Background.
In organ transplantation, the University of Wisconsin (UW) solution has been the gold standard for organ preservation. Quercetin (Que) has numerous antioxidant and anti-inflammatory activities, and sucrose (Suc) may be effective for cold storage (CS). This study aimed to investigate the in vitro protective effect of Que and Suc on cold injury to the kidney and to determine whether Que + Suc could improve ischemia-reperfusion injury during CS and hypothermic oxygenated perfusion (HOPE) in autologous transplantation models.
Methods.
BHK-21 cells were stored at 4°C for 3 days in UW solution for CS/machine perfusion (CS/MP-UW) with Que (33.1 μM, 3.3 μM, 0.33 μM) and Suc (0.1 M). In a porcine model of renal autologous transplantation, left kidney grafts were preserved under 3 conditions: group 1, CS preservation for 24 hours; group 2, CS preservation for 22 hours and HOPE with CS/MP-UW solution for 2 hours; and group 3, identical preservation as group 2, with Que and Suc added to the solution. Animals were euthanized on day 7 after autologous transplantation.
Results.
After 3 days of CS preservation, the CS/MP-UW solution with Que (33.1 μM, 3.3 μM) and Suc showed significant cell protection against cold injury. In the porcine model of renal autologous transplantation, the last blood Cre level and the blood lipid hydroperoxide on posttransplantation day 2 were significantly different between group 1 and group 3. Moreover, the total endothelial, glomerular, tubular, interstitial (EGTI) histology score in the kidney tissue was also significantly different. Regarding the change in renal resistance in HOPE, the decrease observed in group 3 was significantly larger than that in group 2.
Conclusions.
Our results suggest that the addition of Que and Suc to a UW solution can improve kidney preservation and could potentially enhance the outcome of kidney transplantation.
INTRODUCTION
The shortage of organ donors is a serious problem for transplantation throughout the world. The University of Wisconsin (UW) solution has been the gold standard preservation solution for the liver, kidneys, pancreas, and small bowel since its development by Belzer and Southard,1 and considerable efforts are underway to improve its efficacy. Paralleling the introduction of the UW solution, advances in machine perfusion technology and techniques have rendered machine perfusion a safe and effective method for preserving the kidneys for transplantation.2 Among the available machine perfusion techniques, hypothermic oxygenated perfusion (HOPE) is a promising preservation strategy as it may have comparable effects to immunosuppressive drugs.3,4 However, the technology of preservation solution used for machine perfusion is inferior to that used for cold storage (CS).
Quercetin (Que), one of the most widely used flavonoids, reportedly exerts numerous effects, including antioxidative,5 anti-inflammatory,6 and anti-apoptotic7 activities, and certain flavonoids attenuate cell injury to the renal tubules during CS in the Euro-Collins and UW solution.8 The beneficial effect of a preservation solution may result from its ability to prevent tissue edema during CS. Various studies have investigated the efficacy of different impermeants during CS of organs in solution. Among these, sucrose (Suc) may be an effective impermeant for CS.9 Several studies have shown the positive effects of preserving animal organs in a solution containing Que or Suc.7,8,10-12 However, studies wherein organs are preserved and transplanted using a preservation solution containing Que or Suc for clinical application are lacking. This study aimed to investigate the protective effects of Que and Suc on cold injury inflicted on the kidney in vitro and evaluate whether their use improves ischemia-reperfusion (I/R) injury during CS and HOPE in autologous transplantation models.
MATERIALS AND METHODS
Reagents and Preparation of Preservation Solutions Containing Que and Suc
Physiological saline solution (Otsuka Normal Saline; Otsuka Pharmaceutical Factory, Naruto, Japan) and the UW (CS-UW) solution (Belzer UW Cold Storage Solution; Bridge to Life Ltd., Columbia, SC) or the UW (MP-UW) solution (Machine Perfusion Solution-Belzer UW; Bridge to Life (Europe) Ltd., London, United Kingdom) were used as preservation solutions. Suc (Nacalai Tesque, Inc., Kyoto, Japan) was prepared in CS-UW or MP-UW solution to a concentration of 0.1 M. Que (Que hydrate, ≥95%; Sigma Aldrich, St. Louis, MO) dissolved in dimethylsulfoxide (DMSO; Nacalai Tesque, Inc.) was prepared in these solutions to a final concentration of 0.33–33.1 μM. For the in vitro study, 10% fetal bovine serum (GE Healthcare HyClone, South Logan, UT) was added to DMEM (DMEM-high glucose; Sigma Aldrich).
Cell Culture and Experimental Protocol
BHK-21 cells (Syrian hamster, kidney, fibroblast-like) were grown in 10% FCS/DMEM at 37°C under a humidified atmosphere with 5% CO2. For experimental studies, cells were seeded in 96-well plates at a density of 10.0 × 103 cells/well and cultured in an incubator for 3 hours at 37°C under a humidified atmosphere with 5% CO2. After replacement of the regular cell culture medium by either preservation solution (100 μL/well; Saline, Culture medium, CS/MP-UW solution alone, CS/MP-UW solution + Suc 0.1 M + Que 33.1/3.3/0.33 μM, or CS/MP-UW solution + Suc 0.1 M + DMSO), the 96-well plate was stored in a cooling container at 4°C for 3 days.
Water-Soluble Tetrazolium Assay
Protection against cold injury was evaluated in BHK-21 cells using the Cell Counting-8 kit method. After storage for 3 days, each preservation solution on the 96-well plate was replaced by 10% FCS/DMEM (100 μL/well), and cells were cultured in an incubator for 1 h at 37°C under a humidified atmosphere with 5% CO2. Next, Water-Soluble Tetrazolium-8 solution (Cell Count Reagent SF; Nacalai Tesque, Inc.) was added to the cells at 0.1 mL/well, and cells were cultured for another 3 hours. Absorbance was measured at 450 nm using the EnSpire Multimode Plate Reader (PerkinElmer Inc., Waltham, MA). In an experiment performed in advance, the absorbance at various cell concentrations was measured to create a calibration curve. Using this curve, the cell number was calculated based on the measured absorbance (Figure 1).
FIGURE 1.
