Supplemental Digital Content is available in the text.
Keywords: cause of death, hypercholesterolemia, insurance, myocardial infarction, statins
Background:
Atherosclerotic cardiovascular disease remains a major cause of death and disability, especially for high-risk familial hypercholesterolemia individuals. PCSK9i (proprotein convertase subtilisin kexin type 9 inhibitors) reduce low-density lipoprotein cholesterol levels and cardiovascular event rates. However, PCSK9i prescriptions are rejected at high rates by payers, and use is often delayed or eventually abandoned as a treatment option. We tested the hypothesis that acute coronary syndromes, coronary interventions, stroke, and cardiac arrest are more prevalent in patients with rejected or abandoned PCSK9i prescriptions than for those with paid PCSK9i prescriptions.
Methods and Results:
We identified 139 036 individuals aged ≥18 years who met the following 3 criteria: prescribed PCSK9i between August 2015 and December 2017, had claims history, and had an established date of exposure for paid, rejected, or abandoned status. To compare the effects of rejected versus paid and abandoned versus paid status, propensity score matching was performed to minimize confounding because of baseline differences in patient groups. Cox regression analyses and incidence density rates for cardiovascular events were estimated on the propensity score-matched cohorts. Patients who received 168 or more days of paid PCSK9i medication within a 12-month period were defined as paid. The hazard ratios for composite cardiovascular events outcome in propensity score-matched analyses were 1.10 (95% CI, 1.01–1.19; P=0.02) for rejected versus paid and 1.12 (95% CI, 1.01–1.24; P=0.03) for abandoned versus paid. In a stricter analysis where paid patients were defined by receiving 338 or more days of therapy within 12-months, hazard ratio was 1.16 (95% CI, 1.02–1.30; P=0.04) for rejected versus paid and 1.21 (95% CI, 1.04–1.38; P=0.03) for the abandoned versus paid status. Higher PCSK9i rejection rates were observed with women, racial minorities, and lower-income groups.
Conclusions:
Individuals in the rejected and abandoned cohorts had significantly increased risk of cardiovascular events compared with those in the paid cohort. Rejection, abandonment, and disparities related to PCSK9i prescriptions are related to higher cardiovascular outcome rates.
WHAT IS KNOWN
In large cardiovascular outcomes trials, PCSK9is (proprotein convertase subtilisin kexin 9 inhibitors) were shown to significantly reduce major acute cardiovascular events.
Though safe and efficacious, these medicines are costly, resulting in substantial potential budget constraints for payers and high out-of-pocket costs for patients.
WHAT THE STUDY ADDS
We measure the impact on cardiovascular outcomes of high rates of prescription denials and abandonment using a comprehensive electronic healthcare dataset with over 221 million patients.
Using propensity score–matched patient cohorts, we demonstrate that individuals in PCSK9i rejected and abandoned cohorts had significantly increased risk of cardiovascular events compared with those in the paid cohort.
We demonstrate that individuals with primary prevention familial hypercholesterolemia and secondary prevention atherosclerotic cardiovascular disease statistically have no difference in risk of future cardiovascular events, and both high-risk cohorts were denied access to PCSK9is at the same rate as the general population.
See Editorial by Nasir et al
PCSK9is (proprotein convertase subtilisin kexin 9 inhibitors) alirocumab and evolocumab are monoclonal antibodies that were approved in 2015 by the US Food and Drug Administration for treatment of hypercholesterolemia in individuals with either familial hypercholesterolemia (FH) or clinical atherosclerotic cardiovascular disease (ASCVD) who require additional lowering of low density lipoprotein - cholesterol.1,2 When PCSK9i is added to existing therapy with statins alone or in combination with ezetimibe (a cholesterol absorption inhibitor), it substantially lowers low density lipoprotein - cholesterol levels.3–5 In large cardiovascular outcomes trials, evolocumab and alirocumab were each proven to significantly reduce major acute cardiovascular events.3,5–7 The safety of PCSK9is has been well established.8–17
Though safe and efficacious, these medicines are costly, resulting in substantial potential budget constraints for payers and high out-of-pocket costs for patients.18–20 Up to 63% of PCSK9i prescriptions are rejected by payers.21 In addition, claims abandonment, in which the prescription is approved by the payer but the patient does not collect or refill the prescription, may also occur at a high rate.17 The impact of rejected or abandoned PCSK9i prescriptions on cardiovascular outcomes has not been systematically examined.
Using a large comprehensive healthcare database, the FH Foundation performed a propensity score (PS)-matched cohort study to estimate the effects of paid coverage for PCSK9i prescriptions compared with prescription rejection and prescription abandonment on cardiovascular outcomes.22,23 We further stratified the cohorts to determine relative event rates for those with diagnosis of FH and ASCVD versus individuals who had no history of a diagnosis code of FH at any time and no previous history of ASCVD.
Methods
Study Design and Oversight
This retrospective PS-matched cohort study used a healthcare claims dataset (n=221138729 persons) from Symphony Health (Symphony Health, Blue Bell, PA) consisting of diagnosis, procedure, and prescription claims. Laboratory result data (eg, low density lipoprotein - cholesterol levels) were not available for this analysis. Our analysis was conducted on a subset of patients identified with hypercholesterolemia who were prescribed a PCSK9i (n=161 181) between August 2015 and December 2017. A subgroup of those patients (n=139 036) comprised individuals who also had diagnosis and procedure claims and met other inclusion criteria allowing for tracking their cardiovascular events. Patients included in the study were identified from retrospective anonymized claims data, thus neither informed consent nor IRB approval was not required. The claims data details are provided in the Appendix in the Data Supplement. The authors accept responsibility for the accuracy, completeness, analyses, and interpretation of the data. Because of the sensitive nature of the data in this study, requests to access the dataset from qualified researchers may be sent to Kelly D. Myers at the FH Foundation.
Two separate analyses were used to calculate the hazard ratios (HRs) and incidence density rates reported in this study. A Cox proportional hazard regression analysis was performed on post-propensity-matched patient cohorts. A stepwise Cox hazard regression analysis was performed on the entire nonpropensity-matched patient cohorts. This method tests all the baseline characteristics in a stepwise manner, where each step considers a single covariate for addition/subtraction from the final set of variables in the model. HRs from the second model are discussed in the text.
Patients
Patient records included in the study were ≥18 years old, prescribed at least 1 PCSK9i during the defined study period, and had a claims history that included diagnosis and procedure claims. Adjudication of prescription coverage as paid (PD), rejected (RJ), or abandoned (AB) was performed. Patients for whom such an adjudication status could not be made were excluded from the analyses. Patients were qualified as PD status if they received 168 or more days of paid PCSK9i medication within a 12-month period. We chose this definition to identify patients who received adequate PCSK9i therapy to potentially impact cardiovascular events while still allowing for access challenges.3 Patients not qualified as PD status were qualified as RJ status if their initial PCSK9i prescription claims had been rejected and as AB status if their initial claims indicated they had abandoned their approved PCSK9i prescription. Date of the first prescription that qualified the patient for PD, RJ, or AB status was defined as the final adjudication status (FAS) date. Patients who did not meet these criteria were not allocated into the categories and were excluded from the analyses. There were 22 145 patients who met 1 or more of the above criteria and were thus were excluded from the main analysis.
