Abstract
Objectives
To evaluate the analytical and clinical performance of the Truvian Easy Check coronavirus disease 2019 (COVID-19) IgM/IgG anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody test.
Serologic assays have become increasingly available for surveillance through the Food and Drug Administration emergency use authorization in the ongoing COVID-19 global pandemic. However, widespread application of serologic assays has been curbed by reports of faulty or inaccurate tests. Therefore, rapid COVID-19 antibody tests need to be thoroughly validated prior to their implementation.
Methods
The Easy Check device was analytically evaluated and its performance was compared with the Roche Elecsys anti-SARS-CoV-2 antibody assay. The test was further characterized for cross-reactivity using sera obtained from patients infected by other viruses. Clinical performance was analyzed with polymerase chain reaction-confirmed samples and a 2015 prepandemic reference sample set.
Results
The Easy Check device showed excellent analytical performance and compares well with the Roche Elecsys antibody assay, with an overall concordance of 98.6%. Clinical performance showed a sensitivity of 96.6%, a specificity of 98.2%, and an overall accuracy of 98.1%.
Conclusions
The Easy Check device is a simple, reliable, and rapid test for detection of SARS-CoV-2 seropositivity, and its performance compares favorably against the automated Roche Elecsys antibody assay.
Keywords: SARS-CoV-2 antibodies, COVID-19, Coronavirus, Point-of-care, Lateral flow, IgM, IgG, Diagnostics, Surveillance
Key Points.
We evaluated the analytical and clinical performance of the Truvian Easy Check COVID-19 IgM/IgG antibody test designed to detect the nucleocapsid and S1 spike protein RBD epitopes of SARS-CoV-2.
The Easy Check device showed excellent clinical and analytical performance; the test compares well with the Roche Elecsys anti-SARS-CoV-2 antibody assay.
This study demonstrates that the Easy Check device is easy to use, fast, and a reliable platform for detection of seropositivity of SARS-CoV-2 at 10 minutes after test initiation for serum and possibly fingerstick blood samples.
Newly developed laboratory assays have become increasingly available worldwide to help guide both clinical management and public health measures in the ongoing coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1,2 Although the rapid development of many serologic assays has sought to address the paucity of information on the epidemiologic spread of the virus, recent reports of potential inaccuracy or inapplicability have called into question their reliability as dependable diagnostic tools in certain clinical settings.3-5 The cause of unsatisfactory assay performance is typically multifactorial; however, withdrawal of usage by the US Food and Drug Administration (FDA) includes noncompliance of Emergency Use Authorization (EUA) requirements and delayed validation studies.6-9 In response to such concerns, the FDA made several updates (March 16, May 4, and May 11 of 2020) to tighten validation guidelines in its initial policy statement on February 29, 2020, for manufacturers developing antibody-based tests against SARS-CoV-2.
Quantitative and qualitative reverse transcription polymerase chain reaction (RT-PCR) remain the gold standard testing for diagnosing active infection by SARS-CoV-2 and is applied by direct detection and amplification of viral genetic material.10 In contrast, serologic assays are indirect tests that detect the presence of IgM, IgG, and total antibodies in patient plasma or sera, which typically develop several weeks after initial infection.11-13 Although IgM, IgG, and total antibody levels are thought to peak after 2 to 3 weeks, 3 to 6 weeks, and 2 to 3 weeks, respectively,14-17 both IgM and IgG levels can vary considerably so most serologic assays aim to detect both IgM and IgG simultaneously for improved sensitivity.18
The Easy Check is a rapid diagnostic test that utilizes an immunochromatographic-based, lateral flow platform to detect both IgM and IgG antibodies against SARS-CoV-2 applied in a stand-alone device format. Whereas RT-PCR requires at least several to 24 hours of processing and turnaround time, lateral flow devices provide results rapidly to allow for near-immediate assessment of a patient’s exposure history.19 Therefore, particularly as large segments of the US population remain undertested and with the number of COVID-19 cases still continuing to rise several months after the first confirmed US case in January 2020,20 availability of the Easy Check device will help address current gaps by providing real-time information of the seroconversion status of individuals who have been infected by SARS-CoV-2.21-23
In this study, the Easy Check was evaluated analytically and clinically and the basis of its technology is briefly described. The clinical performance of the Easy Check was validated using prepandemic, SARS-CoV-2-negative, and SARS-CoV-2-positive, PCR-confirmed patient samples and compared against the performance of the Roche Elecsys anti-SARS-CoV-2 antibody assay.24,25 The Easy Check demonstrated excellent analytical performance, high sensitivity and specificity, as well as minimal cross-reactivity against plasma or sera obtained from patients infected by other viruses, and is therefore a reliable modality for widespread and rapid serologic testing of SARS-CoV-2 infection.
