INTRODUCTION
Acute T cell-mediated rejection (TCMR) is currently thought to have little effect on allograft survival in kidney transplantation (KT) because of the favorable response to corticosteroids and lymphocyte-depleting agents.1,2 However, the recent 2017 Banff classification update has introduced the category of chronic-active TCMR (CA-TCMR), which is mainly represented by features of interstitial inflammation in sclerotic cortical parenchyma (i-IF/TA) and tubulitis.3 The prognosis of allograft survival with this diagnosis according to the current criteria for CA-TCMR, including the presence of interstitial fibrosis and tubular atrophy (IF/TA) and moderate-to-severe grades of i-IF/TA, is considered unfavorable. Our previous study4 reported that the revision of criteria from Banff 2015 to 2017 had increased the incidence of CA-TCMR by 1-y protocol biopsy from 1% to 8%. Additionally, the revised CA-TCMR criteria are associated with a 5.42-fold risk of composite endpoint that is defined as a 2-fold increase in serum creatinine or death-censored graft loss compared with normal tissue. CA-TCMR is the second-worst diagnosis for allograft prognosis after antibody-mediated rejection. However, data regarding CA-TCMR treatment are scarce, and treatment strategies have not been established for CA-TCMR. This report aims to demonstrate the effect of CA-TCMR treatment on pathological findings in 3 patients.
CASE PRESENTATION
All patients received induction therapy with basiliximab (20 mg on d 0 and 4) and were maintained on everolimus (EVR, target trough concentration 3–8 ng/mL, standard dose once-daily), a prolonged-release formulation of tacrolimus (Tac-QD, target trough concentration 5–8 ng/mL), and methylprednisolone (MP, 4 mg/d). All cases underwent 3-mo and 1-y protocol allograft biopsies. Patient characteristics are shown in Table 1. Pathological features of each kidney allograft biopsy are shown in Figure 1, and Banff lesion scores and diagnoses according to the 2017 Banff classification are shown in Table 2. A summary of the 3 patients’ disease courses is shown in Figure 2.
TABLE 1.
Patient characteristics
| No | Age at Dx | Sex | Age at KT | Primary kidney disease | Previous biopsy | Biopsy when diagnosed with CA-TCMR | Number of HLA mismatch | ABO | Donor | Period from KT to Dx |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | Male | 45 | Nephrosclerosis | 3-mo protocolBorder line change | 1-y protocol | 1 | Identical | Living69 y oldmale | 378 d |
| 2 | 62 | Female | 61 | Polycystic kidney disease | 3-mo protocolNo rejection | 1-y protocol | 3 | Incompatible | Living70 y oldmale | 376 d |
| 3 | 56 | Male | 55 | Chronic glomerular nephritis | 3-mo protocolNo rejection | 1-y protocol | 2 | Identical | Deceased54 y oldmale | 404 d |
CA-TCMR, chronic-active T cell-mediated rejection; Dx, diagnosis as chronic-active T cell-mediated rejection; KT, kidney transplantation.
FIGURE 1.