Sensitivity analyses were performed with stricter criteria for patients in PD, RJ, and AB cohorts to evaluate possible differences in outcomes for patients who gained more consistent and longer-term access to PCSK9i therapy. In the sensitivity analyses, patients were qualified as PD status if they received 338 or more days of paid PCSK9i medication within a 12-month period. Patients who had no paid PCSK9i medications at any time were qualified as RJ status if their initial PCSK9i prescription claims had been rejected and as AB status if their initial claims indicated they had abandoned their approved PCSK9i prescription. A fourth cohort of patients who received fewer than 338 days of PCSK9i therapy was excluded from the sensitivity analyses. There were 79 026 patients who met 1 or more of the above criteria and thus were excluded from the sensitivity analyses.
ASCVD-specific analyses were performed on non-ASCVD and ASCVD patient cohorts to evaluate possible differences in outcomes for patients with the highest baseline risk. In these analyses, we further divided each of the PD, RJ, and AB patient populations into 2 subgroups based on whether a documented history of ASCVD is found in a patient’s records or not.
All diagnostic and procedural codes used in this study were based on the International Classification of Diseases, Ninth and Tenth Revisions-Clinical Modification (ICD-9-CM and ICD-10-CM), Current Procedural Terminology and Healthcare Common Procedure Coding System. These codes are provided in the Appendix in the Data Supplement.24 Occurrence of 8 prespecified cardiovascular events: myocardial infarction, unstable angina, acute ischemic heart disease, ischemic stroke, percutaneous coronary intervention, coronary artery bypass graft surgery, cardiac arrest and heart failure (HF) was determined by the above codes for PS matching if the event occurred before the FAS date. Seven of the above cardiovascular events (myocardial infarction, unstable angina, acute ischemic heart disease, ischemic stroke, percutaneous coronary intervention, coronary artery bypass graft, and cardiac arrest) were used after the FAS date as predetermined composite outcomes. Coding practices of HF presented challenges in differentiating acute episodes of HF versus documenting HF as a chronic condition; therefore, HF was not used as a study outcome.
For each type of cardiovascular event, occurrences were studied for 2 cases: those occurring before FAS date and those after. For each occurrence, the number of days before or after FAS was documented. The location(s) of the occurrence of the cardiovascular event (eg, emergency room, outpatient, or inpatient), the number of healthcare professionals involved, the number and types of diagnoses, procedures, and medication codes used were incorporated in an algorithm to authenticate the occurrences and dates of each cardiovascular event. For occurrences of cardiovascular events after FAS date, the number of days between FAS date and the initial cardiovascular event was noted as days at risk. For patients who did not have any occurrence of cardiovascular event after FAS date, the first of the following events was recorded as censored: (1) the patient therapy was exhausted plus 6 months, (2) the patient was still observed in the data up to at least October 1, 2017 without a cardiovascular event and, therefore, deemed to have reached the end of study, or (3) the patient was no longer observed in the data and, therefore, considered to be lost to follow-up. The days at risk for these patients was calculated as the number of days from FAS date to the occurrence of any of the scenarios described above.
After the application of these rules, the mean follow-up duration of the patients in this study was 411.5±170.5, 337.0±219.4, and 310.5±212.7 days for the PD, RJ, and AB cohorts, respectively. In the case of the PD versus RJ propensity-matched cohorts, the mean follow-up duration was 411.3±170.4 and 341.4±219.0 days for the PD and RJ, respectively. In the case of the PD versus AB propensity-matched cohorts, the mean follow-up duration was 408.6±166.7 and 314.1±213.7 days for the PD and RJ, respectively.
PS Matching
PS matching was performed using nonparsimonious multivariable logistic regression models to reduce the impact of confounding because of baseline differences in patients when estimating and comparing the unbiased effect of RJ versus PD and AB versus PD status on outcomes.22,23 For each logistic regression (RJ versus PD and AB versus PD), PSs were estimated using demographic and clinical characteristics, including history of any 8 cardiovascular events occurring before FAS and the duration between their respective occurrences and FAS date as explanatory variables. The variables considered are listed in Tables 1 and 2 and are the same for the full, sensitivity, and ASCVD-specific analyses. Case-controlled PS matching without replacement was performed in a 1:1 ratio for RJ versus PD and AB versus PD cohorts and was tested to assess patient characteristics in the matched pairs, given no significant difference, and within 10% SD.22,23,25 Greedy matching was used and the caliper width was <0.2 of the SD of the PSs. After PS matching for each analysis, 65 278 patients (32 639 RJ and 32 639 PD) were in the RJ and PD dataset (1:1 for RJ versus PD) and 38 890 patients (19 445 AB and 19 445 PD) were in the AB and PD data set (1:1 for AB versus PD). In the RJ versus PD case, this represents 38.2% (99.2%) of the total available RJ (PD) patients. In the AB versus PD case, this represents 93.6% (59.1%) of the total available AB (PD) patients. For the sensitivity analyses, there were 82 155 patients including 10 362 (12.6%) PD, 58 740 (71.5%) RJ, and 13 053 (15.9%) AB status. The number of patients who met the definition of cardiovascular outcome was 2502 (3.0%). After propensity matching, 20 386 patients (10 193 RJ and 10 193 PD) were in the RJ and PD dataset (1:1 for RJ versus PD) and 15 700 (7850 AB and 7850 PD) patients were in the AB and PD dataset (1:1 for AB versus PD). The distribution of the PSs before and after matching are provided in the Supplementary Appendix.
Table 1.