Materials and Methods
Patient Samples
Banked heparinized plasma or sera from PCR-confirmed COVID-19 inpatients hospitalized at the University of Chicago Medical Center were retrieved from the Clinical Chemistry and Immunology Laboratories for this evaluation. For long-term storage, samples were frozen at −25°C until they were ready for use. Samples were collected under a quality assurance protocol that qualifies for institutional review board (IRB) waiver, as no patient identifiers were used. For the paired fingerstick capillary and venous samples collected from COVID-19 patients, oral consent was obtained under an approved IRB protocol (IRB16-0555).
The Truvian Easy Check COVID-19 IgM/IgG Device
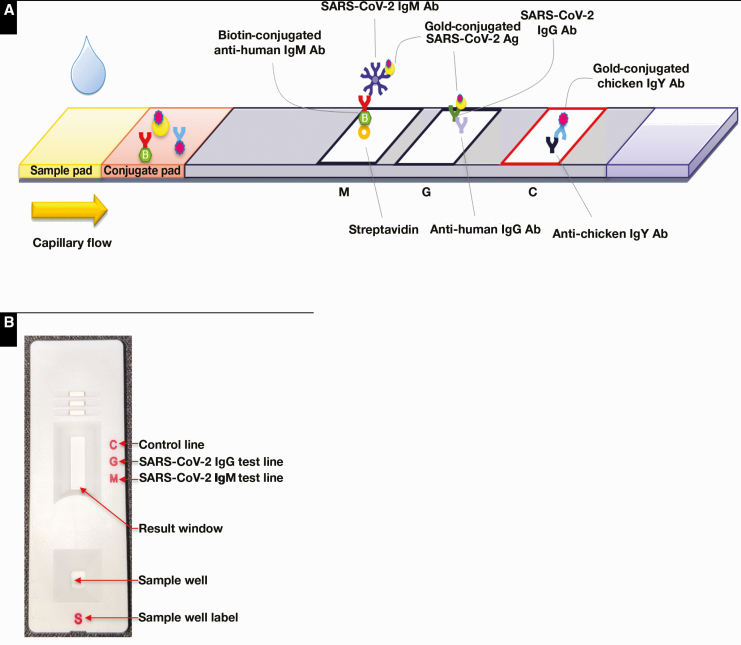
The Easy Check is a standalone lateral flow device manufactured by Access Bio who received EUA by the US FDA on July 24, 2020 (publicly available via the FDA website at https://www.fda.gov/media/140444/download) for the detection of anti-SARS-CoV-2 IgM and/or IgG antibodies in human blood specimens. As shown in Figure 1, control anti-chicken IgY, anti-human IgG, and streptavidin are immobilized onto a nitrocellulose membrane to form the internal control line, IgG test line, and the IgM test line, respectively. Blood, serum, or plasma samples in the amount of 10 µL is added to the sample well of the test device followed by a drop of the reagent buffer to initiate a test. The SARS-CoV-2 antibodies in sample specimens bind to recombinant antigens (the SARS-CoV-2 nucleocapsid and the S1 receptor binding domain of the spike protein) that are conjugated to colloidal gold nanobeads to form an immune complex as the sample migrates through the conjugate pad. IgM reacts with the gold-conjugated SARS-CoV-2 antigens and biotinylated anti-human IgM, while IgG only reacts with the gold-conjugated SARS-CoV-2 antigens to generate their respective purple-colored lines to indicate positivity. The color intensity in the test region will vary depending on the amount of IgM and IgG present in the sample. The red internal control line will appear as gold-conjugated chicken IgY binds to the anti-chicken IgY in the control region.26 The results were read visually 10 mins after test initiation, and for a valid result, the internal control line must be present. In this study, the device test results were interpreted as either “positive” (+) or “negative” (–) since the Easy Check was strictly authorized as a qualitative test where the appearance of any IgM or IgG line indicates positivity.
Figure 1.
Design schematic of the Truvian Easy Check COVID-19 IgM/IgG test device. A, The device is an implementation of immunochromatography that relies on capillary flow of sample across immobilized anti-human IgM, anti-human IgG, and control (anti-chicken IgY) antibodies (Ab). Detection is achieved by secondary binding via gold-conjugated antigens (Ag). B, Macro design of the test device. Figure used with permission from Truvian.
Precision Studies
A PCR-confirmed, COVID-19-positive heparinized plasma sample that showed positive IgM and IgG lines was used as a positive quality control (QC) specimen. Similarly, a heparinized plasma sample from a healthy volunteer that showed negative IgM and IgG lines was used as a negative QC sample. Both positive and negative QC samples were run (n = 8) during a period of 10 consecutive days to assess for between-day precision according to the manufacturer’s instructions.
Cross-Reactivity Studies
In total, 25 samples from patients with PCR-positive, non-SARS-CoV-2 respiratory infections (via the BioFire FilmArray Respiratory Panel 2), 5 HIV-positive samples, 5 hepatitis B surface antigen Ab-positive samples, and 5 hepatitis C virus Ab-positive samples were assayed for anti-SARS-CoV-2 IgM and/or IgG reactivity using the Easy Check device. Additionally, these 40 samples were confirmed to be negative for SARS-CoV-2 by real-time RT-PCR (Roche cobas).