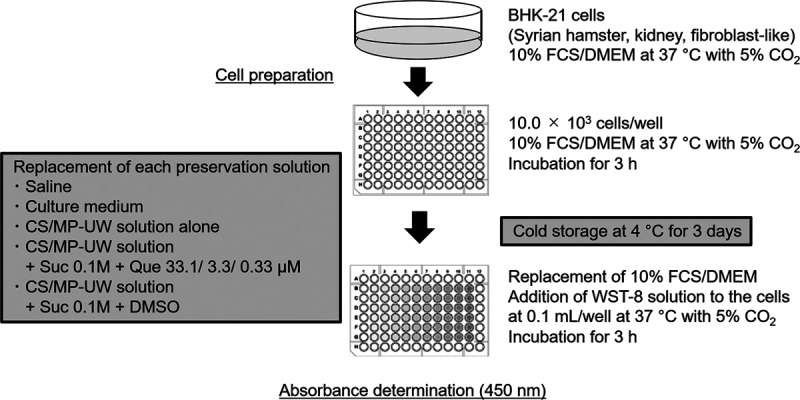
Study design of cold storage of BHK-21 cells. CS, cold storage; DMSO, dimethylsulfoxide; DMEM, Dulbecco’s modified Eagle’s medium; FCS, fetal calf serum; MP-UW, University of Wisconsin for machine perfusion; Que, quercetin; Suc, sucrose; WST, water-soluble tetrazolium.
Animals
Domestic female pigs crossbred with Large Yorkshire, Landrace, and Duroc female pigs (weight, approximately 20 kg; age, 2–3 months) were used. Animal care was provided in accordance with the Declaration of Helsinki. All animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals at Asahikawa Medical University and approved by the Animal Care and Use Committee of Asahikawa Medical University (permission number: 14 172). Procuring procedures were conducted under anesthesia.
Procurement and HOPE
Under general anesthesia, the abdomen was opened, and a catheter was placed into the internal jugular vein. The ureter, artery, and vein of the left kidney were identified, and the blood flow was cut off. The left kidney remained in a warm ischemic condition for 30 minutes (WIT 30 minutes), and a graft was then retrieved as a donation after cardiac death (DCD). The DCD kidney graft was washed from the renal artery with 200 mL of each preservation solution at 4–8°C as a back-table procedure. The DCD kidney grafts were preserved under CS with or without HOPE for 22–24 hours. The grafts were perfused using an originally developed machine perfusion system,13,14 consisting of a renal artery perfusion circuit. The circuit consisted of a roller-type pump (MasterFlex7520-40; Cole-Parmer, Vernon Hills, IL), an electrical flow meter for the renal arteries (FD-SS02; Keyence, Osaka, Japan), a ceramic capacitance pressure sensor (KL76; Nagano Keiki, Nagano, Japan), and an in-house-developed air trap. An oxygenator (HP0-06 H-C; Senko Medical Instrument, Tokyo, Japan) was installed in the circuit for the renal artery. The perfusate temperature was controlled by a heat exchanger and chilled water. The oxygenated perfusate supplied oxygen (O2) to the kidney grafts during machine perfusion under machine perfusion conditions using an oxygenator. Oxygen concentrations were controlled by adjusting the volumes of O2 and air using the air blender of the oxygenator. Machine perfusion started at a flow rate of 2.0 mL/min with 40% O2. The upper-pressure limits were adjusted within a renal artery pressure of 30 mm Hg. The temperature of the perfusate for machine perfusion was set at 8°C.15,16
Study Design
The kidney grafts were preserved under 3 conditions for 24 hours: group 1 (G1), preserved with CS (CS × 24h, n = 5); group 2 (G2), preserved with CS for 22 hours and perfused with HOPE with the CS/MP-UW solution for 2 hours (CS × 22h + HOPE × 2h, n = 5); and group 3 (G3), preserved with CS for 22 hours and perfused with HOPE for 2 hours with the supplemented CS/MP-UW solution (CS/MP-UW solution containing Que 33.1 μM and Suc 0.1 M) (supCS × 22h + supHOPE × 22h, n = 5).
Autologous transplantation was performed after a right nephrectomy. First, side-to-end anastomosis of the renal vein and the inferior vein was performed, followed by side-to-end anastomosis of the renal artery and the abdominal artery, and reperfusion was initiated. Finally, side-to-end anastomosis of the ureter and the bladder was performed. After transplantation, blood samples were taken from the internal jugular vein catheter each day. On day 7, the animals were euthanized by injecting KCl (10 mEq), and samples were obtained (Figure 2).
FIGURE 2.
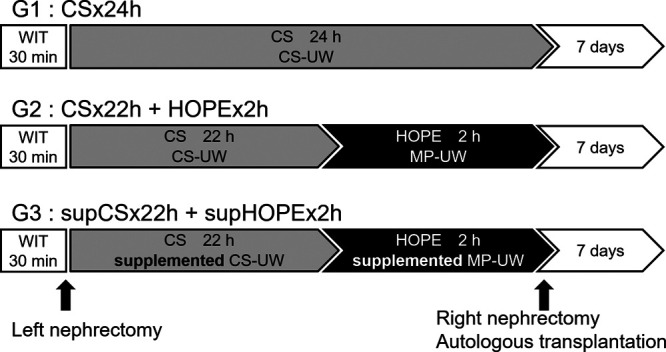
Study design of preservation method in a porcine model of renal autologous transplantation. CS, cold storage; CS-UW, University of Wisconsin for cold storage; MP-UW, University of Wisconsin for machine perfusion; HOPE, hypothermic oxygenated perfusion; WIT, warm ischemic time.
Assessment of Blood Creatinine Levels
In the porcine model of renal autologous transplantation, the creatinine (Cre) levels were measured in a core laboratory before the right nephrectomy, before transplantation, and daily after transplantation, as an index of the degree of kidney damage. Moreover, the data obtained on day 7 posttransplantation for the euthanized animals and the last data obtained for the animals that died during the observation period were used to compare the last Cre levels of each group.
Assessment of Blood LPO Activity
In the porcine model of renal autologous transplantation, the blood lipid hydroperoxide (LPO) levels as an oxidative stress marker were measured on days 1 and 2 posttransplantation using the TBA assay (code: KLP-004K).
Histopathological Evaluation
Animals were euthanized on day 7 posttransplantation, and tissue samples were taken. All kidneys were simultaneously immobilized using a 10% aqueous solution of phosphate-buffered formalin and embedded in paraffin according to the standard method. Each sample was sectioned into 4-mm slices, and hematoxylin-eosin staining was performed. The degree of renal damage was scored according to the EGTI scoring system suggested by Khalid et al17 as a novel and more reliable method for assessing kidney histology after I/R injury. In a blinded fashion, the histopathology of all sections of the kidney was scored as the average score for 4 individual components (EGTI) in 10 randomly selected fields on each section under an optical microscope at 400× magnification. The sum of all numerical scores in each group was taken as the total score.
