Abstract
The high mortality rate of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection is a critical concern of the coronavirus disease 2019 (COVID-19) pandemic. Strikingly, men account for the majority of COVID-19 deaths, with current figures ranging from 59% to 75% of total mortality. However, despite clear implications in relation to COVID-19 mortality, most research has not considered sex as a critical factor in data analysis. Here, we highlight fundamental biological differences that exist between males and females, and how these may make significant contributions to the male-biased COVID-19 mortality. We present preclinical evidence identifying the influence of biological sex on the expression and regulation of angiotensin-converting enzyme 2 (ACE2), which is the main receptor used by SARS-CoV-2 to enter cells. However, we note that there is a lack of reports showing that sexual dimorphism of ACE2 expression exists and is of functional relevance in humans. In contrast, there is strong evidence, especially in the context of viral infections, that sexual dimorphism plays a central role in the genetic and hormonal regulation of immune responses, both of the innate and the adaptive immune system. We review evidence supporting that ineffective anti-SARS-CoV-2 responses, coupled with a predisposition for inappropriate hyperinflammatory responses, could provide a biological explanation for the male bias in COVID-19 mortality. A prominent finding in COVID-19 is the increased risk of death with pre-existing cardiovascular comorbidities, such as hypertension, obesity, and age. We contextualize how important features of sexual dimorphism and inflammation in COVID-19 may exhibit a reciprocal relationship with comorbidities, and explain their increased mortality risk. Ultimately, we demonstrate that biological sex is a fundamental variable of critical relevance to our mechanistic understanding of SARS-CoV-2 infection and the pursuit of effective COVID-19 preventative and therapeutic strategies.
Keywords: COVID-19, SARS-CoV-2, Sex differences, Inflammation, Cardiovascular comorbidities
Introduction
Millions of people have been infected with the novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) virus worldwide, hundreds of thousands have been killed by coronavirus disease 2019 (COVID-19), and the numbers are still increasing dramatically. Mortality in patients is mostly determined by respiratory failure; however, more recent evidence highlights the significant contribution of cardiovascular complications in COVID-19 related deaths.1 One potential clue to a better understanding of COVID-19 is the striking dominance of male sex in the overall mortality of SARS-CoV-2 infections. Notably, sex is a key determinant of disease susceptibility and outcome in general and, in particular, in cardiovascular diseases (CVD), which often demonstrate increased prevalence and mortality in men.2 As recently commented on by the Centres for Disease Control and Prevention, psychological, social, and behavioural differences between men and women may play important roles in exposure to SARS-CoV-2, existence of comorbidities (e.g. smoking), treatment initiation, compliance, and ultimately, COVID-19 mortality.3 For example, there is evidence that older men (65–81 years old) make less behavioural changes related to infection control, such as wearing a mask, than older women.4 In this review, we will focus on the differences between males and females based on their genetics (sexual dimorphism) and the potential implications of this sexual dimorphism for COVID-19. We focus on sex differences in angiotensin-converting enzyme 2 (ACE2) expression, inflammation, the immune system, and cardiovascular comorbidities, such as hypertension, obesity, and age, all known to play crucial roles in the outcome of COVID-19.
COVID-19 mortality—male sex is a risk factor
Men and women appear to have similar susceptibility to SARS-CoV-2 infection, with sex-disaggregated data demonstrating that 45.7% female vs. 54.3%5 male cases as of September 2020 which is consistent with published data.6–8 It is important to note that these data do not take into account any potentially important corrections, such as the percentage of men vs. women in each location, lifestyle, or other social or behavioural differences, which could influence the interpretation of this data.3,4 However, a wealth of clinical and epidemiological data has now demonstrated that almost twice as many men with COVID-19 suffer severe symptoms or death in comparison to women.6 Figures from China, South Korea, a National Institute of Health report from Italy, and autopsy findings from Germany have reported that males accounted for 59–75% of COVID-19 deaths.8–12 The most extensive study to date, OpenSAFELY, has assessed data from over 17 million patients in the UK and identified that males have a 59% increase in risk of death in comparison to females.11 Data from five European countries (France, Italy, Spain, Switzerland, and Germany) highlight further sex differences, with men 50% more likely to be admitted to ICU than women and a male predominance for mortality with the ratio of male:female case fatality ranging from 1.7 to 1.8.6 This increase in male mortality is consistent across all age groups, with pooled data from 227 219 confirmed COVID-19 cases indicating the highest case fatality ratio in middle-aged men.6 Thus, this important report clearly confirms an imbalance of COVID-19 severity, hospitalization, and mortality between men and women. Cumulatively, this makes male sex a strong risk factor for increased mortality, alongside other factors, such as immunoincompetence, age, and comorbidities including cardiovascular disease.8,13,14
Sexual dimorphism in COVID-19 should not come as a surprise because it is known that men and women respond to viral infections differently.15,16 Sex differences were recorded during the Spanish flu outbreak (1918–19), with males experiencing increased mortality.17,18 Also in the context of SARS-CoV (2002–04), males had higher mortality compared with females, 21.9% vs. 13.2%, respectively.19 Similarly, with MERS-CoV (2012, 2015, and 2018), deaths were biased towards males.20 Interestingly, the 2008 H1N1 pandemic reported that women were less susceptible to infection as compared to men, but middle-aged women experienced higher mortality.21 These significant differences between the sexes during viral infections provide a compelling argument to identify the mechanisms of sexual dimorphism in SARS-CoV-2, as this may provide important insights into the currently mostly enigmatic pathology of COVID-19. There is clearly an urgent need to understand who is most at risk of severe outcomes of COVID-19 and how to adjust therapeutic interventions accordingly.
The role of the X chromosome and hormones in sexual dimorphism of infection
In humans, the X and Y sex chromosomes contain diverse genes, with the Y chromosome being much smaller and coding mainly for sex-specific effects and testis development. Notably, many genes that play key roles in immune responses are present on the X chromosome, including those involved in both innate and adaptive immune responses to viral infections, e.g. pattern recognition receptors (PRR), such as toll-like receptors (TLR), costimulatory molecules, and transcription factors.22,23 Given that most males have a single X chromosome, if they inherit an X-related gene mutation, they will manifest the respective phenotype. In contrast, as females express two X chromosomes, they are generally protected from such mutations, as the paternal X chromosome can compensate the maternal, and vice versa. Moreover, due to the presence of two X chromosomes, a process known as X chromosome inactivation occurs in females to prevent the overexpression of X-linked genes. Some genes, including those controlling immune responses, can escape this silencing, which can lead to their increased expression and direct functional consequences. Notably, the gene encoding the receptor mainly responsible for SARS-CoV-2 cellular entry, ACE2 (discussed further below), is present on the X chromosome and, therefore, may be susceptible to increased expression in females as a result of ineffective X chromosome inactivation. Current evidence demonstrates that the differential expression and regulation of X-linked genes between males and females play significant roles in sexual dimorphic responses to infection.16
In addition to these ‘gene-dosage’ effects, many sex differences in the manifestation of infectious diseases have long been attributed to the influence of sex hormones. For example, endogenous oestrogens can ameliorate the severity of influenza infections in mice by reducing chemokine and pro-inflammatory cytokine release, including interferon (IFN) γ, tumour necrosis factor-α (TNFα), and C-C chemokine ligand-2 (CCL2).24–27 In the case of SARS-CoV, it has been shown that male mice were more susceptible to infection and showed higher mortality compared with female mice. Importantly, ovariectomy increased female mortality in these mice, reversing protection in females.28 This suggests that the balance between oestrogen and testosterone is likely crucial to anti-viral responses in coronavirus infections. Interestingly, men with prostate cancer on androgen deprivation therapy appear to be protected from SARS-CoV-2 infections, highlighting the potential for androgens to modulate disease susceptibility and progression.29–31 Furthermore, the Sry gene family located on the Y chromosome can upregulate the activity of components of the renin–angiotensin system that downregulate ACE2 via decreased promotor activity.32 Also, as shown in experiments in mice, oestrogen and testosterone can both modulate ACE2, with increased activity in oestrogen deficiency and decreased activity in testosterone deficiency.33–36 Ultimately, the biological influence of sex hormones and sexually dimorphic gene expression in viral infections, alongside the male-biased mortality in COVID-19, support the concept that sexual dimorphism is also a central feature of SARS-CoV-2 infection.
