in the studies performed to date.
Strategies for Reducing Household Air Pollution
A number of strategies have been attempted to reduce pollutant exposure in diverse settings. Interventions have been attempted to reduce a wide range of indoor pollutants including, particulate matter, gases, allergens, and mold (Table 2). Certain strategies outlined focus on source reduction (i.e LPG stove interventions and smoking bans) while others focus on secondary reduction (air cleaner interventions).
Table 2.
Common Strategies for Reducing Household Pollutants
| Pollutant | Interventions |
|---|---|
| Particulate Matter (PM2.5, PM10) | Cookstove Exchanges (In homes burning biomass fuels), Air Cleaner Interventions, Woodstove Replacement |
| Gases (NO2) | Air Cleaners (HEPA with carbon filter), Gas Stove Replacement, Vented Hoods for Stoves |
| Allergens | Air Cleaners, Integrated Pest Management, Pest Education |
| Molds | Remediation of household dampness and humidity |
| Environmental Tobacco Smoke | Smoking Cessation, Indoor Smoking Bans |
Cookstove Interventions
Over the past decade, a number of studies have attempted to lower the levels of HAP through cleaner burning biomass stoves. RESPIRE was one of the first randomized controlled trials to demonstrate a reduction in deleterious health outcomes, in their case severe pediatric pneumonia96. Romieu and colleagues demonstrated reduced respiratory symptoms (odds ratio 0.29, 95% CI 0.11– 0.77; for wheeze) and declines in lung function (31 mL vs. 62 mL over 1 year, p=0.01) over a 12 month period among those randomized to cook stove intervention, though few other studies have replicated these results despite evidence of increased cookstove uptake (Table 3)97.
Table 3.
| Author/Year | Intervention | Location/Participants | Findings |
|---|---|---|---|
| Romieu et al. 200997 | RCT of chimney stove vs traditional open fire Primary Outcomes: Respiratory symptoms and Lung Function |
Rural Mexico, n=552 women | No statistically significant effect seen in intention-to-treat (ITT) analyses, although a significant effect on both cough and annual rate of decline in forced expiratory volume in 1 second (FEV1; 31 vs. 62 ml) was observed for women who reported using the chimney stove. |
| Smith et al. 201196 | RCT of chimney stove vs traditional open fire Primary Outcomes: Physician diagnosed pneumonia |
Rural Guatemala, n= 518 children under 19 mo age | No statistically significant effect on physician-diagnosed pneumonia was found, although physician-diagnosed severe pneumonia (defined by the presence of hypoxemia) was significantly reduced among children in the intervention group. The chimney stove intervention was reported to improve respiratory symptoms in the mothers of the study children, but not rate of decline in lung function in ITT analyses. A subsequent analysis demonstrated reduced carbon monoxide exposure was associated with a lower rate of decline in FEV1 |
| Tielsch et al. 201698 | Cluster-randomized, step-wedge, community-based trial of a cleaner-burning biomass stove vs traditional open stove Primary outcome: Acute lower respiratory tract infection |
Rural Nepal, n= 5,254 children under the age of 3 yo | No statistically significant effect on the incidence of acute lower respiratory tract infection in the intervention compared with the control group (relative risk = 0.87 [95% confidence interval = 0.67–1.13]). Potentially beneficial effects were seen in selected secondary analyses on cough, wheeze, and burn injury. |
| Asante et al. 201799 | Three-arm household-level RCT of liquefied petroleum gas versus a cleaner-burning biomass-fueled cookstove Primary Outcome: Birthweight and physician-diagnosed severe pneumonia in the first year of life |
Rural Ghana, n = 1,415 pregnant women | Although children born to mothers with higher HAP CO exposures during pregnancy were at increased risk for impaired lung function measured 1 month after birth, no significant difference in birth weight or physician-assessed IMCI pneumonia in either of the intervention arms in IIT analysis |
| Mortimer et al. 2017100 | Community-level C-RCT of two cleaner-burning, biomass-fueled cookstoves with a solar charger vs traditional open fire Primary Outcome: Pneumonia |
Rural Malawi, n = 10,750 children under 5 yo | No effect of the intervention on the primary outcome of WHO Integrated Management of Childhood Illness–defined pneumonia in an ITT analysis. |
Design of stove interventions to reduce the burden of disease has additionally been challenged by a limited understanding of exposure-response relationships101. Many studies were limited by small samples size, limited length of follow up, variability in intervention and level of adoption, as well as protocol deviations102. Importantly, most studies have failed to capture meaningful exposure reduction to gain a complete understanding of exposure-response across a range of relevant and targeted exposures. Few HAP studies have included low exposure groups (i.e., clean fuel users) to demonstrate the maximum benefit that can be expected. Because of financial and technical constraints associated with performing large-scale HAP measurements in LMIC settings, many studies have relied on imprecise, proxy exposure measures. Measurement of fine particulate matter (PM2.5), the best exposure indicator of health risk, has been particularly challenging due to the limitations of affordable, feasible, and reliable instrumentation103,104.
The evidence from clinical trials to date do not support the efficacy of cleaner-burning cook stoves to improve pulmonary health or reduce mortality in LMICs102. Liquified petroleum gas (LPG) stoves may sufficiently reduce HAP levels to deliver meaningful health gains. Ongoing trials utilizing LPG stoves with inclusion of participants across the lifespan (fetal, childhood and elderly participants), sufficient follow up and exposure monitoring may add additional value in understanding the pulmonary benefits of reduction in HAP. A multicenter RCT, funded by the NIH, with sites in Rwanda, Peru, Guatemala and India aims to address additional gaps in evidence105.
Air Cleaner Interventions
Portable air cleaner devices may be effective for reducing household air pollution in high income countries. Air cleaner interventions have been studied in both urban and rural regions of the United States including both HEPA cleaners and HEPA and activated carbon filters to address household gases (i.e. NO2) Table 4.
Table 4.