Determination of Renal Resistance
Renal resistance (RR) refers to vascular resistance and is calculated as pressure (mm Hg)/flow (mL/min). The change in RR (ΔRR), which indicates the decrease in vascular resistance from the start of perfusion, is determined as RRt − RR0, where RR0 is the RR immediately after the start of HOPE, and RRt is the RR immediately before the end of HOPE.
Tissue Weight Determination
The changes in total tissue weight (TTW) during CS were used to assess the effect of Que and Suc on tissue edema. The preservation solutions of CS-UW in G1-2 and supCS-UW (supplemented CS-UW solution) in G3 were compared. After 22–24 hours of CS, the weight change (%) was determined as follows: % = (Wt − W0)/W0 × 100%, where W0 is TTW before CS, and Wt is TTW after CS.
Statistical Analyses
All data are presented as the mean ± SD. Student’s t-test with 2-tailed distribution or Dunnett’s test for multiple comparisons following the Bartlett test was performed with the EZR software program (version 2.2-3). Statistical significance was considered at P < 0.05.
RESULTS
CS of BHK-21 Cells for 3 Days in CS/MP-UW Solutions
When the BHK-21 cells were stored at 4°C for 3 days, the cell viability decreased from 10.0 × 103 cells/well (initial) to 0.92 ± 0.07 × 103 cells/well in the CS-UW solution alone and to 2.31 ± 0.07 × 103 cells/well in the MP-UW solution alone. In addition, the cell viability was 0.01 ± 0.01 × 103 cells/well in Saline, 0.09 ± 0.03 × 103 cells/well in Culture medium, 0.58 ± 0.11 × 103 cells/well in CS-UW + Suc + DMSO, and 1.33 ± 0.10 × 103 cells/well in MP-UW + Suc + DMSO, and these 3 solutions were less effective for cell protection than CS/MP-UW solution alone.
In contrast, when Que and Suc were both added during CS, cell viability increased compared to that in the absence of either compound (CS-UW + Que [33.1 μM] + Suc 3.83 ± 0.21 × 103 cells/well [versus CS-UW, P < 0.001], CS-UW + Que [3.3 μM] + Suc 4.19 ± 0.16 × 103 cells/well [versus CS-UW, P < 0.001], MP-UW + Que [33.1 μM] + Suc 3.57 ± 0.21 × 103 cells/well [versus MP-UW, P < 0.001], MP-UW + Que [3.3 μM] + Suc 3.83 ± 0.38 × 103 cells/well [versus MP-UW, P < 0.001]). The highest cell viability was obtained when the concentration of Que was 3.3 μM in CS/MP-UW solution. These results revealed that cell protection against cold injury in the BHK-21 cells was significantly greater in the CS/MP-UW solution supplemented with Que (33.1 or 3.3 μM) and Suc (0.1 M) than in the CS/MP-UW solution alone (Figure 3).
FIGURE 3.
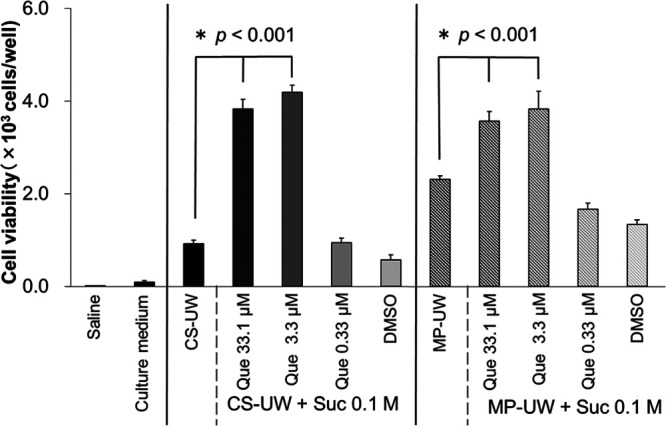
Protective effects against cold injury in BHK-21 cells preserved at 4°C for 3 d. Data are the mean ± SD (n = 3). Data were compared by the Bartlett test, followed by Dunnett’s test for multiple comparisons. After 3 d of cold storage, the CS-UW solution with Que and Suc significantly protected the BHK-21 cells against cold injury in comparison with the CS-UW solution alone (P < 0.001). In addition, the MP-UW solution with Que and Suc significantly protected the BHK-21 cells against cold injury in comparison with the MP-UW solution alone (P < 0.001). CS-UW, University of Wisconsin for cold storage; DMSO, dimethylsulfoxide; MP-UW, University of Wisconsin for machine perfusion; Suc, sucrose; Que, quercetin.
Blood Cre Activity as a Measure of Renal Dysfunction in the Porcine Model of Renal Autologous Transplantation
We assessed renal dysfunction through the blood Cre levels over time in each group. The survival rate of each group at posttransplantation day 7 was 40% (2/5) in G1, 80% (4/5) in G2, and 80% (4/5) in G3. The survival days of each group after transplantation were 5.4 ± 1.8 days in G1, 6.4 ± 1.3 days in G2, and 6.8 ± 0.4 days in G3. Uremia was considered to be the cause of death before sacrifice. In G1, blood Cre levels gradually increased until day 7, whereas the blood Cre levels of G3 peaked on day 3. The blood Cre levels on posttransplantation day 7 were 19.3 ± 11.6 mg/dL in G1, 12.8 ± 9.7 mg/dL in G2, and 7.4 ± 2.8 mg/dL in G3. Blood Cre levels did not exhibit significant differences in any group (Figure 4A). The last blood Cre levels were 17.2 ± 8.6 mg/dL in G1, 13.6 ± 9.9 mg/dL in G2, and 7.3 ± 2.9 mg/dL in G3, and there was a significant difference between G1 and G3 (P < 0.05) (Figure 4B).
FIGURE 4.