ACE2 and cellular entry of SARS-CoV-2: is there a role for sex?
To facilitate cellular entry SARS-CoV-2 binds to specific cell surface receptors, such as ACE2, via its spike protein.37–40 The spike protein has two subunits. The S1 subunit binds to a cellular receptor, e.g. ACE2. The S2 subunit undergoes priming (cleavage of the spike protein) by a cellular protease, e.g. Transmembrane Serine Protease 2 (TMPRSS2), allowing S2 to mediate fusion of viral and cellular membranes. In addition to ACE2, several cell surface receptors (CD26, CD147, and ITGA5) and proteases [furin protease (PCKSK3), dipeptidyl peptidase 4 (DPP4), cathepsin B, L (CTSB, CTSL), trypsin, human airway trypsin-like protease (HAT)] have been implicated in the cellular entry of coronaviruses.41–53 These receptors and/or proteases may also be relevant for COVID-19 pathology, but whether they play a significant role in SARS-CoV-2 infection, particularly differential roles between the sexes, is currently unknown. Rightfully, ACE2 and TMPRSS2 have attracted major interest as targets for prevention and treatment of SARS-CoV-2 infections.
Initial reports suggest that ACE2 expression levels may influence COVID-19 outcomes, as transcriptional analysis of lung samples from patients with comorbidities that predispose to severe COVID-19 infection (e.g. hypertension, diabetes, chronic lung disease) demonstrate higher ACE2 expression.54 However, human data comparing ACE2 expression between men and women are rare. One report has suggested that ACE2 expression is similar in both sexes across a range of tissues.55 Single-cell sequencing of ACE2 in the adult human heart demonstrated that male hearts have less ACE2 expressing cells compared to females.56 Contrastingly, integration of single-cell atlas data has shown an association between male sex and increased expression of TMPRSS2, the main protease involved in SARS-CoV-2 cellular entry, as well as ACE2.41,57 In addition, transcription of TMPRSS2 is regulated by an androgen receptor binding element in its promotor and androgenic ligands.58,59 Thus, as TMPRSS2 is regulated by androgens and more highly expressed in men, this may contribute to the increased COVID-19 severity in males. Although the relationship between ACE2 expression, plasma ACE2 concentrations and COVID-19 severity is currently unknown, it has been shown that plasma ACE2 concentrations are higher in men than women.60 Genome-wide association studies identified three loci associated with increased plasma ACE2 concentration in men but not in women, providing further evidence for a sex-specific genetic regulation of ACE2 concentration.61 Consequently, sex may be critical to the regulation of ACE2 expression and, potentially, SARS-CoV-2 viral entry. Given the importance sexual dimorphism in ACE2 expression could play in COVID-19, and the importance of ACE2 and the SARS-CoV-2 spike protein as therapeutic targets, further research on the role of ACE2 expression in COVID-19 and potential differences between male and females is warranted.
Patients with non-ischaemic dilated cardiomyopathy have recently been shown to exhibit increased expression of ACE2 and ITGA5, the integrin subunit α5 that can bind to both ACE2 and SARS-CoV-2, the latter via an RGD docking site on the spike protein, and may act as an alternative cellular entry mechanism. This may contribute to the worse outcome of COVID-19 in patients with pre-existing heart disease and also suggests that reducing ACE2 upregulation may be a therapeutic approach in these patients.49,52 Another therapeutic aspect, widely discussed by cardiologists, is related to angiotensin-converting enzyme inhibitors (ACE-I), which are widely prescribed in patients with CVD and have been shown to increase ACE2 expression in the lung.62 While it may be tempting to withdraw ACE-I treatment from COVID-19 patients, clinical data suggest this may lead to exacerbation of cardiovascular risk/injury and it is currently not recommended.63 However, the current consensus is also to avoid newly starting ACE-I medication out of concern that a potential increase in ACE2 expression may predispose to or worsen COVID-19.64–66
Sexual dimorphism in the COVID-19 immune response
Given the consistently increased mortality observed in males during SARS-CoV, MERS-CoV, and now the SARS-CoV-2 pandemic, a fundamental inability to mount an appropriate immune response against coronavirus infections could explain the significant bias towards male COVID-19 deaths. It is well described that males generally have a less robust immune response to some viral illnesses; there is a scientific basis to the ‘man flu’, as men may experience more significant symptoms due to a weaker immune response.67 While our understanding of the exact immunological mechanisms underlying these differences is in its infancy, evidence to-date supports divergence in the composition, epigenetic regulation, and function of immune cell populations between men and women.68–71 A gene expression study of the immune system in mice by Gal-Oz et al.71 indicated that this sexual dimorphism is restricted mainly to macrophages. More specifically, of the differentially expressed genes identified in the macrophages, 63% of these were found to be upregulated in female cells. These genes included complement pathway-related genes (e.g. Fcgr2, Fxgr3a, and Lrg1) and IFN-stimulated genes (e.g. Irf7 and Klra2). The authors conclude that the stronger immune response of females may be due to more activated innate response pathways prior to infection with a pathogen. Importantly, age has also been shown to expose sexual dimorphism. In a comprehensive study, Márquez et al. describe an increasing divergence in gene expression and chromatin accessibility with age. Comparing older men and women (>65 years old), who are at highest risk of COVID-19, females were found to display increased frequency of T and B cells. Furthermore, gene enrichment analysis of ATAC (Assay for Transposase-Accessible Chromatin) and RNA sequencing data annotated several inflammation modules to men, with enhanced monocyte-related gene expression and chromatin accessibility. In contrast, females demonstrated enrichment in T and B cells modules, as well as IFN-inducible pathways. These findings indicate that older men have higher innate and pro-inflammatory activity and lower adaptive immunity. While there are some discrepancies between these two studies, indeed highlighting the significant work still to be done to understand sexual dimorphism in the immune system, both Gal-Oz et al. and Márquez et al. report clear differences in the underlying epigenetic landscape of immune cells in males and females.
There are notable further examples of inherent sexual dimorphism in immune cells, including increased IFNα expression by female plasmacytoid dendritic cells following TLR7 stimulation, with oestrogen receptor 1 dependent IRF5 gene expression implicated in this pathway.72,73 In respect to the adaptive immune system, a recent study has highlighted B-cell intrinsic sexual dimorphism, whereby expression of GPR174 in male, but not female, B cells resulted in sub-optimal cellular positioning within B-cell follicles. Ultimately, this results in poorer antibody responses in male mice.74 T cells also demonstrate inherent sexual dimorphism, with T cells from women expressing increased CXCR3 and CD40L, both of which are important regulators of T-cell function.75,76 It should be noted that a limitation of most human studies of sexual dimorphism, and indeed many mouse studies, is the focus on peripheral blood mononuclear cells. It is important to appreciate than immune cell phenotype and behaviour differs significantly depending on the environment, including between the circulation and within tissues. Bain et al.,68 for example, recently demonstrated significant differences in peritoneal macrophage phenotype and function between male and female mice, including in their transcriptional states (e.g. increased expression of Celc4g, Cd209a, and Cd209b in female mice). Importantly, they discovered that these differences were not cell intrinsic, and instead were driven by differing peritoneal microenvironments in males vs. females. A similar pattern of findings was identified by Vasanthakumar et al.,69 who described that an increased inflammatory status in the adipose tissue of male mice corresponded with an IL-33-dependent expansion of regulatory T cells. Again, these differences were determined by the respective tissue niche, as opposed to being cell intrinsic. Consequently, in interpreting data on sexual dimorphism both location and context need to be considered, as well as other important factors, such as age. Despite these complexities, based on our current understanding, it appears likely that sexual dimorphism in immune responses plays a major role in the response to SARS-CoV-2 infection.
