Abstract
Background
The efficacy of convalescent plasma (CP) for the treatment of coronavirus disease 2019 (COVID-19) remains unclear.
Methods
In a matched cohort analysis of hospitalized patients with severe COVID-19, the impact of CP treatment on in-hospital mortality was evaluated using univariate and multivariate Cox proportional-hazards models, and the impact of CP treatment on time to hospital discharge was assessed using a stratified log-rank analysis.
Results
In total, 64 patients who received CP a median of 7 days after symptom onset were compared to a matched control group of 177 patients. The incidence of in-hospital mortality was 12.5% and 15.8% in the CP and control groups, respectively (P = .52). There was no significant difference in the risk of in-hospital mortality between the 2 groups (adjusted hazard ratio [aHR] 0.93, 95% confidence interval [CI] .39–2.20). The overall rate of hospital discharge was not significantly different between the 2 groups (rate ratio [RR] 1.28, 95% CI .91–1.81), although there was a significantly increased rate of hospital discharge among patients 65-years-old or greater who received CP (RR 1.86, 95% CI 1.03–3.36). There was a greater than expected frequency of transfusion reactions in the CP group (2.8% reaction rate observed per unit transfused).
Conclusions
We did not demonstrate a significant difference in risk of mortality or rate of hospital discharge between the CP and control groups. There was a signal for improved outcomes among the elderly, and further adequately powered randomized studies should target this subgroup when assessing the efficacy of CP treatment.
Keywords: convalescent plasma, COVID-19, SARS-CoV-2, efficacy
Treatment of severe COVID-19 with convalescent plasma was not associated with a significantly different risk of in-hospital mortality or rate of hospital discharge as compared to standard care. Elderly patients may be most likely to benefit from convalescent plasma.
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to a global pandemic with millions of infections reported across over 200 countries less than 6 months after the first case was reported [1–3]. Many therapeutic agents are being evaluated, some of which are already in clinical use despite varying levels of evidence to support their efficacy [4]. One such widely used agent is convalescent plasma (CP), the transfusion of plasma collected from individuals who have recovered from coronavirus disease 2019 (COVID-19) to currently infected patients, in an attempt to provide some degree of passive humoral immunity to the recipient via the transfer of antibodies directed against SARS-CoV-2 [5]. This approach has been used to treat infections for centuries, and more recent experiences with CP for other emerging viral infections suggest that CP may also be an effective therapy for SARS-CoV-2 [6, 7].
Clinical evidence describing the efficacy of CP for patients with COVID-19 remains limited. Early clinical reports from China were encouraging [8–11], although another report suggests limited efficacy when used late in the course of disease [12]. Multiple randomized clinical trials are ongoing [13]. A report of early safety data from 20 000 patients given CP through a large expanded access protocol is reassuring [14].
This study describes the clinical outcomes of a cohort of hospitalized patients with severe COVID-19 who received CP. Notably, the study is one of the first to analyze the clinical outcomes of a large group of patients who received CP in comparison to a closely matched group receiving standard of care treatment.
METHODS
Study Setting and Data Collection
We studied adult patients admitted to three hospitals within the Lifespan health system, Rhode Island Hospital (RIH) and The Miriam Hospital, both in Providence, Rhode Island, USA, and Newport Hospital, in Newport, Rhode Island, USA. This matched cohort study was an electronic chart review approved by the Institutional Review Board of RIH. All data were extracted from the electronic health record.
CP Characteristics and Use
All patients who received CP at our institution did so through the expanded access protocol [15]. Due to limitations in locally available serologic testing at the time, CP was given to patients prior to knowing the SARS-CoV-2 antibody content. Instead, SARS-CoV-2 antibody content was assessed retrospectively on thawed segments (if available) using the Abbott Architect SARS-CoV-2 immunoglobulin G (IgG) assay (Abbott, Abbott Park, IL, USA). All patients were prescribed 2 units of plasma, and patients were included even if they only received 1 of the units.
In addition to the broad eligibility requirements set out in the CP expanded access protocol [16], patients were eligible to receive CP treatment if they also fulfilled the following local inclusion criteria: (1) symptom onset ≤10 days prior, (2) requiring supplemental oxygen (but not invasive ventilation), (3) no evidence of current hypercoagulability (D-dimer > 1000 µg/L, clinical signs of thrombosis).
Control Group Patient Selection
All adult patients with a positive molecular test for COVID-19 admitted to the hospital prior to 31 May 2020 who did not receive CP were reviewed for potential inclusion in the control group. To capture a similar case mix to those patients eligible for CP (see above), additional inclusion criteria for the control group included the following: (1) symptom onset ≤10 days prior to admission, (2) hospital admission ≥48 hours, (3) required supplemental oxygen (but not invasive ventilation) within 48 hours of hospitalization, (4) D-dimer obtained within 48 hours of hospitalization and < 1000 µg/L.