Interference Studies
Positive and Negative Plasma Samples
A heparinized COVID-19-positive patient’s plasma sample with both positive IgM and IgG and a heparinized COVID-19-negative volunteer’s plasma sample with no IgM and IgG lines were used for spiking with the following interferents: biotin (0-12,000 ng/mL); bilirubin (0-70 mg/dL); hemoglobin (0-1,890 mg/dL); and triglycerides (0-3,845 mg/dL), respectively.
Preparation of Stock Solutions
Six mg of biotin (Sigma-Aldrich) was dissolved in 10 mL of deionized water and further diluted 10-fold to yield a stock biotin solution of concentration of 60,000 ng/mL. A hemolysate stock solution was prepared from 5 mL of type O-negative RBC that was centrifuged at 3,000 rpm to pack the cells, resuspended with 5 mL of isotonic saline, and subsequently washed three times. After the final centrifugation and removal of the isotonic saline, 2 mL of deionized water was added, vortexed vigorously to lyse the RBCs, and stored frozen overnight at −80°C. The stock hemolysate solution was thawed the following day and the total hemoglobin concentration was measured on a GEM5000 (Instrumentation Laboratory) co-oximeter to be 18,900 mg/dL. An amount of 6 mg of conjugated bilirubin (Sigma-Aldrich) was dissolved in 1 mL of deionized water, then diluted 10-fold with deionized water; the concentration of total bilirubin was determined on the Cobas 702 analyzer (Roche Diagnostics) to be 350 mg/dL. An Intralipid solution (20% emulsion, Fresenius Kabi) was used as the stock lipid solution and the triglycerides concentration was determined to be 18,900 mg/dL using a 10-fold isotonic saline-diluted sample measured on the Cobas 702 analyzer (Roche Diagnostics).
Preparation of Interference Sets
For hemolysis and lipemia sets, 2 µL and 5 µL of each respective stock solution was added to both the positive and negative plasma samples to make up to a total volume of 50 µL. For biotin and bilirubin sets, 5 µL and 10 µL of each respective stock solution was used to make up to a total volume of 50 µL. The positive and negative plasma samples were considered to be the baseline sample without interferents present.
Method Comparison Studies
A total of 99 heparinized plasma samples from PCR-positive COVID-19 patients and 41 prepandemic plasma samples, obtained from a subset of 56 volunteers of a 2015 reference range study, were used for the comparison study between Easy Check and the Roche Elecsys anti-SARS-CoV-2 total antibody assay.
Clinical Performance Studies
A total of 56 unique heparinized plasma samples from 56 volunteers from a 2015 reference range study were run on the Easy Check device and used to calculate the clinical specificity of the device. A total of 99 unique COVID-19 heparinized plasma samples from 99 patients were assessed on the Easy Check device for calculation of clinical sensitivities at 0 to 6 days (n = 22); 7 to 13 days (n = 18) and 14 days or after (n = 59) post-PCR positivity. Calculations of sensitivity, specificity, positive predictive, and negative predictive values were performed, assuming a 5%, 10%, or 20% prevalence, using the MedCalc statistical software (https://www.medcalc.org/calc/diagnostic_test.php).
Results
Precision Studies
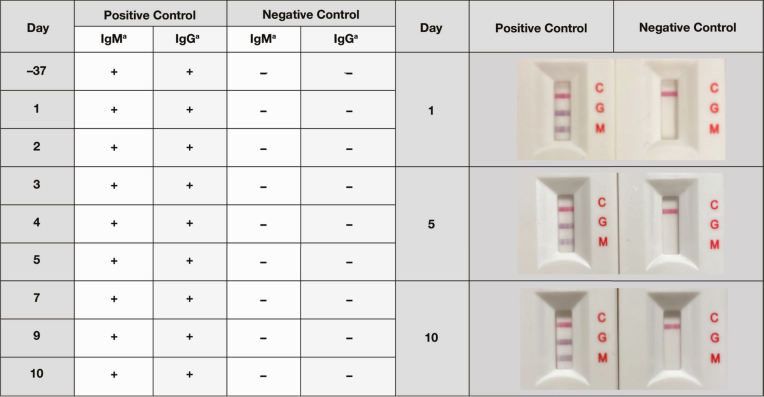
A between-day precision analysis of the Easy Check device was performed by serial testing of a positive control sample and negative control sample. The Easy Check device performed reproducibly well by showing 100% consistency in all days tested for both control samples Figure 2.
Figure 2.
Between-run precision analysis of the Truvian Easy Check test. The same positive and negative banked control samples were tested serially by the Easy Check test. The results of an initial test performed on the control samples 37 days prior to testing conducted during a 10-day period (n = 8) is included. aThe presence of a line indicates positive reactivity and is denoted by “+,” whereas nonreactivity is denoted by “–.”
