Abstract
Background
Warfarin use can trigger the occurrence of bleeding independently or as a result of a drug–drug interaction when used in combination with nonsteroidal anti-inflammatory drugs (NSAIDs).
Objectives
This article examines the risk of bleeding in individuals exposed to concomitant warfarin and NSAID compared with those taking warfarin alone (Prospero Registry ID 145237).
Methods
PubMed, EMBASE, Scopus, and Web of Science were searched. The primary outcome of interest was gastrointestinal bleeding and general bleeding. Summary effects were calculated to estimate average treatment effect using random effects models. Heterogeneity was assessed using Cochran’s Q and I2. Risk of bias was also assessed using the Agency for Healthcare Research and Quality bias assessment tool.
Results
A total of 651 studies were identified, of which 11 studies met inclusion criteria for meta-analysis. The odds ratio (OR) for gastrointestinal bleeding when exposed to warfarin and an NSAID was 1.98 (95% confidence interval [CI]: 1.55–2.53). The risk of gastrointestinal bleeding was also significantly elevated with exposure to a COX-2 inhibitor and warfarin relative to warfarin alone (OR = 1.90, 95% CI: 1.46–2.46). There was an increased risk of general bleeding with the combination of warfarin with NSAIDs (OR = 1.58, 95% CI: 1.18–2.12) or COX-2 inhibitors (OR = 1.54, 95% CI: 0.86–2.78) compared with warfarin alone.
Conclusion
Risk of bleeding is significantly increased among persons taking warfarin and a NSAID or COX-2 inhibitor together as compared with taking warfarin alone. It is important to caution patients about taking these medications in combination.
Keywords: warfarin, nonsteroidal anti-inflammatory drug, cyclooxygenase-2 inhibitors, gastrointestinal hemorrhage, drug interactions
Introduction
Warfarin remains one of the most commonly used anticoagulants worldwide, despite the availability of new oral anticoagulants. The effectiveness of warfarin in preventing and reducing the occurrence of thromboembolic events in patients at risk of thromboembolism and in those with prosthetic implants is widely established.1,2 However, warfarin use can trigger the occurrence of bleeding individually or as a result of a drug–drug interaction (DDI) when used in combination with other medications, especially those with a propensity to also result in bleeding.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also commonly used and have been associated with gastrointestinal (GI) bleeding.3 NSAIDs reduce prostaglandin (PG) synthesis, with differences in the extent of inhibition of the cyclooxygenase enzymes COX-1 and COX-2. While all NSAIDs can inhibit COX-1 and COX-2 enzymes, nonselective NSAIDs have varying effects on bleeding and cardiovascular risk, mostly related with treatment dose and utilization time; Likewise, selective COX-2 inhibitors may increase the occurrence of various cardiovascular events. The Food and Drug Administration has determined that an increased risk of serious adverse cardiovascular events may be a class consequence for all NSAIDs.4,5
Bleeding risk is considerably higher in older people, many of whom take both warfarin and an NSAID or COX-2 inhibitor. The mechanisms of this DDI are believed to be related to an effect on platelet function and gastric mucosal damage thorough inhibition of COX-1. These consequences in combination with the anticoagulant effect of warfarin raises the risk of bleeding without producing changes in the international normalized ratio.6 Also, there is some evidence suggesting that COX-2 inhibitors might not be a safer option in patients requiring an NSAID and warfarin.7,8
The specific aim of this study is to systematically evaluate the risk of bleeding in individuals exposed to concomitant warfarin and a NSAID compared with warfarin alone.
Methods
This study was a systematic literature review and meta-analysis of studies evaluating bleeding events among individuals exposed to warfarin and NSAIDs compared with warfarin alone. The null hypothesis of this study was that exposure to the combination of warfarin and NSAIDs did not increase the risk of bleeding relative to warfarin alone.
Study Identification
This study was conducted according to the Meta-analysis Of Observational Studies in Epidemiology guidelines.9 We identified published studies of DDIs involving warfarin, NSAIDs, and selective COX-2 inhibitors, focusing on GI bleeding and general bleeding, using four abstracting databases: PubMed, EMBASE, Scopus, and Web of Science, using university-affiliated Internet access to these databases. The search strategy incorporated the following search terms and combinations: “warfarin,” “NSAID,” “NSAIDs,” “non-steroidal anti-inflammatory drugs,” “anti-inflammatory agents,” “COX-2 selective inhibitors,” “coxibs,” and each individual product common name within the therapeutic classes of NSAIDs and COX-2 inhibitors. These terms were combined with “bleeding,” “gastrointestinal bleeding,” “gastrointestinal risk,” “NSAID gastropathy,” “upper gastrointestinal complications,” “gastrointestinal hemorrhage,” “drug–drug interaction,” “interaction,” and “drug interaction” to identify relevant articles. No restriction in publication dates was applied and articles published in other than English were evaluated by multilingual researchers. Searches using the aforementioned databases were completed in April 2019. The electronic search was complemented by a manual review of references cited by included articles. Searches were conducted by clinical pharmacists and reviewed by researchers with experience in both meta-analyses and drug interactions. There was no need to contact study authors for clarification of reported data.