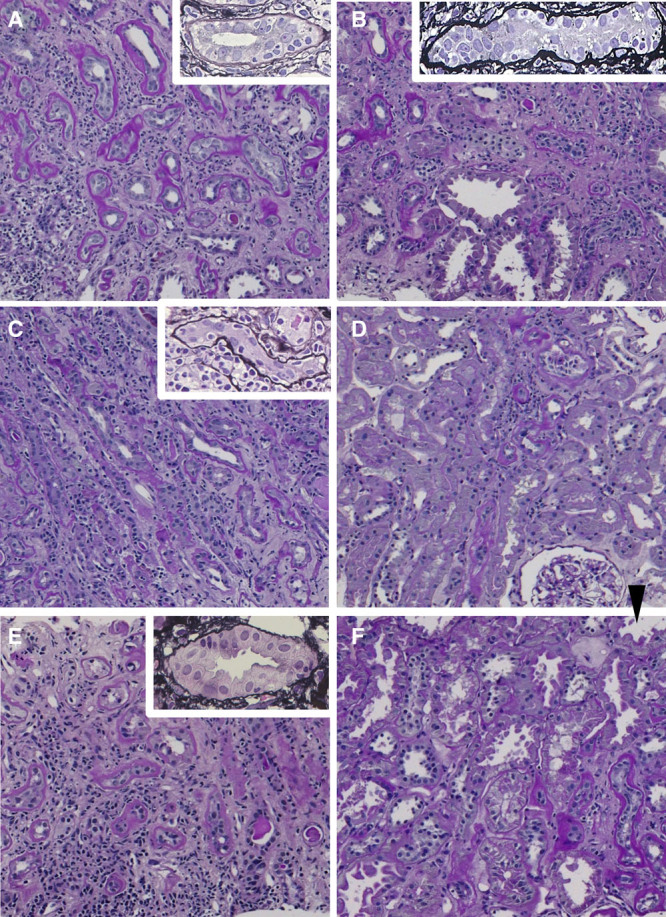
Light microscopic findings from 1-y protocol biopsies (A, C, E) and confirmed biopsies (B, D, F) of 3 cases (case 1: A and B, case 2: C and D, and case 3: E and F). A–F (periodic acid-Schiff stain, original magnification ×100); and A–C, E (insert, silver stain, original magnification ×400). (A) Presence of IF/TA, severe i-IF/TA (i-IF/TA3), and moderate tubulitis in mildly atrophic tubules (t2, insert). (B) Moderate i-IF/TA (i-IF/TA2) and severe tubulitis in moderately atrophic tubules (t3, insert). (C) Moderate i-IF/TA (i-IF/TA2) and moderate tubulitis in moderately atrophic tubules (t2, insert). (D) Small areas of IF/TA and slight interstitial infiltration in IF/TA (i-IF/TA0). (E) Presence of IF/TA, severe i-IF/TA (i-IF/TA3), and moderate tubulitis in mildly atrophic tubules (t2, insert). (F) Small areas of IF/TA. No i-IF/TA (i-IF/TA0) and tubulitis only in moderately-to-severe atrophic tubules (arrowhead). IF/TA, interstitial fibrosis and tubular atrophy; i-IF/TA, interstitial inflammation in sclerotic cortical parenchyma.
TABLE 2.
Pathological and immunological findings
| Case 1 | Case 2 | Case 3 | ||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Pathological diagnosis | CA-TCMR IA | CA-TCMR IB | CA-TCMR IA | No rejection | CA-TCMR IA | No rejection |
| Postoperative d | 378 | 574 | 376 | 571 | 404 | 815 |
| Banff lesion score (0–3) | ||||||
| ti | 2 | 2 | 2 | 0 | 3 | 0 |
| i | 0 | 1 | 1 | 0 | 1 | 0 |
| i-IF/TA | 3 | 2 | 2 | 0 | 3 | 0 |
| t | 2 | 3 | 2 | 0 | 2 | 0 |
| g | 0 | 0 | 0 | 0 | 0 | 0 |
| v | 0 | 0 | 0 | 0 | 0 | 0 |
| ci | 3 | 2 | 3 | 0 | 3 | 0 |
| ct | 3 | 2 | 3 | 1 | 3 | 1 |
| cv | 2 | 3 | 0 | 0 | 3 | 3 |
| ah | 0 | 0 | 1 | 0 | 1 | 1 |
| aah | 0 | 0 | 0 | 0 | 1 | 0 |
| ptc | 0 | 0 | 0 | 0 | 0 | 0 |
| C4d | 1 | 0 | 1 | 0 | 0 | 0 |
| PRA class I/II | 0%/0% | 0%/0% | 0%/0% | 0%/0% | 1.8%/0% | 1.8%/0% |
| De novo DSA | Negative | Negative | Negative | Negative | Negative | Negative |
Pathological diagnoses were based on the 2017 Banff classification.