Air Cleaner Interventions to Improve Respiratory Health
| Author/Year | Intervention | Location/Participants | Findings |
|---|---|---|---|
| Ward et al. 2017 | Randomized placebo-controlled trial of woodstove change out and air filtration Primary Outcomes: PM2.5 and CO concentration |
Missoula Montana, n=98 households | Homes randomized to the air purifier intervention had a 63% (95% CI 47–75%) reduction in household PM2.5. The air purifier intervention arm was more efficacious and less expensive than a employing a woodstove changeout, which resulted in no significant change in household PM2.5. |
| Butz et al. 2011 | RCT of health coaching and high-efficiency particle air (HEPA) filter vs HEPA filter vs control Primary Outcomes: Change in PM, air nicotine, urine cotinine concentration and symptom-free days |
Baltimore MD, n=125 children with asthma who resided with smokers | Homes randomized to receive an air cleaner observed a 50% reduction in PM2.5, representing a change of ~20ug/m3 for children living in smoking homes. These improvements were observed with only modest adherence (59%) to the intervention itself. No reduction was observed in markers of tobacco exposure; air nicotine levels or cotinine measurements. The intervention still led to an improvement in symptom free days (1.36; P=0.03), though did not result in a significant improvement in nocturnal symptoms or a reduction in acute asthma events |
| Paulin et al. 2014 | RCT of gas stove replacement with electric stoves vs instillation of hood over exisiting stoves vs placement of HEPA and carbon purifiers in the house Primary Outcomes: Indoor NO2 concentrations |
Baltimore MD, n= 100 households | Homes where the air cleaners were placed in the kitchen and bedroom of homes using gas stoves had an immediate decrease in median NO2 concentrations in both the kitchen (27%; P<0.01) and bedroom (22%, P=0.02). However, at 3-month follow-up improvements were only observed in the kitchen (20%; P=0.05). Notably in this study adherence data was missing from the bedroom air purifier, which potentially could account for the lack of long-term benefit |
While air purifiers have been shown to improve indoor air quality and reduce household PM2.5 concentrations, the long-term health benefits of these devices are still being uncovered. A study of healthy adults in woodsmoke-impacted community, demonstrated improved endothelial function (based on the reactive hyperemia index) and decreased concentrations of inflammatory biomarkers (serum CRP) after a weeklong HEPA filter intervention106. As noted previously, studies of air purifier interventions have shown promise for improving childhood asthma outcomes. Two trials in urban settings have shown improvements in symptom free days and healthcare utilization with air cleaner interventions107,108. In contrast for adults with COPD the effects of an air cleaner intervention remain unknown, given a lack of high-quality intervention trials. The effects of an area cleaner intervention for adults with COPD remains an area in need of ongoing research and studies are underway109.
Integrated Approaches for Pollutant and Allergen Reduction
Integrated approaches have additionally been attempted that combine education on allergen reduction and remediation, pest management, and air cleaners to reduce household particulates. These studies have primarily focused on children with asthma, and their effects on other populations are not yet known. One multi-center randomized trial of 937 inner-city children with asthma demonstrated improvements with a 12 month environment intervention centered around the bedroom that included HEPA air cleaners, allergen remediation and allergen prevention education for caretakers110. Children who underwent this intervention saw an improvement in the symptom free days during the intervention year (3.39 vs 4.20 days, P<0.001) and the year after (2.62 vs 3.21 days, P<0.001). This was accompanied by a significant reduction in dust mite and cockroach allergens. A smaller study that employed air cleaner use and cockroach extermination among 100 asthmatic children, demonstrated a 51% reduction in cockroach allergen along with a 36% reduction in PM2.5 at one-year followup111. In this study participants who received the intervention reported fewer daytime symptoms (OR 0.55, 95% CI 0.31–0.97). Studies that have focused solely on pest allergen reduction have had mixed results29,79,112,113. A more recent randomized trial did not demonstrate a benefit of an integrated pest management intervention over providing pest management education alone to families of children with asthma29. However, a post-hoc analysis demonstrated that mouse allergen reduction was associated with greater increases in lung function over a year, suggesting that allergen exposure reduction may improve lung growth114. In this study pest management education alone (providing written material and demonstration about and pest management and housekeeping practices) resulted in a 65% reduction in household mouse antigen, suggesting that detailed education may provide benefit in reducing environmental exposures.
Community Level Interventions
Community level strategies, bolstered by policy, have been utilized to reduce sources of pollution in high income countries. Successful campaigns have been implemented to reduce pollution from solid fuels and address the use of wood stoves, in rural communities. One community-wide wood stove exchange program in Libby Montana led to a 27.6% (95% CI 3.0% to 44.5%) decrease in ambient PM2.5 levels, with an associated decrease in odds of both wheeze and respiratory infections among school children in this community115. A similar study in Tasmania, Australia observed a significant reduction in outdoor PM10 (from 43.6ug/m3 to 27.0ug/m3), after community wide efforts to replace wood burning stoves with cleaner electric appliances. Community level woodstove exchange programs have also been effective in reducing ambient concentration of phenolics and PAHs116. Despite observed improvements in ambient pollutant levels and potential health benefits, there has not been a consistent long-term decrease in household PM2.5 from these efforts alone117. Given that significant legislative and regulatory action is often required to implement similar community level interventions, further research is needed in order to confirm their long-term benefits.
Smoke-free legislation provides examples of successful interventions with the potential to impact health at a population level. For example, meta analyses of studies investigating the effect of smoke-free legislation in North America and Europe have demonstrated reductions in emergency department visits for asthma and hospitalizations for asthma and respiratory tract infections118,119. Recent legislation on smoking bans in public housing more specifically addresses residential smoke exposure. Effective February 2017, the U.S. Department of Housing and Urban Development published a rule requiring each public housing agency to implement a smoke-free policy within 18 months120. Implementation of this rule poses challenges and studies investigating health effects are not yet available.