An analysis of the transcriptional response by Blanco-Melo et al.77 combining human data with animal (ferret) and in vitro models of the SARS-CoV-2 infection has highlighted an atypical and potentially inappropriate anti-viral response. Notably, a poor type I and III IFN response to SARS-CoV-2, but significant induction of other inflammatory genes, such as interleukin 6 (IL-6), CCL2, and C-C chemokine ligand 8 (CCL8), were observed. This study was supported by Hadjadj et al.,78 also identifying a weak type I IFN response coupled with increased IL-6 and TNFα in a cohort of 50 COVID-19 patients. Importantly, emerging evidence suggests that type I IFNs are effective at reducing the virus’s replication.79 As such, the lack of an appropriate IFN response may be a significant deficit in the immune system’s ability to combat SARS-CoV-2 and prevent severe infection.
TLR7, a PRR recognizing single-stranded RNA and leading to IFN production has been identified as a potentially important receptor for the recognition of and response to coronavirus infections. Highlighting this likely critical role of TLR7 in SARS-CoV-2 infection is the association of rare TLR7 mutations with severe COVID-19.80 Comprising of two unrelated families, each with a different mutation, this study assessed four male patients with loss of function mutations in TLR7. In comparison with TLR7 competent individuals, including a wildtype and also TLR7 heterezygous parent from one the families; peripheral blood mononuclear cells isolated from male patients with TLR7 mutations were unresponsive to the TLR7 agonist imiquimod, with this stimulation failing to induce key components of the TLR7 signalling pathway (IRF7, ISG15, and IFNB1) which lead to IFN production. All four patients with loss of function in TLR7 we are young, ranging from 21 to 32 years old, and all experienced severe COVID-19 symptoms requiring mechanical ventilation; one patient did not to recover. While this study cannot conclude a direct link between these mutations and COVID-19 severity; the presence of two different loss of function mutations in TLR7 in unrelated families associated with severe COVID-19 implicates TLR7 as a likely central node in the response to SARS-CoV-2 infection.
Of great relevance to sexual dimorphism in COVID-19, TLR7 is found to be more highly expressed in females, with increased copy numbers of the TLR7 gene correlating with both increased TLR7 signalling and the resulting type I IFN production.76,81,82 In addition to this gene-dosage effect, it has been shown that oestrogen also increases TLR7 expression. A potential mechanism behind the described low IFN response in COVID-19 may be related to the virus’s ability to evade detection via PRRs, such as TLR7, or downstream products of their signalling. Many viruses, including SARS-CoV and MERS-CoV, possess an array of mechanisms to evade or subvert the immune response, including those which specifically disrupt IFN signalling pathways.83 In the context of SARS-CoV-1, structural and non-structural viral proteins have been identified that target PRR and IFN pathways, ultimately antagonizing IFN by preventing IRF3, NF-кB, STAT-1, STING, MAVS, TRAF3, and TRAF 6 signalling.84–89 Moreover, the non-structural protein 1 of both SARS-CoV-1 and SARS-CoV-2 has been demonstrated to suppress host gene expression and inhibit IFN production.90,91 Unsurprisingly, MERS-CoV demonstrates similar IFN antagonism.92 While research on SARS-CoV-2 is in its infancy, it has already been identified that open reading frames 3b, 6, and 9b (ORF3, ORF6, and ORF9b, respectively) appear to be critical to the virus’s ability to downregulate host IFN responses, at least in vitro.93–95 Consequently, enhanced expression of TLR7 by females may improve the likelihood of generating a strong IFN response, limiting PRR and IFN evasion by SARS-CoV-2, and achieving viral clearance. Supporting this concept is the fact that females appear to clear SARS-CoV-2 infection faster than men, whom experience increased viral persistence.96,97 It has also been determined that women have a conserved enrichment of IFN response pathways in comparison with men even into old age.70 Moreover, significantly higher IFNα2 levels have been identified in women infected with SARS-CoV-2 in a longitudinal analysis of 98 patients (47 men and 51 women) admitted to Yale-New Haven Hospital.98 Though further investigations are required, the evidence supports that enhanced IFN responses may provide women with a significant immunological advantage over men in the response to SARS-CoV-2.
IFN treatment is one of several therapeutics being explored currently, with the results from these studies highly anticipated.99 Given the evidence highlighting sexual dimorphism in IFN responses, it is important to consider the differential effects IFN treatment may have on males and females. To draw a parallel with another RNA virus, a clinical trial exploring the treatment of hepatitis C with IFN has suggested that females respond more effectively to lower doses of IFN than males. This may mean that males and females require different IFN dosages for optimal therapy and again highlights the need to consider the potential for inherent sexual variance.100 To fully explore the efficacy of IFN treatment, sexual dimorphism in basal levels of IFN and any fundamental variation in the response to IFN therapy should be taken into consideration. In addition, given the general protective role oestrogen plays in anti-viral immune responses, the use of drugs that upregulate oestrogen-receptor activation may also be capable of improving treatment outcomes during COVID-19 infection in both males and females.101 Such an approach may provide a broader response than IFN alone and provide a useful adjunct therapy.
Sexual dimorphism in COVID-19 hyperinflammation
As well as mediating anti-viral responses, the immune system has been implicated in driving detrimental and dysregulated inflammation in COVID-19, a feature also present in SARS-CoV and MERS-CoV infections.102,103 More specifically, the occurrence of respiratory failure, acute respiratory distress syndrome (ARDS), and adverse clinical outcomes correlates with elevated IL-6, C-reactive protein (CRP), and a phenotype likened to cytokine release syndrome.104,105
It is perhaps surprising that males are at greater risk of COVID-19 and the associated hyperinflammation considering females have been described to mount stronger immune responses to viral infections and are also prone to many autoimmune and inflammatory pathologies (Figure 1).106 It has been noted that the hyperinflammatory response observed in severe COVID-19 is similar to secondary haemophagocytic lymphohistiocytosis (sHLH), a condition often triggered by infection.107 Interestingly, evidence suggests that male sex is also associated with poor outcomes in sHLH.108,109 To provide a contrast to the male-biased pathology in SARS-CoV-2; in the context of influenza infections, increased inflammatory responses have been correlated with poorer outcomes in females.24 This again highlights the complexity of differences to be appreciated between the male and female immune system and their responses to infection, compounding the need for the mechanisms of sexual dimorphism likely present in SARS-CoV-2 infection to be understood.
