INTRODUCTION:
Hereditary hemochromatosis is an autosomal recessive disorder of iron absorption, leading to organ dysfunction. C282Y gene homozygosity is implicated in 80%–95% of cases of hereditary hemochromatosis. The clinical penetrance of this genotype remains unclear. The purpose of the study was to better describe the clinical penetrance and disease progression of C282Y homozygotes.
METHODS:
This is a retrospective study of all individuals in Newfoundland and Labrador, Canada, homozygous for the C282Y mutation from 1999 to 2009. Using electronic health records, laboratory values, phlebotomy status, radiologic reports, and clinic records were recorded up to November 2017. Iron overload status was classified via the HealthIron study. SPSS Version 19.0 (IBM Corporation) was used for descriptive statistics. Predictors of disease penetrance were assessed with logistic regression; a Student t test was used for continuous variables, and χ2 tests were used for categorical variables.
RESULTS:
Between 1999 and 2009, 360 individuals tested positive for C282Y/C282Y. The mean age of diagnosis was 49.1 years. Three hundred six individuals had adequate follow-up for analysis (mean 11.6 years). End-organ damage was observed in 18.3%, with 5.8% developing liver disease. End-organ damage was more frequently observed in men 24.3% vs 10.5% (P < 0.05). Clinical penetrance in postmenopausal women approached that of men 18.3%.
DISCUSSION:
This is the largest reported cohort of C282Y homozygotes, followed for an extended duration of time in North America. The findings reflect outcomes in routine clinical practice and suggest that C282Y homozygosity uncommonly causes end-organ damage and liver disease.
INTRODUCTION
Hereditary hemochromatosis (HH) is the most common autosomal recessive disorder among individuals of Northern European descent, with a 1:250 homozygote frequency (1,2). In 1996, Feder et al. identified the HFE gene (3). Missense alterations to this particular gene are most commonly implicated in HH. It was later found that approximately 80% of iron overload-related diseases are due to C282Y homozygosity in the HFE gene (2). Genes encoding hemojuvelin, hepcidin, TFR2, and FPN are some examples of other non-HFE mutations implicated in hemochromatosis (4). An observational study (5) in Newfoundland showed that compared with the wild-type HFE gene, both H63D and C282Y homozygotes had a significantly higher transferrin saturation, and this was an independent predictor of higher iron saturation levels. Regarding primary and secondary prevention of disease, some observational studies (6,7) have found that therapeutic phlebotomy may minimize disease onset and severity, especially if performed before the onset of end-organ damage.
Initially, C282Y homozygosity was regarded as highly penetrant with significant morbidity and mortality if not identified and treated in a timely manner (1,8). Some investigators concluded that although time to progression was variable, iron overload was almost inevitable (9). HH has been highlighted as an important condition in primary care that has been frequently under recognized (10).
However, there are some studies that suggest otherwise: An analysis (11) of multiple-cause mortality in the United States from 1979 to 1992 suggested a low clinical penetrance of the HH mutations and/or a lower rate of recognition of disease. Another study (12) at a liver transplant center found that subjects with C282Y homozygosity or C282Y/H63D compound heterozygosity accounted for less than 1% of all patients seen for cirrhosis over 10 years. The purpose of our study was to better describe the clinical penetrance of C282Y homozygosity in Newfoundland and Labrador, Canada.
METHODS
This study was approved by Research Ethics Board of Memorial University of Newfoundland (St. John's, Newfoundland and Labrador, Canada) before commencement. Approval was also obtained from respective regional health authorities to obtain medical records of study subjects.
Study population
This retrospective analysis was conducted in the province of Newfoundland and Labrador, Canada, where most people are Whites of Irish or British descent with a total population of 525,073 in the year 2018. Subjects included in the study were identified through Medical Genetics. The subjects were referred for HFE genotyping for a variety of HH-related indications, such as biochemical evidence of iron overload and/or first-degree relatives of patients homozygous for C282Y. However, the indication for genotyping was not specified for these patients. All individuals who had genotyping in the province between 1999 and 2009 were included for initial case identification. There is only one laboratory that performs genotyping for HH, enabling us to acquire all cases in the province. Only subjects homozygous for C282Y were included in this study.
Using electronic health records, the following information for each subject was collected at time of genotyping: age, sex, serum ferritin (SF), serum iron, transferrin saturation, aspartate aminotransferase, alanine aminotransferase, total bilirubin, international normalized ratio, and pathology reports including liver biopsies, radiologic imaging reports, and clinic letters. This information was used to assess baseline characteristics. For patients who did not have these investigations performed at the time of genotyping, bloodwork that was obtained within 6 months of genotyping was used. No data collected beyond 6 months were used for baseline characteristics to avoid including information for partially treated individuals. In addition, any evidence of active treatment for iron overload such as phlebotomies or any history of blood donations was collected.