CP Group Patient Selection
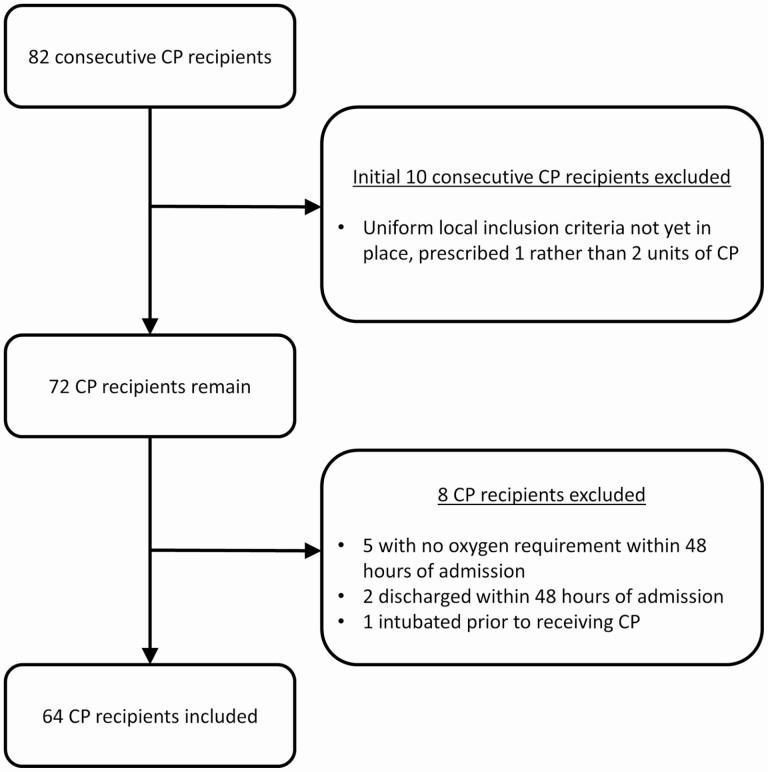
All patients who received CP prior to 31 May 2020 were considered for potential inclusion in the CP group. Of note, our local practice regarding CP use (including implementation of our local inclusion criteria and increasing the dose of CP from 1 to 2 units) changed quickly after we first began using CP. The initial 10 patients given CP before this change were not included in this analysis. Several other patients who received CP were also excluded from the analysis after applying the control group inclusion criteria to the CP group. The decision to exclude these CP recipients was an effort to preserve uniformity within the CP group and between the CP and control groups and was made prior to any further data analysis.
Study Outcomes
The primary outcome of this study was the impact of CP treatment on all cause in-hospital mortality; the secondary outcome was the impact of CP treatment on the time to hospital discharge. All outcomes were censored at day 28.
Statistical Analysis
We compared patients’ characteristics between the 2 groups using Pearson χ 2 test for categorical variables, and Student t test or Mann-Whitney-Wilcoxon test for continuous variable as means (with standard deviation [SD]) or medians (with interquartile range [IQR]), respectively. All analyses were performed using Stata v15.1 (Stata Corporation, College Station, TX, USA). The statistically significant level was set at .05.
To evaluate the impact of CP treatment on all cause in-hospital mortality, we utilized both univariate and multivariate Cox proportional-hazards models. To evaluate the impact of CP treatment on time to hospital discharge, we used a stratified log-rank test and calculated the Mantel-Cox rate ratios. We also performed a subgroup analysis of in-hospital mortality and time to hospital discharge based on the antibody content of the CP units.
RESULTS
Study Population and CP Use
Of the 82 consecutive patients who received CP during the study period, 64 were included in the analysis. Excluded patients either received CP prior to the implementation of our local inclusion criteria or (in retrospect) did not meet the structured inclusion criteria for the matched control group (Figure 1). Three patients included in the CP group received only 1 unit of CP (1 withdrew due to clinical improvement and hospital discharge, 2 had transfusion-related acute lung injury [TRALI] reactions associated with the first unit). The remainder of the patients received 2 units of CP. The control group included 177 patients who did not receive CP (Table 1).
Figure 1.
Flowchart for study inclusion of patients receiving CP. Abbreviation: CP, convalescent plasma.
Table 1.