Baseline Patient Characteristics Before Propensity Score Matching
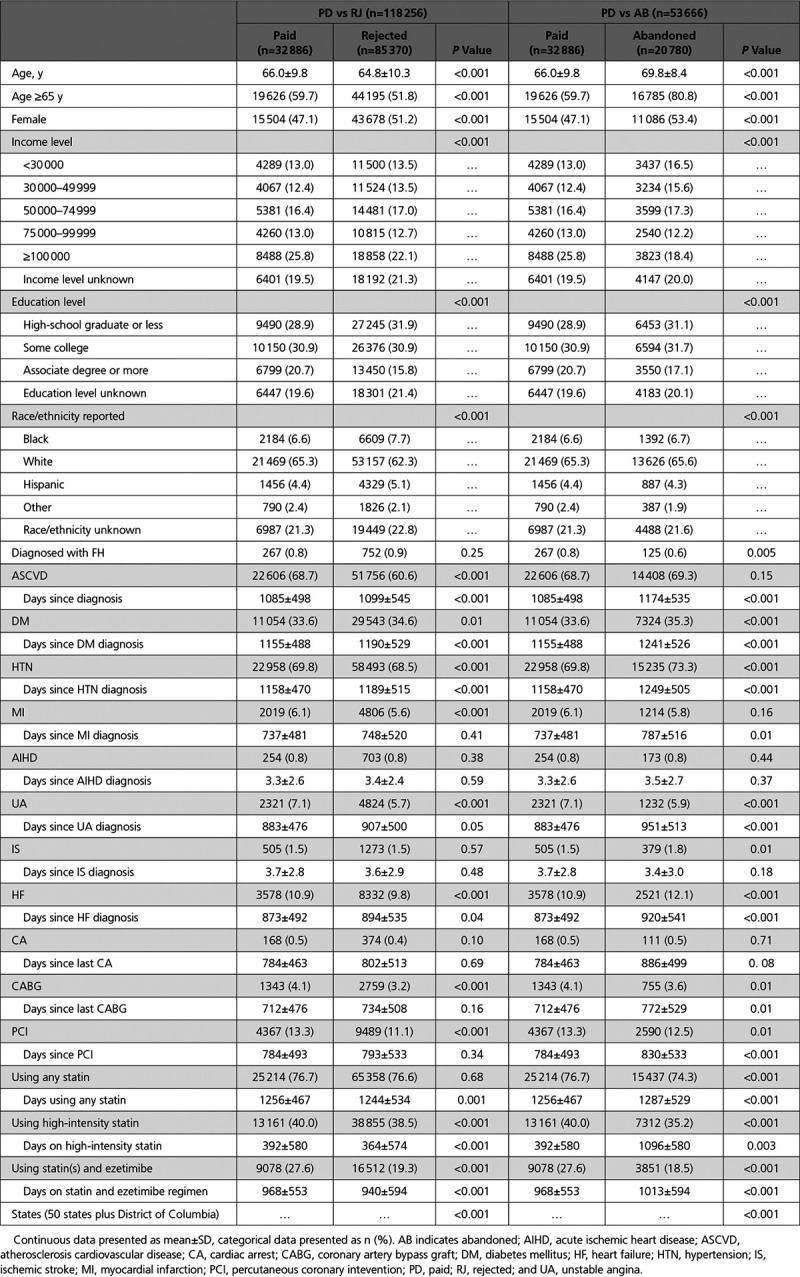
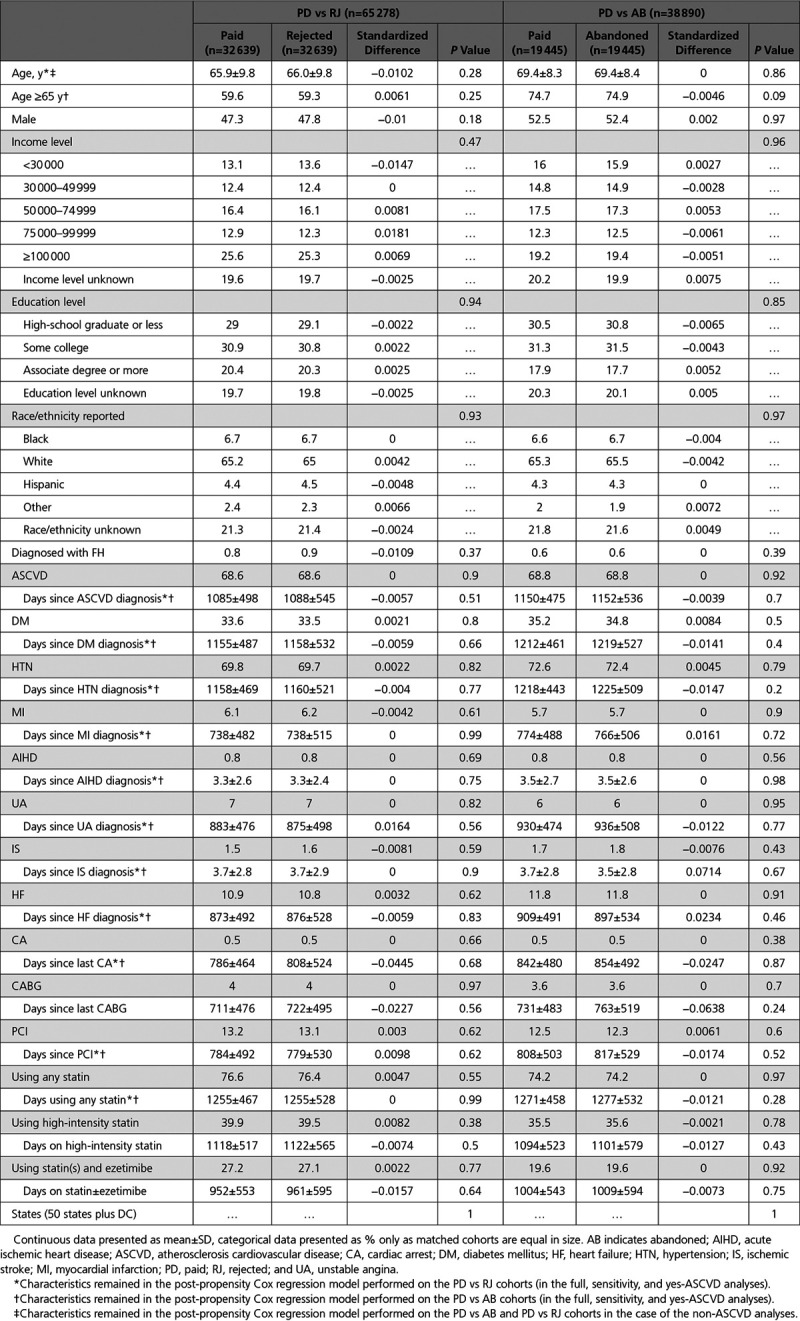
Table 2.
Baseline Patient Characteristics After Propensity Score Matching
Outcomes
Study outcome was a predetermined composite outcome of the earliest diagnosis of any one of the 7 cardiovascular events: myocardial infarction, unstable angina, acute ischemic heart disease, ischemic stroke, cardiac arrest, percutaneous coronary intervention, or coronary artery bypass graft after FAS date. HRs and incidence density rates (IDRs) for the outcome were calculated and compared by payment status for the PS-matched RJ versus PD cohorts and for AB versus PD cohorts. The number of new cases of cardiovascular events occurring after FAS date within the study period was divided by the total person-time at risk for the patients in the corresponding cohort to determine and report IDR for each cohort.
Statistical Analyses
We compared the characteristics of the unmatched RJ versus PD and AB versus PD cohorts using Student t test and χ2 test for continuous variables and categorical variables, respectively (Table 1). Differences in baseline characteristics in the respective PS-matched cohort pairs were re-tested with paired Student t test and McNemar test for continuous variables and categorical variables, respectively (Table 2). Statistical significance was considered when 2-sided P values were <0.05. For the post-PS-matching dataset, the standardized differences were <10% and P values were larger than 0.05 for all matched variables in AB versus PD dataset (Table 2).
Cox-proportional hazard regression analysis was performed, adjusting for any significant differences in baseline characteristics that persisted (P<0.2) in the post-PS-matched cohorts, to estimate the unbiased HRs indicating the effect of RJ or AB status compared with PD status on cardiovascular outcomes (characteristics that persisted in both analyses are listed in Table 2). In addition, a second risk-adjusted Cox-proportional hazard regression analysis in the presence of multiple confounders was performed without a prior-PS matching as a cross check. Finally, IDR analyses were performed to estimate and compare the incidence density rates across the post-PS-matched cohorts.
For the sensitivity and ASCVD-specific analyses, a Cox-proportional hazard regression analysis was performed, adjusting for any significant differences in baseline characteristics that persisted (P<0.2) in the post-PS-matched cohorts, to estimate the unbiased HRs indicating the effect of RJ or AB status compared with PD status on cardiovascular outcomes. For the sensitivity analysis, the characteristics that persisted are the same as in the full analysis (listed in Table 2). For the ASCVD-specific analysis, the characteristics that persisted are different between the ASCVD and non-ASCVD cohorts and listed in Table 2. IDR analyses were performed in both cases to estimate and compare the incidence density rates across the post-PS-matched cohorts.