Schools represent another venue for community-level interventions. Despite the strong evidence base for health effects of air pollution, including indoor air pollution, on children’s respiratory health, there has been relatively little investigation of interventions in schools. Children spend a substantial proportion of their time in schools and thus, school environments represent an opportunity to improve health at a population level by reducing harmful exposures. The School Inner-City Asthma Study demonstrated that mouse allergen exposure in schools was associated with increased asthma symptoms and decreased lung function80. Studies of school-based environmental interventions are logistically challenging but studies, some of which are currently underway, are needed in order to provide evidence of the magnitude of potential health benefits for children121,122.
Future Areas for Study
Today there is robust data to suggest that indoor air pollution is associated with a multitude of respiratory effects across the lifespan, including impaired lung development in childhood, greater risk for acute lower respiratory tract infections, risk for developing chronic lung disease and increased morbidity related to asthma and COPD. While longitudinal cohort studies of participants followed for decades has informed our understanding of the effects of outdoor air pollution and the benefits of pollution reduction, analogous studies of the long-term health effects of indoor air pollution are lacking123. While there is a relatively limited number of studies investigating interventions to reduce household pollutants, existing evidence has been inconsistent in demonstrating benefit. Challenges of studying the health effects of indoor air pollution include the need for individual exposure assessment, and interventions that require long-term implementation at the household level. Opportunities to longitudinally study the health effects of legislation aimed at improving indoor air quality and interventions at community levels with the potential to improve health of local populations provide a means of efficiently understanding the health benefits of improved indoor air quality. As individuals spend the majority of their time indoors and most of their time in the home environment, this continues to be an important target for improving respiratory health. Ongoing investigations are needed in order to more clearly identify targetable mechanisms by which indoor pollutants may influence respiratory morbidity, interventions with long term benefit, and susceptible populations who are most likely to benefit from these interventions.
Summary
Indoor air pollution, from sources that include indoor tobacco smoke, the burning of solids fuels, and noxious gases from cooking and heating, is associated with greater risk for chronic lung disease development and respiratory morbidity worldwide. While the impact is most pronounced in Low- and Middle Incomes countries, significant health effects are still observed at lower pollutant concentrations typical of high-income settings. It is important to raise awareness of common sources of pollution among clinicians, policy makers, and patients with chronic respiratory disease alike, in order to improve health education and promote efforts that can reduce pollutant exposure across households and address environmental health disparities. While the long-term benefits of efforts to reduce indoor air pollution are yet to be defined, multiple intervention strategies at the household and community levels (including indoor smoking bans and air cleaner interventions) have been effective in reducing pollutant exposure. Future research may help to define optimal strategies for reducing indoor air pollution at the individual, household, community and population level to improve long-term lung health and reduce health disparities.
Key Points:
Over 4 million people die prematurely annually due to household air pollution.
A large number of factors contribute to household air pollution including household characteristics (ventilation, dampness, humidity), behaviors (cooking practices and use of solid fuels), as well as allergens related to pests.
Indoor air pollution is associated with impaired lung development, increased risk for respiratory tract infection, and increased prevalence of and morbidity attributable to asthma and chronic obstructive pulmonary disease.
Research is ongoing into effective strategies to reduce pollutant exposure and improve lung health.
Acknowledgments
Disclosures: TS is supported through the NHLBI (1K23HL146946). MCM is supported by NIMHD P50MD010431/EPA 83615001, NIEHS P50ES018176/EPA 83615201, and EPA 83563901. SR is supported by NHLBI K12HL143957 and NIAID P30AI094189
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. World Health Organization: Air Pollution. https://www.who.int/health-topics/air-pollution#tab=tab_1. Accessed December 15, 2019.
- 2.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet Lond Engl. 2012;380(9859):2224–2260. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson G, Eick S, Jones R. How is the indoor environment related to asthma?: literature review. J Adv Nurs. 2005;52(3):328–339. 10.1111/j.1365-2648.2005.03591.x [DOI] [PubMed] [Google Scholar]
- 4.Ho LA, Kuschner WG. Respiratory Health in Home and Leisure Pursuits. Occup Pulmonol. 2012;33(4):715–729. 10.1016/j.ccm.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 5.Smith Kirk R, Peel Jennifer L Mind the Gap. Environ Health Perspect. 2010;118(12):1643–1645. 10.1289/ehp.1002517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sood A, Assad NA, Barnes PJ, et al. ERS/ATS workshop report on respiratory health effects of household air pollution. Eur Respir J. 2018;51(1):1700698 10.1183/13993003.00698-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balmes JR. Household air pollution from domestic combustion of solid fuels and health. J Allergy Clin Immunol. 2019;143(6):1979–1987. 10.1016/j.jaci.2019.04.016 [DOI] [PubMed] [Google Scholar]
