INTRODUCTION:
The aim of this study was to determine the role of hepatitis E virus (HEV) infection in a large cohort of prospectively enrolled patients with severe acute liver injury (ALI).
METHODS:
Serum samples from 594 consecutive adults enrolled between 2008 and 2018 in the US Acute Liver Failure Study Group ALI registry were tested for anti-HEV IgM and anti-HEV IgG levels. Those with detectable anti-HEV IgM underwent further testing for HEV RNA using real-time polymerase chain reaction.
RESULTS:
The median age of patients was 38 years; 41% were men and 72% Caucasian. Etiologies of ALI included acetaminophen hepatotoxicity (50%), autoimmune hepatitis (8.9%), hepatitis B virus (8.9%), and idiosyncratic drug-induced liver injury (7.9%). Overall, 62 patients (10.4%) were negative for anti-HEV IgM but positive for IgG, whereas only 3 men (0.5%) were positive for both anti-HEV IgM and IgG. These 3 cases were initially diagnosed as having indeterminate, HEV, and hepatitis B virus-related ALI. One of these patients had detectable HEV RNA genotype 3, and another anti-HEV IgM+ patient had detectable HEV antigens by immunohistochemistry on liver biopsy. On multivariate modeling, older (odds ratio: 1.99) and non-Caucasian subjects (odds ratio: 2.92) were significantly more likely to have detectable anti-HEV IgG (P < 0.0001).
DISCUSSION:
Acute HEV infection is an infrequent cause of ALI in hospitalized North American adults. The anti-HEV IgG+ patients were significantly older and more likely to be non-Caucasian. These data are consistent with other population-based studies that indicate exposure to HEV in the general US population is declining over time and might reflect a cohort effect.
INTRODUCTION
Hepatitis E virus (HEV) is an enterically transmitted single-stranded RNA virus that can lead to a symptomatic illness with self-limited jaundice (1). Acute HEV infection is associated with detectable anti-HEV IgM antibodies and HEV RNA by polymerase chain reaction (PCR) in the serum of 50%–66% of infected patients during the acute illness and eventual development of anti-HEV IgG antibodies (2). In endemic areas of the world, acute HEV infection with genotypes 1 and 2 has been associated with the development of acute liver failure (ALF), particularly in pregnant women (1,2). It is estimated that there are nearly 20 million infections, 3 million symptomatic cases, and over 70,000 deaths globally each year attributed to HEV infection (3). In addition, subjects with underlying chronic liver disease can develop acute-on-chronic liver failure with prolonged cholestasis after acute HEV infection (4).
Acute HEV infection is very uncommon in the United States unless someone has a history of recent travel to endemic areas such as Asia, Africa, or South America (5,6). However, as many as 20% of individuals in the general US population have detectable anti-HEV IgG indicative of previous exposure with no antecedent history of acute hepatitis (7). In the United States, acute HEV infection is exceedingly rare with only 15 cases reported to the Centers for Disease Control and Prevention (CDC) over a 7-year period (5). Sporadic acute HEV infection in nonendemic areas is believed to be a zoonosis wherein genotype 3 HEV is transmitted through consumption of undercooked pork or wild game (8,9). In addition, recent reports from the United Kingdom, Australia, and the United States have suggested that HEV infection might account for up to 5%–10% of cases of presumed idiosyncratic drug-induced liver injury (DILI) (10–12). These data and those collected by the CDC suggest that sporadic acute HEV infection leading to jaundice and hospitalization does occur in the United States albeit at a very low rate (5).
The US Acute Liver Failure Study Group (ALFSG) has been prospectively studying the etiology and outcomes of adults with ALF and enrolling patients with severe acute liver injury (ALI) since 2008 (13,14). ALI was defined for study purposes as a hospitalized patient with an acute hepatic illness and an international normalized ratio (INR) of >2 but no encephalopathy at enrollment (14). Acetaminophen overdose is the leading cause of both ALI and ALF, followed by idiosyncratic DILI, autoimmune hepatitis, and indeterminate etiologies (13,14). We recently reported that only 3 of 681 consecutive patients with ALF (0.41%) had evidence of acute HEV infection, although 43.4% of them were positive for anti-HEV IgG (15).
Based on the aforementioned information, we hypothesized that acute HEV infection might be involved in some cases of ALI and, particularly, in pregnant women or other indeterminate patients with ALI enrolled in the US ALFSG (15–17). It is also of interest to determine whether some cases of DILI-related ALI might actually be due to misdiagnosed acute HEV infection (10–12). In this study, we report the results of testing for acute and previous HEV infection in a large cohort of 594 adults prospectively enrolled in the North American ALI registry study.
METHODS
ALFSG study protocol
This consortium of academic medical centers, funded by the National Institutes of Health, has been conducting a prospective observational study of consecutive patients with ALF and ALI since 1998 (13,14). Eligible patients with ALI have symptoms of new-onset jaundice or hepatitis of less than 26 weeks duration before admission and an INR > 2.0 with no encephalopathy at enrollment (14). In addition to baseline demographics and presenting features, detailed clinical information, research blood samples, and clinical data and outcome data were obtained through 21 days after admission. In addition, long-term clinical data were collected at 1 year and 2 years after enrollment in a subgroup of patients (18,19). The study protocol was reviewed and approved by the Institutional Review Board of participating sites, and written informed consent was obtained from the patient and next of kin of eligible patients.
HEV testing
Day 1 sera (or the earliest day with available serum) from patients with ALI enrolled between 2008 and 2018 were stored at −80 °C before shipment for testing for anti-HEV IgM and anti-HEV IgG. Overall, 80% of the serum samples tested was obtained on study day 1, and the remaining 20% was obtained on study day 2 or later. The anti-HEV IgM, anti-HEV IgG, and HEV-RNA testing were performed in the research laboratory of Dr. Patrizia Farci at the National Institute of Allergy and Infectious Diseases (NIAID). Anti-HEV IgM was detected by a class-capture enzyme linked immunoassay (ELISA) assay using 10 μL of serum in duplicate (20,21). A modification of the ELISA assay was used to test all samples for anti-HEV IgG using 10 μL of serum in duplicate, and positive controls were run on each plate (20). In addition, all samples that were positive for anti-HEV IgG using the ELISA assay were also tested with the Wantai HEV IgM Elisa (Wantai, Beijing), which showed a 100% concordance with the in-house class-capture anti-HEV IgM tests. An independent serum sample from all anti-HEV IgM+ patients was also tested for HEV RNA, using consensus primers, from 140 μL of serum by real-time PCR with a lower limit of detection of 50 genome equivalents per reaction or 3.3 log10 genome equivalent per milliliter (22,23). Available long-term follow-up serum samples collected at 6, 12, and 24 months after enrollment underwent testing for anti-HEV IgM and quantitative anti-HEV IgG titers.