Individuals with an ICD-10 diagnosis of FH with a history of ASCVD were also evaluated for developing cardiovascular events. To assess the HR of the highest risk cohort of patients with FH and ASCVD, we performed 2 analyses: first, a Cox proportional hazard regression analyses over post-propensity-matched patients with adjustments for baseline covariates, and second, a stepwise Cox hazard regression analysis over the entire nonpropensity-matched patient cohorts.
We performed 2 additional cross-check analyses. First, to determine if insurance type is a major factor in the HR results, we performed a dedicated Cox regression analysis using these data fields. Second, to determine if missing data introduced any bias in the HR results, we performed a dedicated multiple imputation sensitivity analysis. Analyses were performed with the use of SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Study Population
In our dataset, a total of 161 181 patients were prescribed a PCSK9i. Of those, 30 patients were younger than 18 or age was unknown, 10 904 individuals had only prescription claims making it impossible to measure cardiovascular events, and 12 725 patients did not meet the definition of either PD, RJ, or AB and were thus excluded (Figure 1). Also, 66 patients had a cardiac event on the FAS date and were excluded. Some individuals met more than 1 exclusion criteria. Of the remaining 139 036 patients (Table 1), the average age was 66 years, 51% were females, 63% were white, 7% were black, 5% were Hispanic, 2% were classified as other, and 22% were unreported or unknown. Seventeen percent had an associate degree or higher, 31% had some college education, 31% were high school graduates or less, and for 21%, the education level was unknown. These and other baseline characteristics are shown by payment status in Table 1. The fraction of patients without education, ethnicity, and income data represents the only incomplete data included in this study. To account for this, these data fields included an unknown categorization in the propensity-matching procedure. Women, minorities, and those with lower education or lower income levels were less likely to receive approval for a PCSK9i prescription and were less likely to fill an approved prescription.
Figure 1.
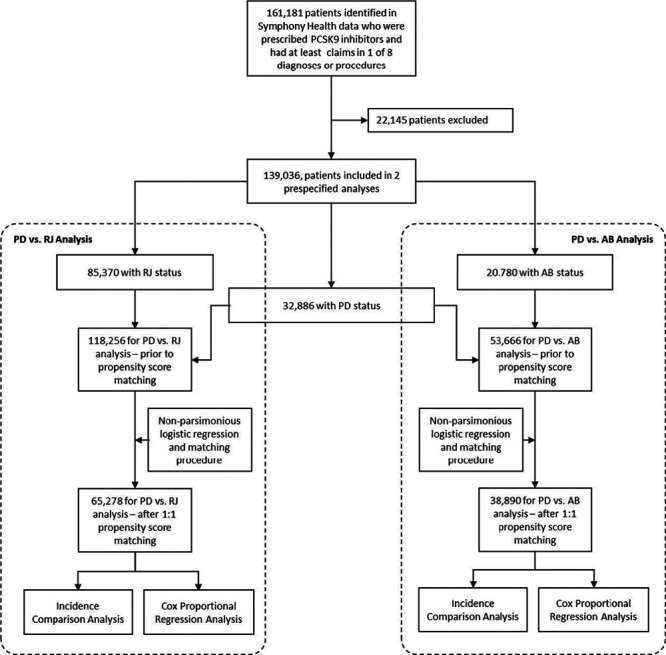
Patient attrition diagram depicting paid (PD) versus rejected (RJ) and PD versus abandoned (AB) propensity score-matched analyses to Cox proportional hazard regressions and incidence density rate analyses. PCSK9 indicates proprotein convertase subtilisin kexin type 9.
Of the 139 036 patients prescribed a PCSK9i, exposure cohorts were 32 886 (24%) for paid (PD) group, 85 370 (61%) for rejected (RJ) group, and 20 780 (15%) for abandoned (AB) group. Also, among those prescribed PCSK9is, 88 770 (63.8%) had a history of ASCVD before their FAS date and 2889 (2.1%) had a documented diagnosis of FH. Of this latter group, 1944 (1.4%) also had a history of ASCVD before their FAS date. A total of 49 321 individuals (35%) had no diagnosis of FH or preFAS ASCVD.
Composite Outcome (HR and IDR)
The total number of patients prescribed a PCSK9i meeting the definition of composite cardiovascular outcome was 4702 (3.4%). Both RJ and AB status were associated with a significantly higher probability of a cardiovascular event compared with PD status. The adjusted HR for the composite cardiovascular event outcome was 1.10 (95% CI, 1.02–1.18; P=0.02) for RJ versus PD status and 1.12 (95% CI, 1.02–1.23; P=0.03) for AB versus PD status (Table 3). The IDRs for the propensity-matched RJ and PD cohorts were 4.08 and 3.49 cases per 100 person-years (P=0.02), respectively, whereas those values in the matched AB and PD cohorts were 3.94 and 3.44 cases per 100 person-years, respectively (P=0.03).
Table 3.
HRs of Outcomes for Propensity Score–Matched Cohorts in PD vs RJ and PD vs AB Analyses
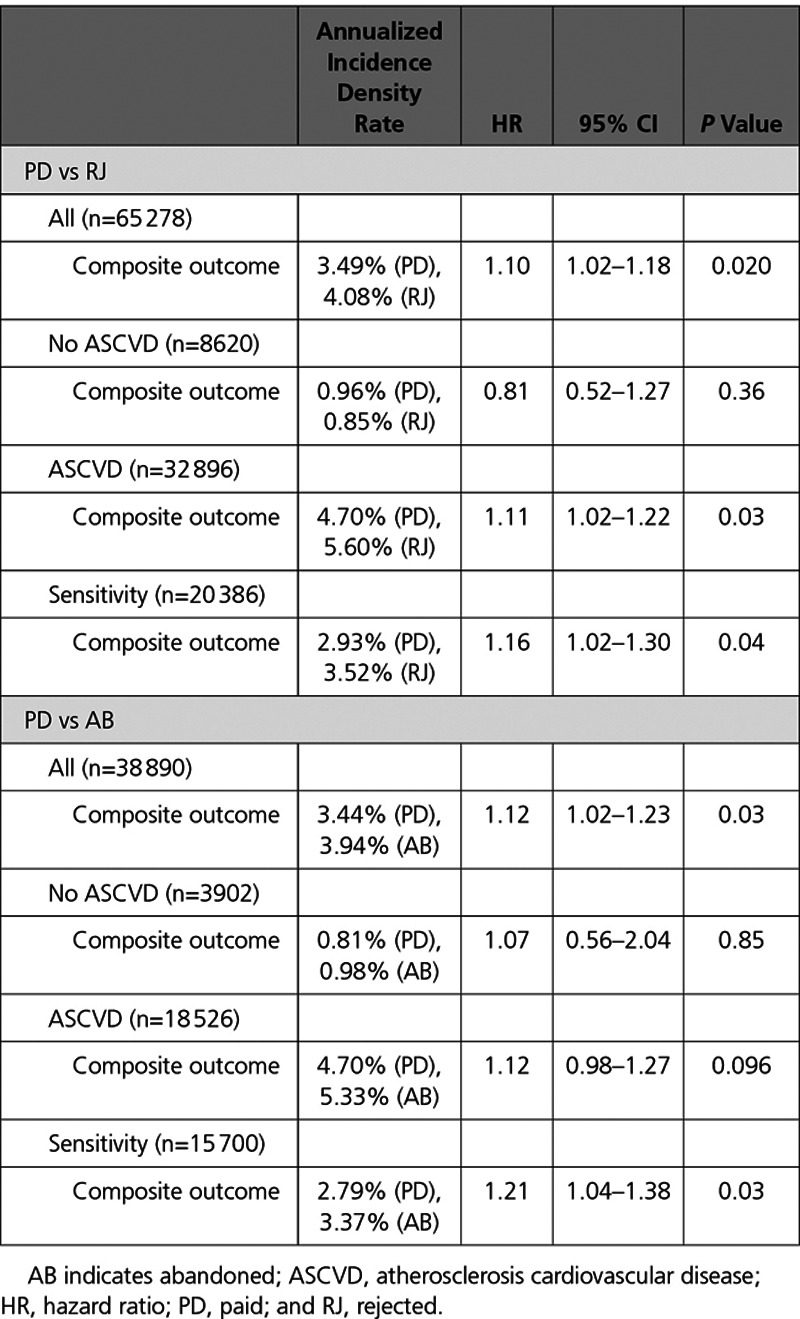
For the sensitivity analyses in which patients had to meet stricter criteria to be qualified for PD status (338 days or more on PCSK9i therapy over a 1-year period), the adjusted HR for freedom from the composite cardiovascular event outcome was 1.16 (95% CI, 1.02–1.30; P=0.04) for RJ versus PD status and 1.21 (95% CI, 1.04–1.38; P=0.03) for the AB versus PD status. IDRs for the matched RJ versus PD cohorts were 3.52 and 2.93 cases per 100 person-years (P=0.04), respectively, and the IDRs in the matched AB versus PD cohorts were 3.37 and 2.79 cases per 100 person-years, respectively (P=0.03).