On every patient, annual follow-up data were collected whenever available, up to November 2017. Follow-up data were used to examine progression of iron overload and related diseases. Iron overload-related disease was classified as per the HealthIron Study (13) (Table 1). History of diabetes, hypopituitarism, obesity, and alcohol use was not collected. Clinical penetrance was defined as meeting criteria for Health Iron (HI) Classification 4 (Table 1), excluding endocrinopathies such as diabetes or hypopituitarism. Female subjects were divided into premenopausal and postmenopausal on the basis of being younger than or equal to 50 years vs older than 50 years, respectively.
Table 1.
Iron overload categories adapted from the HealthIron study
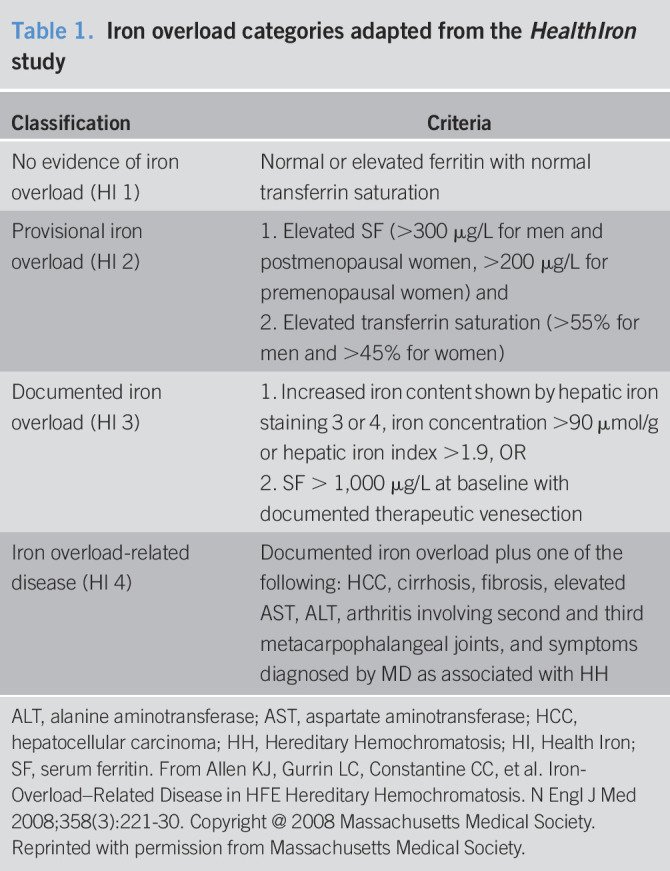
| Classification | Criteria |
| No evidence of iron overload (HI 1) | Normal or elevated ferritin with normal transferrin saturation |
| Provisional iron overload (HI 2) | 1. Elevated SF (>300 μg/L for men and postmenopausal women, >200 μg/L for premenopausal women) and 2. Elevated transferrin saturation (>55% for men and >45% for women) |
| Documented iron overload (HI 3) | 1. Increased iron content shown by hepatic iron staining 3 or 4, iron concentration >90 μmol/g or hepatic iron index >1.9, OR 2. SF > 1,000 μg/L at baseline with documented therapeutic venesection |
| Iron overload-related disease (HI 4) | Documented iron overload plus one of the following: HCC, cirrhosis, fibrosis, elevated AST, ALT, arthritis involving second and third metacarpophalangeal joints, and symptoms diagnosed by MD as associated with HH |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; HCC, hepatocellular carcinoma; HH, Hereditary Hemochromatosis; HI, Health Iron; SF, serum ferritin. From Allen KJ, Gurrin LC, Constantine CC, et al. Iron-Overload–Related Disease in HFE Hereditary Hemochromatosis. N Engl J Med 2008;358(3):221-30. Copyright @ 2008 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Incidence and prevalence of iron overload were calculated using the number of patients with complete data as the denominator. SPSS version 19.0 was used for descriptive statistics. Values are expressed as means or percentage. To compare differences between male and female C282Y homozygotes, the Student t test was used to assess continuous variables and χ2 tests were used for categorical variables. Binomial logistic regression was used to assess for potential predictors of end-organ damage. A P value ≤ 0.05 was considered statistically significant.