CA-TCMR, chronic-active T cell-mediated rejection; DSA, donor-specific anti-HLA antibody; i-IF/TA, interstitial inflammation in sclerotic cortical parenchyma; PRA, panel reactive antibody.
FIGURE 2.
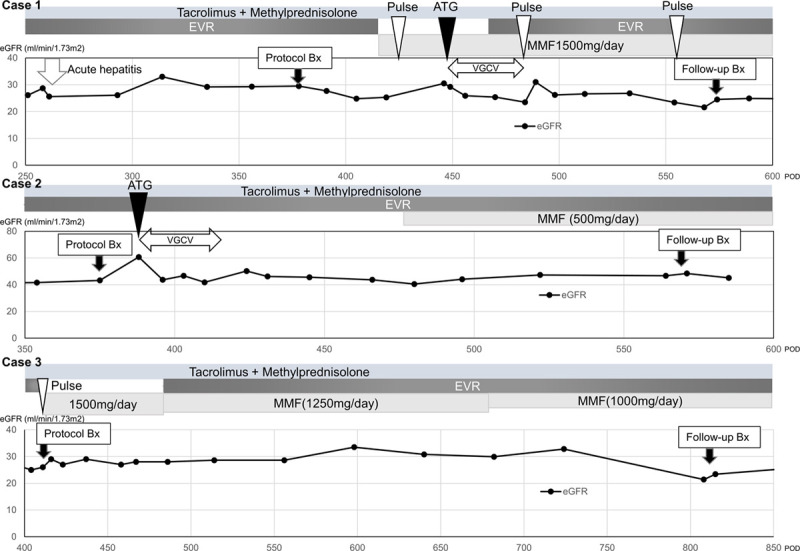
Disease course summary of 3 cases of chronic-active T cell-mediated rejection. ATG, rabbit antithymoglobulin; Bx, biopsy; eGFR, estimated glomerular filtration rate; EVR, everolimus; MMF, mycophenolate mofetil; POD, postoperative d; pulse, steroid pulse therapy; VGCV, valganciclovir.
Case 1
A 46-y-old man weighing 75 kg was diagnosed with CA-TCMR IA by protocol biopsy 1 y after living-donor KT. Because of his acute hepatitis B, the treatment was delayed. Two mo after biopsy, the patient received steroid pulse therapy followed by rabbit antithymocyte globulin (ATG) with 1-mo prophylactic valganciclovir (VGCV) and escalating doses of 4 maintenance oral immunosuppressant agents: Tac-QD (C0 range, 4.6–6.4 ng/mL), mycophenolate mofetil (MMF, 1500 mg/d), MP (4 mg/d), and EVR (C0 range, 2.2–2.5 ng/mL). Six months after commencement of therapy, MMF was ceased because of cytomegalovirus (CMV) antigenemia. The patient’s estimated glomerular filtration rate (eGFR) did not deteriorate during treatment. One hundred ninety-six days after CA-TCMR diagnosis, the patient underwent confirmed biopsy. The Banff lesion score of i-IF/TA improved from 3 to 2, but tubulitis in scarred areas was persistent.
Case 2
A 62-y-old woman weighing 42 kg was diagnosed with CA-TCMR IA by protocol biopsy 1 y after living-donor KT. The patient received ATG with 1-mo prophylactic VGCV. Two months after ATG administration, the patient received escalating doses of 4 maintenance oral immunosuppressant agents: Tac-QD (C0 range, 5–8 ng/mL), MMF (500 mg/d), MP (4 mg/d), and EVR (C0 range, 2–4 ng/mL). The patient’s eGFR did not deteriorate during treatment. Then, 195 d after CA-TCMR diagnosis, the patient underwent confirmed biopsy. Although some areas of IF/TA remained, most of the pathological findings of CA-TCMR were improved.