Definitions
Acute HEV infection was defined as a patient with anti-HEV IgM+ on repeated testing with or without detectable HEV RNA or a patient with repeatedly positive HEV RNA, regardless of the presence of anti-HEV IgM and IgG levels. Previous exposure to HEV infection was defined as a patient with anti-HEV IgG+ alone (i.e., anti-HEV IgM−). For characterization of HEV genotype and detection of HEV antigens in liver tissue, see Supplementary Methods (Supplementary Digital Content 2, http://links.lww.com/CTG/A443).
Data analyses
Statistical analyses were performed using SAS Version 9.3 (SAS Institute, Cary, NC) at the data coordinating center in Charleston, South Carolina. Differences between the anti-HEV IgG positive and negative test result groups were tested using the Student t test for continuous measures and the χ2 test for categorical measures. All tests were assessed at the 0.05 significance level. Logistic regression analysis was used to determine whether the probability of testing anti-HEV IgG positive was affected by prespecified prognostic variables.
RESULTS
Acute HEV infection
A total of 594 adult patients with ALI enrolled between 2008 and 2018 from 21 sites underwent testing for HEV infection (Figure 1). The median time between initial hospitalization and enrollment into the ALI study was 2 days. Overall, only 3 patients of the 594 tested were found to be positive for both anti-HEV IgM (0.5%) and anti-HEV IgG, whereas 62 patients (10.4%) were found to be anti-HEV IgG positive but IgM negative. The 3 male patients who tested positive for anti-HEV IgM were enrolled at 3 different sites in calendar years 2009, 2010, and 2016. None of them were immunosuppressed, and none progressed to ALF or underwent liver transplantation.
Figure 1.
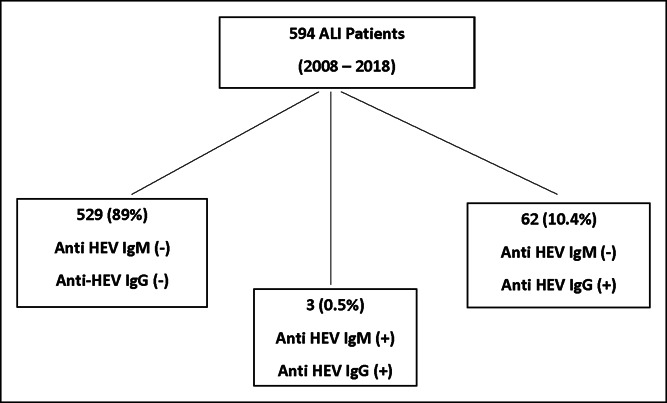
Overview of study population. Among the 594 patients with ALI who underwent testing, only 3 patients had detectable anti-HEV IgM (and IgG), whereas 62 patients were positive for HEV IgG but negative for IgM. The other 529 patients with ALI did not have detectable anti-HEV IgG or anti-HEV IgM. ALI, acute liver injury; HEV, hepatitis E virus.
Case 1
A 19-year-old previously healthy Asian man was hospitalized for nausea and vomiting 2 weeks after returning from Pakistan in 2009. His initial serum alanine aminotransferase (ALT) level was 2,210 U/L, total bilirubin 14.8 mg/dL, and INR 2.0. During his hospitalization, evaluations for hepatitis A, B, and C viruses (HAV, HBV, and HCV respectively) and Epstein-Barr virus showed negative results, as was autoantibody testing. Abdominal imaging revealed mild splenomegaly. A liver biopsy obtained on hospital day 1 demonstrated marked panlobular cholestatic hepatitis with lymphocytes, macrophages, plasma cells, eosinophils, and scattered neutrophils infiltrating the portal areas and parenchyma (Figure 2a, b). He was discharged after 6 days with a diagnosis of indeterminate ALI. His day 1 serum sample was positive for both anti-HEV IgM and IgG, but there was no detectable HEV RNA. Immunohistochemical staining of his day 1 liver biopsy confirmed the presence of HEV antigens by immunohistochemistry (Figure 2c).
Figure 2.