RESULTS
Overall results
A total of 4,138 individuals underwent HFE genotyping in Newfoundland and Labrador between 1999 and January 2009. There were 366 C282Y homozygotes, 170 H63D homozygotes, 758 C282Y heterozygotes, 858 H63D heterozygotes, 267 compound heterozygotes, and 1719 wild type.
There were 333 C282Y homozygotes with available baseline data, 22.8% of which were premenopausal women (younger than 50 years), 19.2% were postmenopausal women, and 58.0% were men. At the time of genotyping, the mean age was 49.1 years, 73.5% had an elevated SF (mean 767.4 μg/L), and 79.1% had an elevated serum transferrin saturation (mean 0.66). The mean baseline biochemical values are listed in (Table 2).
Table 2.
Baseline characteristics of the study population
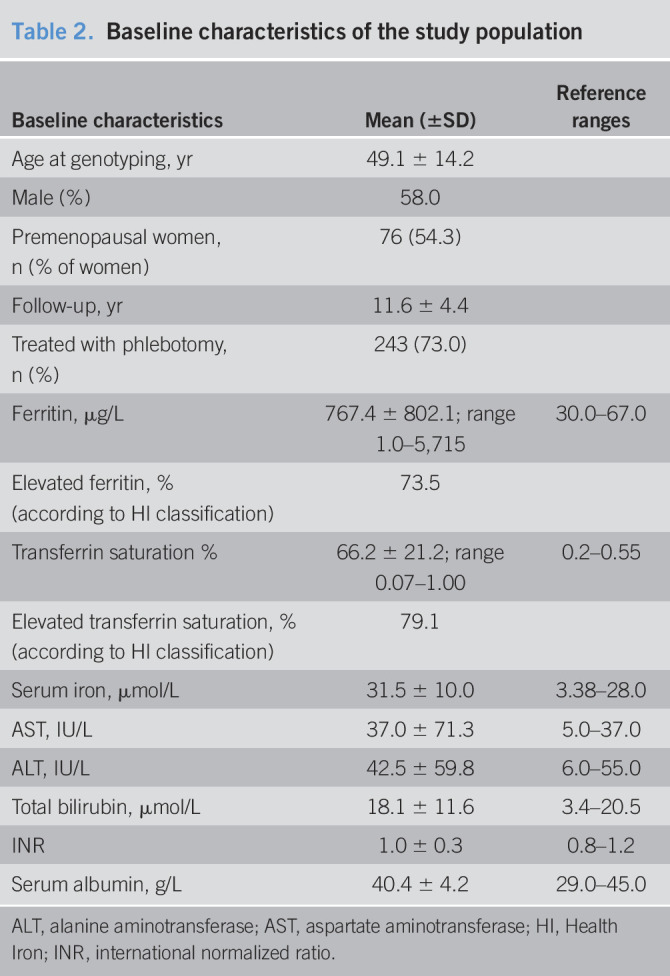
| Baseline characteristics | Mean (±SD) | Reference ranges |
| Age at genotyping, yr | 49.1 ± 14.2 | |
| Male (%) | 58.0 | |
| Premenopausal women,n (% of women) | 76 (54.3) | |
| Follow-up, yr | 11.6 ± 4.4 | |
| Treated with phlebotomy, n (%) | 243 (73.0) | |
| Ferritin, μg/L | 767.4 ± 802.1; range 1.0–5,715 | 30.0–67.0 |
| Elevated ferritin, % (according to HI classification) | 73.5 | |
| Transferrin saturation % | 66.2 ± 21.2; range 0.07–1.00 | 0.2–0.55 |
| Elevated transferrin saturation, % (according to HI classification) | 79.1 | |
| Serum iron, μmol/L | 31.5 ± 10.0 | 3.38–28.0 |
| AST, IU/L | 37.0 ± 71.3 | 5.0–37.0 |
| ALT, IU/L | 42.5 ± 59.8 | 6.0–55.0 |
| Total bilirubin, μmol/L | 18.1 ± 11.6 | 3.4–20.5 |
| INR | 1.0 ± 0.3 | 0.8–1.2 |
| Serum albumin, g/L | 40.4 ± 4.2 | 29.0–45.0 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; HI, Health Iron; INR, international normalized ratio.
HI status at the time of genotyping
At time of genotyping, 10.5% of subjects were HI 4, 15.0% were HI 3, 38.7% were HI 2, and 35.7% were HI 1 (Figure 1). Three hundred six of 333 cases had an adequate follow-up to study end point or death. Fifty-six percent were followed up to at least 60 years of age. The mean follow-up duration was 11.6 years. 2.3% died (7/306) within 3 years of diagnosis. For the 306 cases that had adequate follow-up, their baseline HI status was 11.4% HI 4, 15.7% HI 3, 40.2% HI 2, and 32.7% HI 1.