Specificity and Cross-Reactivity
The specificity of the Easy Check device was further assessed using a total of 40 samples derived from patients infected with hepatitis B, hepatitis C, HIV, or common, non-SARS-CoV-2 respiratory viruses and confirmed by either antibody or PCR positivity. Zero cross-reactivity was observed as the Easy Check test showed negativity in both IgM and IgG reactivity for all 40 samples, which is also in complete agreement with independent testing performed using the automated Roche Elecsys anti‑SARS‑CoV‑2 test system Table 1. The Easy Check device is designed to qualitatively detect and distinguish both anti-SARS‑CoV‑2 IgM and IgG antibodies, whereas the Roche Elecsys system qualitatively detects total anti-SARS‑CoV‑2 antibodies (IgG, IgM, and IgA) in relation to a cutoff index (COI).
Table 1.
Cross-Reactivity of the Truvian Easy Check Test Against Other Common Virusesa
| Sera Sample | Truvian IgM Reactivity | Truvian IgG Reactivity | Roche Elecsys | |||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | COI or Mean COI | Resultb | |
| OC43 CVc | 0 | 8 | 0 | 8 | 0.097 | Negative |
| 229E CVc | 0 | 2 | 0 | 2 | 0.095 | Negative |
| OC43 CV + 229E CVc | 0 | 1 | 0 | 1 | 0.108 | Negative |
| NL63 CVc | 0 | 7 | 0 | 7 | 0.091 | Negative |
| HKU1 CVc | 0 | 4 | 0 | 4 | 0.093 | Negative |
| HKU1 CV + RSVc | 0 | 1 | 0 | 1 | 0.101 | Negative |
| Rhinovirusc | 0 | 2 | 0 | 2 | 0.151 | Negative |
| HepBSd | 0 | 5 | 0 | 5 | 0.090 | Negative |
| HCVd | 0 | 5 | 0 | 5 | 0.088 | Negative |
| HIVd | 0 | 5 | 0 | 5 | 0.086 | Negative |
| Total | 0 | 40 | 0 | 40 | 0.095 |
COI, cutoff index ; CV, coronavirus; HCV, hepatitis C virus; HepBS, hepatitis B surface antigen; PCR, polymerase chain reaction; RSV, respiratory syncytial virus.
aZero cross-reactivity was observed with 40 samples from patients infected with hepatitis B, hepatitis C, HIV, or a common, non-SARS-CoV-2 respiratory virus and confirmed by either antibody or PCR positivity. OC43, 229E, NL63, and HKU1 are common strains of other CVs.
bNegative result is defined as both individual and mean COIs < 1.0.
cPCR-positive.
dAntibody-positive.
Interference Studies
The performance and robustness of the Easy Check device were evaluated in the presence of common potential interferents such as biotin, bilirubin, hemoglobin, and triglycerides Table 2. Biotin interference was assessed due to the biotin-based anti-IgM test line design. Two representative samples (one as a positive control for both IgM and IgG and another as a negative control) were tested with increasing concentrations of each interferent. Both positive and negative readings were unaffected by up to 12,000 ng/mL of biotin, 70 mg/dL of bilirubin, 1,890 mg/dL of hemoglobin, or 3,845 mg/dL of triglycerides, thereby demonstrating that the performance of the Easy Check device remains unaffected by very high concentrations of circulating biotin or by interference from hyperbilirubinemia, hemolysis, or lipemia, respectively.
Table 2.
Interference Analysis of the Truvian Easy Check Testa
| Interferent | Positive Control | Negative Control | ||
|---|---|---|---|---|
| IgMb | IgGb | IgMb | IgGb | |
| Biotin (ng/mL) | ||||
| 0 | + | + | – | – |
| 6,000 | + | + | – | – |
| 12,000 | + | + | – | – |
| Bilirubin (mg/dL) | ||||
| 0 | + | + | – | – |
| 35 | + | + | – | – |
| 70 | + | + | – | – |
| Hemoglobin (mg/dL) | ||||
| 0 | + | + | – | – |
| 756 | + | + | – | – |
| 1,890 | + | + | – | – |
| Triglycerides (mg/dL) | ||||
| 0 | + | + | – | – |
| 1,538 | + | + | – | – |
| 3,845 | + | + | – | – |
aPositive and negative control samples were tested following introduction of increasing interferent.
bThe presence of a line indicates positive reactivity and is denoted by “+,” whereas nonreactivity is denoted by “–.”