Patient Characteristics
| Total (N = 241) | Convalescent Plasma (N = 64) | Control (N = 177) | P value | |
|---|---|---|---|---|
| Median age (IQR), y | 61 (48–73) | 61 (47–70) | 61 (50–75) | .17 |
| Sex | .57 | |||
| Female | 109 (45.2%) | 27 (42.2%) | 82 (46.3%) | |
| Male | 132 (54.8%) | 37 (57.8%) | 95 (53.7%) | |
| Race/ethnicity | .28 | |||
| Black or African American | 27 (11.2%) | 9 (14.1%) | 18 (10.2%) | |
| Hispanic or Latino | 89 (36.9%) | 27 (42.2%) | 62 (35.0%) | |
| Other/unknown | 25 (10.4%) | 8 (12.5%) | 17 (9.6%) | |
| White or Caucasian | 100 (41.5%) | 20 (31.3%) | 80 (45.2%) | |
| Congestive heart failure | 33 (13.7%) | 11 (17.2%) | 22 (12.4%) | .34 |
| Cardiac arrhythmias | 42 (17.4%) | 8 (12.5%) | 34 (19.2%) | .23 |
| Valvular disease | 8 (3.3%) | 2 (3.1%) | 6 (3.4%) | .92 |
| Pulmonary circulation disorders | 4 (1.7%) | 2 (3.1%) | 2 (1.1%) | .28 |
| Peripheral vascular disorders | 15 (6.2%) | 3 (4.7%) | 12 (6.8%) | .55 |
| Hypertension | 98 (40.7%) | 22 (34.4%) | 76 (42.9%) | .23 |
| Paralysis | 2 (0.8%) | 0 (0.0%) | 2 (1.1%) | .39 |
| Other neurological disorders | 29 (12.0%) | 6 (9.4%) | 23 (13.0%) | .45 |
| Chronic pulmonary disease | 36 (14.9%) | 8 (12.5%) | 28 (15.8%) | .52 |
| Diabetes | 57 (23.7%) | 16 (25.0%) | 41 (23.2%) | .77 |
| Hypothyroidism | 15 (6.2%) | 2 (3.1%) | 13 (7.3%) | .23 |
| Renal failure | 26 (10.8%) | 11 (17.2%) | 15 (8.5%) | .054 |
| Liver disease | 3 (1.2%) | 2 (3.1%) | 1 (0.6%) | .11 |
| Peptic ulcer disease excluding bleeding | 2 (0.8%) | 0 (0.0%) | 2 (1.1%) | .39 |
| HIV/AIDS | 3 (1.2%) | 3 (4.7%) | 0 (0.0%) | .004 |
| Lymphoma | 2 (0.8%) | 0 (0.0%) | 2 (1.1%) | .39 |
| Metastatic cancer | 2 (0.8%) | 0 (0.0%) | 2 (1.1%) | .39 |
| Solid tumor without metastasis | 11 (4.6%) | 1 (1.6%) | 10 (5.6%) | .18 |
| Rheumatoid arthritis/collagen vascular disease | 4 (1.7%) | 1 (1.6%) | 3 (1.7%) | .94 |
| Coagulopathy | 18 (7.5%) | 5 (7.8%) | 13 (7.3%) | .90 |
| Obesity | 95 (39.4%) | 26 (40.6%) | 69 (39.0%) | .82 |
| Weight loss | 12 (5.0%) | 2 (3.1%) | 10 (5.6%) | .43 |
| Fluid and electrolyte disorders | 74 (30.7%) | 17 (26.6%) | 57 (32.2%) | .40 |
| Blood loss anemia | 2 (0.8%) | 1 (1.6%) | 1 (0.6%) | .45 |
| Deficiency anemia | 7 (2.9%) | 1 (1.6%) | 6 (3.4%) | .46 |
| Alcohol abuse | 7 (2.9%) | 3 (4.7%) | 4 (2.3%) | .32 |
| Drug abuse | 4 (1.7%) | 2 (3.1%) | 2 (1.1%) | .28 |
| Psychoses | 4 (1.7%) | 1 (1.6%) | 3 (1.7%) | .94 |
| Depression | 28 (11.6%) | 9 (14.1%) | 19 (10.7%) | .48 |
| Weighted Elixhauser score (van Walraven) | .72 | |||
| <0 | 46 (19.1%) | 15 (23.4%) | 31 (17.5%) | |
| 0 | 60 (24.9%) | 15 (23.4%) | 45 (25.4%) | |
| 1–4 | 35 (14.5%) | 10 (15.6%) | 25 (14.1%) | |
| ≥5 | 100 (41.5%) | 24 (37.5%) | 76 (42.9%) | |
| Baseline oxygen requirements | .27 | |||
| Low flow supplemental oxygen | 171 (71.0%) | 42 (65.6%) | 129 (72.9%) | |
| NIPPV or HFNC | 70 (29.0%) | 22 (34.4%) | 48 (27.1%) | |
| Mean D-dimer (SD), μg/L | 360 (192) | 367 (181) | 357 (196) | .74 |
| Mean CRP (SD), mg/L | 112 (76.9) | 125 (78.7) | 106 (75.8) | .096 |
| Mean peripheral lymphocyte % (SD) | 13.8 (7.84) | 13.8 (8.52) | 13.8 (7.60) | .97 |
| Mean creatinine clearance (SD), mL/min | 101 (62.6) | 108 (64.3) | 98.6 (61.9) | .33 |
| Corticosteroid use | 66 (27.4%) | 26 (40.6%) | 40 (22.6%) | .006 |
| Hydroxychloroquine use | 19 (7.9%) | 3 (4.7%) | 16 (9.0%) | .27 |
| Remdesivir use | 77 (32.0%) | 18 (28.1%) | 59 (33.3%) | .44 |
| Admission to ICU | 85 (35.3%) | 27 (42.2%) | 58 (32.8%) | .18 |
| Invasive ventilation | 28 (11.6%) | 7 (10.9%) | 21 (11.9%) | .84 |
| Deceased | 36 (14.9%) | 8 (12.5%) | 28 (15.8%) | .52 |
| Median hospital length of stay (IQR), days | 8 (5–12) | 8 (5–10.5) | 8 (5–13) | .76 |
| Median time from symptom onset to hospital admission (IQR), days | 5 (2–7) | 6 (3–7) | 5 (2–7) | .060 |
| Median time from symptom onset to CP transfusion (IQR), days | 7 (5–9) | 7 (5–9) |
Abbreviations: HFNC, high-flow nasal cannula; HIV, human immunodeficiency virus; ICU, intensive care unit; IQR, interquartile range; NIPPV, noninvasive positive pressure ventilation; SD, standard deviation.