Figure 1.
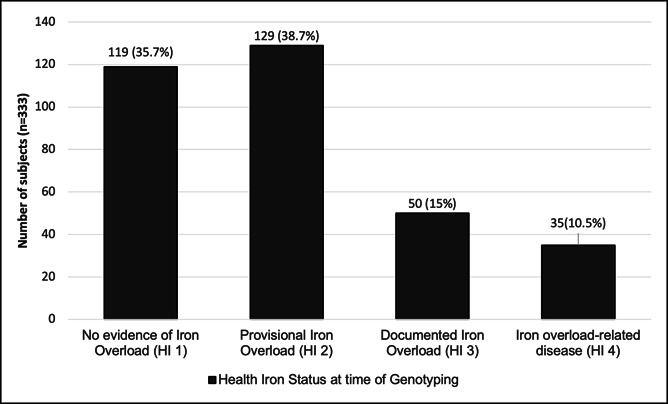
Baseline HI status n = 333. HI, Health Iron.
HI status and iron indices at the end of the follow-up period
At the end of follow-up, 18.3% of subjects were HI 4, 16.0% were HI 3, 43.8% were HI 2, and 21.9% were HI 1 (Figure 2).
Figure 2.
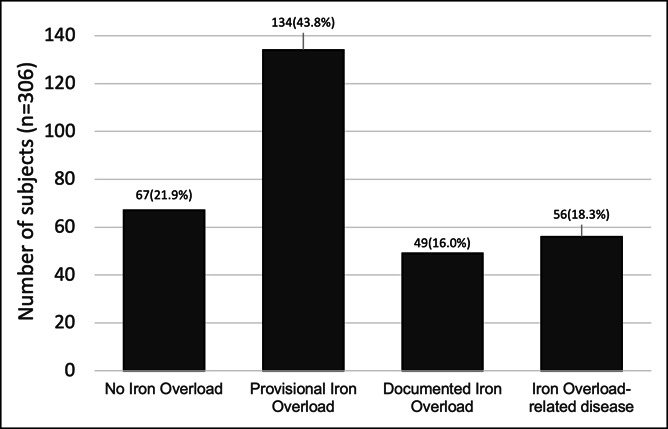
Health Iron status at the end of follow-up (n = 306).
The 20 subjects who had progression to end-organ damage had elevated SF (mean = 1,039.1 μg/L, SD = 910.8) compared with the 225 subjects who did not (mean = 565.6 μg/L, SD = 510.7) meet the criteria for end-organ damage (P < 0.005).
End-organ damage was observed in 18.3% (56/306) of subjects. This is described in greater detail under “Discussion—Subjects classified as HI 4 at the end of follow-up.”
In subjects with a SF lower than 1,000 μg/L at the time of genotyping, 6.3% developed liver fibrosis or cirrhosis. This is in contrast to 18.3% of all subjects overall. The only predictor for development of end organ damage identified through logistic regression was for an elevated SF at baseline (P < 0.005). χ2 tests found that patients receiving therapeutic phlebotomy tended to be more likely to have end-organ damage (9.7% vs 3.1%) (P = 0.066).
Subjects divided by biological sex
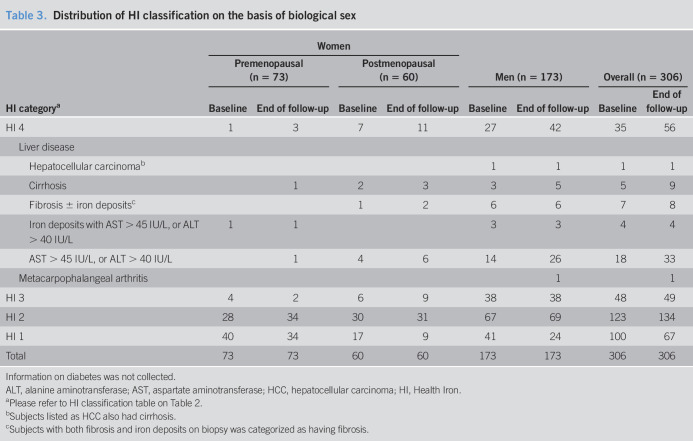
At baseline, 34.7% of men vs 12.9% of women were classified as HI 3 or higher (P < 0.005). At the end of follow-up, HI 4 classification was met by 24.3% of men and 10.5% of women. When divided into pre- vs post-menopausal groups for women, HI 4 was met by 18.3% of postmenopausal women, and 4.1% of premenopausal women (P < 0.05).