Comparisons With the Roche Elecsys Antibody Assay
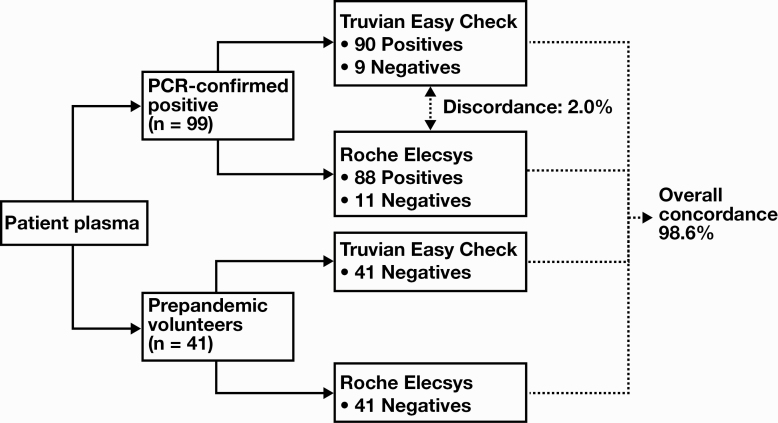
The performance of the Easy Check device showed high concordance to that of the Roche Elecsys system Figure 3. Of 99 patient samples that were positively confirmed by PCR for SARS-CoV-2, antibodies against the virus were detected by both tests in 88 of the samples, whereas 9 of the 99 samples eluded detection by both testing modalities. In the two cases where there was discordance between the two serologic assays, both samples tested positive with the Easy Check device but tested negative with the Roche Elecsys assay with measured COI values below the threshold of 1.0. Of note, the Easy Check device only detected a faint IgG line for the sample with a COI = 0.126 measured by the Roche Elecsys assay, whereas for the sample with a COI = 0.849 measured by the Roche Elecsys assay, the Easy Check device detected both faint IgM and IgG lines. However, since the Roche Elecsys detects total anti-SARS‑CoV‑2 antibodies, it is not possible to determine the relative contribution of each immunoglobulin class to the measured COI values and thereby make direct comparisons with the Easy Check results.
Figure 3.
Comparison of the Truvian Easy Check and Roche Elecsys anti-SARS-CoV-2 antibody assays. A total of 140 patient samples (99 COVID-19-positive, PCR-confirmed samples and 41 prepandemic volunteer samples) were tested using both the Easy Check and Roche Elecsys assays, yielding an overall concordance rate of 98.6% between the two serologic tests. Among the 99 COVID-19-positive, PCR-confirmed samples, there were 2 samples for which the Easy Check assay resulted as positive, whereas the Roche Elecsys assay resulted as negative.
Against a 2015 prepandemic sample subset, which by definition were negative for SARS-CoV-2, the Easy Check device also compared well to that of the Roche Elecsys system. All 41 prepandemic samples tested negative with both the Easy Check device and the Roche Elecsys system. Therefore, across all 140 samples with which both assay performances were compared, the overall concordance rate was 98.6% (Figure 3).
Fingerstick vs Serum Samples
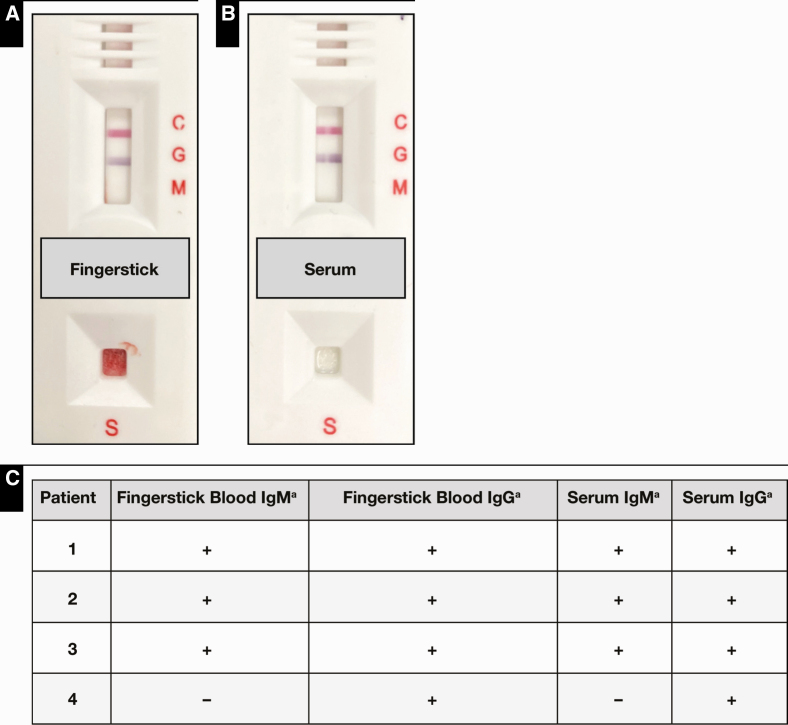
Potential differences in the Easy Check readings due to the use of capillary fingerstick vs serum samples were assessed using paired samples obtained from four patients Figure 4. Although there were between-patient variations in the IgM and IgG line intensities, the test results were interpreted qualitatively as either positive or negative per the FDA EUA of the device. Complete concordance between each of the four pairs of patient samples was observed. These data suggest that fingerstick blood and serum samples may be equivalent and interchangeable when used on the Easy Check and warrants further investigation given its potential use as a point-of-care device.
Figure 4.