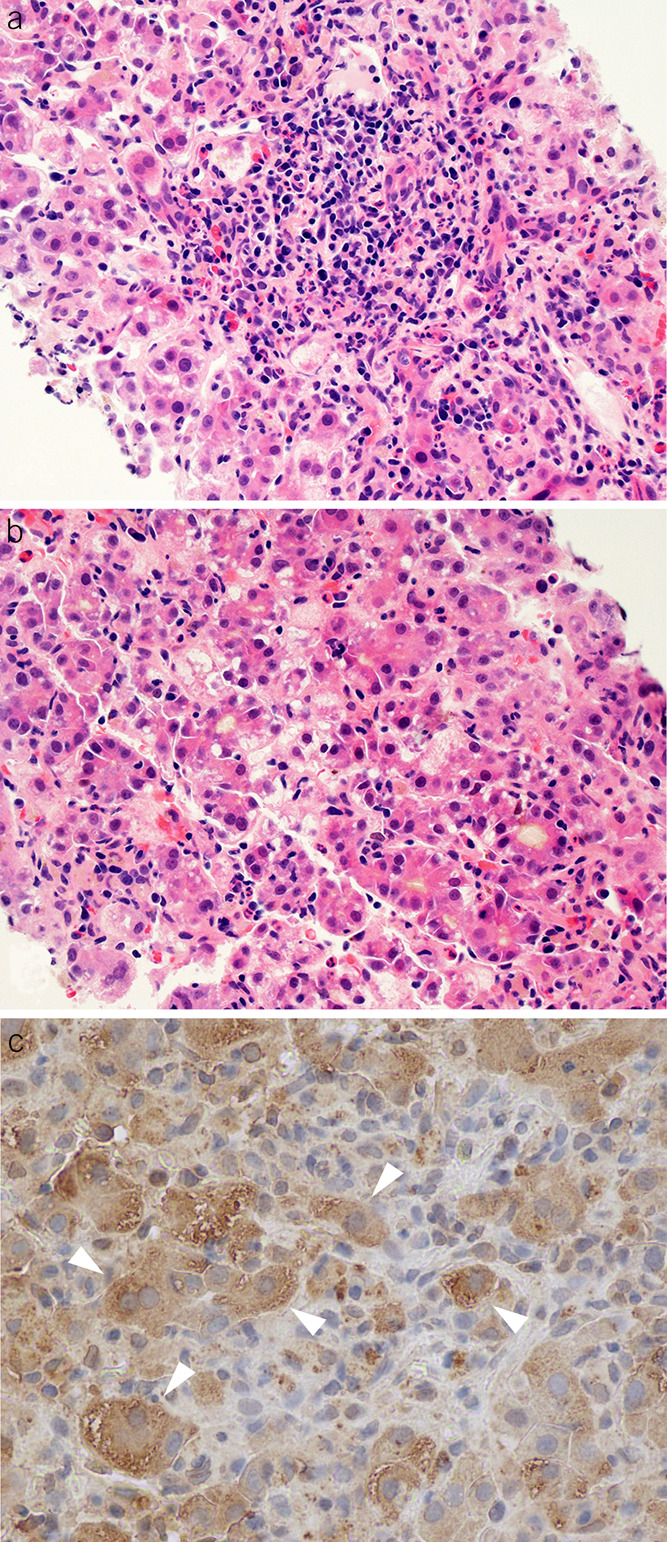
Histopathologic features of a patient with acute hepatitis E virus (HEV) infection. (a) A mixed portal inflammation with lymphocytes, plasma cells, and eosinophils (hematoxylin and eosin, ×400). (b) Lobular disarray with cholestatic rosettes and numerous foci of inflammation (hematoxylin and eosin, original magnification ×400). (c) Immunohistochemical staining of HEV antigen using an antibody against the ORF2 of HEV. The white arrowheads indicate positive cytoplasmic staining for HEV antigen.
Case 2
A 64-year-old Hispanic man presented with fever, diarrhea, and anorexia after a vacation in southern California in 2010. He denied any sick contacts or ingestion of wild game but had been consuming up to 3 or 4 alcoholic beverages per day. However, in the hospital, his ALT level rose from 300 IU/L to 2,000 IU/L. An evaluation for HAV, HBV, and HCV showed negative results, and he was initially treated with ciprofloxacin and metronidazole. Abdominal imaging demonstrated diffuse hepatic steatosis and mild splenomegaly. Because of worsening of his clinical status, he was transferred to a tertiary care center, with an ALT of 2,745 IU/L, total bilirubin of 13.5 mg/dL, and INR of 2.7. Serological testing using commercial assays demonstrated detectable anti-HEV IgM and IgG. He was treated with antiemetics and supportive care and did not require any blood products or progressed to ALF. He was eventually discharged in stable condition with a final diagnosis of acute HEV-related ALI. Testing of his serum at NIAID demonstrated anti-HEV IgM+, anti-HEV IgG+, and low levels of HEV-RNA of 2.15 log10. PCR amplification followed by direct sequencing analysis revealed that this patient was infected by HEV genotype 3.
Case 3
A 46-year-old Caucasian man with morbid obesity and hypertension incurred a puncture wound while cleaning a rabbit cage in 2016. Two weeks after his hand swelling had subsided, he was hospitalized with pale stools and dark urine with an ALT of 1,233 U/L, total bilirubin 30 mg/dL, and INR 2.1. Serological testing demonstrated detectable hepatitis B surface antigen, anti-hepatitis B core antibody IgM and IgG, whereas anti-HBsAg was negative. Testing for acute HAV and HCV infections showed negative results; ceruloplasmin was within the normal range. He was discharged in stable condition with an initial diagnosis of acute self-limited HBV-related ALI. Follow-up laboratory testing 10 days later showed ALT of 254 IU/L, total bilirubin of 14.0 mg/dL, and HBV DNA of 521 IU/mL. Testing of his serum at the NIAID revealed that he was positive for both anti-HEV IgM and IgG but negative for HEV RNA. Repeat testing for HBsAg and anti-HB core antibody at the NIAID confirmed the initial positivity detected for these markers, whereas antibodies testing against hepatitis D virus and HCV were found to be negative.
Seroprevalence of anti-HEV IgG
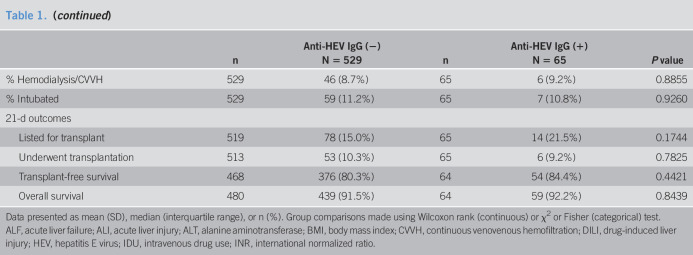
The median age of the overall cohort was 38 years, 72.1% were Caucasian, and 40.9% were men. The most common etiology of ALI was APAP overdose (50%), followed by autoimmune hepatitis (8.9%), hepatitis B (8.9%), and idiosyncratic DILI (7.9%). In addition, 8.4% had indeterminate ALI, and 0.5% of infections were pregnancy related. A total of 65 (11%) subjects tested positive for anti-HEV IgG. The median duration of follow-up in the protocol was 364 days (range, 1–720 days). As summarized in Table 1, patients with detectable anti-HEV IgG were significantly older (53.6 vs 38.1), more likely to be non-Caucasian (47.7% vs 25.5%), and found to have a non–APAP-related ALI (70.8% vs 47.3%). In addition, the median presenting ALT was lower, whereas the median alkaline phosphatase and total bilirubin levels were higher but the use of intubation, pressors, and dialysis were similar in the 2 groups. In addition, the proportion of patients progressing to ALF, listed for liver transplant, and actually undergoing transplantation was similar, as was transplant-free survival and overall 21-day survival. Potential risk factors of acute HEV infection such as intravenous drug use were similar in the 2 groups, whereas those with detectable anti-HEV IgG were more likely to receive a blood transfusion during their ALI admission.