Progression to HI 3 or 4 tended to be more frequent in men than in women (13% vs 6.1%, P = 0.079) (Table 3). In women who were HI 1 or 2 at the time of genotyping, progression to HI 4 was higher in postmenopausal women than in premenopausal women, 8.5% vs 0% (P < 0.05) (Table 3). The one premenopausal woman who progressed to HI 4 over the course of follow-up was HI 3 at baseline.
Table 3.
Distribution of HI classification on the basis of biological sex
| HI categorya | Women | Men (n = 173) | Overall (n = 306) | |||||
| Premenopausal (n = 73) | Postmenopausal (n = 60) | |||||||
| Baseline | End of follow-up | Baseline | End of follow-up | Baseline | End of follow-up | Baseline | End of follow-up | |
| HI 4 | 1 | 3 | 7 | 11 | 27 | 42 | 35 | 56 |
| Liver disease | ||||||||
| Hepatocellular carcinomab | 1 | 1 | 1 | 1 | ||||
| Cirrhosis | 1 | 2 | 3 | 3 | 5 | 5 | 9 | |
| Fibrosis ± iron depositsc | 1 | 2 | 6 | 6 | 7 | 8 | ||
| Iron deposits with AST > 45 IU/L, or ALT > 40 IU/L | 1 | 1 | 3 | 3 | 4 | 4 | ||
| AST > 45 IU/L, or ALT > 40 IU/L | 1 | 4 | 6 | 14 | 26 | 18 | 33 | |
| Metacarpophalangeal arthritis | 1 | 1 | ||||||
| HI 3 | 4 | 2 | 6 | 9 | 38 | 38 | 48 | 49 |
| HI 2 | 28 | 34 | 30 | 31 | 67 | 69 | 123 | 134 |
| HI 1 | 40 | 34 | 17 | 9 | 41 | 24 | 100 | 67 |
| Total | 73 | 73 | 60 | 60 | 173 | 173 | 306 | 306 |
Information on diabetes was not collected.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; HCC, hepatocellular carcinoma; HI, Health Iron.
Please refer to HI classification table on Table 2.
Subjects listed as HCC also had cirrhosis.
Subjects with both fibrosis and iron deposits on biopsy was categorized as having fibrosis.
Subjects classified as HI 4 at the end of follow-up
Of the 56 HI 4 subjects, 17.8% developed cirrhosis, 1.8% developed hepatocellular carcinoma, 14.3% developed liver fibrosis, 5.4% were found to have iron deposits on biopsy (indication: elevated liver transaminases) but no fibrosis or cirrhosis, 58.9% developed elevated transaminases, and 1.8% (1/56) developed metacarpophalangeal joint arthritis (Table 3). 14.3% died of the following: 1 cirrhosis, 1 cirrhosis + hepatocellular carcinoma, 2 metastatic lung cancer, 1 metastatic colon cancer, and 3 unknown cause.
Cirrhosis and fibrosis
Of all 306 subjects, 5.8% developed liver fibrosis or cirrhosis associated with iron overload. 2.6% developed fibrosis, and 3.3% were diagnosed with cirrhosis. Of the subjects diagnosed with cirrhosis, 12 were men (mean age 50.3), 1 was premenopausal woman (age 45), and 5 were postmenopausal women (mean age 66.8). Postmenopausal women were more likely to develop cirrhosis than premenopausal women (P < 0.005). One subject developed cirrhosis but did not have elevated SF > 1,000 μg/L and therefore did not meet the criteria for HI 4.
Treated population
78.1% of subjects received therapeutic phlebotomy. Mean age at diagnosis was 51.5 years, mean follow-up duration was 9.98 years, and 61.9% were men. The subjects who received phlebotomy had a higher SF (mean = 833.9 μg/L) at the time of diagnosis than those who did not (mean = 226.7 μg/L) (P < 0.001). At the time of genotyping, 21.3% were HI 1, 45.2% were HI 2, 19.7% were HI 3, and 13.8% were HI 4. At the end of follow-up, 13.0% were HI 1, 44.8% were HI 2, 20.1% were HI 3, and 22.2% were HI 4. Of those who received phlebotomy, 56.0% achieved a SF of <100 μg/L. Four of 159 patients (2.5%) who received phlebotomy developed cirrhosis over the course of follow-up.