All data are no. (%) unless otherwise specified.
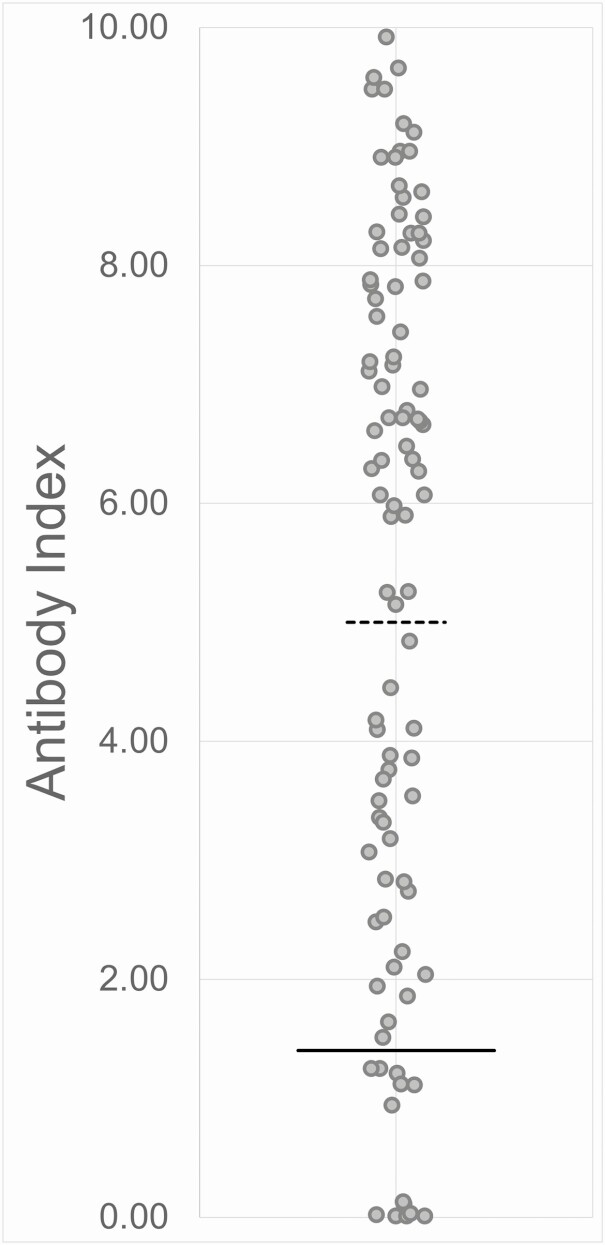
Patients received CP at a median of 7 days after symptom onset. SARS-CoV-2 antibody testing was retrospectively performed on 97 (89.0%) of the 109 CP units that were transfused; 13 (13%) of these units had an antibody index (AI) below the cutoff for a positive result (AI < 1.4) (Figure 2). All patients in the CP group except 3 received at least 1 unit of CP with an AI ≥ 1.4 and 18 patients received 2 units of CP both with AI ≥ 5 (Table 2).
Figure 2.
Distribution of AI values detected in the convalescent plasma units transfused in this study. The red bar indicates the cutoff for a positive assay result (AI = 1.4), and the black bar indicates the cutoff for an arbitrarily high positive result (AI = 5). Abbreviation: AI, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG) antibody index.
Table 2.
Convalescent Plasma Characteristics
| Units of CP transfused | 109 |
| Units of CP with AI results available | 97 |
| AI < 1.4 | 13 (13%) |
| 1.4 ≤ AI < 5 | 27 (28%) |
| AI ≥ 5 | 57 (59%) |
| Patients transfused CP | 64 |
| No units with AI ≥ 1.4 | 3 (4.7%) |
| At least 1 unit with AI ≥ 1.4a | 32 (50%) |
| Two units both with AI ≥ 5.0 | 18 (28%) |
| Missing data | 11 (17%) |
Abbreviations: AI, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG) antibody index; CP, convalescent plasma.
All data are no. (%) unless otherwise specified.
aAt least 1 unit with AI ≥ 1.4 but not 2 units both with AI ≥ 5.