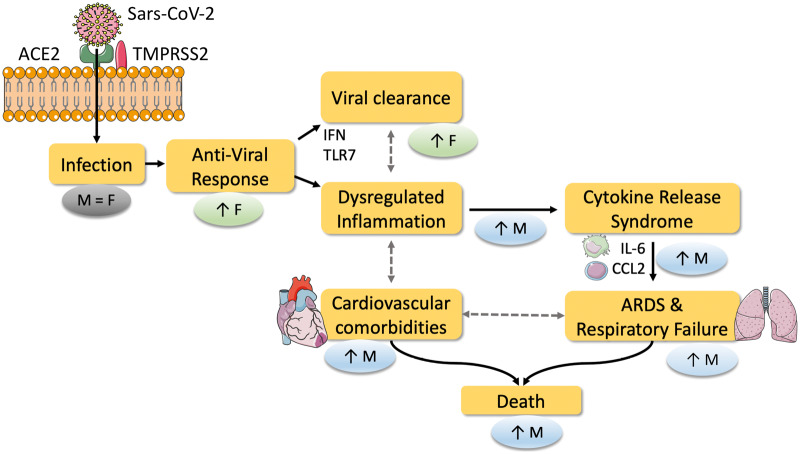
Figure 1.
Potential mechanisms of how sexual dimorphism results in higher mortality of COVID-19 in males. SARS-CoV-2 enters cells via binding to the cellular ACE2 receptor and subsequent spike protein cleavage by TMPRSS2 mediating fusion of viral and cellular membranes. Current data suggest that ACE2 expression levels are the same in both sexes. Infection rates are similar between men and women, however, the response to infection differs between the sexes. Anti-viral responses and viral clearance, mediated by IFN and TLR7, are postulated to be increased in females and may be one of the key mechanisms behind the reduced mortality observed in women compared with men in COVID-19. Dysregulated inflammation and increased cytokine release are potential links to increased ARDS, respiratory failure, and cardiovascular comorbidities in males, culminating in increased mortality. ACE2, angiotensin-converting enzyme 2; ARDS, acute respiratory distress syndrome; CCL2, C-C chemokine ligand 2; F, female; IFN, interferon; IL-6, interleukin 6; M, male; TLR7, toll-like receptor 7; TMPRSS2, Transmembrane Serine Protease 2.
Based on the observation of a poor IFN response in patients with severe COVID-19, it is possible that ineffective anti-viral immunity precedes or contributes to the hyperinflammatory responses seen in severe COVID-19. This again may be particularly relevant in men as a diminished ability to generate effective anti-viral responses (discussed above) in the early stage of disease could increase the likelihood of enhanced infection, dysregulated immunity, and poorer outcomes.
Supporting the likely importance of early anti-viral responses in the prevention of severe COVID-19 is the observation that delayed IFN signalling manifested in lethal pneumonia in mice infected with SARS-CoV, while early IFN ameliorated this phenotype.110 Similarly in humans; a retrospective clinical study of 446 patients has demonstrated a significant improvement in survival in COVID-19 patients treated with early IFN-α2b (≤5 days post-admission).111 In contrast, late administration of IFN treatment (≥6 days post-admission) was associated with longer hospital admission, slower recovery of lung function, and higher mortality. While further studies are required to correlate effective anti-viral immunity to the hyperinflammation associated with COVID-19, these data highlight a potentially critical importance of timely and effective immunity to SARS-CoV-2 in order to prevent severe disease and likely the associated hyperinflammatory responses.
One of the severe consequences of a hyperactive immune system are prothrombotic effects, potentially resulting in stroke, deep vein thrombosis, and pulmonary embolism and microthrombosis and consequently micro-obstruction, e.g. in the lung, heart, and kidney.112,113 Through this link between inflammation and (micro)thrombosis, sex differences could contribute to sex-associated differences in COVID-19 mortality. However, clinical data reported so far often does not provide sex differentiation that would allow drawing definite conclusions. There is clearly an urgent need to report sex-specific COVID-19 outcome and clinical complication data.
Ultimately, a failure to clear the SARS-CoV-2 infection due to ineffective anti-viral immunity could lead to the male-biased propagation of hyperactive inflammatory responses which appear to contribute directly to severe COVID-19. Cumulatively, evidence supports the concept that females have a distinct immunological advantage over men in the context of SARS-CoV-2 infection, producing a more robust anti-viral response that may prevent the initiation of dysregulated immune response and COVID-19 related immunopathology. This leads us on to the connection between cardiovascular comorbidity and COVID-19, where the described sexual dimorphism in immune-inflammatory responses play a key role.
Cardiovascular comorbidity in COVID-19 and potential sex differences
It has become clear that cardiovascular comorbidities are a significant risk factor for COVID-19 hospitalization and mortality.1,114,115 The cardiovascular community is attuned to sex differences in CVD.116 Given biological sex plays a significant role in the immune system and, likely, the response to SARS-CoV-2; we suggest that sexual dimorphism in immune-inflammatory responses may directly influence the relationship between cardiovascular diseases and COVID-19 risk and therefore attribute to the higher mortality in men.
It is notable that CRP and inflammatory cytokines, such as IL-1β and IL-6, that have been implicated in COVID-19 are also central to CVD risk.105,117 The importance of the inflammasome/IL-1β axis in CVD was recently highlighted by the reduction of cardiovascular events in several clinical trials, achieved either by IL-1β inhibition via the application of the blocking antibody canakinumab or by the proposed inhibition of the inflammasome via the anti-inflammatory drug colchicine.118–121 IL-1β is downstream of the inflammasome and upstream of IL-6, and this entire pathway has now been implicated in the severity of COVID-19.122,123 Accordingly, IL-1β and IL-6 have both been identified as potential therapeutic targets in severe COVID-19, with retrospective cohort studies suggesting that anakinra, an IL-1 receptor antagonist that blocks the function of IL-1α and IL-1β, may be efficacious.124,125 Retrospective studies of IL-6 blockade have also suggested this to be an effective treatment for COVID-19, however a randomized clinical trial did not demonstrate a therapeutic benefit.126,127 Further investigation will be needed to explore whether targeting either of these cytokines is indeed a worthwhile approach, with patient inclusion criteria and treatment strategy likely to have significant bearing on the success or failure of these trials. Ultimately, the use of immunomodulatory drugs which dampen immunity are likely to be beneficial for those with an excessive inflammatory response, but only in the context where they do not compromise host immunity; therefore, careful patient stratification is likely to be required to optimize both patient care and our understanding of causal pathways in severe COVID-19. In respect to cardiovascular comorbidity in COVID-19, it should be considered that increased basal expression of inflammatory proteins in CVD patients, including IL-1β and IL-6, could ultimately contribute to the severity of COVID-19. Likewise, the inflammation resulting from SARS-CoV-2 infection could provide a direct mechanistic link to increased incidences of cardiovascular events.
Arterial hypertension is an excellent example linking CVD immunity, sex and COVID-19. The immune system is a significant mechanistic determinant of hypertension, which is clearly a male-biased disease.1,128–130 A retrospective observational study by Gao et al.131 has observed a two-fold increase of COVID-19 mortality in hypertensive individuals, highlighting a clear relationship between hypertension and COVID-19. This finding is further supported by the OpenSAFELY study, which identified increased COVID-19 mortality in hypertensive patients under the age of 70. Given the observation of hyperinflammation in COVID-19, it is of interest that monocytes from patients with hypertension display increased inflammatory activity and produce elevated levels of Interleukin 1 Beta (IL-1β) and IL-6 when stimulated in vitro compared to monocytes from non-hypertensive individuals.128 Moreover, hypertensive patients show an increased activation/inflammatory phenotype of circulating blood cells such as monocytes and platelets, the latter is of particular interest as COVID-19 mortality appears to be associated with (micro)thrombotic events.112,132,133 With respect to the adaptive immune system, there is also evidence that CD8+ T cells, which are important mediators of anti-viral immunity, are dysfunctional in hypertensive patients.134 It is important to consider that women mount robust CD8+ T-cell responses during SARS-CoV-2 irrespective of their age,98 while, men produce weaker CD8+ T-cell responses, which declined with age and are predictive of poorer COVID-19 outcomes. Consequently, CD8+ T-cell dysfunction and heightened inflammatory responses in patients with hypertension may compound the already discussed potentials for deficient anti-viral immunity and increased inflammatory responses in men, leading to reciprocally increased risk of severe COVID-19 and exacerbated cardiovascular complications.