- 8.Bonjour S, Adair-Rohani H, Wolf J, et al. Solid fuel use for household cooking: country and regional estimates for 1980–2010. Environ Health Perspect. 2013;121(7):784–790. 10.1289/ehp.1205987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith KR, Bruce N, Balakrishnan K, et al. Millions Dead: How Do We Know and What Does It Mean? Methods Used in the Comparative Risk Assessment of Household Air Pollution. Annu Rev Public Health. 2014;35(1):185–206. 10.1146/annurev-publhealth-032013-182356 [DOI] [PubMed] [Google Scholar]
- 10.Gordon SB, Bruce NG, Grigg J, et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med. 2014;2(10):823–860. 10.1016/S2213-2600(14)70168-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace LA, Mitchell H, O’Connor GT, et al. Particle concentrations in inner-city homes of children with asthma: the effect of smoking, cooking, and outdoor pollution. Environ Health Perspect. 2003;111(9):1265–1272. 10.1289/ehp.6135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormack MC, Breysse PN, Hansel NN, et al. Common household activities are associated with elevated particulate matter concentrations in bedrooms of inner-city Baltimore pre-school children. Environ Res. 2008;106(2):148–155. 10.1016/j.envres.2007.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansel NN, Breysse PN, McCormack MC, et al. A Longitudinal Study of Indoor Nitrogen Dioxide Levels and Respiratory Symptoms in Inner-City Children with Asthma. Environ Health Perspect. 2008;116(10):1428–1432. 10.1289/ehp.11349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baldacci S, Maio S, Cerrai S, et al. Allergy and asthma: Effects of the exposure to particulate matter and biological allergens. Respir Med. 2015;109(9):1089–1104. 10.1016/j.rmed.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. “The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General.” Atlanta, GA: US Department of Health and Human Services; (2006). [PubMed] [Google Scholar]
- 16.Quinto KB, Akinbami LJ. Environmental Tobacco Smoke Exposure in Children Aged 3‒19 Years With and Without Asthma in the United States, 1999‒2010. 2013;(126):8. [PubMed] [Google Scholar]
- 17.Rogalsky Derek K, Mendola Pauline, Metts Tricia A, Martin William J. Estimating the Number of Low-Income Americans Exposed to Household Air Pollution from Burning Solid Fuels. Environ Health Perspect. 2014;122(8):806–810. 10.1289/ehp.1306709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noonan CW, Ward TJ. Asthma randomized trial of indoor wood smoke (ARTIS): rationale and methods. Contemp Clin Trials. 2012;33(5):1080–1087. 10.1016/j.cct.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward T, Boulafentis J, Simpson J, et al. Lessons learned from a woodstove changeout on the Nez Perce Reservation. Sci Total Environ. 2011;409(4):664–670. 10.1016/j.scitotenv.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 20.Bunnell JE, Garcia LV, Furst JM, et al. Navajo coal combustion and respiratory health near Shiprock, New Mexico. J Environ Public Health. 2010;2010:260525–260525. 10.1155/2010/260525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulin LM, Williams D ‘Ann L, Peng R, et al. 24-h Nitrogen dioxide concentration is associated with cooking behaviors and an increase in rescue medication use in children with asthma. Environ Res. 2017;159:118–123. 10.1016/j.envres.2017.07.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinrich J Influence of indoor factors in dwellings on the development of childhood asthma. Int J Hyg Environ Health. 2011;214(1):1–25. 10.1016/j.ijheh.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 23.Barnes B, Mathee A, Moiloa K. Assessing child time – activity patterns in relation to indoor cooking fires in developing countries: A methodological comparison. Int J Hyg Environ Health. 2005;208(3):219–225. 10.1016/j.ijheh.2005.01.022 [DOI] [PubMed] [Google Scholar]
- 24.Qian Z, Zhang J (Jim), Korn LR, Wei F, Chapman RS. Factor analysis of household factors: are they associated with respiratory conditions in Chinese children? Int J Epidemiol. 2004;33(3):582–588. 10.1093/ije/dyg278 [DOI] [PubMed] [Google Scholar]
- 25.Smith KR, Samet JM, Romieu I, Bruce N. Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax. 2000;55(6):518–532. 10.1136/thorax.55.6.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regalado J, Pérez-Padilla R, Sansores R, et al. The Effect of Biomass Burning on Respiratory Symptoms and Lung Function in Rural Mexican Women. Am J Respir Crit Care Med. 2006;174(8):901–905. 10.1164/rccm.200503-479OC [DOI] [PubMed] [Google Scholar]
- 27.Samet JM, Spengler JD. Indoor environments and health: moving into the 21st century. Am J Public Health. 2003;93(9):1489–1493. 10.2105/ajph.93.9.1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ Health Perspect. 2011;119(6):748–756. 10.1289/ehp.1002410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsui EC, Perzanowski M, Peng RD, et al. Effect of an Integrated Pest Management Intervention on Asthma Symptoms Among Mouse-Sensitized Children and Adolescents With Asthma: A Randomized Clinical Trial. JAMA. 2017;317(10):1027–1036. 10.1001/jama.2016.21048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roche N, Chinet T, Huchon G. Allergic and nonallergic interactions between house dust mite allergens and airway mucosa. Eur Respir J. 1997;10(3):719. [PubMed] [Google Scholar]