Study Selection and Outcome Measures
Study selection was accomplished by first evaluating study titles, abstracts, and then the full text of reports. Two researchers identified potential studies with final determination made by a third researcher. Studies were included if: (1) data were provided on cohorts exposed to warfarin alone and those receiving the combination of warfarin and an NSAID or selective COX-2 inhibitor; (2) publications reported the time for the concomitant use of the drug–drug combination; (3) reported number of patients experiencing GI bleeding or general bleeding was defined through the International Classification of Diseases codes (ICD-9 or ICD-10) as an outcome; and (4) authors reported a crude or adjusted measure of association between exposure and outcome (e.g., odds ratio [OR]) or reported the number of patients receiving warfarin alone and warfarin with NSAID or selective COX-2 inhibitor and the number of bleeding events by exposure cohort, allowing for a measure of association to be calculated.
Studies were excluded if they did not report original findings (e.g., review articles), if warfarin was not the comparator agent for bleeding events, or if insufficient information was provided to determine the risk of bleeding. For each study, details about design, year of publication, country, number of cases, age or age range of participants, type and duration of drug therapy, measure of association, and 95% confidence interval (95% CI) were extracted. For each study, the authors examined characteristics of exposed and unexposed subjects to identify potential confounding.
Risk of Bias
The inclusion of observational studies in systematic reviews substantially increases the challenges in establishing causal inference because, by design, they do not assure randomization and allocation concealment. To assess risk of bias, a method proposed by the Agency for Healthcare Research and Quality was used.10 Studies included were analyzed considering the following items: allocation, selection, performance, detection, and reporting bias, as well as inadequate study size and study efficiency. Each item was categorized as having a low risk of bias, unclear risk of bias, or high risk of bias.
Statistical Analysis
Summary effects were calculated as an estimation of the average treatment effect of 95% CI. Using conventional metaanalyses methods, pooled ORs were calculated. Two-sided p-value of < 0.05 was considered statistically significant. In addition, a random-effects model that assumes different underlying true effects was used. This model was selected because of the nature of the studies included—observational studies—that are expected to have varying study designs, including different types of patients, slightly different approaches in measuring bleeding events, and unique sources of data.11 Statistical heterogeneity was evaluated using the I2 statistic, with values of 25, 50, and 75% considered low, intermediate, and high inconsistency, respectively. Cochran’s Q statistics was reported for each analysis. Significant heterogeneity was assumed when the Cochran’s Q statistic p-value was less than 0.10 and the I2 was greater than 50%.11 Publication bias was assessed by composing a funnel plot for the rate of GI bleeding and general bleeding using Egger’s test.12 A lack of publication bias was defined by a symmetric funnel-shaped distribution and by a two-tailed significance level of p > 0.05 in Egger’s test.
To evaluate robustness of the findings, sensitivity analyses were performed using the leave-one-out approach by iteratively removing one study at a time and recalculating the pooled OR. Combined ORs remaining stable is indicative that results were not driven by any single study and similar results could be obtained after excluding that study. The meta-analysis and the corresponding graphical visualization through a forest plot were performed with Comprehensive Meta-Analysis statistical software, version 3 (Biostat, Englewood, New Jersey, United States).
Patient Involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in the design and implementation of the study. No patients were asked to advice on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
Results
Study Characteristics
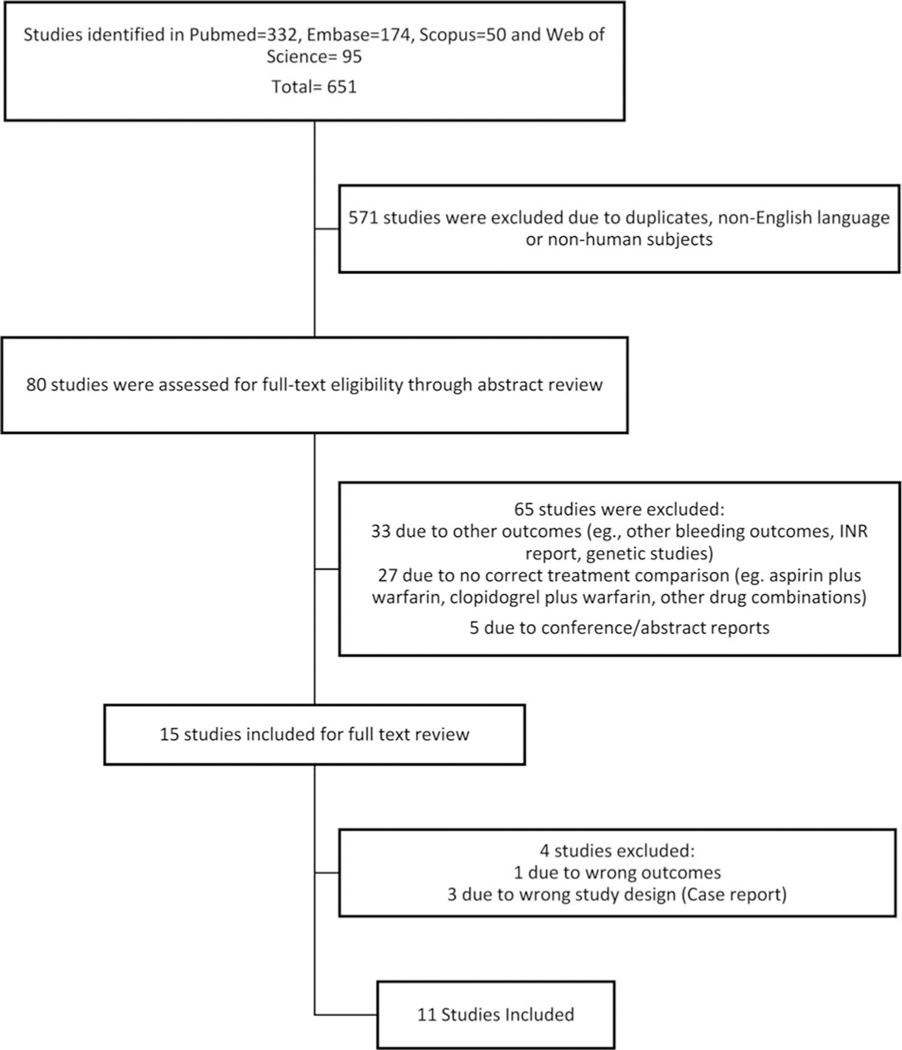
Based on the search strategy, a total of 651 studies were identified. After reviewing title and abstract, 571 studies were excluded because of duplication, publication in language other than English, or nonhuman subjects (►Fig. 1). An abstract review was conducted for the remaining 80 articles, and of these, a full-text review was conducted for 15 studies, with 11 studies selected to be included in the meta-analysis (2% of the studies initially considered). Seven of the included studies were conducted in North America, three in Europe, and one in Oceania. Five studies used retrospective observational cohort design while the other six used a case–control design. Seven reported GI bleeding and five reported general bleeding. Five articles reported results for the combination of warfarin and selective COX-2 inhibitor; of these, three reported GI bleeding and three reported general bleeding. Other demographic characteristics as well as variables considered in each model are summarized in ►Table 1.