Truvian Easy Check testing using fingerstick blood vs serum samples. Fingerstick blood (A) and serum (B) samples obtained from the same patient who tested positive for SARS-CoV-2 by polymerase chain reaction yields comparable results by Easy Check testing. C, Comparability of fingerstick capillary blood and serum positive samples from four COVID-19 patients. aThe presence of a line indicates positive reactivity and is denoted by “+,” whereas nonreactivity is denoted by “–.”
Clinical Performance of the Easy Check Device
The clinical performance of the Easy Check device was evaluated using a total of 155 clinical samples collected at the University of Chicago Medical Center, consisting of 99 PCR-confirmed, SARS-CoV-2-positive patient samples from 2020 and 56 prepandemic samples, collected from healthy volunteers for a 2015 reference range study. All test results were read after a 10-minute incubation period, consistent with manufacturer specifications. Overall, the device showed excellent performance, and was most sensitive at 96.61% (95% CI of 88.29%-99.59%) when used to test samples derived from patients at 14 or more days since initial PCR positivity. The sensitivity of the Easy Check device for anti-SARS-CoV-2 IgM or IgG in patient samples obtained between 0 to 6 days and between 7 to 13 days since initial PCR positivity were 77.27% (95% CI of 54.63%-92.18%) and 88.89% (95% CI of 65.29%-98.62%), respectively. The specificity of the device was 98.21% (95% CI of 90.45%-99.95%). Assuming a 5.0% prevalence, in accordance with FDA review for EUA,27 the positive and negative predictive values of the Easy Check test were between 69% to 74% and approximately 99%, respectively, with an associated overall accuracy of 97% to 98% Table 3.
Table 3.
Clinical Performance of the Truvian Easy Check COVID-19 IgM/IgG Test Devicea
| Test | Prepandemic | PCR-Positive | |||||
|---|---|---|---|---|---|---|---|
| 0-6 d | 7-13 d | ≥14 d | |||||
| No Disease | Disease | Disease | Disease | ||||
| Truvian Ab positive | 1 | 17 | 16 | 57 | |||
| Truvian Ab negative | 55 | 5 | 2 | 2 | |||
| Total | 56 | 22 | 18 | 59 | |||
| Statistic | Value | (95% CI) | Value | (95% CI) | Value | (95% CI) | |
| Sensitivity (%) | 77.27 | (54.63-92.18) | 88.89 | (65.29-98.62) | 96.61 | (88.29-99.59) | |
| Specificity (%) | 98.21 | (90.45-99.95) | 98.21 | (90.45-99.95) | 98.21 | (90.45-99.95) | |
| Disease prevalence (%) | 5.0 | 5.0 | 5.0 | ||||
| Positive predictive value (%) | 69.49 | (24.37-94.15) | 72.37 | (27.17-94.85) | 74.01 | (28.98-95.21) | |
| Negative predictive value (%) | 98.80 | (97.43-99.44) | 99.41 | (97.85-99.84) | 99.82 | (99.30-99.95) | |
| Accuracy (%) | 97.17 | (90.64-99.60) | 97.75 | (91.26-99.80) | 98.13 | (93.67-99.75) |
Ab, antibody; CI, confidence interval; PCR, polymerase chain reaction.
aA total of 155 clinical samples consisting of 99 PCR-confirmed, SARS-CoV-2-positive patient sera samples from 2020 and 56 prepandemic patient sera samples from 2015 were used to evaluate the point-of-care testing performance of the Easy Check device.
Discussion
The COVID-19 global pandemic caused by the SARS-CoV-2 virus remains a pressing challenge that is still afflicting many parts of the world more than half a year since its emergence from Wuhan, China, at the end of 2019.21,28-31 In countries still experiencing rapid spread of the virus, accurate and timely information on populational immunity and seroprevalence remains sorely lacking but is much needed to better guide public health policies and to thereby curb continued spread of the virus.21,32 Although the increasing availability of serologic assays will help address this paucity of information, useful tests need to be accurate, reliable, and both readily available and accessible for effective widespread application.33
In this study, we analytically and clinically evaluated the Truvian Easy Check COVID-19 IgM/IgG test device, which recently received EUA by the FDA to detect SARS-CoV-2 seroconversion27 on July 24, 2020. We also showed that fingerstick blood likely gives identical results as serum collected in parallel via venipuncture in a small pilot study, which will further expand the applicability of the Easy Check upon further evaluation with a large sample size study and regulatory approval for that sample type. Furthermore, the test device itself is simple, compact, and easily deployable in many clinical settings with the potential of remote applications and at point-of-care services. Once initiated with a 10 μL sample, Easy Check results are available after a 10-minute incubation period, which differs from many conventional serologic assays for COVID-19 testing that require advanced instrumentation and greater sample volumes, such as the automated Roche Elecsys platform.