- 31.Ohman JL, Hagberg K, MacDonald MR, Jones RR, Paigen BJ, Kacergis JB. Distribution of airborne mouse allergen in a major mouse breeding facility. J Allergy Clin Immunol. 1994;94(5):810–817. 10.1016/0091-6749(94)90147-3 [DOI] [PubMed] [Google Scholar]
- 32.Matsui EC, Simons E, Rand C, et al. Airborne mouse allergen in the homes of inner-city children with asthma. J Allergy Clin Immunol. 2005;115(2):358–363. 10.1016/j.jaci.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 33.Ahluwalia SK, Peng RD, Breysse PN, et al. Mouse allergen is the major allergen of public health relevance in Baltimore City. J Allergy Clin Immunol. 2013;132(4):830–5. 10.1016/j.jaci.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsui EC, Eggleston PA, Buckley TJ, et al. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann Allergy Asthma Immunol. 2006;97(4):514–520. 10.1016/S1081-1206(10)60943-X [DOI] [PubMed] [Google Scholar]
- 35.Rinne S, Rodas E, Rinne M, Simpson J, Glickman L. Use of biomass fuel is associated with infant mortality and child health in trend analysis. Am J Trop Med Hyg. 2007;76:585–591. 10.4269/ajtmh.2007.76.585 [DOI] [PubMed] [Google Scholar]
- 36.Loke J, Paul E, Virgulto JA, Smith GJW. Rabbit Lung After Acute Smoke Inhalation: Cellular Responses and Scanning Electron Microscopy. Arch Surg. 1984;119(8):956–959. 10.1001/archsurg.1984.01390200074017 [DOI] [PubMed] [Google Scholar]
- 37.Feldbaum DM, Wormuth D, Nieman GF, Paskanik M, Clark WR, Hakim TS. Exosurf treatment following wood smoke inhalation. Burns. 1993;19(5):396–400. 10.1016/0305-4179(93)90060-L [DOI] [PubMed] [Google Scholar]
- 38.Kurmi OP, Semple S, Simkhada P, Smith WCS, Ayres JG. COPD and chronic bronchitis risk of indoor air pollution from solid fuel: a systematic review and meta-analysis. Thorax. 2010;65(3):221 10.1136/thx.2009.124644 [DOI] [PubMed] [Google Scholar]
- 39.Stockfelt L, Sallsten G, Olin A-C, et al. Effects on airways of short-term exposure to two kinds of wood smoke in a chamber study of healthy humans. Inhal Toxicol. 2012;24(1):47–59. 10.3109/08958378.2011.633281 [DOI] [PubMed] [Google Scholar]
- 40.Barregard L, Sällsten G, Andersson L, et al. Experimental exposure to wood smoke: effects on airway inflammation and oxidative stress. Occup Environ Med. 2008;65(5):319 10.1136/oem.2006.032458 [DOI] [PubMed] [Google Scholar]
- 41.Fick RB, Paul ES, Merrill WW, Reynolds HY, Loke JSO. Alterations in the Antibacterial Properties of Rabbit Pulmonary Macrophages Exposed to Wood Smoke. Am Rev Respir Dis. 1984;129(1):76–81. 10.1164/arrd.1984.129.1.76 [DOI] [PubMed] [Google Scholar]
- 42.Rylance J, Fullerton DG, Scriven J, et al. Household Air Pollution Causes Dose-Dependent Inflammation and Altered Phagocytosis in Human Macrophages. Am J Respir Cell Mol Biol. 2014;52(5):584–593. 10.1165/rcmb.2014-0188OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Susanne Becker JMS. EXPOSURE TO URBAN AIR PARTICULATES ALTERS THE MACROPHAGE-MEDIATED INFLAMMATORY RESPONSE TO RESPIRATORY VIRAL INFECTION. J Toxicol Environ Health A. 1999;57(7):445–457. 10.1080/009841099157539 [DOI] [PubMed] [Google Scholar]
- 44.Danielsen PH, Møller P, Jensen KA, et al. Oxidative Stress, DNA Damage, and Inflammation Induced by Ambient Air and Wood Smoke Particulate Matter in Human A549 and THP-1 Cell Lines. Chem Res Toxicol. 2011;24(2):168–184. 10.1021/tx100407m [DOI] [PubMed] [Google Scholar]
- 45.Berry CE, Billheimer D, Jenkins IC, et al. A Distinct Low Lung Function Trajectory from Childhood to the Fourth Decade of Life. Am J Respir Crit Care Med. 2016;194(5):607–612. 10.1164/rccm.201604-0753OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez FD. Early-Life Origins of Chronic Obstructive Pulmonary Disease. N Engl J Med. 2016;375(9):871–878. 10.1056/NEJMra1603287 [DOI] [PubMed] [Google Scholar]
- 47.Bui DS, Lodge CJ, Burgess JA, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018;6(7):535–544. 10.1016/S2213-2600(18)30100-0 [DOI] [PubMed] [Google Scholar]
- 48.Belgrave DCM, Granell R, Turner SW, et al. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med. 2018;6(7):526–534. 10.1016/S2213-2600(18)30099-7 [DOI] [PubMed] [Google Scholar]
- 49.Bui DS, Walters HE, Burgess JA, et al. Childhood Respiratory Risk Factor Profiles and Middle-Age Lung Function: A Prospective Cohort Study from the First to Sixth Decade. Ann Am Thorac Soc. 2018;15(9):1057–1066. 10.1513/AnnalsATS.201806-374OC [DOI] [PubMed] [Google Scholar]
- 50.Latzin P, Röösli M, Huss A, Kuehni CE, Frey U. Air pollution during pregnancy and lung function in newborns: a birth cohort study. Eur Respir J. 2009;33(3):594 10.1183/09031936.00084008 [DOI] [PubMed] [Google Scholar]
- 51.Gauderman WJ, Urman R, Avol E, et al. Association of Improved Air Quality with Lung Development in Children. N Engl J Med. 2015;372(10):905–913. 10.1056/NEJMoa1414123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee AG, Kaali S, Quinn A, et al. Prenatal Household Air Pollution Is Associated with Impaired Infant Lung Function with Sex-Specific Effects. Evidence from GRAPHS, a Cluster Randomized Cookstove Intervention Trial. Am J Respir Crit Care Med. 2018;199(6):738–746. 10.1164/rccm.201804-0694OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M, Bruce N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ. 2008;86(5):390–398C. 10.2471/blt.07.044529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jary H, Simpson H, Havens D, et al. Household Air Pollution and Acute Lower Respiratory Infections in Adults: A Systematic Review. PloS One. 2016;11(12):e0167656–e0167656. 10.1371/journal.pone.0167656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horne BD, Joy EA, Hofmann MG, et al. Short-Term Elevation of Fine Particulate Matter Air Pollution and Acute Lower Respiratory Infection. Am J Respir Crit Care Med. 2018;198(6):759–766. 10.1164/rccm.201709-1883OC [DOI] [PubMed] [Google Scholar]