Fig. 1.
Flow chart of study selection according to Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines.
Table 1.
Summary table of studies reporting gastrointestinal bleeding and general bleeding for warfarin alone and warfarin combined with NSAID or selective COX-2 inhibitor
| First author, publication year, country | Study design | Age (±SD or range) in years Warfarin indicationa | Outcome considered | No. cases | Concurrent therapy | Time with concomitant use | Reported multivariate odds ratio (95% CI) | Potential confounding variables |
|---|---|---|---|---|---|---|---|---|
| Shorr et al, 1993, United States14 | Retrospective cohort | Over 65 | GI bleeding | 8 | NSAID | 30 d | 2.66 (1.12–6.27) | Adjusted for history of ulcer disease, aspirin use, cigarette smoking, and heavy alcohol use |
| Johnsen et al, 2001, Denmark15 | Retrospective cohort | Over 16 | GI bleeding | 269 | NSAID | 90 d | 3.38 (1.21–10.17) | Adjusted for concomitant drugs |
| Battistella et al, 2005, Canada7 | Case–control | 79.8 ± 6.9 | GI bleeding | 24 22 |
NSAID COX-2 |
90 d | 1.90 (1.16–3.11) 1.90 (1.40–2.40) |
Adjusted by age, gender, potential interaction with other medications, prior GI bleeding, comorbidities, use of other gastrotoxic medications, and antiulcer agents (e.g., glucocorticoids) |
| Chung et al, 2005, United States20 | Case–control | Average: 61.6 | GB | 123 | COX-2 | 30 d | 1.34 (0.70–2.57) | Adjusted for age and concomitant medications |
| Zhang et al, 2006, United States19 | Retrospective cohort | 64.29 ± 14.6 | GB | 700 | NSAID | At least 7 d | 1.88 (1.69–2.11) | Adjusted for age, gender, average dose of warfarin, and physician specialty |
| Hauta-Aho et al, 2009, Finland16 | Case–control | NSAID: 68 (17–97) COX-2: 68 (24–92) |
GI bleeding GB GI bleeding GB |
8 44 2 12 |
NSAID COX-2 |
At least 7 d | 2.79 (0.88–8.91) 2.57 (1.56–4.23) 2.91 (0.33–25.79) 3.10 (1.44–6.67) |
Adjusted for age, sex, ward, proton-pump inhibitor, and oral glucocorticoid medications |
| Cheetham et al, 2009, United States13 | Case–control | NSAID: 66.2 ± 12.8 Coxib: 67.9 ± 11.9 Coronary artery disease Congestive heart failure |
GI bleeding | 44 6 |
NSAID COX-2 |
NSAID + warfarin: 71 d COX-2 + warfarin: 113 d |
3.58 (2.3–5.5) 1.71 (0.6–4.8) |
Adjusted for medication used (warfarin and selective COX-2), baseline diseases, and prior GI bleeding |
| Wallerstedt et al, 2009, Sweden22 | Case–control | Over 55 Atrial fibrillation |
GB | 2 | NSAID | 14 d | 1.97 (0.44–8.74) | Adjusted for age, sex, and concomitant medications |
| Schelleman et al, 2011, United States17 | Case–control | Over 18 | GI bleeding | 721 | NSAID | 30 d | 1.68 (1.55–1.81) | Adjusted for index date, date, and state. Eligible controls consisted of warfarin users who had not been hospitalized with diagnosis of gastrointestinal bleeding |
| Vitry et al, 2011, Australia21 | Retrospective cohort | Over 65 | GB | 69 27 |
NSAID COX-2 |
28 d | 1.19 (0.90–1.59) 1.07 (0.69–1.68) |
Adjusted for age, gender, socioeconomic index, number of comorbidities, number of prescribers, previous 1-y bleeding before first warfarin prescription, other medications |
| Mosholder et al, 2013, United States18 | Retrospective cohort | Over 18 Heart failure Ischemic stroke |
GI bleeding GB |
NS NS |
NSAID | 14 d | 1.39 (1.14–1.66) 1.24 (1.08–1.43) |
Adjusted for age, gender, geographical region, income, hospitalization, emergency room visit, other medications |
Abbreviations: CI, confidence interval; COX-2, selective cyclooxygenase-2 inhibitors; GB, general bleeding; GI, gastrointestinal; NS, nonspecified; NSAID, nonsteroidal anti-inflammatory drug; SD, standard deviation.