Case 3
A 56-y-old man weighing 58 kg was diagnosed with CA-TCMR IA by protocol biopsy 1 y after circulatory death donor KT. The patient received steroid pulse therapy followed by escalating doses of 4 maintenance oral immunosuppressant agents: Tac-QD (C0 range, 5–8 ng/mL), MMF (1500 mg/d), MP (4 mg/d), and EVR (C0 range, 2–4 ng/mL). Two months after commencement of treatment, MMF was reduced to 1250 mg/d because of anemia. During the treatment, the patient’s serum creatinine levels did not increase. Four hundred eleven days after CA-TCMR diagnosis, the patient underwent confirmed biopsy. Although some areas of IF/TA remained, most of the pathological findings of CA-TCMR were improved.
DISCUSSION
In this report, 2 patients treated for CA-TCMR showed improved graft pathological findings. The other patient treated for CA-TCMR showed an improvement in the extent of i-IF/TA, but some CA-TCMR pathological features remained. Therefore, intensive treatment including ATG, steroid pulse therapy, and escalating doses and types of oral maintenance immunosuppressants might improve graft survival after CA-TCMR diagnosis.
CA-TCMR occurs as a persistent or recurrent form of acute TCMR. Acute tubulitis in acute TCMR causes irritation of surrounding fibroblasts via crosstalk with cytokines,5 leading to persistent interstitial fibrosis.6 Thus, owing to inadequate immunosuppression during acute TCMR, persistent immune activation may result in T-cell invasion of renal fibrotic parenchyma and initiation of i-IF/TA and tubulitis, the pathological components of CA-TCMR. Considering that CA-TCMR is related to chronic underimmunosuppression,3 a long-acting approach, including administration of ATG and escalating doses and types of maintenance, oral immunosuppressants appears promising. Because ATG is not approved for induction use but for steroid-resistant TCMR use in KT recipients in Japan, the prompt use of ATG is encouraged upon CA-TCMR diagnosis. However, the high incidence of adverse events including CMV antigenemia and neutropenia often result in the discontinuation of escalated maintenance immunosuppression. This necessitates a personalized treatment strategy, especially for patients at high risk of infection. In particular, ATG often requires CMV prophylaxis with VGCV,7 which causes further neutropenia. However, compared with MMF, EVR has fewer adverse side effects including infection and leukopenia when both drugs are administered in combination with similar tacrolimus exposure.8 Therefore, the addition of EVR to a conventional MMF-based regimen might reduce the dosage of each immunosuppressant.
Because the 3 cases followed a complex and nonuniform treatment course, it is not possible to determine whether this result indicates a synergistic effect of all the drugs or a subset of these drugs. Furthermore, the length of time between the protocol biopsy and follow-up biopsy in these patients makes interpretation of potential treatment responses difficult. However, if the most important aspect of CA-TCMR treatment is intensive immunosuppression that is long-acting and well-tolerated, then our strategy of combining the 4 immunosuppressive agents appeared to be a viable treatment option that might enhance immunosuppression and reduce toxicity by reducing the dosage of individual drugs. Approaches applying the same concept to pancreas transplantation alone9 and posterior islet transplantation10 have been reported.
All the CA-TCMRs diagnosed in this report were subclinical, and it was difficult to prove an improvement in any way other than by histologic diagnosis. Moreover, bias due to problems such as biopsy sampling in an area of cortical scarring when diagnosed with CA-TCMR cannot be ruled out. Notably, the ci and ct scores were markedly decreased from the index biopsy to the follow-up biopsy in cases 2 and 3 (Table 2). However, each graft was biopsied 2 or 3 times, which might reduce potential bias. Scar tissue from repetitive biopsies is more likely to appear in follow-up biopsies, but in our case, a protocol biopsy was performed rather than a follow-up biopsy. Although bias due to sampling issues cannot be ruled out, the current histologic examination might accurately reflect the pathology. Moreover, EVR has recently been reported to be effective for IF/TA suppression.11 Although sample bias must be taken into account, it is noteworthy that not only tubulitis, interstitial infiltration, and i-IF/TA but also the extent of IF/TA was improved in 2 of 3 cases. Although data on CA-TCMR treatment remain scarce, a multiple immunosuppressant regimens that includes both EVR and MMF might be an optimal strategy for CA-TCMR treatment.