Further supporting the concept that CVD and COVID-19 driven inflammation/immune dysregulation might combine to increase risk associated with both diseases, are the findings of an elegant study by Mathew et al.135 who observed associations between cardiovascular risk factors, mainly hyperlipidaemia, and distinct immune features in COVID-19. Using a well-characterized cohort of 125 COVID-19 patients to comprehensively profile adaptive immune responses in SARS-CoV-2 infection, and correlating their findings with the patients’ clinical features, the authors identified that hyperlipidemia was associated with a more rapid decline in important antibody producing cells (plasmablasts) in COVID-19 patients. It was also observed that hyperlipidaemic COVID-19 patients were more likely to have stable or decreasing T-cell populations; ∼60% and ∼75% of COVID-19 patients with decreasing CD4+ or CD8 T+ cells, respectively, possessed hyperlipidaemia as a comorbidity. Given lymphopenia has been associated with severe COVID-19 and, in the latter study, decreased T-cell numbers were associated with increased inflammation (hsCRP, D-dimer, and ferritin levels), a relationship between hyperlipidemia and T-cell deficiency may contribute to more severe COVID-19 outcomes in individuals with cardiovascular comorbidities. Notably, due to robust anti-viral immunity, including T-cell responses to SARS-CoV-2, females may be more protected from such detrimental effects.98
Older age, another classical CVD risk factor, is most profoundly implicated in COVID-19-associated mortality. The OpenSAFELY study identified that hazard ratios increase with age, increasing from 2.40 in those over 60 years old to 20.61 in individuals over 80 years old.11 It is now well known that the immune system ages differently in males and females. A comprehensively assessment of these differences using a multi-omic approach has highlighted that, in comparison to females, males over the age of 65 have enrichments in pro-inflammatory genomic signatures and plasma concentrations of inflammatory cytokines such as IL-6.70 Current evidence would support that this increase in inflammatory burden in aged males may play a key role in COVID-19 risk (and also CVD risk), highlighting further important interactions between sexual dimorphism and significant risk factors for both COVID-19 and CVD outcomes.
Obesity, which clearly demonstrates sexual dimorphism, has been associated with COVID-19 (re)admission, severity, need for ventilation, and death.136–138 OpenSAFELY described increases in hazard ratio (1.05) for those with a BMI above 30, to 1.40 and 1.92 in individuals with BMI’s over 35 and 40, respectively. The mechanisms underpinning the role of obesity in COVID-19 risk remain to be described. However, the relationship between obesity and heightened levels of basal inflammation is highly likely to contribute to a greater risk of dysregulated inflammatory responses and poor COVID-19 outcomes.136,139 It is well documented that obesity varies between males and females with respect to both fat distribution and function. In fact, adipose inflammation in itself is sexually dimorphic, as the immune cell niches within the adipose tissue differ between males and females.140 As mentioned previously, Vasanthakumar et al.,69 recently identified that male adipose tissue in mice was not only more inflammatory than female adipose tissue, but had a markedly different stromal compartment, altering the phenotype of the immune cells present. Ultimately, these data highlight a critical need to consider that any immune-inflammatory mechanisms by which obesity influences the risk of viral infections may differ significantly between males and females.
Conclusions
Male patients with COVID-19 are more symptomatic and exhibit increased disease severity, higher complication rates, and ultimately higher mortality. Potential sexual dimorphism in the expression of ACE2, as the docking site used by SARS-CoV-2 to enter cells, has attracted significant attention. Nevertheless, preclinical evidence that ACE2 expression is regulated in a sex-dependent manner has not yet been validated in humans and, although initially postulated, no clinically relevant influence of medication such as ACE-I has yet been documented. However, as a most fascinating area, sexual dimorphism in the genetic and hormonal regulation of the immune response may hold the answer to the bias seen towards male mortality. Differences in inflammatory responses to viral infections between the sexes alongside different inflammatory/immune statuses associated with cardiovascular comorbidities, such as obesity, hypertension, and age, offer potential explanations for the worse outcomes in men with COVID-19. Further research into sex differences in COVID-19 is necessary; we, and others,141 argue that both preclinical and clinical studies should include sex as a variable and, where possible, present datasets stratified by sex. The significant bias towards male deaths in COVID-19 and the clear interaction with CVD highlights a poorly understood biological phenomenon that is difficult to investigate, but it also provides a unique opportunity to better understand and treat SARS-CoV-2 infections.
Conflict of interest: none declared.
Funding
L.B. was supported by a National Heart Foundation (NHF) of Australia Postdoctoral Fellowship. X.W. was supported by an NHF Future Leader Fellowship and a Baker Fellowship. K.P. was supported by a National Health and Medical Research Council Investigator Fellowship.
Contributor Information
Laura A Bienvenu, Atherothrombosis and Vascular Biology Laboratory, Baker Heart and Diabetes Institute, 75 Commercial Rd, Melbourne, VIC 3004, Australia; Molecular Imaging and Theranostics Laboratory, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia; Department of Cardiometabolic Health, University of Melbourne, VIC, Australia.
Jonathan Noonan, Atherothrombosis and Vascular Biology Laboratory, Baker Heart and Diabetes Institute, 75 Commercial Rd, Melbourne, VIC 3004, Australia; Department of Cardiometabolic Health, University of Melbourne, VIC, Australia; Deparment of Immunology, Monash University, Melbourne, VIC, Australia; Centre for Immunobiology, College of Medical, Veterinary and Life Sciences, Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow, UK.
Xiaowei Wang, Atherothrombosis and Vascular Biology Laboratory, Baker Heart and Diabetes Institute, 75 Commercial Rd, Melbourne, VIC 3004, Australia; Molecular Imaging and Theranostics Laboratory, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia; Department of Cardiometabolic Health, University of Melbourne, VIC, Australia; Department of Medicine, Monash University, Melbourne, VIC, Australia.
Karlheinz Peter, Atherothrombosis and Vascular Biology Laboratory, Baker Heart and Diabetes Institute, 75 Commercial Rd, Melbourne, VIC 3004, Australia; Department of Cardiometabolic Health, University of Melbourne, VIC, Australia; Deparment of Immunology, Monash University, Melbourne, VIC, Australia; Department of Medicine, Monash University, Melbourne, VIC, Australia.
References
- 1. Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, Madhur MS, Tomaszewski M, Maffia P, D’Acquisto F, Nicklin SA, Marian AJ, Nosalski R, Murray EC, Guzik B, Berry C, Touyz RM, Kreutz R, Wang DW, Bhella D, Sagliocco O, Crea F, Thomson EC, McInnes IB. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res 2020;116:1666–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev 2017;97:1–37. [DOI] [PubMed] [Google Scholar]
- 3. Griffith DM. Men and COVID-19: a biopsychosocial approach to understanding sex differences in mortality and recommendations for practice and policy interventions. Prev Chronic Dis 2020;17:E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barber SJ, Kim H. COVID-19 worries and behavior changes in older and younger men and women. J Gerontol B Psychol Sci Soc Sci 2020; doi:10.1093/geronb/gbaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 sex-disaggregated data tracker—Global Health 50/50; https://globalhealth5050.org/covid19 (29 July 2020, date last accessed).