- 56.Croft DP, Zhang W, Lin S, et al. The Association between Respiratory Infection and Air Pollution in the Setting of Air Quality Policy and Economic Change. Ann Am Thorac Soc. 2019;16(3):321–330. 10.1513/AnnalsATS.201810-691OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vork Kathleen L, Broadwin Rachel L, Blaisdell Robert J Developing Asthma in Childhood from Exposure to Secondhand Tobacco Smoke: Insights from a Meta-Regression. Environ Health Perspect. 2007;115(10):1394–1400. 10.1289/ehp.10155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Po JYT, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax. 2011;66(3):232 10.1136/thx.2010.147884 [DOI] [PubMed] [Google Scholar]
- 59.Kumar R, Nagar JK, Raj N, et al. Impact of domestic air pollution from cooking fuel on respiratory allergies in children in India. Asian Pac J Allergy Immunol. 2008;26(4):213–222. [PubMed] [Google Scholar]
- 60.Siddharthan T, Grigsby MR, Goodman D, et al. Association between Household Air Pollution Exposure and Chronic Obstructive Pulmonary Disease Outcomes in 13 Low- and Middle-Income Country Settings. Am J Respir Crit Care Med. 2018;197(5):611–620. 10.1164/rccm.201709-1861OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Semple S, Devakumar D, Fullerton DG, et al. Airborne endotoxin concentrations in homes burning biomass fuel. Environ Health Perspect. 2010;118(7):988–991. 10.1289/ehp.0901605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Behrendt H, Kasche A, Ebner von Eschenbach C, Risse U, Huss-Marp J, Ring J. Secretion of Proinflammatory Eicosanoid-Like Substances Precedes Allergen Release from Pollen Grains in the Initiation of Allergic Sensitization. Int Arch Allergy Immunol. 2001;124(1–3):121–125. 10.1159/000053688 [DOI] [PubMed] [Google Scholar]
- 63.Barry AC, Mannino DM, Hopenhayn C, Bush H. Exposure to Indoor Biomass Fuel Pollutants and Asthma Prevalence in Southeastern Kentucky: Results From the Burden of Lung Disease (BOLD) Study. J Asthma. 2010;47(7):735–741. 10.3109/02770903.2010.485661 [DOI] [PubMed] [Google Scholar]
- 64.Noonan CW, Ward TJ. Environmental Tobacco Smoke, Woodstove Heating and Risk of Asthma Symptoms. J Asthma. 2007;44(9):735–738. 10.1080/02770900701595675 [DOI] [PubMed] [Google Scholar]
- 65.Breysse PN, Diette GB, Matsui EC, Butz AM, Hansel NN, McCormack MC. Indoor Air Pollution and Asthma in Children. Proc Am Thorac Soc. 2010;7(2):102–106. 10.1513/pats.200908-083RM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCormack MC, Breysse PN, Matsui EC, et al. In-home particle concentrations and childhood asthma morbidity. Environ Health Perspect. 2009;117(2):294–298. 10.1289/ehp.11770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCormack MC, Breysse PN, Matsui EC, et al. Indoor particulate matter increases asthma morbidity in children with non-atopic and atopic asthma. Ann Allergy Asthma Immunol. 2011;106(4):308–315. 10.1016/j.anai.2011.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kattan M, Gergen PJ, Eggleston P, Visness CM, Mitchell HE. Health effects of indoor nitrogen dioxide and passive smoking on urban asthmatic children. J Allergy Clin Immunol. 2007;120(3):618–624. 10.1016/j.jaci.2007.05.014 [DOI] [PubMed] [Google Scholar]
- 69.Gaffin JM, Hauptman M, Petty CR, et al. Nitrogen dioxide exposure in school classrooms of inner-city children with asthma. J Allergy Clin Immunol. 2018;141(6):2249–2255. 10.1016/j.jaci.2017.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu KD, Breysse PN, Diette GB, et al. Being overweight increases susceptibility to indoor pollutants among urban children with asthma. J Allergy Clin Immunol. 2013;131(4):1017–1023. 10.1016/j.jaci.2012.12.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holguin F, Fitzpatrick A. Obesity, asthma, and oxidative stress. J Appl Physiol. 2009;108(3):754–759. 10.1152/japplphysiol.00702.2009 [DOI] [PubMed] [Google Scholar]
- 72.Keaney John F, Larson Martin G, Vasan Ramachandran S, et al. Obesity and Systemic Oxidative Stress. Arterioscler Thromb Vasc Biol. 2003;23(3):434–439. 10.1161/01.ATV.0000058402.34138.11 [DOI] [PubMed] [Google Scholar]
- 73.Wu TD, Brigham EP, Peng R, et al. Overweight/obesity enhances associations between secondhand smoke exposure and asthma morbidity in children. J Allergy Clin Immunol Pract. 2018;6(6):2157–2159. 10.1016/j.jaip.2018.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Codispoti CD, LeMasters GK, Levin L, et al. Traffic pollution is associated with early childhood aeroallergen sensitization. Ann Allergy Asthma Immunol. 2015;114(2):126–133. 10.1016/j.anai.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carlsten C, Blomberg A, Pui M, et al. Diesel exhaust augments allergen-induced lower airway inflammation in allergic individuals: a controlled human exposure study. Thorax. 2016;71(1):35–44. 10.1136/thoraxjnl-2015-207399 [DOI] [PubMed] [Google Scholar]
- 76.Biagioni BJ, Tam S, Chen Y-WR, Sin DD, Carlsten C. Effect of controlled human exposure to diesel exhaust and allergen on airway surfactant protein D, myeloperoxidase and club (Clara) cell secretory protein 16. Clin Exp Allergy. 2016;46(9):1206–1213. 10.1111/cea.12732 [DOI] [PubMed] [Google Scholar]
- 77.Morgenstern V, Zutavern A, Cyrys J, et al. Atopic Diseases, Allergic Sensitization, and Exposure to Traffic-related Air Pollution in Children. Am J Respir Crit Care Med. 2008;177(12):1331–1337. 10.1164/rccm.200701-036OC [DOI] [PubMed] [Google Scholar]
- 78.Wang I-J, Tung T-H, Tang C-S, Zhao Z-H. Allergens, air pollutants, and childhood allergic diseases. Int J Hyg Environ Health. 2016;219(1):66–71. 10.1016/j.ijheh.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 79.Eggleston PA. Cockroach allergy and urban asthma. J Allergy Clin Immunol. 2017;140(2):389–390. 10.1016/j.jaci.2017.04.033 [DOI] [PubMed] [Google Scholar]