Of 32 subjects who were classified as HI 4 on the basis of elevated transaminases, 59.4% had normalization of transaminases with phlebotomy. Normalization of transaminases was associated with a SF of <100 μg/L (P < 0.028). We did not find any difference in progression to end-organ damage between phlebotomized subjects who achieved SF < 100 μg/L and those who received phlebotomies but did not achieve an SF < 100 μg/L. However, there was a trend toward end-organ damage in subjects undergoing phlebotomy and achieving SF < 100 μg/L compared with those who never required therapeutic phlebotomy (8% vs 3.2%, P = 0.332). The duration of time for which subjects underwent therapeutic phlebotomy was not recorded.
For the 159 subjects with baseline HI 1 or 2 status who received phlebotomies, 8.2% developed end-organ damage by the end of follow-up. Four subjects had cirrhosis or fibrosis, 8 had elevated liver enzymes, and 1 had metacarpophalangeal arthritis. The mean age was 56.3 years compared with 47.9 years in those who did not (P = 0.034).
Untreated population
There were 62 subjects who never received therapeutic phlebotomy. 33.9% were men, mean age of diagnosis was 47 years with a mean follow-up duration of 9.98 years.
At the time of genotyping, 72.6% were HI 1, 25.8% were HI 2, 0% were HI 3, and 1.6% was HI 4. At the end of the follow-up period, 54.8% were HI 1, 40.3% were HI 2, 0% were HI 3, and 4.8% were HI 4 (Figure 3). The one patient categorized as HI 4 at baseline was found to have cirrhosis and SF >1,000 μg/L at the time of genotyping. He died secondary to decompensated liver disease before receiving therapeutic phlebotomy.
Figure 3.
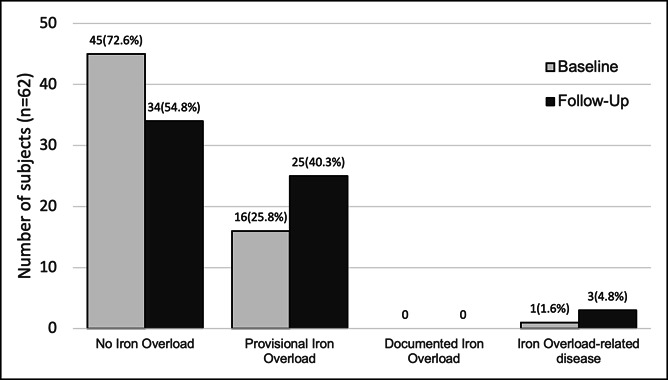
Progression of disease in the untreated population (n = 62).
Within this population, 13/62 had progression of the disease. Overall, only 2/62 (3.2%) met the criteria for HI 4—both were men. The first subject was diagnosed with cirrhosis but also had autoimmune hepatitis based on liver biopsy at age 50 years. The other subject developed elevated transaminases after 7 years of follow-up at age 57 years with no radiologic evidence of cirrhosis.
DISCUSSION
We present a real-world experience of a large group of C282Y homozygotes from a single North American center with a mean 11.6 years of follow-up.
Our results suggest an overall low clinical penetrance of C282Y homozygosity. End-organ damage was observed in 18.3% of the population, most of which developed only transient elevated transaminases, with only 5.8% developing liver fibrosis or cirrhosis. Development of end-organ damage was even less frequent (6.3%) in subjects with a SF lower than 1,000 μg/L at the time of genotyping. In this population, only 2.2% developed liver fibrosis or cirrhosis.
Although it can be argued that the low degree of progression may be related to the fact that most of our patients received therapeutic phlebotomy, our findings show similar rates of progression as those of the previous studies (13–15).
We observed that clinical penetrance was higher in patients whose SF was higher at the time of genotyping. Similarly, it has been shown in other studies that a SF greater than 1,000 μg/L may confer a higher risk in developing end-organ damage (13,16). When compared with H63D homozygotes in Newfoundland, where only one of 170 subjects developed iron overload-related end-organ damage (17), C282Y homozygosity seems to have a higher penetrance.
The HealthIron study from Melbourne (13) followed 203 patients over 12 years and found the proportion of C282Y homozygotes with documented iron overload-related disease was 28.4% for men and 1.2% for women. They concluded that iron overload-related disease develops in men but not in women. They also suggested that physiologic blood loss may play a role in lower iron indices and consequent end-organ damage in women. The Busselton study (18) identified 4 men and 6 women C282Y homozygotes with follow-up duration of 17 years and found only one patient meeting the criteria for end-organ damage associated with a SF >1,000 μg/L. Another study in Copenhagen (19) identified 7 male and 16 female C282Y homozygotes and found that none of their subjects developed end-organ damage during the 25 years of follow-up.
From our study, looking at the treated and untreated populations in patients with SF <1,000 μg/L at baseline, only 4/159 subjects (2.5%) who underwent therapeutic phlebotomy and 1/62 (1.6%) of untreated subjects developed cirrhosis over the course of follow-up.