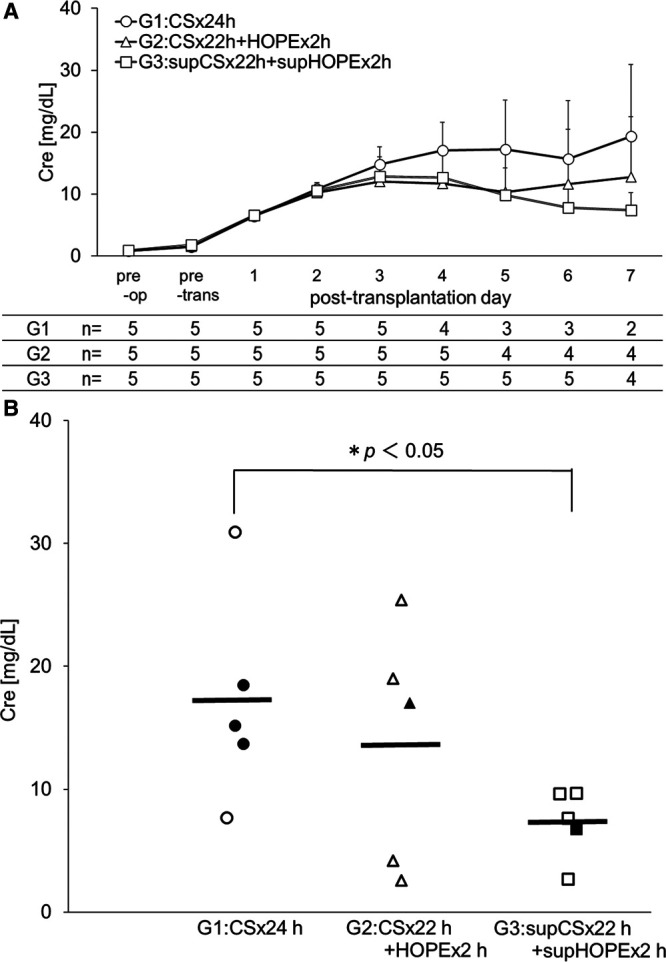
Renal dysfunction in a porcine model of renal autologous transplantation as assessed by blood Cre levels over time (A), and last Cre levels by scatter plot (B). Data are the mean ± SD (n of each group is shown below the graphs). Data were compared by 2-tailed t-test. In G1, blood Cre levels gradually increased until day 7, whereas the blood Cre levels in G3 peaked on day 3. The last Cre levels were significantly different between G1 and G3 (P < 0.05). The frame marker indicates the data obtained on day 7 after transplantation for the euthanized animals. The fill marker indicates the last data obtained for the animals that died during the observation period. The horizontal line indicates the mean level for each group. Cre, blood creatinine. G1:CS × 24h, G2:CS × 22h + HOPE × 2h, G3:supCS × 22h + supHOPE × 2h. pre-op, preoperative; pre-trans, pretransplantation.
Blood LPO Activity as a Marker of Oxidative Stress in the Porcine Model of Renal Autologous Transplantation
LPO levels were assessed in the blood as an index of lipid peroxidation. The blood LPO levels on posttransplantation day 1 were 4.7 ± 0.58 nmol/mL in G1 (n = 5), 4.5 ± 0.28 nmol/mL in G2 (n = 5), and 4.1 ± 0.59 nmol/mL in G3 (n = 5). The blood LPO levels on posttransplantation day 2 were 5.3 ± 0.67 nmol/mL in G1 (n = 5), 4.7 ± 0.58 nmol/mL in G2 (n = 5), and 4.3 ± 0.46 nmol/mL in G3 (n = 5), and there was a significant difference between G1 and G3 (P < 0.05) (Figure 5).
FIGURE 5.
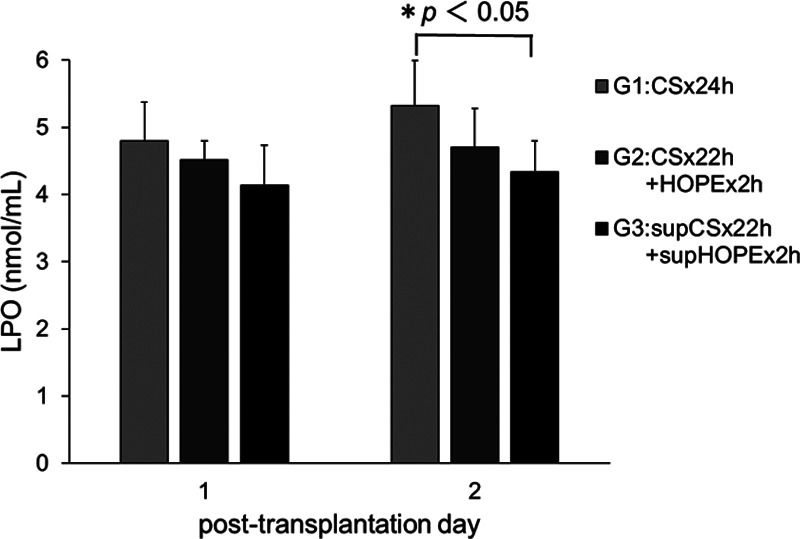
Antioxidant effect of quercetin in a porcine model of renal autologous transplantation as assessed by blood LPO levels. Data are the mean ± SD (n = 5). Data were compared by 2-tailed t-test. Blood LPO levels were significantly different between G1 and G3 on posttransplantation day 2 (P < 0.05). LPO, lipid hydroperoxide. G1:CS × 24h, G2:CS × 22h + HOPE × 2h, G3:supCS × 22h + supHOPE × 2h. pre-op, preoperative; pre-trans, pretransplantation.
The Degree of Renal Damage in the Porcine Model of Renal Autologous Transplantation
The EGTI scoring system following sacrifice at posttransplantation day 7 was employed to investigate renal damage in the porcine model of renal autologous transplantation. The EGTI histology score of each individual component for each group (G1/G2/G3) was 2.80 ± 0.14/1.85 ± 0.42/1.35 ± 0.29 in Endothelial score, 2.00 ± 0.00/1.55 ± 0.74/1.13 ± 0.62 in Glomerular score, 3.50 ± 0.00/2.85 ± 0.41/2.18 ± 0.64 in Tubular score, and 3.40 ± 0.71/2.53 ± 0.63/2.10 ± 0.77 in Interstitial score. The total EGTI score was 11.70 ± 0.85/8.78 ± 1.73/6.75 ± 2.17 (G1/G2/G3), and there was a statistically significant difference between G1 and G3 (P < 0.05) (Figure 6A–D).
FIGURE 6.