Overall, the Easy Check device showed excellent performance by all standard measures and compares well with other serologic tests authorized by EUA by the FDA.27 A 5% disease prevalence was assumed in our positive predictive value, negative predictive value, and overall accuracy calculations; however, presently the true prevalence of COVID-19 remains unknown. Therefore, for comparison, if the disease prevalence is instead 10% or 20%, the negative predictive value and overall accuracy of the Easy Check test remains at greater than 99% and 98%, respectively, whereas the positive predictive value increases dramatically to 86% and 93%, respectively Table 4. Furthermore, in anticipation that the performance of the device may ultimately differ when applied to a general population vs in a controlled laboratory setting due to possibly greater variations in patient immune status and antibody levels, our study specifically compared the Easy Check device performance with that of the automated Roche Elecsys antibody assay, which is now in widespread use in many medical centers despite having received EUA relatively recently (on May 2, 2020) as well.
Table 4.
Effect of Prevalence on Positive Predictive Value (PPV), Negative Predictive Value (NPV), and Overall Accuracy
| Prevalence (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|
| 5 | 74.01a | 99.82 | 98.13 |
| 10 | 85.74 | 99.62 | 98.05 |
| 20 | 93.12 | 99.14 | 97.89 |
aAt 5% prevalence, if the sensitivity is 100%, the PPV increases to only 75%.
Calculations assume a sensitivity of 96.61% and a specificity of 98.21%.
The Easy Check showed high concordance with the Roche Elecsys test for both COVID-19 positive and negative samples with an overall agreement of 98.6%. In fact, for samples derived from patients at 14 or more days since initial PCR positivity (consisting of 57 seropositive and 2 seronegative cases out of a total of 59 PCR-confirmed COVID-19 samples tested by both antibody assays), the two antibody tests are 100% concordant, which is remarkable when considering the apparent simplicity and compactness of the Easy Check device when compared with the more intricate Roche Elecsys system. Medical record review of the patients from which the two PCR-positive SARS-CoV-2 samples that tested negative by both Easy Check and the Roche Elecsys revealed that both patients were immunocompromised. The 2% discordance between the Easy Check and the Roche Elecsys (Figure 3) resulted from two PCR-positive samples obtained from patients at 7 to 13 days post initial PCR positivity that tested positive with the Easy Check and negative with the Roche Elecsys, which likely reflects differences in the antigen choice and/or cutoffs of the two detection systems. In particular, the Easy Check detects and distinguishes IgM and IgG anti-SARS‑CoV‑2 antibodies, whereas the Roche Elecsys detects total anti-SARS‑CoV‑2 antibodies, which limits direct comparison of their performance characteristics to some degree. Additionally, the Easy Check showed high specificity in detecting antibodies elicited by the SARS-CoV-2 virus, suggesting that false-positive results arising from other viral infection is highly unlikely. The device was also largely unaffected by common interferents (in settings of bilirubin, lipemia, and hemolysis)–an important characteristic, especially when these interferents would not be easily visualized when whole blood sample is used.
The Easy Check device is presently authorized for use in moderately or highly complex laboratory settings via FDA EUA; however, its potential application to other clinical settings will be particularly significant in scenarios where more advanced testing may otherwise be limited. Although careful planning and training for proper use, such as ensuring the device results are read between 10 and 15 minutes post test initiation, will certainly be needed for deploying it as a point-of-care testing (POCT) device meaningfully in any mail-dependent or home testing scenarios,34 their availability will inevitably allow for more comprehensive and large-scale epidemiologic examination of the dynamics of SARS-CoV-2 transmission. Our results suggest that the robustness of the Easy Check device has the potential to fill this critical and unmet gap in POCT for SARS-CoV-2 seroconversion. However, availability as a POCT device will require Truvian to file for FDA approval for this indication, ideally as a waived test device using capillary fingerstick whole blood.
On a global scale, potential widespread deployment of Easy Check would provide an additional means to help elucidate the seroprevalence of SARS-CoV-2 in different communities and countries as we confront the global pandemic, ideally with concerted efforts. Due to the shortages of reagents and supplies, access to PCR-based, direct SARS-CoV-2 testing has often been significantly restricted—thus availability of COVID-19 antibody devices such as the Easy Check is an attractive option to assess populational exposure history to SARS-CoV-2, especially for geographic regions that are more economically challenged and cannot afford expensive analyzer systems. In contrast, self-contained and portable devices like the Easy Check, packaged in a complete kit with minimal reagents that do not require refrigeration and can be stored at room temperature, can become a valuable tool in determining the incidence of infection, thereby elucidating better estimates of disease prevalence and, by extension, mortality rates in these scenarios. Such valuable data will inform and direct public health resources to mitigate and contain the spread of infection. As serologic testing options become increasingly available, numerous potential future applications have also been identified in addition to their clear value in seroprevalence studies for public health planning and responses, which include identification of convalescent plasma donors, exposure history assessment of essential workers and schoolchildren, and assessment of vaccine immunogenicity.2 The Easy Check, in particular, adds to our arsenal of tools for tackling these endeavors in the form of an easy-to-use, fast, and reliable antibody testing device.