Clinical Presentation and Course
The demographics and summative preexisting comorbidities score of the patients in the CP group and control groups were generally similar, although there were 3 patients with human immunodeficiency virus (HIV)/AIDS in the CP group (all virally suppressed with baseline CD4 > 700) and none in the control group (Table 1).
Both the CP group and control group had a similar percentage of patients who were treated with remdesivir (28.1% vs 33.3%, P = .44), but the CP group had significantly more patients treated with corticosteroids (40.6% vs 22.6%, P = .006). Overall in-hospital mortality in this study was 14.9%, with 35.3% of patients admitted to the intensive care unit, and 11.6% requiring invasive ventilation. There was no significant difference between the groups in any of these categories (P = .52, P = .18, P = .84, respectively).
Clinical Outcomes and Adverse Events
The incidence of in-hospital mortality in the CP and control groups were not significantly different (12.5% vs 15.8%, P = .52) and a multivariate analysis also found no significant difference between the groups (adjusted hazard ratio [HR] 0.93, 95% confidence interval [CI] .39–2.20) (Table 3). In a subgroup analysis examining only those patients who received 2 units of CP with AI ≥ 5, there was a lower risk for in-hospital mortality as compared to the control group, although this difference was also not significant (adjusted HR 0.39, 95% CI .05–3.08).
Table 3.
Multivariate Analysis of the Impact of Convalescent Plasma Treatment on All Cause In-hospital Mortality as Compared to the Control Group
| Adjusted HR (95% CI) | |||
|---|---|---|---|
| All Cause In-hospital Mortality | Unadjusted HR (95% CI) | Model 1a | Model 2b |
| Overall (n = 64) | .73 (.32–1.69) | .91 (.39–2.15) | .93 (.39–2.20) |
| AI ≥ 1.4c (n = 32) | 1.08 (.41–2.80) | 1.09 (.41–2.86) | 1.17 (.43–3.19) |
| AI ≥ 5d (n = 18) | .35 (.05–2.62) | .38 (.05–2.98) | .39 (.05–3.08) |
Abbreviations: AI, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG) antibody index; CI, confidence interval; HR, hazard ratio.
aAdjusted for age, sex, race, and baseline oxygen requirements.
bAdjusted for age, sex, race, baseline oxygen requirements, remdesivir use, and corticosteroid use.
cAt least 1 unit with AI ≥ 1.4 but not 2 units both with AI ≥ 5.
dTwo units both with AI ≥ 5.
The median length of stay in both the CP and control groups was 8 days. There was no significant difference in the overall rate of hospital discharge between the 2 groups using a stratified log-rank test (rate ratio [RR] 1.28, 95% CI .91–1.81) (Table 4). There was also no significant difference in the rate of hospital discharge in subgroup analyses examining only those patients who received 2 units of CP with AI ≥ 5 (RR 1.63, 95% CI .92–2.88) or only those patients who had 5 days of symptoms or fewer prior to hospital admission (RR 1.31, 95% CI .79–2.16).
Table 4.
Stratified Log-rank Analysis of the Impact of Convalescent Plasma Treatment on Time to Hospital Discharge as Compared to the Control Group
| All CP Recipients (n = 64) RR (95% CI) | AI ≥ 1.4a (n = 32) RR (95% CI) | AI ≥ 5b (n = 18) RR (95% CI) | |
|---|---|---|---|
| Overall | 1.28 (.91–1.81) | 1.14 (.72–1.83) | 1.63 (.92–2.88) |
| Stratification subgroups | |||
| Sex | |||
| Female | 1.28 (.75–2.19) | 1.31 (.66–2.60) | 1.21 (.40–3.68) |
| Male | 1.27 (.80–2.00) | 1.00 (.52–1.91) | 1.85 (.94–3.64) |
| Age group, y | |||
| 18–49 | .90 (.48–1.70) | 1.77 (.70–4.48) | .81 (.29–2.29) |
| 50–64 | .82 (.43–1.55) | .80 (.37–1.75) | 1.57 (.25–9.93) |
| ≥ 65 | 1.86 (1.03–3.36) | 1.28 (.58–2.85) | 2.70 (1.16–6.28) |
| Race/ethnicity | |||
| Black or African American | 1.49 (.56–3.93) | 1.19 (.34–4.14) | 3.00 (.67–13.4) |
| Hispanic or Latino | .88 (.51–1.54) | 1.09 (.48–2.51) | .95 (.44–2.05) |
| Other/Unknown | 1.38 (.54–3.51) | 1.75 (.44–6.91) | 1.20 (.14–10.5) |
| White or Caucasian | 1.51 (.82–2.76) | 1.05 (.52–2.14) | 6.67 (1.39–32.1) |
| Baseline oxygen requirements | |||
| Low flow supplemental oxygen | 1.34 (.89–2.03) | 1.15 (.68–1.94) | 2.03 (.90–4.56) |
| NIPPV or HFNC | 1.52 (.78–2.96) | 1.00 (.33–3.02) | 2.36 (.97–5.74) |
| Days from symptom onset to admission | |||
| ≤5 | 1.31 (.79–2.16) | 1.03 (.52–2.04) | 1.82 (.66–5.06) |
| >5 | 1.16 (.71–1.89) | 1.21 (.63–2.35) | 1.30 (.64–2.65) |
| Remdesivir use | |||
| No | 1.20 (.79–1.82) | 1.04 (.60–1.78) | 1.66 (.83–3.33) |
| Yes | 1.41 (.75–2.66) | 1.68 (.63–4.50) | 1.37 (.50–3.77) |
| Corticosteroid use | |||
| No | 1.25 (.81–1.93) | .97 (.52–1.83) | 1.94 (.94–4.01) |
| Yes | 1.66 (.90–3.05) | 1.74 (.82–3.69) | 1.66 (.63–4.37) |
Abbreviations: AI, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG) antibody index; CI, confidence interval; CP, convalescent plasma; HFNC, high-flow nasal cannula; NIPPV, noninvasive positive pressure ventilation; RR, rate ratio.