For the ASCVD-specific analyses, where the PD, RJ, and AB patient populations were subdivided into propensity-matched ASCVD and non-ASCVD cohorts, the adjusted HR for freedom from the composite cardiovascular event outcome was 1.11 (95% Cl, 1.02–1.22; P=0.03) for RJ versus PD and 1.12 (95% Cl, 0.98–1.27; P=0.096) for AB versus PD for the ASCVD case. Conversely, for the non-ASCVD case, the adjusted HR for freedom from the composite cardiovascular event outcome was 0.81 (95% Cl, 0.52–1.27; P=0.36) for RJ versus PD and 1.07 (95% Cl, 0.56–2.04; P=0.85) for AB versus PD. The ASCVD/non-ASCVD IDRs for the matched RJ versus PD cohorts were 5.60/0.85 and 4.70/0.96 cases per 100 person-years (P=0.03/0.36), respectively, and the IDRs in the matched AB versus PD cohorts were 5.33/0.98 and 4.70/0.81 cases per 100 person-years (P=0.096/0.85), respectively.
The adjusted HR for freedom from the composite cardiovascular event outcome with no prior PS matching was 1.11 (95% CI, 1.03–1.18; P=0.003) for RJ versus PD analysis and 1.13 (95% CI, 1.03–1.24; P=0.01) for AB versus PD analysis. For the sensitivity analyses on the entire cohort, the HR was 1.2 (95% CI, 1.07–1.34; P=0.001) for RJ versus PD and 1.2 (95% CI, 1.05–1.4; P=0.009) for AB versus PD analyses.
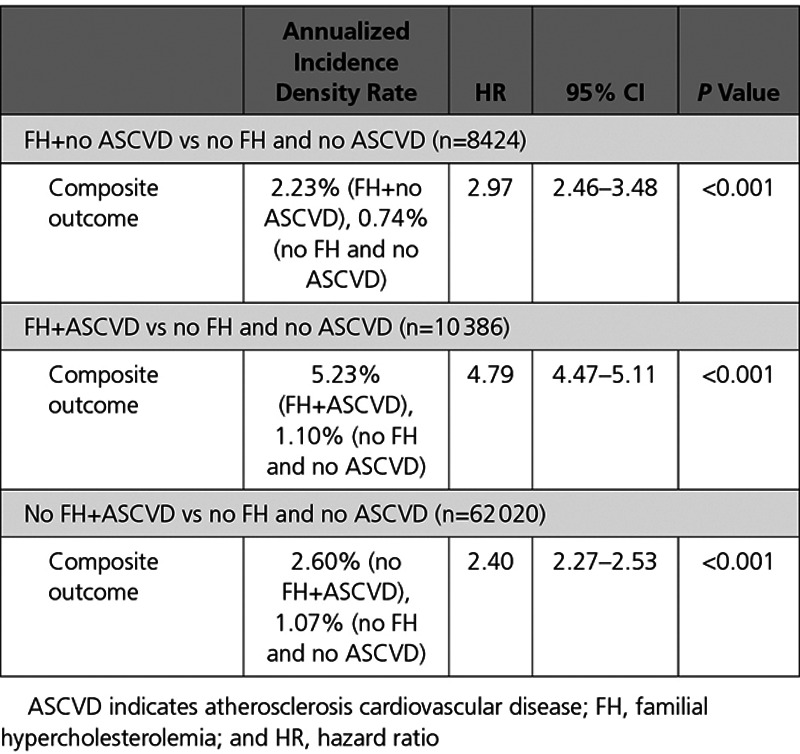
In addition, the relative risk of cardiovascular events was evaluated for individuals with FH, ASCVD, and all combinations of the 2 groups. Previous reports have found variable elevated risk of cardiovascular events when comparing FH and ASCVD cohorts.26 Primary prevention FH cohorts (FH diagnosis and no history of ASCVD) was compared with cohorts who had no FH or ASCVD diagnosis and ASCVD without a diagnosis of FH. Secondary cardiovascular event risk was evaluated for individuals with ASCVD and no history of FH and risk of cardiovascular events was evaluated for individuals with FH and ASCVD. Regression-adjusted HRs for the outcome of no composite cardiovascular event were explicitly calculated for a number of relevant combinations. We found a HR of 2.8 (95% CI, 1.78–4.42; P<0.001) for the FH cohort versus individuals who did not have a documented diagnosis of FH or preFAS ASCVD, 2.12 (95% CI, 1.86–2.43; P<0.001) for ASCVD versus non-FH and non-ASCVD, 1.75 (95% CI, 1.48–2.08; P<0.001) for FH+ASCVD versus ASCVD alone, 5.4 (95% CI, 4.28–6.9; P<0.001) for FH+ASCVD versus non-FH and non-ASCVD, and no statistical difference in risk between primary prevention FH and secondary prevention ASCVD. It is of note that PCSK9i claims rejection rates for FH and ASCVD individuals were 58.5% and 58.3%, respectively. Comparable results were observed using propensity-matched HR analyses (Table 4).
Table 4.
HRs of Outcomes for Propensity Score–Matched High-Risk Cohorts
To determine if insurance type is a major factor in the HR analysis, we performed a dedicated stepwise Cox hazard regression analysis on PD versus RJ with payment type included in the model to evaluate the impact on the HRs. The type of insurance (payment type) is broken down in the data into Cash, Medicare, Managed Medicaid, Assistance Program, and Commercial. The result was nonsignificant with a P value of 0.7, rendering this variable not eligible for entry (SLENTRY=0.25). Therefore, insurance type was not a determining factor in the hazard rate differences that we observe between the 2 cohorts.