In conclusion, we report 3 patients who received treatment for CA-TCMR. Our results show that intensive treatment might improve the graft pathological findings of patients with CA-TCMR. However, studies with larger patient numbers are required to reach a more definitive conclusion.
ACKNOWLEDGMENTS
The authors would like to thank Ms Yasuka Ogawa (medical assistant) for data management. We thank Dr Alla Bradley and J. Ludovic Croxford, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this article.
Footnotes
Published online 10 November, 2020.
The authors declare no funding.
The Department of Surgery and Oncology, Graduate School of Medical Sciences, Kyushu University received scholarship donations from Novartis Pharma and Astellas Pharma. This study was conducted under the regulation of the Kyushu University Policy of Conflicts of Interest.
H.N. contributed to writing the initial draft of the article. K.N., K.U., and A.T. contributed to the pathological evaluation. K.K. and Y.O. contributed to data collection and interpretation. M.N. contributed to the revision of the article. All authors discussed the results and commented on the article.
REFERENCES
- 1.Halloran PF, Chang J, Famulski K, et al. Disappearance of T cell-mediated rejection despite continued antibody-mediated rejection in late kidney transplant recipients. J Am Soc Nephrol. 2015; 26:1711–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sellarés J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012; 12:388–399 [DOI] [PubMed] [Google Scholar]
- 3.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018; 18:293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masutani K, Tsuchimoto A, Kurihara K, et al. ; Japan Academic Consortium of Kidney Transplantation (JACK) investigators. Histological analysis in ABO-compatible and ABO-incompatible kidney transplantation by performance of 3- and 12-month protocol biopsies. Transplantation. 2017; 101:1416–1422 [DOI] [PubMed] [Google Scholar]
- 5.Tan RJ, Zhou D, Liu Y. Signaling crosstalk between tubular epithelial cells and interstitial fibroblasts after kidney injury. Kidney Dis (Basel). 2016; 2:136–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takaori K, Nakamura J, Yamamoto S, et al. Severity and frequency of proximal tubule injury determines renal prognosis. J Am Soc Nephrol. 2016; 27:2393–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksson M, Jokinen JJ, Söderlund S, et al. Low-dose valganciclovir prophylaxis is efficacious and safe in cytomegalovirus seropositive heart transplant recipients with anti-thymocyte globulin. Transpl Infect Dis. 2018; 20:e12868. [DOI] [PubMed] [Google Scholar]
- 8.Sommerer C, Suwelack B, Dragun D, et al. ; Athena Study Group. An open-label, randomized trial indicates that everolimus with tacrolimus or cyclosporine is comparable to standard immunosuppression in de novo kidney transplant patients. Kidney Int. 2019; 96:231–244 [DOI] [PubMed] [Google Scholar]
- 9.Fridell JA, Mangus RS, Chen JM, et al. Steroid-free three-drug maintenance regimen for pancreas transplant alone: comparison of induction with rabbit antithymocyte globulin +/- rituximab. Am J Transplant. 2018; 18:3000–3006 [DOI] [PubMed] [Google Scholar]
- 10.Wisel SA, Gardner JM, Roll GR, et al. Pancreas-after-islet transplantation in nonuremic type 1 diabetes: a strategy for restoring durable insulin independence. Am J Transplant. 2017; 17:2444–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noguchi H, Tsuchimoto A, Ueki K, et al. One-year outcome of everolimus with standard-dose tacrolimus immunosuppression in de novo ABO-incompatible living donor kidney transplantation: a retrospective, single-center, propensity score matching comparison with mycophenolate in 42 transplants. Transplant Direct. 2020; 6:e514. [DOI] [PMC free article] [PubMed] [Google Scholar]