Despite observing a low overall disease penetrance, it is noteworthy that although approximately half of treated subjects achieved a target SF below 100 μg/L (56%), patients requiring phlebotomy had more progression of liver disease compared with those who never required phlebotomy. This is because of the fact that treated patients were identified as higher risk for disease progression based on other factors, demonstrated by the higher mean SF at diagnosis in the treated group. Logistic regression confirmed that the only predictor of disease progression was elevated SF at baseline.
The 62 patients in our cohort who never received phlebotomy adds to the existing literature that not every C282Y homozygote will develop clinical disease. These findings are similar to those of the previous studies (18,20). Within this population, only 13 had any progression of disease with only 2 developing end-organ damage. The first subject was diagnosed with cirrhosis but also had autoimmune hepatitis based on liver biopsy at the age 50 years. The other subject developed elevated transaminases after 7 years of follow-up at the age of 57 years, with no radiologic evidence of cirrhosis.
As has been shown previously, men were more likely to have elevated iron indices, require therapeutic phlebotomy, and develop end-organ damage than women. Previous studies (12) have suggested that few female C282Y homozygotes develop clinical manifestations of HH. However, our study suggests that clinical penetrance in postmenopausal women may be higher than previously believed. 10.5% of our female population was found to be HI 4. We noted significantly less end-organ damage in premenopausal women (4.1%) vs postmenopausal women (18.3%) (P < 0.05), with only 1 premenopausal woman developing liver cirrhosis. Conversely, we found virtually no progression of iron overload in premenopausal women who were HI 1 at the time of genotyping. Therefore, it may be reasonable to defer phlebotomy and perform less frequent laboratory monitoring in some female patients until they approach menopause.
Strengths
This is the largest cohort of C282Y homozygotes in North America. Apart from health records privy to Western Health region in Newfoundland leading to exclusion of approximately 10% of cases, all C282Y homozygotes confirmed by genotyping in Newfoundland and Labrador were included. At least 50 percent of subjects were followed to greater than the age of 60 years with a mean follow-up duration over 11 years. Although the study was retrospective in nature, data were collected prospectively through electronic health records.
Limitations
The main limitation to this study was that 78% of patients received therapeutic phlebotomy at some point during their follow-up. Although appropriate for patient care, we recognize that this would have attenuated the natural history of disease. Patients who received phlebotomy were those at higher risk for disease progression. In addition, we were unable to determine the frequency or duration of therapeutic phlebotomy of these subjects.
Because of the lack of information on obesity, diabetes, and alcohol use, we were unable to assess other risk factors for the development of cirrhosis. This could lead to over attributing the cause of liver disease to HH. If so, the clinical penetrance of C282Y homozygosity may actually be overestimated in this study. The cumulative incidence of liver disease in this study is low, suggesting that these other factors did not play an important role.
Liver biopsies were not routinely performed. This could underestimate the frequency of liver disease. Similarly, FibroScan was not readily available during the study period so fibrosis may have been underestimated. The definition of clinical penetrance excluded endocrinopathies, which may underestimate the clinical penetrance of these manifestations.
Finally, the indication for index genotyping was not available and therefore could not be controlled for (e.g., screening vs abnormal biochemistries).
This is the largest cohort of C282Y homozygotes followed for an extended duration of time in North America. The findings suggest a low clinical penetrance of C282Y homozygosity. This study describes outcomes when routine clinical practice is taken into account and may mirror outcomes in similar populations.
CONFLICTS OF INTEREST
Guarantor of the article: Mark Borgaonkar, MD, MSc, FRCPC, FACG.
Specific author contributions: D.L.: primary author, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and statistical analysis. G.V.: acquisition of data. C.P.: acquisition of data. M.B.: supervisor, study concept and design, and critical revision of the manuscript for important intellectual content.
Financial support: None to report.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ The C282Y homozygous mutation is implicated in most phenotypic HH.
✓ The penetrance of C282Y homozygosity is variable across studies.
✓ Men are at much greater risk of developing iron overload-related disease compared with women, with the latter having as low as 1.2% in some studies (13).
WHAT IS NEW HERE
✓ We present a large (n = 306) cohort of subjects homozygous for the C282Y mutation over a decade of clinical follow-up in a North American center.
✓ Postmenopausal women have a clinical penetrance that approaches that of men. We noted much less cirrhosis and fibrosis in premenopausal women as compared to postmenopausal women (1.3% vs 8.3%, P < 0.005)—suggesting that these subjects may not require as frequent follow-up, especially if their ferritin is <200 at time of genotyping.