- 6. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ 2020;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020;382:1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jin J-M, Bai P, He W, Wu F, Liu X-F, Han D-M, Liu S, Yang J-K. Gender Differences in Patients With COVID-19: focus on Severity and Mortality. Front Public Health 2020;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Korean Society of Infectious Diseases, Korean Society of Pediatric Infectious Diseases, Korean Society of Epidemiology, Korean Society for Antimicrobial Therapy, Korean Society for Healthcare-Associated Infection Control and Prevention, Korea Centers for Disease Control and Prevention. Report on the Epidemiological Features of Coronavirus Disease 2019 (COVID-19) Outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci 2020;35:e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature 2020;584:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wichmann D, Sperhake J-P, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, Heer G. D, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, Weerth A. D, Paschen H-R, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med 2020; doi:10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang G, Chen W, Jin X, Chen Y. Description of COVID‐19 cases along with the measures taken on prevention and control in Zhejiang. J Med Virol 2020;92:1948–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borges do Nascimento IJ, Cacic N, Abdulazeem HM, Groote T V, Jayarajah U, Weerasekara I, Esfahani MA, Civile VT, Marusic A, Jeroncic AC, Junior N, Pericic TP, Zakarija-Grkovic IM, Guimarães SM, L, Bragazzi N, Bjorklund M, Sofi-Mahmudi A, Altujjar M, Tian M, Arcani DMC, O’Mathúna DP, Marcolino MS. Novel coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis. J Clin Med 2020;9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghosh S, Klein RS. Sex drives dimorphic immune responses to viral infections. J Immunol 2017;198:1782–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16:626–638. [DOI] [PubMed] [Google Scholar]
- 17. Viboud C, Eisenstein J, Reid AH, Janczewski TA, Morens DM, Taubenberger JK. Age- and sex-specific mortality associated with the 1918–1919 influenza pandemic in Kentucky. J Infect Dis 2013;207:721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noymer A, Garenne M. The 1918 influenza epidemic’s effects on sex differentials in mortality in the United States. Popul Dev Rev 2000;26:565–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karlberg J, Chong DSY, Lai WYY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol 2004;159:229–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsuyama R, Nishiura H, Kutsuna S, Hayakawa K, Ohmagari N. Clinical determinants of the severity of Middle East respiratory syndrome (MERS): a systematic review and meta-analysis. BMC Public Health 2016;16:1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eshima N, Tokumaru O, Hara S, Bacal K, Korematsu S, Tabata M, Karukaya S, Yasui Y, Okabe N, Matsuishi T. Sex- and age-related differences in morbidity rates of 2009 pandemic influenza A H1N1 virus of swine origin in Japan. PLoS One 2011;6:e19409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Snell DM, Turner JMA. Sex chromosome effects on male-female differences in mammals. Curr Biol 2018;28:R1313–R1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schurz H, Salie M, Tromp G, Hoal EG, Kinnear CJ, Möller M. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genomics 2019;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog 2011;7:e1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nguyen DC, Masseoud F, Lu X, Scinicariello F, Sambhara S, Attanasio R. 17β-Estradiol restores antibody responses to an influenza vaccine in a postmenopausal mouse model. Vaccine 2011;29:2515–2518. [DOI] [PubMed] [Google Scholar]
- 26. Pazos MA, Kraus TA, Muñoz-Fontela C, Moran TM. Estrogen mediates innate and adaptive immune alterations to influenza infection in pregnant mice. PLoS One 2012;7:e40502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vermillion MS, Ursin RL, Attreed SE, Klein SL. Estriol reduces pulmonary immune cell recruitment and inflammation to protect female mice from severe influenza. Endocrinology 2018;159:3306–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol 2017;198:4046–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, Carbone GM, Cavalli A, Pagano F, Ragazzi E, Prayer-Galetti T, Alimonti A. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol 2020;31:1040–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chakravarty D, Nair SS, Hammouda N, Ratnani P, Gharib Y, Wagaskar V, Mohamed N, Lundon D, Dovey Z, Kyprianou N, Tewari AK. Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Commun Biol 2020;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mjaess G, Karam A, Aoun F, Albisinni S, Roumeguère T. COVID-19 and the male susceptibility: the role of ACE2, TMPRSS2 and the androgen receptor. Prog Urol 2020;30:484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Milsted A, Underwood AC, Dunmire J, DelPuerto HL, Martins AS, Ely DL, Turner ME. Regulation of multiple renin-angiotensin system genes by Sry. J Hypertens 2010;28:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xie X, Xudong X, Chen J, Junzhu C, Wang X, Xingxiang W, Zhang F, Furong Z, Liu Y, Yanrong L. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci 2006;78:2166–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dalpiaz PLM, Lamas AZ, Caliman IF, Ribeiro RF, Abreu GR, Moyses MR, Andrade TU, Gouvea SA, Alves MF, Carmona AK, Bissoli NS. Sex hormones promote opposite effects on ACE and ACE2 activity, hypertrophy and cardiac contractility in spontaneously hypertensive rats. PLoS One 2015;10:e0127515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Araujo FC, Milsted A, Watanabe IKM, Del Puerto HL, Santos RAS, Lazar J, Reis FM, Prokop JW. Similarities and differences of X and Y chromosome homologous genes, SRY and SOX3, in regulating the renin-angiotensin system promoters. Physiol Genomics 2015;47:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu J, Ji H, Zheng W, Wu X, Zhu JJ, Arnold AP, Sandberg K. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol Sex Diff 2010;1:6–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020;581:221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020;581:215–220. [DOI] [PubMed] [Google Scholar]
- 39. Chen L, Hao G. The role of angiotensin-converting enzyme 2 in coronaviruses/influenza viruses and cardiovascular disease. Cardiovasc Res 2020; doi: 10.1093/cvr/cvaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Latini A, Agolini E, Novelli A, Borgiani P, Giannini R, Gravina P, Smarrazzo A, Dauri M, Andreoni M, Rogliani P, Bernardini S, Helmer-Citterich M, Biancolella M, Novelli G. COVID-19 and genetic variants of protein involved in the SARS-CoV-2 entry into the host cells. Genes 2020;11:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A 2020;117:11727–11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coutard B, Valle C, X de L, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res 2020;176:104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Millet JK, Whittaker GR. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res 2015;202:120–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vankadari N, Wilce JA. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect 2020;9:601–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang K, Chen W, Zhou Y-S, Lian J-Q, Zhang Z, Du P, Gong L, Zhang Y, Cui H-Y, Geng J-J, Wang B, Sun X-X, Wang C-F, Yang X, Lin P, Deng Y-Q, Wei D, Yang X-M, Zhu Y-M, Zhang K, Zheng Z-H, Miao J-L, Guo T, Shi Y, Zhang J, Fu L, Wang Q-Y, Bian H, Zhu P, Chen Z-N. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein | bioRxiv https://www.biorxiv.org/content/10.1101/2020.03.14.988345v1 (4 September 2020); doi:10.1101/2020.03.14.988345. [DOI] [PMC free article] [PubMed]