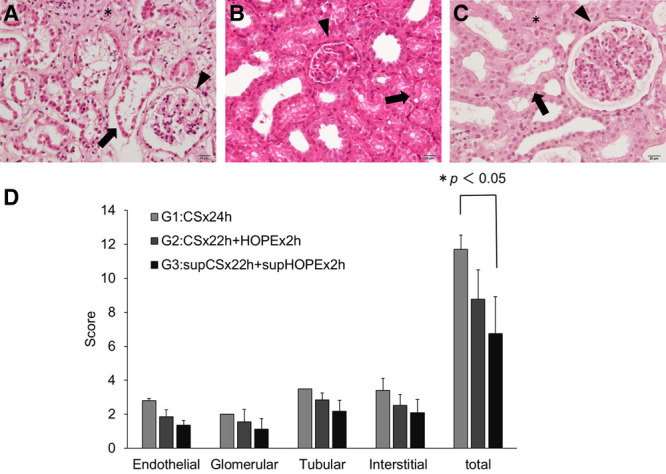
Effects of quercetin and sucrose on the EGTI histology score in kidney tissue. Renal tissues were stained with hematoxylin-eosin (H&E), and pathological changes were evaluated using the EGTI scoring system under a light microscope at 400× magnification. A, H&E stain in G1 shows a glomerular tuft retraction (Glomerular score 2: ▾), complete necrosis in tubular cells (Tubular score 4: →), and inflammation and necrosis within the interstitium (Interstitial score 3: *). B, H&E stain in G2 shows a thickened Bowman’s capsule (Glomerular score 1: ▾) and thickened basal membrane of the tubular cells with loss of the brush border and the presence of cast formation (Tubular score 3: →). C, H&E stain in G3 shows the integrity of the basal membrane of the tubular cells with loss of the brush border in less than 25% of the tubular cells (Tubular score 1: →) and an intact glomerulus with thin-walled Bowman’s capsule (Glomerular score 0: ▾). There is no visible interstitium, signifying no damage/abnormality within the interstitial compartment (Interstitial score 0: *). In (D), the scoring graph shows data as the mean ± SD (G1: n = 2, G2: n = 4, G3: n = 4). Differences between each group were calculated using the Student’s t-test. G1:CS × 24h, G2:CS × 22h + HOPE × 2h, G3:supCS × 22h + supHOPE × 2h. CS, cold storage; EGTI, endothelial, glomerular, tubular, interstitial; HOPE, hypothermic oxygenated perfusion.
RR Changes During HOPE in the Porcine Model of Renal Autologous Transplantation
RR, which is said to reflect the kidney graft viability, was measured immediately after the start of HOPE and before the end of HOPE, and its change (ΔRR) was evaluated. The ΔRR tended to decrease over time in all groups; however, the decrease in G3 was significantly larger than that in G2 (ΔRR = 5.27 ± 3.87 mm Hg/mL/min in G2, 12.59 ± 2.41 mm Hg/mL/min in G3, P < 0.05) (Figure 7).
FIGURE 7.
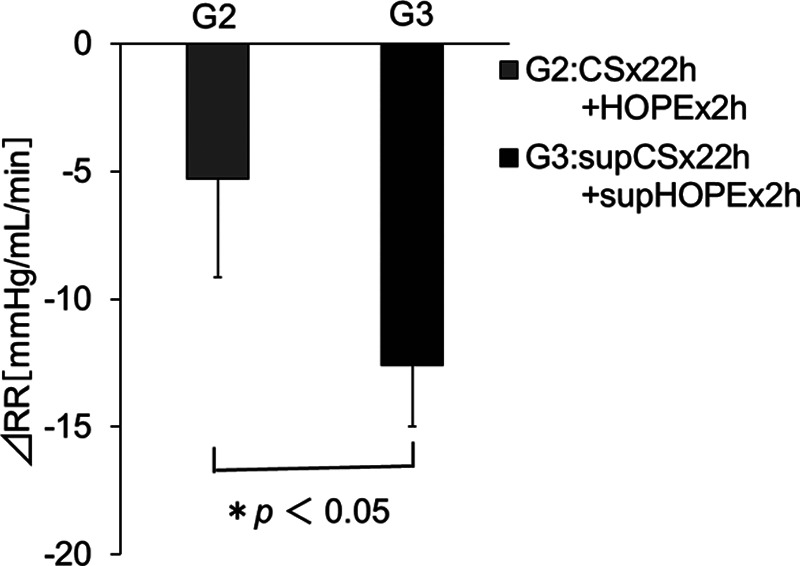
Effects of quercetin and sucrose on renal resistance changes (ΔRR) during HOPE in the porcine model of renal autologous transplantation. Data are the mean ± SD (group 2: n = 4 [a case with sensor trouble was excluded], group 3: n = 5). Differences between the 2 groups were calculated using the Student’s t-test. ΔRR = RRt − RR0, where RR0 is the RR immediately after the start of HOPE, and RRt is the RR immediately before the end of HOPE. RR = pressure (mm Hg)/flow (mL/min). G1:CS × 24h, G2:CS × 22h + HOPE × 2h, G3:supCS × 22h + supHOPE × 2h. CS, cold storage; HOPE, hypothermic oxygenated perfusion.
Effects of Suc on TTW Changes Following CS
To evaluate if Suc in CS-UW solution was effective for the prevention of tissue edema, the change of TTW in kidney tissues after CS was compared with that after preservation in the CS-UW solution alone. The preservation time was 1355 ± 137 minutes (n = 10) for the CS-UW solution alone and 1387 ± 27 minutes (n = 4, excluding unmeasured cases) for the supCS-UW solution. Preservation times were not significantly different among the groups (P = 0.63, t-test). The TTW loss after CS was 10.9 ± 3.0% in the CS-UW solution alone and 19.5 ± 7.5% in the supCS-UW solution. The weight change was statistically significant in both groups (P < 0.001) (Figure 8).
FIGURE 8.
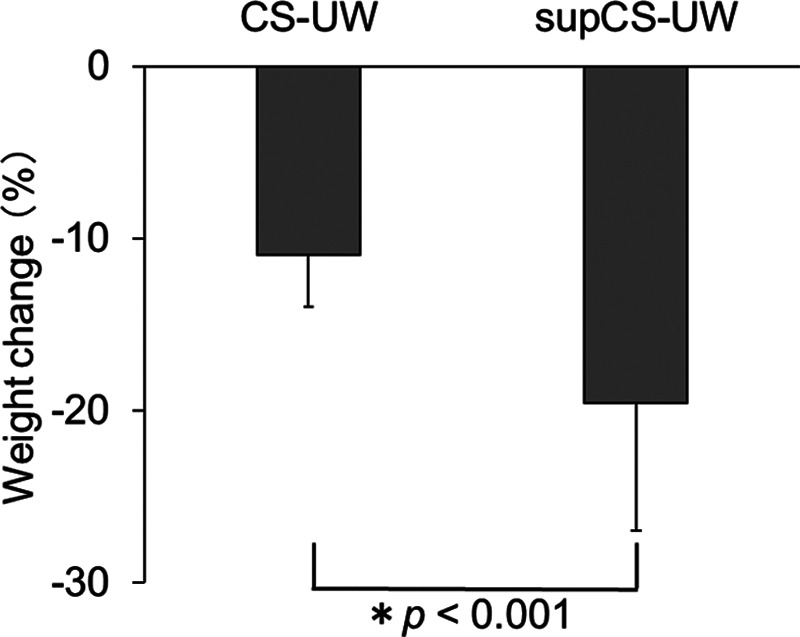
The effects of sucrose on total tissue weight (TTW) changes following CS. Data are the mean ± SD (CS-UW: n = 10, supCS-UW: n = 4 [unmeasured case excluded]). Differences between every 2 groups were calculated using Student’s t-test. The weight change (%) was determined as follows: % = (Wt − W0)/W0 × 100%, where W0 is the tissue weight before CS, and Wt is the weight after CS. CS-UW, Belzer UW Cold Storage Solution; supCS-UW, CS-UW solution with the addition of quercetin and sucrose.