- 80.Sheehan WJ, Permaul P, Petty CR, et al. Association Between Allergen Exposure in Inner-City Schools and Asthma Morbidity Among Students. JAMA Pediatr. 2017;171(1):31–38. 10.1001/jamapediatrics.2016.2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Breysse PN, Buckley TJ, Williams D, et al. Indoor exposures to air pollutants and allergens in the homes of asthmatic children in inner-city Baltimore. Environ Res. 2005;98(2):167–176. 10.1016/j.envres.2004.07.018 [DOI] [PubMed] [Google Scholar]
- 82.Amaral AFS, Patel J, Kato BS, et al. Airflow Obstruction and Use of Solid Fuels for Cooking or Heating. BOLD (Burden of Obstructive Lung Disease) Results. Am J Respir Crit Care Med. 2017;197(5):595–610. 10.1164/rccm.201701-0205OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rinne ST, Rodas EJ, Bender BS, et al. Relationship of pulmonary function among women and children to indoor air pollution from biomass use in rural Ecuador. Respir Med. 2006;100(7):1208–1215. 10.1016/j.rmed.2005.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Camp PG, Ramirez-Venegas A, Sansores RH, et al. COPD phenotypes in biomass smoke-versus tobacco smoke-exposed Mexican women. Eur Respir J. 2014;43(3):725 10.1183/09031936.00206112 [DOI] [PubMed] [Google Scholar]
- 85.Diette GB, Accinelli RA, Balmes JR, et al. OBSTRUCTIVE LUNG DISEASE AND EXPOSURE TO BURNING BIOMASS FUEL IN THE INDOOR ENVIRONMENT. Glob Heart. 2012;7(3):265–270. 10.1016/j.gheart.2012.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pérez-Padilla R, Ramirez-Venegas A, Sansores-Martinez R. Clinical Characteristics of Patients With Biomass Smoke-Associated COPD and Chronic Bronchitis, 2004–2014. Chronic Obstr Pulm Dis Miami Fla. 2014;1(1):23–32. 10.15326/jcopdf.1.1.2013.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Montaño M, Cisneros J, Ramírez-Venegas A, et al. Malondialdehyde and superoxide dismutase correlate with FEV1 in patients with COPD associated with wood smoke exposure and tobacco smoking. Inhal Toxicol. 2010;22(10):868–874. 10.3109/08958378.2010.491840 [DOI] [PubMed] [Google Scholar]
- 88.Sood A, Petersen H, Blanchette CM, et al. Wood smoke exposure and gene promoter methylation are associated with increased risk for COPD in smokers. Am J Respir Crit Care Med. 2010;182(9):1098–1104. 10.1164/rccm.201002-0222OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raju S, Keet CA, Paulin LM, et al. Rural residence and poverty are independent risk factors for COPD in the United States. Am J Respir Crit Care Med. 2018;(ja). [DOI] [PMC free article] [PubMed]
- 90.Hansel NN, McCormack MC, Belli AJ, et al. In-Home Air Pollution Is Linked to Respiratory Morbidity in Former Smokers with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2013;187(10):1085–1090. 10.1164/rccm.201211-1987OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jamieson DB, Matsui EC, Belli A, et al. Effects of Allergic Phenotype on Respiratory Symptoms and Exacerbations in Patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2013;188(2):187–192. 10.1164/rccm.201211-2103OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCormack MC, Belli AJ, Kaji DA, et al. Obesity as a susceptibility factor to indoor particulate matter health effects in COPD. Eur Respir J. 2015;45(5):1248–1257. 10.1183/09031936.00081414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hansel NN, McCormack MC, Kim V. The Effects of Air Pollution and Temperature on COPD. COPD. 2016;13(3):372–379. 10.3109/15412555.2015.1089846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McCormack MC, Paulin LM, Gummerson CE, Peng RD, Diette GB, Hansel NN. Colder temperature is associated with increased COPD morbidity. Eur Respir J. 2017;49(6):1601501 10.1183/13993003.01501-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCormack MC, Belli AJ, Waugh D, et al. Respiratory Effects of Indoor Heat and the Interaction with Air Pollution in Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc. 2016;13(12):2125–2131. 10.1513/AnnalsATS.201605-329OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith KR, McCracken JP, Weber MW, et al. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. The Lancet. 2011;378(9804):1717–1726. 10.1016/S0140-6736(11)60921-5 [DOI] [PubMed] [Google Scholar]
- 97.Romieu I, Riojas-Rodríguez H, Marrón-Mares AT, Schilmann A, Perez-Padilla R, Masera O. Improved Biomass Stove Intervention in Rural Mexico. Am J Respir Crit Care Med. 2009;180(7):649–656. 10.1164/rccm.200810-1556OC [DOI] [PubMed] [Google Scholar]
- 98.Tielsch JM, Katz J, Khatry SK, et al. Effect of an improved biomass stove on acute lower respiratory infections in young children in rural Nepal: a cluster-randomised, step-wedge trial. Lancet Glob Health. 2016;4:S19 10.1016/S2214-109X(16)30024-9 [DOI] [Google Scholar]
- 99.Asante KP, Wylie B, Chilrude S, et al. The Ghana Randomized Air Pollution and Health Study (GRAPHS): a cluster randomized trial of Liquified petroleum gas (LPG) and efficient biomass cookstoves delivered during pregnancy. Environ Epidemiol. 2019;3 https://journals.lww.com/environepidem/Fulltext/2019/10001/The_Ghana_Randomized_Air_Pollution_and_Health.903.aspx. [Google Scholar]