- 48. Ulrich H, Pillat MM. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev Rep 2020;20:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sigrist CJ, Bridge A, Le Mercier P. A potential role for integrins in host cell entry by SARS-CoV-2. Antiviral Res 2020;177:104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bertram S, Glowacka I, Müller MA, Lavender H, Gnirss K, Nehlmeier I, Niemeyer D, He Y, Simmons G, Drosten C, Soilleux EJ, Jahn O, Steffen I, Pöhlmann S. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J Virol 2011;85:13363–13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci U S A 2009;106:5871–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bristow MR, Zisman LS, Altman NL, Gilbert EM, Lowes BD, Minobe WA, Slavov D, Schwisow JA, Rodriguez EM, Carroll IA, Keuer TA, Buttrick PM, Kao DP. Dynamic regulation of SARS-Cov-2 binding and cell entry mechanisms in remodeled human ventricular myocardium. JACC Basic Transl Sci 2020;5:871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gkogkou E, Barnasas G, Vougas K, Trougakos IP. Expression profiling meta-analysis of ACE2 and TMPRSS2, the putative anti-inflammatory receptor and priming protease of SARS-CoV-2 in human cells, and identification of putative modulators. Redox Biol 2020;36:101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pinto BGG, Oliveira AER, Singh Y, Jimenez L, Gonçalves ANA, Ogava RLT, Creighton R, Schatzmann Peron JP, Nakaya HI. ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. J Infect Dis 2020;222:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li M-Y, Li L, Zhang Y, Wang X-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu H, Gai S, Wang X, Zeng J, Sun C, Zhao Y, Zheng Z. Single-cell analysis of SARS-CoV-2 receptor ACE2 and spike protein priming expression of proteases in the human heart. Cardiovasc Res 2020;116:1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Muus C, Luecken MD, Eraslan G, Waghray A, Heimberg G, Sikkema L, Kobayashi Y, Vaishnav ED, Subramanian A, Smilie C, Jagadeesh K, Thu Duong E, Fiskin E, Torlai Triglia E, Ansari M, Cai P, Lin B, Buchanan J, Chen S, Shu J, Haber AL, Chung H, Montoro DT, Adams T, Aliee H, Samuel J, Zaneta Andrusivova A, Angelidis I, Ashenberg O, Bassler K, Bécavin C, Benhar I, Bergenstråhle J, Bergenstråhle L, Bolt L, Braun E, Bui LT, Chaffin M, Chichelnitskiy E, Chiou J, Conlon TM, Cuoco MS, Deprez M, Fischer DS, Gillich A, Gould J, Guo M, Gutierrez AJ, Habermann AC, Harvey T, He P, Hou X, Hu L, Jaiswal A, Jiang P, Kapellos T, Kuo CS, Larsson L, Leney-Greene MA, Lim K, Litviňuková M, Lu J, Maatz H, Madissoon E, Mamanova L, Manakongtreecheep K, Marquette CH, Mbano I, McAdams AM, Metzger RJ, Nabhan AN, Nyquist SK, Ordovas-Montanes J, Penland L, Poirion OB, Poli S, Qi C, Reichart D, Rosas I, Schupp J, Sinha R, Sit RV, Slowikowski K, Slyper M, Smith N, Sountoulidis A, Strunz M, Sun D, Talavera-López C, Tan P, Tantivit J, Travaglini KJ, Tucker NR, Vernon K, Wadsworth MH, Waldmann J, Wang X, Yan W, Zhao W, Ziegler CGK, The NHLBI LungMAP Consortium, and The Human Cell Atlas Lung Biological Network. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. bioRxiv. https://www.biorxiv.org/content/10.1101/2020.04.19.049254v1 (27 July 2020); doi:10.1101/2020.04.19.049254. [Google Scholar]
- 58. Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun X-W, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005;310:644–648. [DOI] [PubMed] [Google Scholar]
- 59. Lucas JM, Heinlein C, Kim T, Hernandez SA, Malik MS, True LD, Morrissey C, Corey E, Montgomery B, Mostaghel E, Clegg N, Coleman I, Brown CM, Schneider EL, Craik C, Simon J, Bedalov T, Nelson PS. The androgen-regulated protease TMPRSS2 activates aProteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov 2014;4:1310–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sama IE, Ravera A, Santema BT, van Goor H, Ter Maaten JM, Cleland JGF, Rienstra M, Friedrich AW, Samani NJ, Ng LL, Dickstein K, Lang CC, Filippatos G, Anker SD, Ponikowski P, Metra M, Veldhuisen DV, Voors AA. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J 2020;41:1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nelson CP, Sama IE, Codd V, Webb Thomas R, Ye S, Lang CC, Voors AA, Ng LL, Samani NJ. Genetic associations with plasma ACE2 concentration: potential relevance to COVID-19 risk. Circulation 2020;142:1117–1119. [DOI] [PubMed] [Google Scholar]
- 62. Wevers BA, Hoek LVD. Renin–angiotensin system in human coronavirus pathogenesis. Future Virol 2010;5:145–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Murray E, Tomaszewski M, Guzik TJ. Binding of SARS-CoV-2 and angiotensin-converting enzyme 2: clinical implications. Cardiovasc Res 2020;116:e87–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Guo J, Huang Z, Lin L, Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc 2020;9:e016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, Widmer AF, Osswald S. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J 2020;41:1801–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Danser AHJ, Epstein M, Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension 2020;75:1382–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sue K. The science behind ‘man flu’. BMJ 2017;359:j5560. [DOI] [PubMed] [Google Scholar]
- 68. Bain CC, Gibson DA, Steers NJ, Boufea K, Louwe PA, Doherty C, González-Huici V, Gentek R, Magalhaes-Pinto M, Shaw T, Bajénoff M, Bénézech C, Walmsley SR, Dockrell DH, Saunders PTK, Batada NN, Jenkins SJ. Rate of replenishment and microenvironment contribute to the sexually dimorphic phenotype and function of peritoneal macrophages. Sci Immunol 2020; doi:10.1126/sciimmunol.abc4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vasanthakumar A, Chisanga D, Blume J, Gloury R, Britt K, Henstridge DC, Zhan Y, Torres SV, Liene S, Collins N, Cao E, Sidwell T, Li C, Spallanzani RG, Liao Y, Beavis PA, Gebhardt T, Trevaskis N, Nutt SL, Zajac JD, Davey RA, Febbraio MA, Mathis D, Shi W, Kallies A. Sex-specific adipose tissue imprinting of regulatory T cells. Nature 2020;579:581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Márquez EJ, Chung C, Marches R, Rossi RJ, Nehar-Belaid D, Eroglu A, Mellert DJ, Kuchel GA, Banchereau J, Ucar D. Sexual-dimorphism in human immune system aging. Nat Commun 2020;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gal-Oz ST, Maier B, Yoshida H, Seddu K, Elbaz N, Czysz C, Zuk O, Stranger BE, Ner-Gaon H, Shay T. ImmGen report: sexual dimorphism in the immune system transcriptome. Nat Commun 2019;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Griesbeck M, Ziegler S, Laffont S, Smith N, Chauveau L, Tomezsko P, Sharei A, Kourjian G, Porichis F, Hart M, Palmer CD, Sirignano M, Beisel C, Hildebrandt H, Cénac C, Villani A-C, Diefenbach TJ, Gall SL, Schwartz O, Herbeuval J-P, Autran B, Guéry J-C, Chang JJ, Altfeld M. Sex differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN-α production in women. J Immunol 2015;195:5327–5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, Wen TF, Lindsay RJ, Orellana L, Mildvan D, Bazner S, Streeck H, Alter G, Lifson JD, Carrington M, Bosch RJ, Robbins GK, Altfeld M. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 2009;15:955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhao R, Chen X, Ma W, Zhang J, Guo J, Zhong X, Yao J, Sun J, Rubinfien J, Zhou X, Wang J, Qi H. A GPR174–CCL21 module imparts sexual dimorphism to humoral immunity. Nature 2020;577:416–420. [DOI] [PubMed] [Google Scholar]
- 75. Wang J, Syrett CM, Kramer MC, Basu A, Atchison ML, Anguera MC. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc Natl Acad Sci Usa U S A 2016;113:E2029–E2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sarmiento L, Svensson J, Barchetta I, Giwercman A, Cilio CM. Copy number of the X-linked genes TLR7 and CD40L influences innate and adaptive immune responses. Scand J Immunol 2019;90:e12776. [DOI] [PubMed] [Google Scholar]
- 77. Blanco-Melo D, Nilsson-Payant BE, Liu W-C, Uhl S, Hoagland D, Møller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA, tenOever BR. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020;181:1036–1045.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, Péré H, Charbit B, Bondet V, Chenevier-Gobeaux C, Breillat P, Carlier N, Gauzit R, Morbieu C, Pène F, Marin N, Roche N, Szwebel T-A, Merkling SH, Treluyer J-M, Veyer D, Mouthon L, Blanc C, Tharaux P-L, Rozenberg F, Fischer A, Duffy D, Rieux-Laucat F, Kernéis S, Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020;369:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lokugamage KG, Schindewolf C, Menachery VD. SARS-CoV-2 is sensitive to type I interferon pretreatment. bioRxiv https://www.biorxiv.org/content/10.1101/2020.03.07.982264v3; doi:10.1101/2020.03.07.982264.