aAt least 1 unit with AI ≥ 1.4, but not 2 units both with AI ≥ 5.
bTwo units both with AI ≥ 5.
Patients 65-years-old or greater who received CP had an increased rate of hospital discharge as compared to those who did not (RR 1.86, 95% CI 1.03–3.36); this increased rate of hospital discharge was even more pronounced in a subgroup analysis of elderly patients who received 2 units of CP with AI ≥ 5 (RR 2.70, 95% CI 1.16–6.28).
Two patients who received CP were judged to have a TRALI reaction. The first, a previously healthy 37-year-old male, had severe chest pain 45 minutes after starting the CP transfusion with associated tachypnea and worsening hypoxemia; the transfusion was stopped and symptoms resolved several hours later. The second, a 36-year-old male who had recently undergone kidney transplantation, also had acute worsening of tachypnea and hypoxemia associated with chest pain approximately 30 minutes after starting the CP transfusion; the transfusion was stopped and symptoms resolved within 24 hours. Both patients remained afebrile and without elevated brain natriuretic peptide (BNP) during these episodes. Additionally, a 64-year-old female with end-stage renal disease on hemodialysis who received CP was judged to have transfusion-associated circulatory overload (TACO) with acutely worsening hypoxemia approximately 3 hours after transfusion of 2 units of CP. There were no other documented adverse events associated with CP use for the patients included in this study.
DISCUSSION
This matched cohort study examined the use of CP in hospitalized patients with severe COVID-19 and found no significant difference in overall in-hospital mortality or time to hospital discharge as compared to a control group who did not receive CP. The CP group and control groups in this study were drawn from a well-defined population, were similar in baseline characteristics and severity of illness, and were followed long enough to allow for adequate analysis of clinical outcomes. We found a signal for efficacy of CP in elderly patients, a signal for a larger effect of CP with a higher quantity of measured SARS-CoV-2 antibody, and a greater than expected frequency of transfusion reactions.
Importantly, a subgroup analysis examining only patients ≥65-years-old who received CP showed a significantly increased rate of hospital discharge as compared to the control group. An effect specific to this age group is not entirely surprising given the increase in morbidity among the elderly with COVID-19, the waning of humoral immunity with age, and the importance of the humoral compartment of the overall immune response in combating this infection [17–19]. If this increased efficacy of CP in the elderly is redemonstrated in other settings, this may impact the design of clinical trials for SARS-CoV-2 monoclonal antibodies or aid in the triage of limited CP resources.
A subgroup analysis examining only those patients who received 2 units of CP with AI ≥ 5 showed an even larger increase in the rate of hospital discharge among the elderly as compared to the control group. There was also a statistically significant increase in the rate of hospital discharge among White/Caucasian patients of all ages, although this may have been primarily driven by the relative increased age of the White/Caucasian patients in our study as compared patients of other races or ethnicities (data not shown). The semiquantitative description of the amount of SARS-CoV-2 anti-nucleocapsid IgG provided by the assay’s antibody index (AI) that was used in this study has been shown to have similar positivity rate as a recombinant neutralizing assay, although the correlation between neutralizing titers and AI was demonstrated to be poor [20]. In a more recent study using a SARS-CoV-2 neutralizing assay, a different SARS-CoV-2 anti-spike IgG assay was very well correlated with the neutralizing titer [21]. Although we cannot draw any direct conclusions about the neutralizing titers of the CP used in our study, the increase in the rate of hospital discharge across multiple stratifications seen in the subgroup with AI ≥ 5 as compared to the entire CP group aligns well with the expected and recently demonstrated dose-dependent effect of CP [22].
There was a greater than expected frequency of transfusion reactions in the CP group. The observed per unit reaction rate of 3/109 (2.8%) is consistent with the 2.5% reported by Li et al [23] and higher than that reported to our blood banks (<1%, data not shown). We classified 2 cases as TRALI reactions. We are aware that CP is manufactured from never-transfused male donors and never-pregnant female donors, or the CP is tested for anti-HLA antibodies. Hence, neither product (donor) was investigated for anti-HLA antibodies. However, the pathophysiology of type 2 TRALI in hospitalized patients is not related to donor alloantibodies but to other compounds with inflammatory evoking properties in the context of an activated pulmonary endothelium, which clearly pertains to COVID-19 patients [24]. Our elevated frequency of transfusion reactions does not align with data reported from the large safety study associated with the expanded access protocol (TRALI 0.10%, TACO 0.18%, severe allergic transfusion reaction 0.13%) [14]. This difference may highlight the difficulty of accurately detecting transfusion reactions in critically ill patients.