DISCUSSION
Kidney transplantation is an established treatment for end-stage kidney disease. However, the shortage of kidney grafts has become a global problem. DCD donors and others have been used to expand the available donor pool.18,19
CS is the easiest and most widely used preservation method in kidney transplantation. However, prolonged CS causes postoperative complications, early graft dysfunction, and poor outcomes after transplantation.20,21 This occurs as ischemia during storage is associated with oxidative stress and apoptosis. Oxidative stress is a known consequence of the imbalance between the production of reactive oxygen species (ROS) and antioxidants, and repair processes.22 Lipid peroxidation, mediated by ROS, has been hypothesized as an important cause of destruction and damage to cell membranes during oxidative stress.23
There is increasing interest in new preservation techniques that resolve these problems, improve organ quality, and decrease severe complications, and various new organ preservation strategies have been examined.3 Among them, HOPE has received particular attention. HOPE is a technique of short-term (1–2 h) hypothermic oxygenated machine perfusion applied after CS in organ transplantation. Kron et al3,4 reported that HOPE-treated rat kidneys showed superb function after transplantation in terms of nuclear injury, macrophage activation, endothelium activation, and tubular damage graft function as well as decreased immune response. We considered HOPE to be a superior preservation strategy, and we examined whether a better preservation effect could be achieved by introducing HOPE and using a preservation solution containing Que and Suc. Currently, the preservation solution for machine perfusion is inferior to solutions used for CS. The UW solution, commonly used for preservation, has limitations in preventing oxidative injury; however, the addition of antioxidants to preservation solutions has resulted in improved cell survival.24
Que is an excellent in vitro antioxidant as it is the most potent scavenger of ROS.25 Inhibition of NF-κB activation required for tissue repair has been suggested to prevent apoptosis associated with renal I/R injury.26,27 Kinaci et al7 reported that NF-κB expression was significantly decreased by Que treatment in an I/R rat model. Thus, it was suggested that Que treatment reduced renal I/R injury by reducing apoptosis. The levels of enzymes such as superoxide dismutase, catalase, and glutathione peroxidase have been reported to become insufficient during I/R in association with abundant ROS formation.11,28 Inal et al11 reported that superoxide dismutase, catalase, and glutathione peroxidase levels in the renal cortex of I/R groups were significantly lower than those of the control group but not significantly different between the Que-treated group and control group. These findings suggest that as an antioxidant, Que may exert a protective effect against ROS in renal I/R. However, the molecular mechanism through which Que provides renal protection during I/R remains unknown.
In our in vitro experiments, CS/MP-UW solution alone showed a higher cytoprotective effect than Saline, Culture medium, and DMSO, but the cytoprotective effect was further enhanced by adding Que and Suc. However, when the Que concentration was reduced to 0.33 μM, the protective effect rapidly weakened. Thus, the Que concentration used in the porcine model of renal autologous transplantation was 33.1 μM, in consideration of its renal metabolism, the stability of the effects, and dilution of the preservation solution with urine or exudate. The peak of blood Cre level, used as an index of renal dysfunction, was earlier in G3, and the last Cre level was significantly lower than that in G1. The LPO level was used as an oxidative stress marker and was significantly lower in G3 than in G1 on posttransplantation day 2. It has been shown that the half-lives of Que metabolites are 11–28 hours.29 Therefore, we considered that the antioxidant effect of Que would last for 28 hours after transplantation and accordingly measured the LPO level until posttransplantation day 2. Moreover, in kidney histology after I/R injury, the G3 score was low in all EGTI components, and the total score showed that G3 had significantly less renal damage than G1. These results suggest that the preservation solution containing Que and Suc improved renal dysfunction and I/R injury via the antioxidant effect of Que as compared with CS. Although no significant difference was observed in comparison with HOPE (not containing Que and Suc), preservation with Que and Suc showed a tendency for lower oxidative stress, better renal function, and lower I/R injury. These results indicate the possibility of clinical application of Que in organ preservation. Moreover, Que is less expensive than other flavonoid compounds; thus, it is considered suitable for clinical application from an economic perspective.
The viability of kidney grafts is a critical part of organ transplantation, and various qualitative, semiquantitative, and quantitative techniques have been used to assess viability. Machine perfusion permits the use of a more quantitative approach using perfusion parameters, such as flow, pressure, and RR. These parameters are dynamic and better reflect the initial and evolving status of renal microcirculation, independent of the device type.30 Among them, RR is an independent risk factor for delayed graft function and poorer graft survival.30-33 In our study, G1 (CS only) had a poor survival rate, and G2 and G3 (HOPE) had a high survival rate. This proved the usefulness of HOPE, consistent with previous studies.3,4 The RR decrease in G3 (MP-UW solution containing Que and Suc) compared with G2 was larger, and kidney graft viability was possibly higher. These results suggest that an MP-UW solution containing Que and Suc may be useful as a preservation solution for HOPE. This has been suggested based on the vasodilation and antioxidant effects of Que. Que reportedly affects both endothelium-dependent vasorelaxation and smooth muscle relaxation.34,35 Thus, it is considered that the vasodilator effect of Que occurred with machine perfusion, and it was associated with RR decrease. It is possible that a significant difference in survival rate and Cre levels would have been observed between G2 and G3 if the study period was extended. However, large animal models are labor-intensive, technically challenging, and carry a high financial burden.36 In addition, long-term breeding is difficult for postoperative management and pain management; thus, it is necessary to overcome these issues.