Warfarin indication reported when available.
Outcomes
Gastrointestinal Bleeding
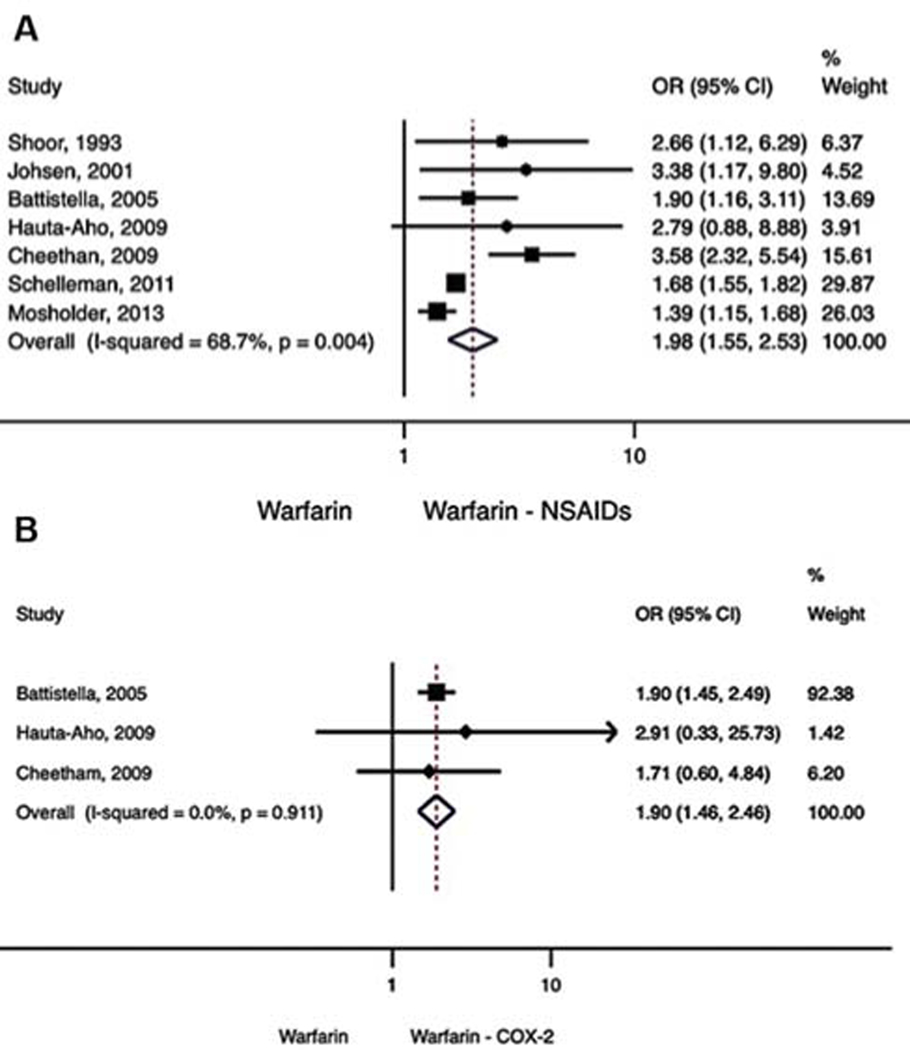
In an analysis of seven studies7,13–18 that examined NSAIDs as a class and bleeding when taken concurrently with warfarin, a significant difference in the risk of GI bleeding was observed (OR = 1.98, 95% CI: 1.55–2.53) (►Fig. 2A), but the degree of heterogeneity was high (Q statistic = 19.2; I2 = 68.7; z-value = 5.51; p < 0.001). In the analysis of warfarin and COX-2 inhibitor combining three studies, a significant difference in the risk of GI bleeding was observed between patients taking warfarin alone compared with warfarin plus COX-2 inhibitor (OR = 1.90, 95% CI: 1.46–2.46, ►Fig. 2B), but no significant heterogeneity was present (Q statistic = 0.19; I2 = 0.00, p = 0.91).
Fig. 2.
(A) Gastrointestinal bleeding. Warfarin – NSAID. (B) Gastrointestinal bleeding. Warfarin – COX-2.