Table 1.
Presenting features and outcomes of patients with ALI with and without detectable anti-HEV IgG
| n | Anti-HEV IgG (−) N = 529 |
n | Anti-HEV IgG (+) N = 65 |
P value | |
| Age (yr) | 529 | 38.1 (14.2) | 65 | 53.6 (15.4) | <0.0001 |
| Men | 529 | 213 (40.3%) | 65 | 30 (46.2%) | 0.3621 |
| Race | 529 | 65 | 0.0021 | ||
| White | 394 (74.5%) | 34 (52.3%) | |||
| Black/African American | 80 (15.1%) | 19 (29.2%) | |||
| Asian | 27 (5.1%) | 6 (9.2%) | |||
| Other | 28 (5.3%) | 6 (9.2%) | |||
| Hispanic or Latino | 526 | 36 (6.8%) | 65 | 8 (12.3%) | 0.1296 |
| BMI (kg/m2) | 485 | 27.4 (7.4) | 60 | 31.3 (19.6) | 0.0660 |
| Median duration of symptoms (d) | 525 | 4 (2–11) | 64 | 6 (4–15) | 0.0273 |
| ALI to ALF converter | 529 | 75 (14.1%) | 65 | 15 (23.1%) | 0.0590 |
| Etiology of ALI | 0.0209 | ||||
| Acetaminophen | 279 (52.7%) | 19 (29.2%) | |||
| Pregnancy | 2 (0.4%) | 1 (1.5%) | |||
| Autoimmune hepatitis | 45 (8.5%) | 8 (12.3%) | |||
| Budd-Chiari | 2 (0.4%) | 0 (0.0%) | |||
| DILI | 39 (7.4%) | 8 (12.3%) | |||
| Hepatitis A virus | 12 (2.3%) | 3 (4.6%) | |||
| Hepatitis B virus | 44 (8.3%) | 9 (13.8%) | |||
| Hepatitis C virus | 2 (0.4%) | 1 (1.5%) | |||
| Hepatitis E virus | 0 (0.0%) | 1 (1.5%) | |||
| Mushroom intoxication | 4 (0.8%) | 0 (0.0%) | |||
| Shock/ischemia | 32 (6.0%) | 6 (9.2%) | |||
| Wilson disease | 6 (1.1%) | 0 (0.0%) | |||
| Indeterminate | 44 (8.3%) | 6 (9.2%) | |||
| Other viruses | 2 (0.4%) | 0 (0.0%) | |||
| Other | 16 (3.0%) | 3 (4.6%) | |||
| HIV (%) | 529 | 12 (2.3%) | 65 | 1 (1.5%) | 1.0 |
| IDU at any time in the past | 529 | 39 (7.4%) | 65 | 2 (3.1%) | 0.2976 |
| Any blood products over 7 study days | 529 | 99 (18.7%) | 65 | 20 (30.8%) | 0.0219 |
| Admission laboratory values | |||||
| ALT (IU/mL) | 522 | 3,725 (3,397) | 65 | 2,906 (2,867) | 0.0573 |
| Alkaline phosphatase (IU/mL) | 522 | 130 (79) | 65 | 151 (76) | 0.0102 |
| Bilirubin (mg/dL) | 521 | 8.9 (9.0) | 64 | 10.8 (8.4) | 0.0176 |
| INR | 511 | 3.0 (1.7) | 64 | 2.9 (1.5) | 0.8933 |
| Creatinine (mg/dL) | 523 | 1.3 (1.3) | 64 | 1.6 (1.7) | 0.1458 |
| Region of enrolling site | 529 | 65 | 0.3224 | ||
| Northeast | 67 (12.7%) | 9 (13.8%) | |||
| South | 184 (34.8%) | 16 (24.6%) | |||
| West | 102 (19.3%) | 19 (23.2%) | |||
| Midwest | 167 (31.6%) | 20 (30.8%) | |||
| Canada | 9 (1.7%) | 1 (1.5%) | |||
| % Hemodialysis/CVVH | 529 | 46 (8.7%) | 65 | 6 (9.2%) | 0.8855 |
| % Intubated | 529 | 59 (11.2%) | 65 | 7 (10.8%) | 0.9260 |
| 21-d outcomes | |||||
| Listed for transplant | 519 | 78 (15.0%) | 65 | 14 (21.5%) | 0.1744 |
| Underwent transplantation | 513 | 53 (10.3%) | 65 | 6 (9.2%) | 0.7825 |
| Transplant-free survival | 468 | 376 (80.3%) | 64 | 54 (84.4%) | 0.4421 |
| Overall survival | 480 | 439 (91.5%) | 64 | 59 (92.2%) | 0.8439 |
Data presented as mean (SD), median (interquartile range), or n (%). Group comparisons made using Wilcoxon rank (continuous) or χ2 or Fisher (categorical) test.
ALF, acute liver failure; ALI, acute liver injury; ALT, alanine aminotransferase; BMI, body mass index; CVVH, continuous venovenous hemofiltration; DILI, drug-induced liver injury; HEV, hepatitis E virus; IDU, intravenous drug use; INR, international normalized ratio.