✓ The overall incidence of end-organ damage observed in this cohort is low, HI 4 classification was met by 18.3%, with 5.8% developing cirrhosis at the end of follow-up.
TRANSLATIONAL IMPACT
✓ The clinical penetrance of C282Y homozygosis is suggested to be low. Biological sex and postmenopausal status in women are important factors in development of end-organ damage.
REFERENCES
- 1.Adams PC, Reboussin DM, Barton JC, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med 2005;352:1769–78. [DOI] [PubMed] [Google Scholar]
- 2.Grosse SD, Gurrin LC, Allen KJ, et al. Clinical penetrance in hereditary hemochromatosis: Estimates of the cumulative incidence of severe liver disease among HFE C282Y homozygotes. Genet Med 2018;20(4):383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feder J, Gnirke A, Thomas A, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet 1996;13:399–408. [DOI] [PubMed] [Google Scholar]
- 4.Wallace DF, Subramaniam VN. The global prevalence of HFE and non-HFE hemochromatosis estimated from analysis of next-generation sequencing data. Genet Med 2016;18(6):618–26. [DOI] [PubMed] [Google Scholar]
- 5.Samarasena J, Windsor W, Borgaonkar M, et al. Individuals Homozygous for the H63D mutation have significantly elevated iron indexes. Dig Dis Sci 2006;51(4):803–7. [DOI] [PubMed] [Google Scholar]
- 6.Niederau C, Fischer R, Sonnenberg A, et al. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med 1985;313(20):1256. [DOI] [PubMed] [Google Scholar]
- 7.Niederau C, Fischer R, Pürschel A, et al. Long-term survival in patients with hereditary hemochromatosis. Gastroenterology 1996;110(4):1107. [DOI] [PubMed] [Google Scholar]
- 8.Phatak PD, Ryan DH, Cappuccio J, et al. Prevalence and penetrance of HFE mutations in 4865 unselected primary care patients. Blood Cells Mol Dis 2002;29:41–7. [DOI] [PubMed] [Google Scholar]
- 9.Edwards CQ, Griffen LM, Ajioka RS, et al. Screening for hemochromatosis: Phenotype versus genotype. Semin Hematol 1998;35(1):72–6. [PubMed] [Google Scholar]
- 10.Borgaonkar M. Hemochromatosis—More common than you think. Can Fam Physician 2003;49(1):36–43. [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Q, McDonnell SM, Khoury MJ, et al. Hemochromatosis-associated mortality in the United States from 1979 to 1992: An analysis of multiple-cause mortality data. Ann Intern Med 1998;129:946–53. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Luna S, Brown K. Clinical burden of liver disease from hemochromatosis at an academic medical center. Hepatol Commun 2017;1(5):453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen KJ, Gurrin LC, Constantine CC, et al. Iron overload-related disease in HFE hereditary hemochromatosis. N Engl J Med 2008;358(3):221–30. [DOI] [PubMed] [Google Scholar]
- 14.Asberg A, Hveem K, Kannelonning K, et al. Penetrance of the C28Y/C282Y genotype of the HFE gene. Scand J Gastroenterol 2007;42:1073–7. [DOI] [PubMed] [Google Scholar]
- 15.Rogowski WH. The cost-effectiveness of screening for hereditary hemochromatosis in Germany: A remodeling study. Med Decis Making 2009;29:224–38. [DOI] [PubMed] [Google Scholar]
- 16.Guyader D, Jacquelinet C, Moirand R, et al. Noninvasive prediction of fibrosis in C282Y homozygous hemochromatosis. Gastroenterology 1998;115:929–36. [DOI] [PubMed] [Google Scholar]
- 17.Kelley M, Joshi N, Borgaonkar M, et al. Iron Overload is rare in patients homozygous for the H63D mutation. Can J Gastroenterol Hepatol 2014;28(4):198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olynyk JK, Hagan SE, Cullen DJ, et al. Evolution of untreated hereditary hemochromatosis in the Busselton population: A 17-year study. Mayo Clin Proc 2004;79:309–13. [DOI] [PubMed] [Google Scholar]
- 19.Andersen RV, Tybjaerg-Hansen A, Appleyard M, et al. Hemochromatosis mutations in the general population: Iron overload progression rate. Blood 2004;103:2914–9. [DOI] [PubMed] [Google Scholar]
- 20.Olynyk JK, Cullen DJ, Aquilia S, et al. A population-based study of the clinical expression of the hemochromatosis gene. N Engl J Med 1999;341:718–24. [DOI] [PubMed] [Google Scholar]