In I/R injury, during ischemia, the osmotic influx of water causes cellular swelling. Moreover, hypothermia is the principal element used to prolong organ viability ex vivo; however, paradoxically, this induces cellular edema through the inhibition of energy-dependent ATPases and an electrolyte imbalance that leads to fluid influx and cellular swelling.37 The UW solution contains lactobionate, raffinose, and hydroxyethyl starch (HES) to prevent cellular edema.38 In the study by Shadan et al,39 rat kidney tissues were stored at 4°C in 4 types of preservation solutions, including UW solution, and TTW as a marker of tissue edema was determined after 24 hours of CS. The TTW in UW solution decreased significantly during 24 hours of CS compared with that in other preservation solutions, likely because HES in the UW solution prevented tissue edema. However, HES is highly viscous, which prolongs the duration of perfusion while compromising microcirculation.40 Phosphate-buffered sucrose (PBS140) has a lower viscosity, which makes it easier to use, allowing rapid and efficient perfusion of the donor organ.28 Several studies have provided novel physiological analyses of edema, which is the principal source of preservation injury.8,12 In the study by Ahmad et al,12 ischemic kidneys were flushed with different solutions, and as a marker of edema in the preserved kidney, the kidneys were weighed. Changes in kidney weight showed that there was a significant increase in all groups of organs perfused with solutions such as UW solution, but not with PBS140, indicating retained fluid or cellular edema in these organs. Moreover, when comparing the influence of preservation solutions on postischemic renal function, according to most of the 15 measured parameters, PBS140-preserved kidneys showed less damage than kidneys preserved with hyperosmolar citrate or UW. The present study suggested that the loss of TTW in the CS-UW solution with Que and Suc was greater in CS and that the prevention of edema and protection of renal function were possibly higher. The molecular size of Suc is large (342), and Suc may effectively act as an impermeant. Although its mechanisms of action remain unclear, simply adding Suc to the CS-UW solution may prevent cellular swelling more effectively.
Our study also showed that the survival of BHK-21 cells during CS in the CS/MP-UW solutions was drastically improved by pretreatment with a certain concentration of Que. Furthermore, it was suggested that the antioxidant effect of Que and the edema-preventing effect of Suc reduce renal dysfunction and I/R injury in porcine renal autologous transplantation. Thus, our study indicated that Que and Suc could improve kidney preservation in UW solution and may potentially enhance the outcome of kidney transplantation. We have previously shown that the preservation of liver in rats may be improved by the addition of Que and Suc.41 Therefore, these additions may also be useful for preservation of other organs. Whether these findings can be transferred to whole organ preservation remains to be demonstrated.
Despite its straightforward results, the present study has several limitations. First, in a porcine model of renal autologous transplantation, surgical bias exists. To reduce this bias, it is necessary to systematize and stabilize the procedure and increase the number of samples. Second, it is necessary to prove that Que exerts antioxidative and anti-apoptotic activities even in autologous transplantation, which would require the evaluation of substances involved in these cascades other than LPO. Finally, quercetin-quinone, the oxidation product of Que, displays various toxic effects due to its arylation of protein thiols. Moreover, Que has also been reported to display genotoxic effects in vitro.42 Therefore, long-term observation is necessary to achieve clinical application of Que. Based on our data, further studies should be performed to investigate the potential application of Que and Suc in kidney transplantation and kidney preservation.
In conclusion, we investigated the utility of flavonoid Que and Suc for organ transplantation for the first time. Our results suggest that the addition of Que and Suc to UW solution can improve kidney preservation and could potentially enhance the outcomes of kidney transplantation.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Published online 10 November, 2020.
F.K. and N.M. designed the research. M.G. wrote the initial draft. M.G., A.T., T.K., S.Y., D.I., M.O., and N.M. performed the research. M.G., F.K., Y.N., H.F., and N.M. analyzed the data. All authors reviewed the draft of the article, provided expertise for revisions, and approved the final version of the article.
The authors declare no funding or conflicts of interest.
REFERENCES
- 1.Southard JH, Belzer FO. Organ preservation. Annu Rev Med. 1995; 46:235–247 [DOI] [PubMed] [Google Scholar]
- 2.Polyak MM, Arrington BO, Stubenbord WT, et al. The influence of pulsatile preservation on renal transplantation in the 1990s. Transplantation. 2000; 69:249–258 [DOI] [PubMed] [Google Scholar]
- 3.Kron P, Schlegel A, Muller X, et al. Hypothermic oxygenated perfusion: a simple and effective method to modulate the immune response in kidney transplantation. Transplantation. 2019; 103:e128–e136 [DOI] [PubMed] [Google Scholar]
- 4.Kron P, Schlegel A, de Rougemont O, et al. Short, cool, and well oxygenated—HOPE for kidney transplantation in a rodent model. Ann Surg. 2016; 264:815–822 [DOI] [PubMed] [Google Scholar]
- 5.Liua C, Zhenga Y, Lua J, Zhanga Z, et al. Quercetin protects rat liver against lead-induced oxidative stress and apoptosis. Environ Toxicol Pharmacol. 2010; 29:158–166 [DOI] [PubMed] [Google Scholar]
- 6.Zielińska M, Kostrzewa A, Ignatowicz E, et al. The flavonoids, quercetin and isorhamnetin 3-O-acylglucosides diminish neutrophil oxidative metabolism and lipid peroxidation. Acta Biochim Pol. 2001; 48:183–189 [PubMed] [Google Scholar]
- 7.Kinaci MK, Erkasap N, Kucuk A, et al. Effects of quercetin on apoptosis, NF-κB and NOS gene expression in renal ischemia/reperfusion injury. Exp Ther Med. 2012; 3:249–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahlenstiel T, Burkhardt G, Köhler H, et al. Bioflavonoids attenuate renal proximal tubular cell injury during cold preservation in Euro-Collins and University of Wisconsin solutions. Kidney Int. 2003; 63:554–563 [DOI] [PubMed] [Google Scholar]
- 9.Kumano K, Wang W, Endo T. Effects of osmotic agents for cold kidney preservation. Nihon Hinyokika Gakkai Zasshi. 1994; 85:925–931 [DOI] [PubMed] [Google Scholar]