General Bleeding Events
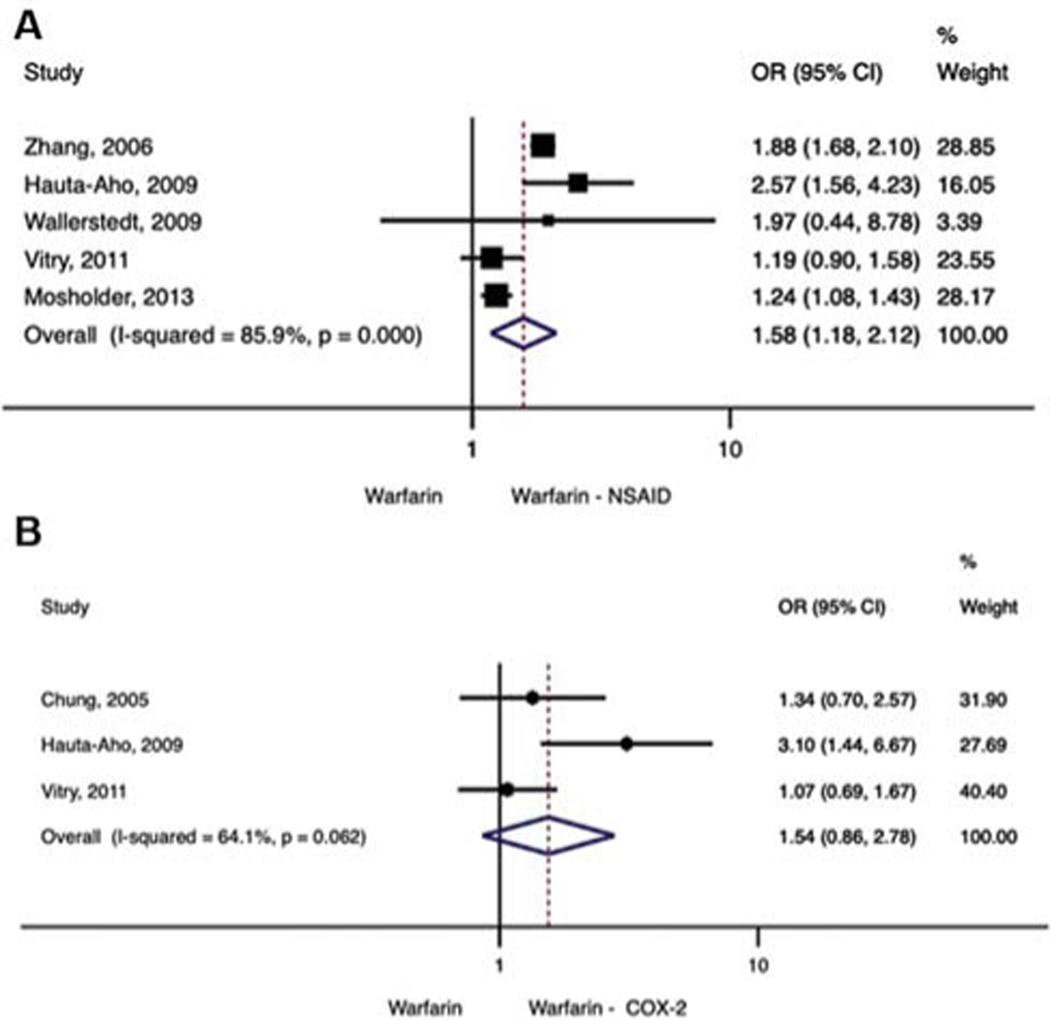
Five studies reported bleeding across multiple sites.18–22 For these studies, there was a significant difference in risk of general bleeding observed between patients taking warfarin alone compared with warfarin plus NSAID (OR = 1.58, 95% CI: 1.18–2.12) (►Fig. 3A). A random-effects meta-analysis was used because there was evidence of heterogeneity among the studies (Q statistic = 28.4, I2 = 85.9, z-value = 3.07, p = 0.002). In an analysis of warfarin and COX-2 inhibitors across three studies, no significant difference in general bleeding was observed between patients taking warfarin alone compared with warfarin plus COX-2 inhibitor (OR = 1.54, 95% CI: 0.86–2.78; Q statistic = 5.54: I2 = 63.9: p = 0.062) (►Fig. 3B).
Fig. 3.
(A) General bleeding, Warfarin – NSAID. (B) General bleeding, Warfarin – COX-2.
Sensitivity Analysis
Results of the sensitivity analysis using the leave-one-out approach are shown in ►Supplementary Appendix S1 (available in the online version). In this figure, each row displays the summary values computed when that row’s study is removed from the meta-analysis rather than the results of a single study. The values in the first row for the outcome of GI bleeding for the warfarin and NSAID combination represent the summary computations for six studies, when the study by Shorr et al was excluded. Results show that the direction and magnitude of the combined studies did not change with the exclusion of individual studies, indicating the results have a high level of reliability.
Publication Bias
A visual inspection of the funnel plots in ►Supplementary Appendix S2 (available in the online version) shows that studies exploring the warfarin and NSAIDs combination are not symmetrical; however, Egger’s test was not significant (p-value = 0.18). In the case of studies including and exploring the warfarin and COX-2 inhibitor combination, there is a symmetrical distribution, also corroborated by Egger’s test, p-value = 0.741. Thus, overall, no statistically significant evidence of publication bias was detected for all outcomes studied (►Supplementary Appendix S2, available in the online version).
Risk of Bias
The results of the risk of bias assessment evaluating seven factors that can influence study results are shown in ►Supplementary Appendix S3 (available in the online version). All studies showed low risk in terms of allocation, selection, performance, and detection bias. Reporting bias risk was low for eight studies (73%) and unclear for three studies (27%). When study size was evaluated, six studies (55%) had low risk of bias, four (36%) showed unclear risk, and one (9%) had high risk of bias. When lack of study efficiency was assessed, seven studies (64%) were evaluated as having low risk of bias, while the four remaining studies (36%) had unclear risk of bias.
Discussion
This study sought to evaluate the existence of a significant DDI between warfarin and NSAIDs, including COX-2 inhibitors, with respect to increased risk in GI bleeding and general bleeding. The principal findings were that the addition of an NSAID, including selective COX-2 inhibitors, to warfarin increased the risk of GI bleeding nearly twofold relative to warfarin alone. Therefore, patients already taking warfarin should avoid these combinations.