Models of anti-HEV IgG seroprevalence
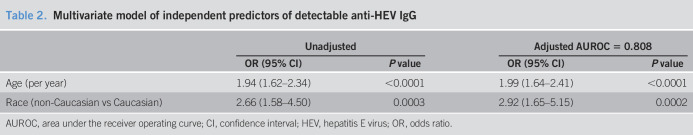
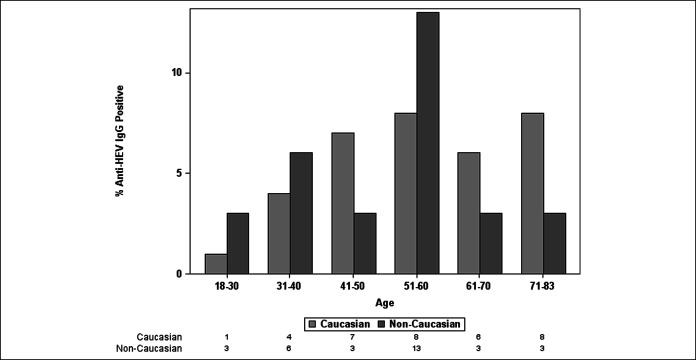
A multivariate model of baseline factors associated with detectable anti-HEV IgG was developed from factors with a P < 0.10 on univariate analysis. Only subject age and race were independently associated with anti-HEV IgG seropositivity (Table 2). When the etiology of ALI was forced into the model, the area under the receiver operating curve did not substantially change, indicating that subject age and race are the independent drivers of the association. Plots of the seroprevalence of anti-HEV IgG by subject age demonstrated a steady increase in anti-HEV IgG seroprevalence with subject age, which is in line with a cohort effect. In addition, stratification of the seroprevalence data by subject race of Caucasian vs other showed significant differences by race as well (Figure 3).
Table 2.
Multivariate model of independent predictors of detectable anti-HEV IgG
| Unadjusted | Adjusted AUROC = 0.808 | |||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (per year) | 1.94 (1.62–2.34) | <0.0001 | 1.99 (1.64–2.41) | <0.0001 |
| Race (non-Caucasian vs Caucasian) | 2.66 (1.58–4.50) | 0.0003 | 2.92 (1.65–5.15) | 0.0002 |
AUROC, area under the receiver operating curve; CI, confidence interval; HEV, hepatitis E virus; OR, odds ratio.
Figure 3.
Seroprevalence of anti-HEV IgG by subject age and race. Plot of anti-HEV IgG stratified by patient age and race (Caucasian vs non-Caucasian). HEV, hepatitis E virus.
Anti-HEV IgG seroprevalence over time and by study location
The seroprevalence of anti-HEV IgG remained stable during the study period varying between 2% and 5% (see Supplementary Figure 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A442). In addition, the seroprevalence of anti-HEV IgG did not substantially vary by site location or region in the United States (Figure 4).
Figure 4.
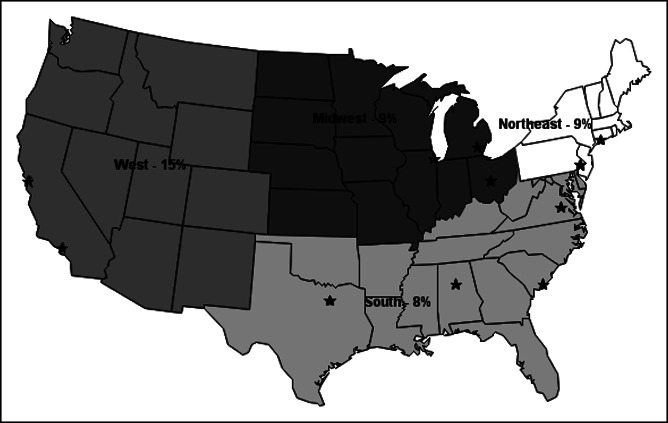
Seroprevalence of antihepatitis E virus (HEV) IgG by Acute Liver Failure Study Group (ALFSG) site and region. There were 21 sites in the ALFSG who contributed cases to the study between 2008 and 2018. One of the 10 cases from Canada had detectable anti-HEV IgG (data not shown). *Represent states with an ALFSG clinical site.
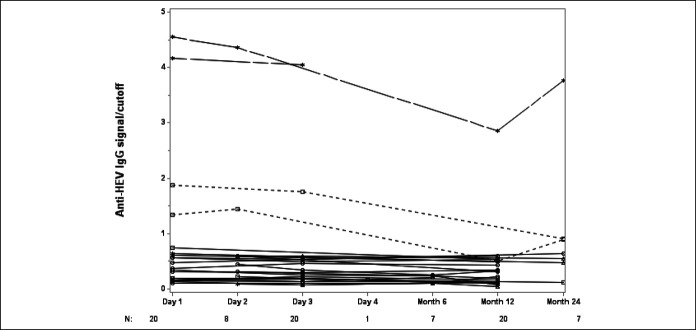
Persistence of anti-HEV IgG during follow-up
Convalescent sera obtained at 6–24 months after enrollment were available for testing in 27 patients. Twenty-two seronegative patients remained negative and 2 seropositive patients remained positive, although the titer of anti-HEV IgG did decline over time and 2 initially seropositive patients became negative (Figure 5).
Figure 5.
Persistence of anti-HEV IgG during follow-up. Solid black lines indicate 23 patients who were negative for anti-HEV IgG negative at all time points tested; black dashed lines indicates 2 patients who were positive for anti-HEV IgG during in-patient treatment but negative for anti-HEV IgG in long-term follow-up; gray dashed lines indicate 2 patients who were anti-HEV IgG positive at all time points tested. HEV, hepatitis E virus.
DISCUSSION
Acute HEV infection with genotype 1 or 2 is a frequent cause of acute hepatitis and jaundice in many parts of the world because of spread of the virus through fecal–oral transmission (1,2,24). By contrast, cases arising from HEV genotypes 3 and 4 infection are zoonotic and account for most autochthonous HEV infections in European countries where pigs and wild boar are the main reservoir. Consumption of raw or undercooked infected meat products and transfusion of contaminated blood are well-established routes of HEV transmission in Western countries. Series from the United Kingdom, Germany, and France report numerous cases of acute hepatitis and jaundice due to HEV infection genotype 3 (9,10,25,26). In some series, up to 20% of patients hospitalized with acute hepatitis had evidence of acute HEV infection (25,26). More recently, acute HEV infection has emerged as the most common cause of acute viral hepatitis in Scotland (27).