- 10.Ahmad N, Hostert L, Pratt JR, et al. A pathophysiologic study of the kidney tubule to optimize organ preservation solutions. Kidney Int. 2004; 66:77–90 [DOI] [PubMed] [Google Scholar]
- 11.Inal M, Altinişik M, Bilgin MD. The effect of quercetin on renal ischemia and reperfusion injury in the rat. Cell Biochem Funct. 2002; 20:291–296 [DOI] [PubMed] [Google Scholar]
- 12.Ahmad N, Pratt JR, Potts DJ, et al. Comparative efficacy of renal preservation solutions to limit functional impairment after warm ischemic injury. Kidney Int. 2006; 69:884–893 [DOI] [PubMed] [Google Scholar]
- 13.Obara H, Matsuno N, Shigeta T, et al. Rewarming machine perfusion system for liver transplantation. J Med Device. 2013; 7:041011. [DOI] [PubMed] [Google Scholar]
- 14.Matsuno N, Obara H, Watanabe R, et al. Rewarming preservation by organ perfusion system for donation after cardiac death liver grafts in pigs. Transplant Proc. 2014; 46:1095–1098 [DOI] [PubMed] [Google Scholar]
- 15.Kanazawa H, Obara H, Yoshikawa R, et al. Impact of machine perfusion on sinusoid microcirculation of liver graft donated after cardiac death. J Surg Res. 2020; 245:410–419 [DOI] [PubMed] [Google Scholar]
- 16.Ishii D, Matsuno N, Gochi M, et al. Applicability of hypothermic oxygenate machine perfusion preservation for split-liver transplantation in a porcine model: an experimental study. Ann Transplant. 2020; 25:e919920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalid U, Pino-Chavez G, Nesargikar P, et al. Kidney ischaemia reperfusion injury in the rat: the EGTI scoring system as a valid and reliable tool for histological assessment. J Histol Histopathol. 2016; 3:1 [Google Scholar]
- 18.Bertuzzo VR, Cescon M, Odaldi F, et al. Actual risk of using very aged donors for unselected liver transplant candidates: a European single-center experience in the MELD era. Ann Surg. 2017; 265:388–396 [DOI] [PubMed] [Google Scholar]
- 19.Flores A, Asrani SK. The donor risk index: a decade of experience. Liver Transpl. 2017; 23:1216–1225 [DOI] [PubMed] [Google Scholar]
- 20.Hoyer DP, Paul A, Gallinat A, et al. Donor information based prediction of early allograft dysfunction and outcome in liver transplantation. Liver Int. 2015; 35:156–163 [DOI] [PubMed] [Google Scholar]
- 21.Agopian VG, Petrowsky H, Kaldas FM, et al. The evolution of liver transplantation during 3 decades: analysis of 5347 consecutive liver transplants at a single center. Ann Surg. 2013; 258:409–421 [DOI] [PubMed] [Google Scholar]
- 22.Elahi MM, Kong YX, Matata BM. Oxidative stress as a mediator of cardiovascular disease. Oxid Med Cell Longev. 2009; 2:259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaçmaz A, Polat A, User Y, et al. Octreotide: a new approach to the management of acute abdominal hypertension. Peptides. 2003; 24:1381–1386 [DOI] [PubMed] [Google Scholar]
- 24.Salahudeen AK, Huang H, Patel P, et al. Mechanism and prevention of cold storage-induced human renal tubular cell injury. Transplantation. 2000; 70:1424–1431 [DOI] [PubMed] [Google Scholar]
- 25.Heijnen CG, Haenen GR, Oostveen RM, et al. Protection of flavonoids against lipid peroxidation: the structure activity relationship revisited. Free Radic Res. 2002; 36:575–581 [DOI] [PubMed] [Google Scholar]
- 26.Seok YM, Kim J, Park MJ, et al. Wen-pi-tang-Hab-Wu-ling-san attenuates kidney fibrosis induced by ischemia/reperfusion in mice. Phytother Res. 2008; 22:1057–1063 [DOI] [PubMed] [Google Scholar]
- 27.Spandou E, Tsouchnikas I, Karkavelas G, et al. Erythropoietin attenuates renal injury in experimental acute renal failure ischaemic/reperfusion model. Nephrol Dial Transplant. 2006; 21:330–336 [DOI] [PubMed] [Google Scholar]
- 28.Barnard ML, Snyder SJ, Engerson TD, et al. Antioxidant enzyme status of ischemic and postischemic liver and ischemic kidney in rats. Free Radic Biol Med. 1993; 15:227–232 [DOI] [PubMed] [Google Scholar]
- 29.Manach C, Williamson G, Morand C, et al. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005; 811 Suppl230S–242S [DOI] [PubMed] [Google Scholar]
- 30.Burgos Revilla FJ, Hevia V, Diez V, et al. Machine perfusion: initial results in an expanded criteria donor kidney transplant program. Transplant Proc. 2015; 47:19–22 [DOI] [PubMed] [Google Scholar]
- 31.Yushkov YY, Stern J, Ying A, et al. Identifying risk factors in renal allografts before transplant: machine-measured renal resistance and posttransplant allograft survival. Prog Transplant. 2012; 22:175–182 [DOI] [PubMed] [Google Scholar]
- 32.Sandal S, Paraskevas S, Cantarovich M, et al. Renal resistance thresholds during hypothermic machine perfusion and transplantation outcomes—a retrospective cohort study. Transpl Int. 2018; 31:658–669 [DOI] [PubMed] [Google Scholar]
- 33.Morii Y, Matsuno N, Morito N, et al. The effectiveness of perfusion using an oxygenated rewarming machine for recovering the function of porcine kidneys donated after cardiac death. Jpn J Transplant. 2018; 53:33–39 [Google Scholar]
- 34.Nishida S, Tshuchida K, Satoh H. The vascular pharmacological effects induced by quercetin contained in Kampo herbal medicine. Nihon Yakurigaku Zasshi. 2015; 146:140–143 [DOI] [PubMed] [Google Scholar]
- 35.Pérez-Vizcaíno F, Ibarra M, Cogolludo AL, et al. Endothelium-independent vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance and resistance arteries. J Pharmacol Exp Ther. 2002; 302:66–72 [DOI] [PubMed] [Google Scholar]
- 36.Yanaga K, Makowka L, Lebeau G, et al. A new liver perfusion and preservation system for transplantation research in large animals. J Invest Surg. 1990; 3:65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LEAF A. Maintenance of concentration gradients and regulation of cell volume. Ann N Y Acad Sci. 1959; 72:396–404 [DOI] [PubMed] [Google Scholar]
- 38.Guibert EE, Petrenko AY, Balaban CL, et al. Organ preservation: current concepts and new strategies for the next decade. Transfus Med Hemother. 2011; 38:125–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shadan L, Bin L, Qiunong G, et al. Cold preservation with hyperbranched polyglycerol-based solution improves kidney functional recovery with less injury at reperfusion in rats. Am J Transl Res. 2017; 9:429–441 [PMC free article] [PubMed] [Google Scholar]
- 40.Tojimbara T, Wicomb WN, Garcia-Kennedy R, et al. Liver transplantation from non-heart beating donors in rats: influence of viscosity and temperature of initial flushing solutions on graft function. Liver Transpl Surg. 1997; 3:39–45 [PubMed] [Google Scholar]
- 41.Kato F, Gochi M, Kawagoe T, et al. The protective effects of quercetin and sucrose on cold preservation injury in vitro and in vivo. Organ Biology. 2020; 27:207–215 [Google Scholar]
- 42.Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008; 585:325–337 [DOI] [PubMed] [Google Scholar]