Limiting the use of NSAIDs is challenging because they are one of the most commonly used medications due to their effectiveness as analgesics, antipyretics, and anti-inflammatory agents. However, it is well known that NSAIDs can inflict damage to gastric and duodenal mucosa, significantly contributing to morbidity and, in some cases, mortality.23–25 While the exact mechanism of this interaction is unknown, it is believed that it involves the blockage of cyclooxygenase (COX-1 and 2) enzymes, limiting the synthesis of PG. PG contributes to the production of mucosa-protective substances, such as epithelial mucin and bicarbonate. A reduction in PG activity induced by NSAIDs may lead to gastric and/or duodenal ulcer formation.26
Unlike NSAIDs, COX-2-selective inhibitors spare the COX-1 enzyme in GI mucosa and in platelets, therefore causing less GI injury and interference with platelet activity.27 As a result, COX-2 inhibitors are believed to carry a lower risk of GI bleeding.28 However, there is evidence that patients with a variant in cytochrome P450 2C9 *2 or *3 alleles who are receiving a selective COX-2 inhibitor may be at increased risk of bleeding. Variants of the *2 and *3 alleles have been identified in 11 and 7% of European populations, respectively, and may alter the pharmacokinetics of warfarin.29 Furthermore, in a nationwide cohort study, it was demonstrated that patients taking COX-2 selective inhibitors have an increased risk of upper GI bleeding especially if they had history of uncomplicated peptic ulcer or Helicobacter pylori infection.30
Our analysis found that patients receiving the combination of warfarin and selective COX-2 inhibitors experience an almost twofold increase risk in GI bleeding, similar to traditional NSAIDs. For those studies evaluating bleeding from any source, no significant increase was observed.
Warfarin is the most widely used vitamin K antagonist. It has a variety of indications for prophylaxis and treatment of thromboembolic diseases and atrial fibrillation. It competitively inhibits a series of coagulation factors, as well as proteins C and S. These factors are biologically activated by the addition of carboxyl groups depending on vitamin K. Warfarin competitively inhibits this chemical reaction, thus depleting functional vitamin K reserves and hence reducing synthesis of active coagulation factors.31 Understanding the pharmacological properties of warfarin prior to use is crucial to maximizing the therapeutic benefit and minimizing patient harm when treating patients with polypharmacy who are at risk of DDIs.32
While patients on warfarin are prone to bleeding from any source, it most frequently occurs from the GI tract. Thus, avoiding NSAIDs and/or limiting and closely monitoring their use is paramount to preventing serious complications from these medications. Other recommendations include avoiding excess alcohol consumption and evaluating and treating H. pylori, if appropriate.33
The safety of warfarin has been tested against novel oral anticoagulants (NOACs) in large randomized controlled studies in patients with stroke and in patients with atrial fibrillation.34–36 These studies suggest that bleeding events with the NOACs are less frequent than with warfarin. However, a study of U.S. veterans that compared rates of GI bleeding of patients receiving the NOACs dabigatran, rivaroxaban, or apixaban with those of patients taking warfarin found the risk of GI bleeding was more than four times higher in patients receiving warfarin, adjusting for prior history of GI bleeding and concomitant use of proton–pump inhibitors (PPIs) and antiplatelet agents.37 Another study of patients with atrial fibrillation has shown that taking warfarin or dabigatran in combination with a nonselective NSAID (no data on individual drugs was provided) as compared with those on warfarin or dabigatran alone was associated with a higher risk of major bleeding, GI bleeding, and stroke.38
In a large population-based study, PPIs reduced gastric acid production and were used to prevent upper GI bleeding in patients receiving warfarin, lowering the incidence of hospitalization.39 A case–control study showed that PPIs reduced the risk of upper GI bleeding in patients taking NSAIDs and also showed a significant advantage for patients taking warfarin and NSAIDs concomitantly.40
DDIs, in general, are associated with an elevated risk of hospitalization and are responsible for an estimated 1 to 5% of all hospitalizations and 14% of adverse events among elderly patients.41 Prescribers and pharmacists often have inadequate knowledge or evidence regarding DDIs and how to properly manage DDIs when patient exposure cannot be avoided.42 Clinical decision support to warn prescribers and pharmacists of potentially harmful medication combinations is necessary, as is summarized evidence on DDIs.43 Despite these safety nets, up to 25% of patients receiving warfarin also receive an NSAID,44 suggesting that the risk of bleeding is not well recognized.
A sensitivity analysis was conducted in this meta-analysis using the leave-one-out approach and showed that estimates of the risk of GI bleeding and general bleeding as result of DDIs between warfarin and NSAIDs and between warfarin and COX-2 inhibitors does not appear to be driven by a single study. No publication bias was observed through Egger’s test or the visual funnel plot assessment. Risk bias assessment showed that in general studies included in this meta-analysis had low risk of bias; however, due to the observational nature of the studies included, not all showed an adequate study size.
Our study has some limitations that should be considered when interpreting the findings. The evidence included in this investigation utilized large administrative databases with large samples to evaluate the coadministration of warfarin and NSAIDs and its association with the occurrence of GI bleeding and overall bleeding. Using claims databases reduces the potential of selection bias that is commonly observed with recruitment in clinical trials. However, observational studies have a disadvantage because assessing causality can be challenging due to potential confounders. All included studies reported their results using multivariate regression models that control for some potential confounders (►Table 1). In our study, we included those studies that provided estimates of risk that had been adjusted by confounders for GI and general bleeding. In this analysis, we could not evaluate the effects of patient demographics, comorbidities, or indications of use on bleeding risk due to the lack of reporting. We also observed moderate to high heterogeneity when all NSAIDs were evaluated, suggesting that there may be underlying differences across the studies. Future studies evaluating bleeding with warfarin and NSAIDs should report more details about risk factors associated with bleeding and also report the results by each NSAID agent. Heterogeneity may have been due to risk of bleeding varying by NSAID product. Furthermore, the definition used for bleeding events varied across the studies and this may have contributed to differences in the observed ORs.