- 100.Mortimer K, Ndamala CB, Naunje AW, et al. A cleaner burning biomass-fuelled cookstove intervention to prevent pneumonia in children under 5 years old in rural Malawi (the Cooking and Pneumonia Study): a cluster randomised controlled trial. The Lancet. 2017;389(10065):167–175. 10.1016/S0140-6736(16)32507-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clark ML, Peel JL, Balakrishnan K, et al. Health and household air pollution from solid fuel use: the need for improved exposure assessment. Environ Health Perspect. 2013;121(10):1120–1128. 10.1289/ehp.1206429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mortimer K, Balmes JR. Cookstove Trials and Tribulations: What Is Needed to Decrease the Burden of Household Air Pollution? Ann Am Thorac Soc. 2018;15(5):539–541. 10.1513/AnnalsATS.201710-831GH [DOI] [PubMed] [Google Scholar]
- 103.Naeher LP, Brauer M, Lipsett M, et al. Woodsmoke Health Effects: A Review. Inhal Toxicol. 2007;19(1):67–106. 10.1080/08958370600985875 [DOI] [PubMed] [Google Scholar]
- 104.Northcross AL, Hwang N, Balakrishnan K, Mehta S. Assessing exposures to household air pollution in public health research and program evaluation. EcoHealth. 2015;12(1):57–67. 10.1007/s10393-014-0990-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Steenland K, Pillarisetti A, Kirby M, et al. Modeling the potential health benefits of lower household air pollution after a hypothetical liquified petroleum gas (LPG) cookstove intervention. Environ Int. 2018;111:71–79. 10.1016/j.envint.2017.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Allen RW, Carlsten C, Karlen B, et al. An Air Filter Intervention Study of Endothelial Function among Healthy Adults in a Woodsmoke-impacted Community. Am J Respir Crit Care Med. 2011;183(9):1222–1230. 10.1164/rccm.201010-1572OC [DOI] [PubMed] [Google Scholar]
- 107.Butz AM, Matsui EC, Breysse P, et al. A randomized trial of air cleaners and a health coach to improve indoor air quality for inner-city children with asthma and secondhand smoke exposure. Arch Pediatr Adolesc Med. 2011;165(8):741–748. 10.1001/archpediatrics.2011.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lanphear BP, Hornung RW, Khoury J, Yolton K, Lierl M, Kalkbrenner A. Effects of HEPA air cleaners on unscheduled asthma visits and asthma symptoms for children exposed to secondhand tobacco smoke. Pediatrics. 2011;127(1):93–101. 10.1542/peds.2009-2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hansel NN. Clinical Trial of Air Cleaners to Improve Indoor Air Quality and COPD Health. https://clinicaltrials.gov/ct2/show/NCT02236858. Accessed March 5, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a Home-Based Environmental Intervention among Urban Children with Asthma. N Engl J Med. 2004;351(11):1068–1080. 10.1056/NEJMoa032097 [DOI] [PubMed] [Google Scholar]
- 111.Eggleston PA, Butz A, Rand C, et al. Home environmental intervention in inner-city asthma: a randomized controlled clinical trial. Ann ALLERGY. 2005;95:7. [DOI] [PubMed] [Google Scholar]
- 112.Gergen PJ, Mortimer KM, Eggleston PA, et al. Results of the National Cooperative Inner-City Asthma Study (NCICAS) environmental intervention to reduce cockroach allergen exposure in inner-city homes. J Allergy Clin Immunol. 1999;103(3):501–506. 10.1016/S0091-6749(99)70477-X [DOI] [PubMed] [Google Scholar]
- 113.Gøtzsche PC, Hammarquist C, Burr M. House dust mite control measures in the management of asthma: meta-analysis. BMJ. 1998;317(7166):1105–1110; 10.1136/bmj.317.7166.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grant T, Phipatanakul W, Perzanowski M, et al. Reduction in mouse allergen exposure is associated with greater lung function growth. J Allergy Clin Immunol. 2020;145(2):646–653. 10.1016/j.jaci.2019.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Noonan CW, Ward TJ, Navidi W, Sheppard L. A rural community intervention targeting biomass combustion sources: effects on air quality and reporting of children’s respiratory outcomes. Occup Environ Med. 2012;69(5):354 10.1136/oemed-2011-100394 [DOI] [PubMed] [Google Scholar]
- 116.Ward TJ, Palmer CP, Houck JE, Navidi WC, Geinitz S, Noonan CW. Community woodstove changeout and impact on ambient concentrations of polycyclic aromatic hydrocarbons and phenolics. Environ Sci Technol. 2009;43(14):5345–5350. 10.1021/es8035253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Noonan CW, Navidi W, Sheppard L, et al. Residential indoor PM2.5 in wood stove homes: follow-up of the Libby changeout program. Indoor Air. 2012;22(6):492–500. 10.1111/j.1600-0668.2012.00789.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Faber T, Kumar A, Mackenbach JP, et al. Effect of tobacco control policies on perinatal and child health: a systematic review and meta-analysis. Lancet Public Health. 2017;2(9):e420–e437. 10.1016/S2468-2667(17)30144-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Been JV, Nurmatov UB, Cox B, Nawrot TS, van Schayck CP, Sheikh A. Effect of smoke-free legislation on perinatal and child health: a systematic review and meta-analysis. The Lancet. 2014;383(9928):1549–1560. 10.1016/S0140-6736(14)60082-9 [DOI] [PubMed] [Google Scholar]
- 120.HUD. Federal Register, U.S. Department of Housing and Urban Development (HUD). Instituting Smoke-FreePublic Housing. 81FR87430.December5,2016www.federalregister.gov/documents/2016/12/05/2016-28986/instituting-smoke-free-public-housing. Accessed February6,2017.
- 121.Phipatanakul W School Inner-City Asthma Intervention Study (SICAS-2) (SICAS-2). https://clinicaltrials.gov/ct2/show/NCT02291302?term=Phipatanakul&draw=2&rank=2.
- 122.Phipatanakul W, Koutrakis P, Coull BA, et al. The School Inner-City Asthma Intervention Study: Design, rationale, methods, and lessons learned. Contemp Clin Trials. 2017;60:14–23. 10.1016/j.cct.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cromar KR, Gladson LA, Ewart G. Trends in Excess Morbidity and Mortality Associated with Air Pollution above American Thoracic Society–Recommended Standards, 2008–2017. Ann Am Thorac Soc. 2019;16(7):836–845. 10.1513/AnnalsATS.201812-914OC [DOI] [PubMed] [Google Scholar]