Acknowledgment
The authors thank Truvian for providing grant support for this project.
Dr Yeo is a member of the Scientific Advisory Board of Truvian and has received honoraria and equities from Truvian.
References
- 1. Tromberg BJ, Schwetz TA, Pérez-Stable EJ, et al. Rapid scaling up of Covid-19 diagnostic testing in the United States - the NIH RADx initiative. N Engl J Med. 2020;383:1071-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng MP, Papenburg J, Desjardins M, et al. Diagnostic testing for severe acute respiratory syndrome-related coronavirus 2: a narrative review. Ann Intern Med. 2020;172:726-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kohmer N, Westhaus S, Rühl C, et al. Brief clinical evaluation of six high-throughput SARS-CoV-2 IgG antibody assays. J Clin Virol. 2020;129:104480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jääskeläinen AJ, Kuivanen S, Kekäläinen E, et al. Performance of six SARS-CoV-2 immunoassays in comparison with microneutralisation. J Clin Virol. 2020;129:104512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deeks JJ, Dinnes J, Takwoingi Y, et al. ; Cochrane COVID-19 Diagnostic Test Accuracy Group Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6:CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. FAQs on testing for SARS-CoV-2. 2020. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/faqs-testing-sars-cov-2#nolonger-ivd. Updated October 8, 2020. Accessed October 10, 2020. [Google Scholar]
- 7. Coronavirus (COVID-19) update: FDA issues new policy to help expedite availability of diagnostics [press release]. FDA; https://www.fda.gov. Published February 29, 2020. [Google Scholar]
- 8. Coronavirus (COVID-19) update: FDA provides more regulatory relief during outbreak, continues to help expedite availability of diagnostics [press release]. FDA; https://www.fda.gov. Published March 16, 2020. [Google Scholar]
- 9. Policy for Coronavirus disease-2019 tests during the public health emergency (revised) [press release]. FDA; https://www.fda.gov. Published May 11, 2020. [Google Scholar]
- 10. D’Cruz RJ, Currier AW, Sampson VB. Laboratory testing methods for novel severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). Front Cell Dev Biol. 2020;8:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jin Y, Wang M, Zuo Z, et al. Diagnostic value and dynamic variance of serum antibody in coronavirus disease 2019. Int J Infect Dis. 2020;94:49-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xiang F, Wang X, He X, et al. Antibody detection and dynamic characteristics in patients with coronavirus disease 2019. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845-848. [DOI] [PubMed] [Google Scholar]
- 15. Lou B, Li TD, Zheng SF, et al. Serology characteristics of SARS-CoV-2 infection since exposure and post symptom onset. Eur Respir J. 2020;56:2000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465-469. [DOI] [PubMed] [Google Scholar]
- 18. To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kubina R, Dziedzic A. Molecular and serological tests for COVID-19 a comparative review of SARS-CoV-2 coronavirus laboratory and point-of-care diagnostics. Diagnostics (Basel). 2020;10:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. 2020. https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2768834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown TS, Walensky RP. Serosurveillance and the COVID-19 epidemic in the US: undetected, uncertain, and out of control. Jama. 2020;324:749-751. [DOI] [PubMed] [Google Scholar]
- 22. Rosenberg ES, Tesoriero JM, Rosenthal EM, et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol. 2020;48:23-29.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weitz JS, Beckett SJ, Coenen AR, et al. Modeling shield immunity to reduce COVID-19 epidemic spread. Nat Med. 2020;26:849-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Favresse J, Eucher C, Elsen M, et al. Clinical performance of the elecsys electrochemiluminescent immunoassay for the detection of SARS-CoV-2 total antibodies. Clin Chem. 2020;66:1104-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang MS, Hock KG, Logsdon NM, et al. Clinical performance of the Roche SARS-CoV-2 serologic assay. Clin Chem. 2020;66:1107-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Truvian easy Check™ COVID-19 IgM/IgG device. 2020. https://truvianhealth.com/easycheck. Accessed October 10, 2020. [Google Scholar]
- 27. EUA authorized serology test performance. 2020. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance. Accessed October 10, 2020. [Google Scholar]
- 28. Chan JF, Kok KH, Zhu Z, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu N, Zhang D, Wang W, et al. ; China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peak CM, Kahn R, Grad YH, et al. Individual quarantine versus active monitoring of contacts for the mitigation of COVID-19: a modelling study. Lancet Infect Dis. 2020;20:1025-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Younes N, Al-Sadeq DW, Al-Jighefee H, et al. Challenges in laboratory diagnosis of the novel coronavirus SARS-CoV-2. Viruses. 2020;12:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pallett SJC, Rayment M, Patel A, et al. Point-of-care serological assays for delayed SARS-CoV-2 case identification among health-care workers in the UK: a prospective multicentre cohort study. Lancet Respir Med. 2020;8:885-894. [DOI] [PMC free article] [PubMed] [Google Scholar]