In conclusion, the concomitant use of warfarin and NSAIDs, including COX-2 inhibitors, should generally be avoided due to an increased risk of DDI leading to GI bleeding or general bleeding compared with taking warfarin alone. These combinations should only be used when the benefits outweigh the risks and for a short period of time under careful monitoring. Patients should be instructed on the need to closely watch for signs of bleeding when taking both agents simultaneously.
Supplementary Material
What is known about this topic?
Many patients use warfarin and NSAIDs concurrently, both types of medications increase the risk of bleeding independently, and some studies suggest there is an increased risk of bleeding when used concurrently. However, not all studies on the topic have shown a significant effect and the overall magnitude of the potential drug-drug interaction was largely unknown.
What does this paper add?
There is twofold risk of gastrointestinal bleeding among individuals who take warfarin and NSAIDs as compared to warfarin alone.
This risk does not appear to be reduced in the presence of a selective Cox-2 inhibitor.
Patients should avoid taking warfarin and NSAIDs concurrently to avoid risk of bleeding.
Acknowledgments
Funding
This project was funded by the Agency for Healthcare Research and Quality, grant numbers R01HS025984 and R21HS023826.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Data Sharing
Data extracted from relevant studies is made available on request.
Conflict of Interest
None declared.
References
- 1.Heneghan C, Alonso-Coello P, Garcia-Alamino JM, Perera R, Meats E, Glasziou P. Self-monitoring of oral anticoagulation: a systematic review and meta-analysis. Lancet 2006;367(9508):404–411 [DOI] [PubMed] [Google Scholar]
- 2.Keeling D, Baglin T, Tait C, et al. Guidelines on oral anticoagulation with warfarin – fourth edition. Br J Haematol 2011;154(03):311–324 [DOI] [PubMed] [Google Scholar]
- 3.Goldstein JL, Cryer B. Gastrointestinal injury associated with NSAID use: a case study and review of risk factors and preventative strategies. Drug Healthc Patient Saf 2015;7:31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh R, Alajbegovic A, Gomes AV. NSAIDs and cardiovascular diseases: role of reactive oxygen species. Oxid Med Cell Longev 2015;2015:536962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meek IL, Van de Laar MAFJ, E Vonkeman H.. Non-steroidal antiinflammatory drugs: an overview of cardiovascular risks. Pharmaceuticals (Basel) 2010;3(07):2146–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter M, Berry H, Pelletier AL; W. P. Clinically relevant drug-drug interactions in primary care. Am Fam Physician 2019;99 (09):558–564 [PubMed] [Google Scholar]
- 7.Battistella M, Mamdami MM, Juurlink DN, Rabeneck L, Laupacis A. Risk of upper gastrointestinal hemorrhage in warfarin users treated with nonselective NSAIDs or COX-2 inhibitors. Arch Intern Med 2005;165(02):189–192 [DOI] [PubMed] [Google Scholar]
- 8.Mamdani M, Juurlink DN, Kopp A, Naglie G, Austin PC, Laupacis A. Gastrointestinal bleeding after the introduction of COX 2 inhibitors: ecological study. BMJ 2004;328(7453):1415–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283(15):2008. [DOI] [PubMed] [Google Scholar]
- 10.Viswanathan M, Berkman ND, Dryden DM, Hartling L. Assessing Risk of Bias and Confounding in Observational Studies of Interventions or Exposures: Further Development of the RTI Item Bank; 2013. Available at: www.ahrq.gov. Accessed July 10, 2019 [PubMed]
- 11.Barili F, Parolari A, Kappetein PA, Freemantle N. Statistical primer: heterogeneity, random- or fixed-effects model analyses? Interact Cardiovasc Thorac Surg 2018;27(03):317–321 [DOI] [PubMed] [Google Scholar]
- 12.Sedgwick P. Meta-analyses: how to read a funnel plot. BMJ 2013; 346(02):13–42 [DOI] [PubMed] [Google Scholar]
- 13.Cheetham TC, Levy G, Niu F, Bixler F. Gastrointestinal safety of nonsteroidal antiinflammatory drugs and selective cyclooxygenase-2 inhibitors in patients on warfarin. Ann Pharmacother 2009; 43(11):1765–1773 [DOI] [PubMed] [Google Scholar]
- 14.Shorr RI, Ray WA, Daugherty JR, Griffin MR. Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants places elderly persons at high risk for hemorrhagic peptic ulcer disease. Arch Intern Med 1993;153(14):1665–1670 [PubMed] [Google Scholar]
- 15.Johnsen SP, Sørensen HT, Mellemkjoer L, et al. Hospitalisation for upper gastrointestinal bleeding associated with use of oral anticoagulants. Thromb Haemost 2001;86(02):563–568 [PubMed] [Google Scholar]
- 16.Hauta-Aho M, Tirkkonen T, Vahlberg T, Laine K. The effect of drug interactions on bleeding risk associated with warfarin therapy in hospitalized patients. Ann Med 2009;41(08):619–628 [DOI] [PubMed] [Google Scholar]
- 17.Schelleman H, Brensinger CM, Bilker WB, Hennessy S. Antidepressant-warfarin interaction and associated gastrointestinal bleeding risk in a case-control study. PLoS One 2011;6(06): e21447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosholder AD, Racoosin JA, Young S, et al. Bleeding events following concurrent use of warfarin and oseltamivir by Medicare beneficiaries. Ann Pharmacother 2013;47(11):1420–1428 [DOI] [PubMed] [Google Scholar]