Studying the efficacy of CP in an adequately powered prospective randomized fashion has historically been difficult. Retrospective or nonrandomized assessments of efficacy cannot provide the same quality of evidence, as highlighted by the contrasting data provided by a nonrandomized cohort study of CP for severe pandemic influenza A (H1N1) 2009, which suggested a mortality benefit of adding CP to the local standard of care [25], and 2 more recent well-powered randomized controlled trials (RCTs) examining the use of high-titer anti-influenza plasma as compared to placebo [26] or low-titer plasma [27] for the treatment of seasonal influenza infection, neither of which showed a significant difference in the primary outcome studied (clinical status at day 7). Considering these findings, a critical analysis of any nonrandomized study assessing the efficacy of CP is imperative.
Although our study does demonstrate a lower incidence of in-hospital mortality in the CP group as compared to the control group, this difference was not statistically significant, and a multivariate analysis also showed no significant difference in the risk for mortality between the groups. Two other propensity-matched cohort studies suggest a mortality benefit of CP for COVID-19 [28, 29]. In contrast, 3 RCTS (each with their own limitations) were unable to demonstrate any mortality benefit [23, 30, 31]. Among other factors, the timing of CP transfusion in relation to symptom onset, the antibody titer of the CP transfused, and the quantity of plasma transfused all varied widely among these studies, making it difficult to analyze their outcomes collectively, a topic explored in several recent meta-analyses [32–34]. The initial outcomes data from over 35 000 patients who received CP under the expanded access protocol reemphasizes the importance of these factors, with a high antibody titer and earlier transfusion corresponding to decreased mortality, as compared to a lower titer or later transfusion [22].
The findings of this study should be generalized with caution. There is the possibility of a known or unknown confounder biasing the composition of the CP group and the control group and thus obscuring (or amplifying) the measured effect of CP treatment. Many of the patients in the control group were hospitalized either before CP was locally available or during the peak of local COVID-19 hospitalizations when logistical constraints may have prevented them from being offered CP. The CP and control groups were generally well matched among all variables examined except corticosteroid use (significantly higher in the CP group), but both the multivariate mortality analysis and stratified rate of hospital discharge analysis adjusted for this difference. Many patients were given multiple potentially efficacious therapies (eg, remdesivir, corticosteroids, convalescent plasma), and this study is not powered to deconvolute any potential synergistic or antagonistic effects of these various therapies. The single center nature of the study allows for a comparison with a well-matched controlled group but also limits enrollment, and thus the study may have been underpowered to show a significant difference in the outcomes studied. To assess the effect that the timing of CP transfusion had on efficacy, rather than use a measure of time from hospital admission to CP transfusion (which does not account for duration of symptoms prior to hospitalization or allow for a comparison to the control group), we instead used a measure of duration of symptoms prior to hospital admission (since most patients received CP within 1–2 days after admission) [Supplementary Table 1]. We also did not specifically analyze an outcome examining time to clinical improvement and using hospital discharge as a surrogate for this measure may be misleading since some patients may have remained hospitalized for unrelated reasons.
In conclusion, this is one of the first analyses comparing CP to standard of care for the treatment of COVID-19. Although our study had several limitations, we found no significant overall difference in the risk for in-hospital mortality or in the rate of hospital discharge for those patients who received CP as compared to those who did not. A secondary analysis showed a significantly increased rate of hospital discharge for CP given to patients ≥65-years-old. Although manufactured hyperimmune globulin or monoclonal antibodies may eventually supplant CP in the developed world, CP may remain a more cost-effective and feasible option in the developing world as it can be manufactured locally. Moving forward, it will be important to keep in mind a relevant transfusion medicine principle: give the right blood (plasma containing an adequate concentration of antibody) to the right patient (perhaps older adults or those with immunocompromising conditions) at the right time (early in the course of disease). We anxiously await the results of several ongoing randomized trials of CP taking place across the globe to help gain further insight into this therapy.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Note
Potential conflicts of interest . E. M. reports grants from Regeneron (R10933-10987-COV-2069; R10933-10987-COV-2067; and R10933-10987-COV-2066), grants from SciClone, during the conduct of the study; grants from the National Institutes of Health (NIH) (P20GM121344-01A1 COBRE and NIH –P01 AI 083214), grants from Chemic Labs, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zhou P, Yang X, Wang X, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. WHO coronavirus disease (COVID-19) dashboard. 2020. Available at: https://covid19.who.int. Accessed 05 August 2020.