In this study of 594 consecutively enrolled patients from 21 North American liver transplant centers, we hypothesized that previously unrecognized acute HEV infection might be responsible for some cases of indeterminate ALI, idiosyncratic DILI, autoimmune hepatitis, or pregnancy-related ALI (Table 1). Even if only a small proportion of these patients had unrecognized acute HEV infection, this finding could have important epidemiological and clinical implications for individual patients. However, only 3 patients met our case definition of acute HEV infection. Of interest, all 3 patients were men of varying age and ethnicity, and 2 of the cases had been initially attributed to an alternative cause of ALI. The HEV infection in case 1 was likely acquired in Pakistan, which is known to have a high incidence of HEV genotype 1 and 2 infections. The detection of anti-HEV IgM in the serum and HEV antigens from the liver biopsy establishes this as an unequivocal case of acute HEV. Patient 3 had evidence of acute HBV infection and acute HEV infection. Of note, 1 of the previously identified patients with ALF from the ALFSG with acute HEV infection also had simultaneous HBV–HEV coinfection (15). Although HEV is commonly implicated as a cause of acute decompensation in patients with chronic HBV, primary presentation with simultaneous acute HEV and HBV coinfection is less well described but still plausible (4,28). A previous study from the United Kingdom demonstrated evidence of acute HEV and HBV coinfection in 2 hospitalized patients with unexplained hepatitis (29). Although we cannot definitively exclude the possibility that case 3 had preexisting HBV infection, we do not believe that this was a case with false-positive anti-HEV IgM because it was repeatedly positive on independent serum samples. In case 2, no identifiable risk factor of acquiring HEV infection was apparent, but HEV genotype 3 viremia was detected and confirmed on repeated testing. Of note, none of the pregnancy-, autoimmune-, or DILI-associated ALI cases had evidence of acute HEV infection. In addition, only 1 of the 50 patients with indeterminate ALI (case 1) was confirmed to be due to acute HEV infection. The low overall incidence of HEV-related ALI in our cohort suggests that previously unrecognized acute HEV infection in North American patients with severe ALI is very uncommon.
Our data on the seroprevalence of previous HEV infection demonstrates a clear relationship of anti-HEV IgG seropositivity with subject age and race (Table 1, Figure 4). An increasing incidence of anti-HEV IgG with patient age has been reported in NHANES and other population-based studies (7,30). Whether the increasing seroprevalence of previous HEV infection with subject age is due to increasing exposure to HEV or other routes of transmission is unknown. The higher proportion of previous HEV infection in ethnic minorities in the United States has also previously been reported, but risk factors of and reasons for these observations remain unclear. A striking finding in our study is the lower overall incidence of anti-HEV IgG in this cohort, enrolled between 2008 and 2018, compared with our previous study in patients with ALF enrolled between 1998 and 2011, wherein the seroprevalence rates were 11% and 45%, respectively (15). The CDC has also reported a marked decline in anti-HEV IgG detection using a consistent ELISA assay (i.e., DiaSorin) in population-based cohorts tested between 1988 and 2010 (26). The significant decline was noted in all subgroups stratified by age, sex, region of residence, and race/socioeconomic status. Contrary to our previous observations in the ALF cohort, there was no apparent relationship between year of study enrollment and anti-HEV IgG positivity in these patients with ALI (see Supplemental Figure 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A442). The persistence of anti-HEV IgG during follow-up in a small subgroup of patients with serial blood samples is consistent with our previous findings of anti-HEV IgG persistence over time in the patients with ALF (15) (Figure 5).
The strengths of our study include the prospective enrollment of consecutive patients with ALI from multiple centers in North America over a 10-year period wherein the etiology and outcomes of ALI were carefully tracked. Furthermore, all of the patients underwent testing at a central laboratory at the NIAID using HEV ELISA assays that have been shown to have excellent reproducibility and accuracy (31,32). Patient 1 clearly had evidence of acute HEV infection with detectable HEV antigens in his liver by immunohistochemistry, and patient 2 had detectable viremia in his blood. Several investigators have suggested that screening for HEV genotype 3 infection in Western patients might be best accomplished using PCR testing because some patients with acute HEV infection do not have detectable anti-HEV IgG or anti-HEV IgM at presentation (26,33). However, resource limitations precluded us from testing all patients for HEV RNA. In addition, a previous study using metagenomic sequencing in 204 adult patients with indeterminate ALF failed to identify any cases of previously unrecognized acute HEV infection (34). Furthermore, previous studies have demonstrated that only 40%–60% of patients with symptomatic acute HEV infection have detectable HEV RNA in the serum (2). Finally, the experience at the NIAID, which helped develop and discover the anti-HEV IgM and anti-HEV IgG serology tests, has indicated that no previous cases of acute HEV infection with detectable HEV RNA were anti-HEV IgM negative. Therefore, we believe that the diagnostic algorithm used in this study is both sensitive and specific for detecting and diagnosing immunocompetent patients with acute HEV infection, as recently proposed by the European Association for the Study of Liver Diseases (6).
In summary, our data demonstrate that previously unsuspected acute HEV infection is an uncommon cause of ALI in North American adults. Furthermore, unsuspected acute HEV infection was not implicated in any case of pregnancy-, DILI-, or autoimmune-related ALI. The presence of viral antigens in the liver tissue of case 1 and detectable HEV RNA in the serum of patient 2 provided confirmatory evidence that these were true acute HEV infections. The only identifiable clinical risk factor in our 3 patients was overseas travel to an endemic area in case 1, whereas neither use of intravenous drug use nor ingestion of undercooked meats or organ meats could be identified in the others. Therefore, we recommend testing for HEV infection using anti-HEV IgM and anti-HEV IgG in immunocompetent patients with unexplained ALI and confirmation with HEV RNA testing by PCR if available as suggested by European Association of the Study of Liver Diseases (6). The modest seroprevalence of previous HEV infection in our study mirrors the demographic and geographic distribution of past HEV infection reported in population-based studies in the United States, wherein the overall prevalence of infection has been declining suggestive of a cohort effect. Clinical outcomes in subjects with anti-HEV IgG were similar to those who were seronegative, demonstrating that previous exposure to HEV is not an independent risk factor of progression to ALF or the need for liver transplantation.
CONFLICTS OF INTEREST
Guarantor of the article: Robert J. Fontana, MD.