- 19.Zhang K, Young C, Berger J. Administrative claims analysis of the relationship between warfarin use and risk of hemorrhage including drug-drug and drug-disease interactions. J Manag Care Pharm 2006;12(08):640–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung L, Chakravarty EF, Kearns P, Wang C, Bush TM. Bleeding complications in patients on celecoxib and warfarin. J Clin Pharm Ther 2005;30(05):471–477 [DOI] [PubMed] [Google Scholar]
- 21.Vitry AI, Roughead EE, Ramsay EN, et al. Major bleeding risk associated with warfarin and co-medications in the elderly population. Pharmacoepidemiol Drug Saf 2011;20(10):1057–1063 [DOI] [PubMed] [Google Scholar]
- 22.Wallerstedt SM, Gleerup H, Sundström A, Stigendal L, Ny L. Risk of clinically relevant bleeding in warfarin-treated patients–influence of SSRI treatment. Pharmacoepidemiol Drug Saf 2009;18 (05):412–416 [DOI] [PubMed] [Google Scholar]
- 23.Lanas A, Perez-Aisa MA, Feu F, et al. ; Investigators of the Asociación Española de Gastroenterología (AEG). A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal antiinflammatory drug use. Am J Gastroenterol 2005;100(08): 1685–1693 [DOI] [PubMed] [Google Scholar]
- 24.Sostres C, Gargallo CJ, Lanas A. Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res Ther 2013;15(03, Suppl 3):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsui H, Shimokawa O, Kaneko T, Nagano Y, Rai K, Hyodo I. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J Clin Biochem Nutr 2011;48(02):107–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang M, Wang H-T, Zhao M, et al. Network meta-analysis comparing relatively selective COX-2 inhibitors versus coxibs for the prevention of NSAID-induced gastrointestinal injury. Medicine (Baltimore) 2015;94(40):e1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knijff-Dutmer EAJ, Van der Palen J, Schut G, Van de Laar MAFJ. The influence of cyclo-oxygenase specificity of non-steroidal anti-inflammatory drugs on bleeding complications in concomitant coumarine users. QJM 2003;96(07):513–520 [DOI] [PubMed] [Google Scholar]
- 28.Wright JM. The double-edged sword of COX-2 selective NSAIDs. CMAJ 2002;167(10):1131–1137 [PMC free article] [PubMed] [Google Scholar]
- 29.Malhi H, Atac B, Daly AK, Gupta S. Warfarin and celecoxib interaction in the setting of cytochrome P450 (CYP2C9) polymorphism with bleeding complication. Postgrad Med J 2004;80(940): 107–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin X-H, Young S- H, Luo J- C, et al. Risk factors for upper gastrointestinal bleeding in patients taking selective COX-2 inhibitors: a nationwide population-based cohort study. Pain Med 2018;19 (02):225–231 [DOI] [PubMed] [Google Scholar]
- 31.Fawzy AM, Lip GYH. Pharmacokinetics and pharmacodynamics of oral anticoagulants used in atrial fibrillation. Expert Opin Drug Metab Toxicol 2019;15(05):381–398 [DOI] [PubMed] [Google Scholar]
- 32.Witt DM, Clark NP, Kaatz S, Schnurr T, Ansell JE. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J Thromb Thrombolysis 2016;41(01): 187–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaduganathan M, Bhatt DL. Gastrointestinal Bleeding With Oral Anticoagulation: Understanding the Scope of the Problem. Clin Gastroenterol Hepatol 2017;15(05):691–693 [DOI] [PubMed] [Google Scholar]
- 34.Patel MR, Mahaffey KW, Garg J, et al. ; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365(10):883–891 [DOI] [PubMed] [Google Scholar]
- 35.Granger CB, Alexander JH, McMurray JJV, et al. ; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365(11):981–992 [DOI] [PubMed] [Google Scholar]
- 36.Connolly SJ, Ezekowitz MD, Yusuf S, et al. ; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361(12): 1139–1151 [DOI] [PubMed] [Google Scholar]
- 37.Cangemi DJ, Krill T, Weideman R, Cipher DJ, Spechler SJ, Feagins LA. A comparison of the rate of gastrointestinal bleeding in patients taking non-vitamin K antagonist oral anticoagulants or warfarin. Am J Gastroenterol 2017;112(05):734–739 [DOI] [PubMed] [Google Scholar]
- 38.Kent AP, Brueckmann M, Fraessdorf M, et al. Concomitant oral anticoagulant and nonsteroidal anti-inflammatory drug therapy in patients with atrial fibrillation. J Am Coll Cardiol 2018;72(03): 255–267 [DOI] [PubMed] [Google Scholar]
- 39.Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA 2018;320(21):2221–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology 2016;151(06): 1105–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dechanont S, Maphanta S, Butthum B, Kongkaew C. Hospital admissions/visits associated with drug-drug interactions: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf 2014;23(05):489–497 [DOI] [PubMed] [Google Scholar]
- 42.Hines LE, Malone DC, Murphy JE. Recommendations for generating, evaluating, and implementing drug-drug interaction evidence. Pharmacotherapy 2012;32(04):304–313 [DOI] [PubMed] [Google Scholar]
- 43.Troiano D, Jones MA, Smith AH, et al. The need for collaborative engagement in creating clinical decision-support alerts. Am J Health Syst Pharm 2013;70(02):150–153 [DOI] [PubMed] [Google Scholar]
- 44.Malone DC, Hutchins DS, Haupert H, et al. Assessment of potential drug-drug interactions with a prescription claims database. Am J Health Syst Pharm 2005;62(19):1983–1991 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.