To determine if the data missing from patient profiles, including those 24% of patients who received PCSK9i prescriptions with unknown education, ethnicity, or income data, introduced bias in the final HR results, we performed a multiple imputation sensitivity analysis. The PSs and HRs were calculated for 5 complete imputed datasets. The averaged HR results from these imputed cohorts remained significant and the HRs and P value agreed with those from the main analysis.
Discussion
These analyses were performed on a large number of individuals prescribed a PCSK9i to assess the impact of access to PCSK9is on risk-adjusted rates of cardiovascular events. This cohort of 139 036 individuals who were prescribed a PCSK9i had significantly higher cardiovascular event rates than individuals found in the general population.27 Although PCSK9is are highly effective in reducing low density lipoprotein - cholesterol levels and cardiovascular risk,8–17,28–32 access to these medicines is restricted for many patients who otherwise might benefit.17 While a previous study by the FH Foundation suggested that patients with rejected claims may be associated with adverse cardiovascular outcomes, the effect was not adequately quantified.21,33 In this study, we found that individuals who were denied access to PCSK9i therapy had a significantly higher incidence density rate of cardiovascular events than those who were provided access. Our results also documented that individuals who were at highest risk (diagnosed FH and ASCVD; HR, 5.4) had a PCSK9i rejection rate of 63.5%. Furthermore, those patients whose prescription was approved but who abandoned attempts to fill the prescription also had higher rates of cardiovascular events. These real-world observational data indicate that access to PCSK9is has an important impact on cardiovascular outcomes in the patients with high risk, who are prescribed these medicines.
For rejected prescriptions, rejection may be because of disagreement between the providers’ diagnoses and the payer policies for coverage, inadequate coding, or information submitted with the prescriptions or noncoverage for the prescribed condition.34 For abandoned prescriptions, patients were approved for coverage of the PCSK9i but did not fill the prescription. Abandonment may be because of economic challenges. According to our data, the average monthly copay for a PD PCSK9i prescription is $103.17, whereas for an abandoned prescription, the copay averages $233.80. About 64.97% of AB prescriptions were Medicare prescriptions versus 42.36% PD and 39.49% RJ. Centers for Medicare and Medicaid Services policy does not allow for copay assistance for Medicare Part D prescriptions, often resulting in higher out-of-pocket costs. The continuous out-of-pocket costs may be prohibitive for some patients, leading to abandonment even if the prescription is approved. As prices for PCSK9is have recently been reduced by manufacturers, lesser costs may impact the results of this study if cost reductions are passed on to patients.
Rejected and abandoned PCSK9i prescriptions represent distinct challenges to medication access and merit further analysis with regard to their impact on cardiovascular outcomes. Prescription approval, as well as the probability of filling an approved prescription are heavily influenced by patient sociodemographic and clinical characteristics. Our datasets do not provide information to further understand these processes. To address some of these systematic differences when estimating the treatment effects on the composite cardiovascular outcome, we used propensity-score matching, which minimized the baseline risk between the statistically compared groups. Despite this matching and controlling for similar background risk, those patients whose PCSK9i prescriptions were either rejected or abandoned had significantly more cardiovascular events than those whose PSCK9i prescriptions were paid and filled. In addition, in the sensitivity analyses with stricter criteria for the comparison groups, the results remained significant.
As expected, patients with FH and ASCVD had markedly increased risk of cardiovascular events compared with those without these conditions and may be among those most likely to benefit from PCSK9i therapy. In our previous analysis, we noted that for presumptive FH patients and ASCVD patients with LDL>100 on maximally tolerated therapy, rejection rates for prescribed PCSK9is were up to 63%.21 In our current analyses, PCSK9i claims rejection rates for FH and ASCVD individuals were 58.5% and 58.3%, respectively.
These analyses were performed on data from within the first 29 months of availability of PCSK9i. The average number of at-risk months for individuals once FAS was established was 11.5. Despite this relatively brief duration, it is notable that there were significantly lower rates of incident adverse cardiovascular events in the PD cohort. In the FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk), ODYSSEY (Long-term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy), and SPIRE-2 (Studies of PCSK9 Inhibition and the Reduction of Vascular Events) trials, follow-up was >2 years and an absolute event rate reduction was about 3× that observed in this analysis (an average of about 1.5% in the trials versus 0.6% here).4,35,36 Given the likely differences between this cohort and the trials’, the average age in our cohort was 66, 58.5 for ODYSSEY, and 62.5 for FOURIER, for example, the results are likely similar to the trials or slightly attenuated.4,36 We plan to continue to follow these cohorts to determine if the difference in event rates continues to diverge over longer follow-up durations.36 The percent lowering of event rates was greater in our PD group compared with the other 2 groups. Health disparities may account partly for the results of this study as the groups less likely to have prescriptions approved are also groups with higher cardiovascular risk related to ethnicity and lower socioeconomic status. It should also be noted that while the trials mentioned above assessed efficacy of PCSK9is, this study is evaluating the impact of access to the PCSK9is.
This study has several limitations. In PS matching, unmeasured factors are not accounted for in minimizing systematic differences in baseline characteristics. In addition, the method dictates that a fraction of the population is propensity matched leading to questions about generalizability of findings to the full population. However, in this study alternative analyses using a 1:1 ratio PS matching for the PD versus RJ and Cox proportional hazard regression analyses with no prior PS matching yielded comparable results. Mortality, or vital status, is often reported as an outcome in cardiovascular event analyses. However, mortality is not explicitly included in this study because coding practices do not consistently include the documentation of mortality data. These patients are categorized as lost to follow-up in the censoring rules, and their days at risk is calculated up until they are no longer observed. Anecdotal evidence indicates that PCSK9i samples may be offered by healthcare providers to help individuals gain access. Claims data do not capture the use of such PCSK9i sample provision; therefore, we were unable to include or measure their impact. Residual confounding could account for results of this study as this analysis could make no attempt to assess the reasons PCSK9i claims were rejected or the appropriateness of therapy. The lack of access to a complete medical history may have led to a missed ASCVD diagnosis that occurred outside the study period, and the lack of availability of laboratory result data prevented further analysis of the appropriateness of PCSK9i therapy. Reverse causality, where PCSK9i treatment is initiated after an additional cardiovascular event in a subject can also not be excluded. Nor can immortal time bias.
This observational study represents a unique collaboration of individuals impacted by FH; the FH Foundation, a research and advocacy organization; physician researchers; practicing clinicians; and data scientists. Biases related to the perspectives of the authors and the FH Foundation should be recognized. Our mission is to raise awareness and save lives by increasing the rate of early diagnosis and encouraging proactive treatment. Study objectives were driven by the personal interest of patients affected by FH who think PCSK9is will be life prolonging for them and have experienced denials by insurance providers. Clinical trials first showing the benefit of PCSK9is were funded by industry to satisfy regulatory requirements for drug approval. The FH Foundation also receives support from these same companies. However, these companies were not involved in any part of the process of research design, data analysis, or article preparation.