- 3. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Institute of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov. Accessed 05 August 2020. [PubMed]
- 5. Devasenapathy N, Ye Z, Loeb M, et al. Efficacy and safety of convalescent plasma for severe COVID-19 based on evidence in other severe respiratory viral infections: a systemic review and meta-analysis. CMAJ 2020; 192:E745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casadevall A, Pirofski L. The convalescent sera option for containing COVID-19. J Clin Invest 2020; 130:1545–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sullivan HC, Roback JD. Convalescent plasma: therapeutic hope or hopeless strategy in the SARS-CoV-2 pandemic. Transfus Med Rev 2020; 34:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang B, Liu S, Tan T, et al. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest 2020; 158:e9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 2020; 323:1582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A 2020; 117:9490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ye M, Fu D, Ren Y, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol 2020;. doi: 10.1002/jmv.25882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeng Q, Yu Z, Gou J, et al. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J Infect Dis 2020; 222:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nature biotechnology news. Convalescent serum lines up as first-choice treatment for coronavirus. Available at: https://www.nature.com/articles/d41587-020-00011-1. Accessed 18 May 2020. [DOI] [PubMed]
- 14. Joyner MJ, Bruno KA, Klassen SA, et al. Safety update: COVID-19 convalescent plasma in 20|000 hospitalized patients. Mayo Clin Proc 2020; 95:1888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mayo Clinic. COVID-19 expanded access program. Available at: https://www.uscovidplasma.org. Accessed 05 Aug 2020.
- 16. Expanded access to convalescent plasma for the treatment of patients with COVID-19. Available at: https://www.uscovidplasma.org/pdf/COVID-19%20Plasma%20EAP.pdf. Accessed 05 August 2020.
- 17. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020; 369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nikolich-Zugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol 2018; 19:10–9. [DOI] [PubMed] [Google Scholar]
- 19. Vabret N, Britton GJ, Gruber C, et al. ; Sinai Immunology Review Project . Immunology of COVID-19: current state of the science. Immunity 2020; 52:910–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meschi S, Colavita F, Bordi L, et al. ; INMICovid-19 laboratory team . Performance evaluation of Abbott architect SARS-CoV-2 IgG immunoassay in comparison with indirect immunofluorescence and virus microneutralization test. J Clin Virol 2020; 129:104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bradfute S, Hurwitz I, Yingling A, et al. SARS-CoV-2 neutralizing antibody titers in convalescent plasma and recipients in New Mexico: an open treatment study in COVID-19 patients. J Infect Dis 2020; doi: 10.1093/infdis/jiaa505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joyner M, Senefeld J, Klassen S, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. 2020. Available at: https://www.medrxiv.org/content/10.1101/2020.08.12.20169359v1. Accessed 02 October 2020.
- 23. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA 2020; 324:460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goshua G, Pine A, Meizlish M, et al. Endotheliopathy in COVID-19 associated coagulopathy: evidence from a single-centre cross-sectional study. Lancet Haematol 2020; 7:e575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hung I, To K, Lee C, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis 2011; 52:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davey R, Fernandez-Cruz E, Markowitz N, et al. Anti-influenza hyperimmune intravenous immunoglobulin for adults with influenza A or B infection (FLU-IVIG): a double-blind, randomized, placebo-controlled trial. Lancet Respir Med 2019; 7:951–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beigel J, Aga E, Elie-Turenne M, et al. Anti-influenza immune plasma for the treatment of patients with severe influenza A: a randomized, double-blind, phase 3 trial. Lancet Respir Med 2019; 7:941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu S, Lin H, Baine I, et al. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med 2020; doi: 10.1038/s41591-020-1088-9 [DOI] [PubMed] [Google Scholar]
- 29. Salazar E, Christensen P, Graviss E, et al. Treatment of COVID-19 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol 2020; 190:2290–303. doi: 10.1016/j.ajpath.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gharbharan A, Jordans C, Geurts-vanKessel C, et al. Convalescent plasma for COVID-19: a randomized clinical trial. 2020. Available at: https://www.medrxiv.org/content/10.1101/2020.07.01.20139857v1. Accessed 02 October 2020.
- 31. Agarwal A, Mukherjee A, Kumar G, et al. Convalescent plasma in the management of moderate COVID-19 in India: an open-label parallel-arm phase II multicentre randomized controlled trial (PLACID Trial). 2020. Available at: https://www.medrxiv.org/content/10.1101/2020.09.03.20187252v2. Accessed 02 October 2020.
- 32. Piechotta V, Chai KL, Valk SJ, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev 2020; 7:CD013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Joyner M, Klassen S, Senefeld J, et al. Evidence favoring the efficacy of convalescent plasma for COVID-19 therapy. 2020. Available at: https://www.medrxiv.org/content/10.1101/2020.07.29.20162917v2. Accessed 02 October 2020.
- 34. Sarkar S, Soni K, Khanna P. Convalescent plasma a clutch at straws in COVID-19 management: a systematic review and meta-analysis. J Med Virol 2020; doi: 10.1002/jmv.26408 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.