Specific author contributions: The design (R.J.F., V.D., W.M.L., and P.F.), conduct, collection, and analysis of the data (R.J.F., R.E.E., M.G., B.H., J.H., V.D., D.E.K., H.N., N.N., W.M.L., and P.F.) were completed along with manuscript drafting (R.J.F., R.E.E., V.D., W.M.L., and P.F.). All authors reviewed the final version of the submitted manuscript.
Financial support: We gratefully acknowledge the support provided by the members of The Acute Liver Failure Study Group. This study was funded by the National Institute of Diabetes, Digestive, and Kidney Diseases (DK U-01-58369). Additional funding was provided by the Tips Fund of Northwestern Medical Foundation and the Jeanne Roberts and Rollin and Mary Ella King Funds of the Southwestern Medical Foundation. This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases.
Potential competing interests: R.J.F. receives research funding from Gilead, Abbvie, and BMS and consults for Sanofi. J.H. reports research support from Salix and Intercept. W.M.L. receives research support from Merck, Conatus, Intercept, Bristol-Myers Squibb, Novo Nordisk, Synlogic, Eiger, Cumberland, Exalenz, Instrumentation Laboratory, and Ocera Therapeutics, now Mallinckrodt Pharmaceuticals. He has consulted for Novartis, Sanofi, Forma Therapeutics, Genentech, and Seattle Genetics. No conflicts of interest reported for R.E.E., M.G., B.H., V.D., D.E.K., H.N., N.N., and P.F.
Study Highlights.
WHAT IS KNOWN
✓ The etiology of ALI is indeterminate in 10%–20% of North American adults.
✓ Worldwide, there are an estimated 20 million acute HEV infections each year leading to more than 70,000 deaths.
✓Previous European studies have suggested that autochthonous acute HEV might account for up to 20% of patients with previously unexplained ALI.
WHAT IS NEW HERE
✓ Three of 594 patients (0.5%) had detectable anti-HEV IgM. Therefore, acute HEV infection is a rare cause of ALI in hospitalized North American patients.
✓ The overall prevalence of anti-HEV IgG was 11%, and older, non-Caucasian patients were most likely to have evidence of previous infection.
✓ These data coupled with those from other studies indicate that exposure to HEV in the general US population is declining over time, consistent with a cohort effect.
TRANSLATIONAL IMPACT
✓ Acute HEV infection is a rare cause of liver injury in hospitalized patients with ALI. Therefore, testing should be limited to patients with unexplained acute hepatitis.
Supplementary Material
ACKNOWLEDGEMENTS
Members and institutions participating in the Acute Liver Failure Study Group 1998–2011 are as follows: W.M. Lee, MD (principal investigator), George A. Ostapowicz, MD, Frank V. Schiødt, MD, Julie Polson, MD, University of Texas Southwestern, Dallas, TX; Anne M. Larson, MD, Iris Liou, MD, University of Washington, Seattle, WA; Timothy Davern, MD, University of California, San Francisco, CA (current address: California Pacific Medical Center, San Francisco, CA), Oren Fix, MD, University of California, San Francisco; Michael Schilsky, MD, Mount Sinai School of Medicine, New York, NY (current address: Yale University, New Haven, CT); Timothy McCashland, MD, University of Nebraska, Omaha, NE; J. Eileen Hay, MBBS, Mayo Clinic, Rochester, MN; Natalie Murray, MD, Baylor University Medical Center, Dallas, TX; A. Obaid S. Shaikh, MD, University of Pittsburgh, Pittsburgh, PA; Andres Blei, MD, Northwestern University, Chicago, IL (deceased), Daniel Ganger, MD, Northwestern University, Chicago, IL; Atif Zaman, MD, University of Oregon, Portland, OR; Steven H.B. Han, MD, University of California, Los Angeles, CA; Robert Fontana, MD, University of Michigan, Ann Arbor, MI; Brendan McGuire, MD, University of Alabama, Birmingham, AL; Raymond T. Chung, MD, Massachusetts General Hospital, Boston, MA; Alastair Smith, MBChB, Duke University Medical Center, Durham, NC; Robert Brown, MD, Cornell/Columbia University, New York, NY; Jeffrey Crippin, MD, Washington University, St Louis, MO; Edwyn Harrison, Mayo Clinic, Scottsdale, AZ; Adrian Reuben, MBBS, Medical University of South Carolina, Charleston, SC; Santiago Munoz, MD, Albert Einstein Medical Center, Philadelphia, PA; Rajender Reddy, MD, University of Pennsylvania, Philadelphia, PA; R. Todd Stravitz, MD, Virginia Commonwealth University, Richmond, VA; Lorenzo Rossaro, MD, University of California Davis, Sacramento, CA; Raj Satyanarayana, MD, Mayo Clinic, Jacksonville, FL; and Tarek Hassanein, MD, University of California, San Diego, CA. The University of Texas Southwestern Administrative Group included Grace Samuel, Ezmina Lalani, Carla Pezzia, and Corron Sanders, PhD, Nahid Attar, the Statistics and Data Management Group included Joan S. Reisch, PhD, Linda S. Hynan, PhD, Janet P. Smith, Joe W. Webster and Mechelle Murray, and the Medical University of South Carolina Data Coordination Unit included Valerie Durkalski, PhD, Wenle Zhao, PhD, Catherine Dillon, and Tomoko Goddard.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A442, http://links.lww.com/CTG/A443
REFERENCES
- 1.Hoofnagle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med 2012;367:1237–44. [DOI] [PubMed] [Google Scholar]
- 2.Patra S, Kumar A, Trivedi SS, et al. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med 2007;147:28–33. [DOI] [PubMed] [Google Scholar]
- 3.Rein DB, Stevens GA, Thaster J, et al. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012;55:988–97. [DOI] [PubMed] [Google Scholar]
- 4.Acharya SK, Sharma SP, Singh R, et al. Hepatitis E virus infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol 2007;46:387–94. [DOI] [PubMed] [Google Scholar]
- 5.Drobeniuc J, Greene-Montfort T, Le NT, et al. Laboratory based surveillance for hepatitis E virus infection, United States, 2005-2012. Emerg Infect Dis 2013;19:218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EASL clinical practice guidelines on hepatitis E virus infection. J Hepatol 2018;68:1256–71. [DOI] [PubMed] [Google Scholar]