This study documents in a real-world analysis the impact of access to PCSK9is on the prevention of ASCVD events in high-risk populations. Those with both FH and prior ASCVD had the highest risk of events but were denied access to PCSK9is to a similar extent. There is a need to collect additional, real-world data prospectively specific to patients who would benefit from PCSK9 is to better understand their long-term effects and any emerging challenges. Health disparities related to sex, race, education, and income among patients prescribed PCSK9is are evident and should be examined and addressed accordingly. Of note, women had higher rejection and abandonment rates than men, those with income of $100 000 and higher and with associate degrees or higher had higher rates of paid prescriptions than the lower groups compared with rejected and abandoned cohorts. In addition, racial minorities had higher rejection rates than whites. Establishment and use of large registries, including patients prescribed PCSK9is, may, in the long run, provide valuable insights and the ability to accurately characterize associated social determinants of disparities, healthcare use, cost and reimbursement burden, impact on comorbidities, and all-cause and cardiovascular mortality.37 Appropriately identifying and characterizing barriers to PCSK9i access, and developing approaches to overcome them, will reduce the clinical and economic burden for patients who are likely to benefit from PCSK9 inhibition and likely result in more cost-effective policies for payers.37
Acknowledgments
K.D. Myers, N. Farboodi, and Drs Mwamburi, Howard, and Staszak contributed to design and analysis of the study and drafting and editing of the manuscript. Dr Gidding, K. Wilemon, Dr Baum, and Dr Rader contributed to drafting and editing of the manuscript.
Sources of Funding
The FH Foundation funded this study. As a 501c3 public charity research and advocacy organization, the FH Foundation receives contributions and sponsorships from individuals, foundations, and pharmaceutical companies.
Disclosures
K. Wilemon, Dr Gidding, and N. Farboodi are employees of the FH Foundation; K.D. Myers, Drs Mwamburi, Howard, and Staszak are paid consultants for the FH Foundation; Drs Baum and Rader are unpaid advisors to the FH Foundation.
Supplementary Material
Footnotes
K.D. Myers and N. Farboodi contributed equally to this work.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCOUTCOMES.118.005404.
References
- 1.U.S. Food & Drug Administration. Approval for Evolocumab (Repatha). https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125522s000lbl.pdf. Accessed May 26, 2019.
- 2.U.S. Food & Drug Administration. Approval for Alirocumab (Praluent). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/125559Orig1s000TOC.cfm. Accessed May 26, 2019.
- 3.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 4.Roth EM. Alirocumab for hyperlipidemia: ODYSSEY phase III clinical trial results and US FDA approval indications. Future Cardiol. 2016;12:115–128. doi: 10.2217/fca.15.78. doi: 10.2217/fca.15.78. [DOI] [PubMed] [Google Scholar]
- 5.Wilson PWF, Polonsky TS, Miedema MD, Khera A, Kosinski AS, Kuvin JT. Systematic Review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1144–e1161. doi: 10.1161/CIR.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 7.Szarek M, White HD, Schwartz GG, Alings M, Bhatt DL, Bittner VA, Chiang CE, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Kimura T, Kiss RG, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Roe MT, Tricoci P, Xavier D, Zeiher AM, Steg PG ODYSSEY OUTCOMES Committees and Investigators. Alirocumab reduces total nonfatal cardiovascular and fatal events: the ODYSSEY OUTCOMES trial. J Am Coll Cardiol. 2019;73:387–396. doi: 10.1016/j.jacc.2018.10.039. doi: 10.1016/j.jacc.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 8.AlHajri L, AlHadhrami A, AlMheiri S, AlMutawa Y, AlHashimi Z. The efficacy of evolocumab in the management of hyperlipidemia: a systematic review. Ther Adv Cardiovasc Dis. 2017;11:155–169. doi: 10.1177/1753944717698925. doi: 10.1177/1753944717698925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bays HE, Leiter LA, Colhoun HM, Thompson D, Bessac L, Pordy R, Toth PP. Alirocumab treatment and achievement of non-high-density lipoprotein cholesterol and apolipoprotein B goals in patients with hypercholesterolemia: pooled results from 10 phase 3 ODYSSEY trials. J Am Heart Assoc. 2017;6:e005639. doi: 10.1161/JAHA.117.005639. doi: 10.1161/JAHA.117.005639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll C, Tappenden P, Rafia R, Hamilton J, Chambers D, Clowes M, Durrington P, Qureshi N, Wierzbicki AS. Evolocumab for treating primary hypercholesterolaemia and mixed dyslipidaemia: an evidence review group perspective of a NICE Single technology appraisal. Pharmacoeconomics. 2017;35:537–547. doi: 10.1007/s40273-017-0492-6. doi: 10.1007/s40273-017-0492-6. [DOI] [PubMed] [Google Scholar]
- 11.Catapano AL, Lee LV, Louie MJ, Thompson D, Bergeron J, Krempf M. Efficacy of alirocumab according to background statin type and dose: pooled analysis of 8 ODYSSEY Phase 3 clinical trials. Sci Rep. 2017;7:45788. doi: 10.1038/srep45788. doi: 10.1038/srep45788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng C, Sun S, Zhou Y, Yang X. Efficacy and safety of different doses of evolocumab in reducing low-density lipoprotein cholesterol levels: a meta-analysis. Biomed Rep. 2016;5:541–547. doi: 10.3892/br.2016.766. doi: 10.3892/br.2016.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray KK, Ginsberg HN, Davidson MH, Pordy R, Bessac L, Minini P, Eckel RH, Cannon CP. Reductions in atherogenic lipids and major cardiovascular events: a pooled analysis of 10 ODYSSEY trials comparing alirocumab with control. Circulation. 2016;134:1931–1943. doi: 10.1161/CIRCULATIONAHA.116.024604. doi: 10.1161/CIRCULATIONAHA.116.024604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson JG, Rosenson RS, Farnier M, Chaudhari U, Sasiela WJ, Merlet L, Miller K, Kastelein JJ. Safety of very low low-density lipoprotein cholesterol levels with alirocumab: pooled data from randomized trials. J Am Coll Cardiol. 2017;69:471–482. doi: 10.1016/j.jacc.2016.11.037. doi: 10.1016/j.jacc.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 15.Stein EA, Giugliano RP, Koren MJ, Raal FJ, Roth EM, Weiss R, Sullivan D, Wasserman SM, Somaratne R, Kim JB, Yang J, Liu T, Albizem M, Scott R, Sabatine MS PROFICIO Investigators. Efficacy and safety of evolocumab (AMG 145), a fully human monoclonal antibody to PCSK9, in hyperlipidaemic patients on various background lipid therapies: pooled analysis of 1359 patients in four phase 2 trials. Eur Heart J. 2014;35:2249–2259. doi: 10.1093/eurheartj/ehu085. doi: 10.1093/eurheartj/ehu085. [DOI] [PubMed] [Google Scholar]
- 16.Toth PP, Descamps O, Genest J, Sattar N, Preiss D, Dent R, Djedjos C, Wu Y, Geller M, Uhart M, Somaratne R, Wasserman SM PROFICIO Investigators. Pooled safety analysis of evolocumab in over 6000 patients from double-blind and open-label extension studies. Circulation. 2017;135:1819–1831. doi: 10.1161/CIRCULATIONAHA.116.025233. doi: 10.1161/CIRCULATIONAHA.116.025233. [DOI] [PubMed] [Google Scholar]
- 17.Zhang XL, Zhu QQ, Zhu L, Chen JZ, Chen QH, Li GN, Xie J, Kang LN, Xu B. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med. 2015;13:123. doi: 10.1186/s12916-015-0358-8. doi: 10.1186/s12916-015-0358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ICER. PCSK9 Inhibitors for Treatment of High Cholesterol: Effectiveness, Value, and Value Based Price Benchmarks. http://resource.nlm.nih.gov/101672684. Accessed May 26, 2019.
- 19.Fonarow GC, Keech AC, Pedersen TR, Giugliano RP, Sever PS, Lindgren P, van Hout B, Villa G, Qian Y, Somaratne R, Sabatine MS. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069–1078. doi: 10.1001/jamacardio.2017.2762. doi: 10.1001/jamacardio.2017.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandra SR, Villa G, Fonarow GC, Lothgren M, Lindgren P, Somaratne R, van Hout B. Cost-Effectiveness of LDL-C lowering with evolocumab in patients with high cardiovascular risk in the united states. Clin Cardiol. 2016;39:313–320. doi: 10.1002/clc.22535. doi: 10.1002/clc.22535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knowles JW, Howard WB, Karayan L, Baum SJ, Wilemon KA, Ballantyne CM, Myers KD. Access to nonstatin lipid-lowering therapies in patients at high risk of atherosclerotic cardiovascular disease. Circulation. 2017;135:2204–2206. doi: 10.1161/CIRCULATIONAHA.117.027705. doi: 10.1161/CIRCULATIONAHA.117.027705. [DOI] [PubMed] [Google Scholar]
- 22.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newgard CD, Hedges JR, Arthur M, Mullins RJ. Advanced statistics: the propensity score–a method for estimating treatment effect in observational research. Acad Emerg Med. 2004;11:953–961. doi: 10.1197/j.aem.2004.02.530. doi: 10.1197/j.aem.2004.02.530. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Medicare & Medicaid Services. ICD-10-CM Codes. https://www.cms.gov/Medicare/Coding/ICD10/. Accessed May 26, 2019. [PubMed]
- 25.Parsons LS. Performing a 1:N Case-Control Match on Propensity Score. https://support.sas.com/resources/papers/proceedings/proceedings/sugi29/165-29.pdf. Accessed May 26, 2019.
- 26.Perak AM, Ning H, de Ferranti SD, Gooding HC, Wilkins JT, Lloyd-Jones DM. Long-Term risk of atherosclerotic cardiovascular disease in US adults with the familial hypercholesterolemia phenotype. Circulation. 2016;134:9–19. doi: 10.1161/CIRCULATIONAHA.116.022335. doi: 10.1161/CIRCULATIONAHA.116.022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White H, Steg P, Szarek M, Bhatt D, Bittner V, Diaz R, Edelberg J, Goodman S, Hantoin C, Harrington R, Jukema J, Lecorps G, Moryusef A, Pordy R, Roe M, Zeiher A, Schwartz G. Cardiovascular outcomes with alirocumab after acute coronary syndrome: results of the ODYSSEY outcomes trial. Heart Lung Circ. 2018;27:s305–s306. [Google Scholar]
- 29.Hassan M. OSLER and ODYSSEY LONG TERM: PCSK9 inhibitors on the right track of reducing cardiovascular events. Glob Cardiol Sci Pract. 2015;2015:20. doi: 10.5339/gcsp.2015.20. doi: 10.5339/gcsp.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koren MJ, Giugliano RP, Raal FJ, Sullivan D, Bolognese M, Langslet G, Civeira F, Somaratne R, Nelson P, Liu T, Scott R, Wasserman SM, Sabatine MS OSLER Investigators. Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the open-label study of long-term evaluation against LDL-C (OSLER) randomized trial. Circulation. 2014;129:234–243. doi: 10.1161/CIRCULATIONAHA.113.007012. doi: 10.1161/CIRCULATIONAHA.113.007012. [DOI] [PubMed] [Google Scholar]
- 31.Koren MJ, Sabatine MS, Giugliano RP, Langslet G, Wiviott SD, Kassahun H, Ruzza A, Ma Y, Somaratne R, Raal FJ. Long-term low-density lipoprotein cholesterol-lowering efficacy, persistence, and safety of evolocumab in treatment of hypercholesterolemia: results up to 4 years from the open-label OSLER-1 extension study. JAMA Cardiol. 2017;2:598–607. doi: 10.1001/jamacardio.2017.0747. doi: 10.1001/jamacardio.2017.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz GG, Bessac L, Berdan LG, Bhatt DL, Bittner V, Diaz R, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Mahaffey KW, Moryusef A, Pordy R, Roe MT, Rorick T, Sasiela WJ, Shirodaria C, Szarek M, Tamby JF, Tricoci P, White H, Zeiher A, Steg PG. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014;168:682–689. doi: 10.1016/j.ahj.2014.07.028. doi: 10.1016/j.ahj.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 33.Baum SJ, Chen CC, Rane P, Patel J, Maya J, Harrison D, Yurgin N, Wade R, Desai N. Cardiovascular risk in patients denied access to PCSK9i therapy. J Am Coll Cardiol. 2018:71(suppl 11):A1760. [Google Scholar]
- 34.Baum SJ, Toth PP, Underberg JA, Jellinger P, Ross J, Wilemon K. PCSK9 inhibitor access barriers-issues and recommendations: Improving the access process for patients, clinicians and payers. Clin Cardiol. 2017;40:243–254. doi: 10.1002/clc.22713. doi: 10.1002/clc.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridker PM, Revkin J, Amarenco P, Brunell R, Curto M, Civeira F, Flather M, Glynn RJ, Gregoire J, Jukema JW, Karpov Y, Kastelein JJP, Koenig W, Lorenzatti A, Manga P, Masiukiewicz U, Miller M, Mosterd A, Murin J, Nicolau JC, Nissen S, Ponikowski P, Santos RD, Schwartz PF, Soran H, White H, Wright RS, Vrablik M, Yunis C, Shear CL, Tardif JC SPIRE Cardiovascular Outcome Investigators. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med. 2017;376:1527–1539. doi: 10.1056/NEJMoa1701488. doi: 10.1056/NEJMoa1701488. [DOI] [PubMed] [Google Scholar]
- 36.Shah SR, Uddin MF, Lateef N, Dharani AM, Shahnawaz W, Kazi AN, Shah SA. Evolocumab to reduce cardiovascular events: results of the (FOURIER) multinational trial. J Community Hosp Intern Med Perspect. 2017;7:199–200. doi: 10.1080/20009666.2017.1340732. doi: 10.1080/20009666.2017.1340732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Brien EC, Roe MT, Fraulo ES, Peterson ED, Ballantyne CM, Genest J, Gidding SS, Hammond E, Hemphill LC, Kindt I, Moriarty PM, Ross J, Underberg JA, Watson K, Pickhardt D, Rader DJ, Wilemon K, Knowles JW. Rationale and design of the familial hypercholesterolemia foundation CAscade SCreening for awareness and DEtection of familial hypercholesterolemia registry. Am Heart J. 2014;167:342–349.e17. doi: 10.1016/j.ahj.2013.12.008. doi: 10.1016/j.ahj.2013.12.008. [DOI] [PubMed] [Google Scholar]