- 7.Kuniholm MH, Purcell RH, McQuillan GM, et al. Epidemiology of hepatitis E virus in the United States: Results from the third NHANES, survey, 1988-1994. J Infec Dis 2009;200:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wichman O, Schimanski S, Koch J, et al. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J Infect Dis 2008;198:1732–41. [DOI] [PubMed] [Google Scholar]
- 9.Peron JM, Bureau C, Poirson H, et al. Fulminant liver failure from acute authochthonous hepatitis E in France: Description of 7 patients with acute hepatitis E and encephalopathy. J Viral Hep 2007;15:293–303. [DOI] [PubMed] [Google Scholar]
- 10.Dalton HR, Fellows HJ, Stableforth W, et al. The role of hepatitis E virus testing in drug-induced liver injury. Aliment Pharmacol Ther 2007;26:1429–35. [DOI] [PubMed] [Google Scholar]
- 11.Dalton HR, Fellows HJ, Gane EJ, et al. Hepatitis E in New Zealand. J Gastro Hepatol 2007;22:1236–40. [DOI] [PubMed] [Google Scholar]
- 12.Davern TJ, Chalasani N, Fontana RJ, et al. Acute hepatitis E infection accounts for some cases of suspected DILI. Gastroenterology 2011;141:1665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reuben A, Tillman H, Fontana RJ, et al. Outcomes in adults with acute liver failure between 1998 and 2013: An observational cohort study. Ann Intern Med 2016;164:724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch DG, Speiser JL, Durkalski V, et al. The natural history of severe acute liver injury. Am J Gastroenterol 2017;112:1389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontana RJ, Engle RE, Scaglione S, et al. The role of hepatitis E virus infection in adult Americans with acute liver failure. Hepatology 2016;64:1870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamar N, Selves J, Mansuy JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med 2008;358:811–7. [DOI] [PubMed] [Google Scholar]
- 17.Haagsma EB, Niesters HG, Van Den Berg AP, et al. Prevalence of hepatitis E virus infection in liver transplant recipients. Liver Transpl 2009;15:149–50. [DOI] [PubMed] [Google Scholar]
- 18.Rangnekar AS, Ellerbe C, Durkalski V, et al. Quality of life is significantly impaired in long-term survivors of acute liver failure and particularly in acetaminophen overdose patients. Liver Transplant 2013;19:991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontana RJ, Ellerbe C, Durkalski VE, et al. Two-year outcomes in initial survivors with acute liver failure: Results from a prospective, multicentre study. Liver Int 2015;35:370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engle RE, Yu C, Emerson SU, et al. Hepatitis E virus capsid antigens derived from viruses of human and swine origin are equally efficient for detecting anti-HEV by Enzyme immunoassay. J Clin Micriobiol 2002;40:4576–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu C, Engle RE, Bryan JP, et al. Detection of immunoglobulin M antibodies to hepatitis E virus by class capture enzyme immunoassay. Clin Diagn Lab Immun 2003;10:579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johne R, Plenge-Bonig A, Hess M, et al. Detection of a novel hepatitis E-like virus in faeces of wild rates using a nested broad-spectrum RT-PCR. J Gen Virol 2010;91:750–8. [DOI] [PubMed] [Google Scholar]
- 23.Shukla P, Nguyen HT, Torian U, et al. Cross-species infections of cultured cells by hepatitis E virus and discovery of an infectious virus-host recombinant. Proc Natl Acad Sci U S A 2011;108:2438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carratala A, Joost S. Population density and water balance influence the global occurrence of hepatitis E epidemics. Sci Rep 2019;9:10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manka P, Bechmann LP, Coombes JD, et al. Hepatitis E virus infection as a possible cause of acute liver failure in Europe. Clin Gastreonterol Hepatol 2015;13:1836–42. [DOI] [PubMed] [Google Scholar]
- 26.Quickert S, Reuken PA, Rose M, et al. Acute Hepatitis E is an underreported cause of severe acute liver injury. Clin Gastro Hepatol 2018;17:1004–7. [DOI] [PubMed] [Google Scholar]
- 27.Wallace SJ, Swann R, Donnelly M, et al. Mortality and morbidity of locally acquired hepatitis E in the national Scottish cohort: A multicentre, retrospective study. Aliment Pharmacol Ther 2020;51:974–86. [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Ziao D, Yu JH, et al. Clinical course of sporadic acute hepatitis E in a hepatitis B virus endemic region. Int J Infect Dis 2018;70:107–14. [DOI] [PubMed] [Google Scholar]
- 29.Genova-Raeva L, Punkova L, Campo DS, et al. Cryptic hepatitis B and E in patients with acute hepatitis of unknown etiology. J Infect Dis 2015;212:1962–9. [DOI] [PubMed] [Google Scholar]
- 30.Teshale EH, Denniston MM, Drobeniuc J, et al. Decline in hepatitis E virus antibody prevalence in the US from 1998-1994 to 2009-2010. J Infect Dis 2015: 211: 366- 373. [DOI] [PubMed] [Google Scholar]
- 31.Mast EE, Alter MJ, Holland PV, et al. Evaluation of assays for antibody to hepatitis E virus by a serum panel: Hepatitis E virus antibody Serum Panel Evaluation Group. Hepatology 1998;27:857–61. [DOI] [PubMed] [Google Scholar]
- 32.Holm DK, Moessner BK, Engle RE, et al. Declining prevalence of hepatitis E antibodies among Danish Blood donors. Transfusion 2015;55:1662–7. [DOI] [PubMed] [Google Scholar]
- 33.Norder H, Galli C, Magnil E, et al. Hepatitis E virus genotype 3 genomes from RNA positive but serologically negative plasma donors have CUG as the start codon for ORF3. Intervirology 2018;61:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Somasekar S, Lee D, Rule J, et al. Viral surveillance in serum samples from patients with acute liver failure by metagenomics next-generation sequencing. Clin Infect Dis 2017;65:1477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.