- 80. Made CVD, Simons A, Schuurs-Hoeijmakers J, Heuvel GVD, Mantere T, Kersten S, Deuren RV, Steehouwer M, Reijmersdal SV, Jaeger M, Hofste T, Astuti G, Galbany JC, Schoot VVD, Hoeven HVD, Have WOT, Klijn E, Meer CVD, Fiddelaers J, Mast QD, Bleeker-Rovers CP, Joosten LAB, Yntema HG, Gilissen C, Nelen M, Meer JVD, Brunner HG, Netea MG, Veerdonk FVD, Hoischen A. Presence of genetic variants among young men with severe COVID-19. JAMA 2020;324:663–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Martin GV, Kanaan SB, Hemon MF, Azzouz DF, El Haddad M, Balandraud N, Mignon-Ravix C, Picard C, Arnoux F, Martin M, Roudier J, Auger I, Lambert NC. Mosaicism of XX and XXY cells accounts for high copy number of Toll like Receptor 7 and 8 genes in peripheral blood of men with Rheumatoid Arthritis. Sci Rep 2019;9:12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Webb K, Peckham H, Radziszewska A, Menon M, Oliveri P, Simpson F, Deakin CT, Lee S, Ciurtin C, Butler G, Wedderburn LR, Ioannou Y. Sex and pubertal differences in the type 1 interferon pathway associate with both X chromosome number and serum sex hormone concentration. Front Immunol 2018;9:3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020;20:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shi C-S, Qi H-Y, Boularan C, Huang N-N, Abu-Asab M, Shelhamer JH, Kehrl JH. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J Immunol 2014;193:3080–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Frieman M, Yount B, Heise M, Kopecky-Bromberg SA, Palese P, Baric RS. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J Virol 2007;81:9812–9824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Frieman M, Ratia K, Johnston RE, Mesecar AD, Baric RS. Severe acute respiratory syndrome coronavirus papain-like protease ubiquitin-like domain and catalytic domain regulate antagonism of IRF3 and NF-kappaB signaling. J Virol 2009;83:6689–6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sun L, Xing Y, Chen X, Zheng Y, Yang Y, Nichols DB, Clementz MA, Banach BS, Li K, Baker SC, Chen Z. Coronavirus papain-like proteases negatively regulate antiviral innate immune response through disruption of STING-mediated signaling. PLoS One 2012;7:e30802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Versteeg GA, Bredenbeek PJ, Worm SVD, Spaan WJM. Group 2 coronaviruses prevent immediate early interferon induction by protection of viral RNA from host cell recognition. Virology 2007;361:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Siu K-L, Chan C-P, Kok K-H, Chiu-Yat Woo P, Jin D-Y. Suppression of innate antiviral response by severe acute respiratory syndrome coronavirus M protein is mediated through the first transmembrane domain. Cell Mol Immunol 2014;11:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Narayanan K, Huang C, Lokugamage K, Kamitani W, Ikegami T, Tseng C-TK, Makino S. Severe acute respiratory syndrome coronavirus nsp1 suppresses host gene expression, including that of type I interferon, in infected cells. J Virol 2008;82:4471–4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Thoms M, Buschauer R, Ameismeier M, Koepke L, Denk T, Hirschenberger M, Kratzat H, Hayn M, Mackens-Kiani T, Cheng J, Straub JH, Stürzel CM, Fröhlich T, Berninghausen O, Becker T, Kirchhoff F, Sparrer KMJ, Beckmann R. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science 2020;369:1249–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kikkert M. Innate immune evasion by human respiratory RNA viruses. J Innate Immun 2020;12:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lei X, Dong X, Ma R, Wang W, Xiao X, Tian Z, Wang C, Wang Y, Li L, Ren L, Guo F, Zhao Z, Zhou Z, Xiang Z, Wang J. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat Commun 2020;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Konno Y, Kimura I, Uriu K, Fukushi M, Irie T, Koyanagi Y, Sauter D, Gifford RJ, Nakagawa S, Sato K. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep 2020;32:108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jiang H, Zhang H, Meng Q, Xie J, Li Y, Chen H, Zheng Y, Wang X, Qi H, Zhang J, Wang P-H, Han Z-G, Tao S. SARS-CoV-2 Orf9b suppresses type I interferon responses by targeting TOM70. Cell Mol Immunol 2020;17:998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Xu K, Chen Y, Yuan J, Yi P, Ding C, Wu W, Li Y, Ni Q, Zou R, Li X, Xu M, Zhang Y, Zhao H, Zhang X, Yu L, Su J, Lang G, Liu J, Wu X, Guo Y, Tao J, Shi D, Yu L, Cao Q, Ruan B, Liu L, Wang Z, Xu Y, Liu Y, Sheng J, Li L. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin Infect Dis 2020;71:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, Xie G, Lin S, Wang R, Yang X, Chen W, Wang Q, Zhang D, Liu Y, Gong R, Ma Z, Lu S, Xiao Y, Gu Y, Zhang J, Yao H, Xu K, Lu X, Wei G, Zhou J, Fang Q, Cai H, Qiu Y, Sheng J, Chen Y, Liang Tet al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ 2020;369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, Silva J, Mao T, Oh JE, Tokuyama M, Lu P, Venkataraman A, Park A, Liu F, Meir A, Sun J, Wang EY, Casanovas-Massana A, Wyllie AL, Vogels CBF, Earnest R, Lapidus S, Ott IM, Moore AJ, Shaw A, Fournier JB, Odio CD, Farhadian S, Dela Cruz C; Yale IMPACT Research Team. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020; doi:10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sallard E, Lescure F-X, Yazdanpanah Y, Mentre F, Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res 2020;178:104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, Nyberg LM, Lee WM, Ghalib RH, Schiff ER, Galati JS, Bacon BR, Davis MN, Mukhopadhyay P, Koury K, Noviello S, Pedicone LD, Brass CA, Albrecht JK, Sulkowski MS; IDEAL Study Team. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med 2009;361:580–593. [DOI] [PubMed] [Google Scholar]
- 101. Suba Z. Prevention and therapy of COVID-19 via exogenous estrogen treatment for both male and female patients. J Pharm Pharm Sci 2020;23:75–85. [DOI] [PubMed] [Google Scholar]
- 102. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, Bucci E, Piacentini M, Ippolito G, Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ 2020;27:1451–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun 2020;111:102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, Li B, Song X, Zhou X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol 2020;127:104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ortona E, Pierdominici M, Maselli A, Veroni C, Aloisi F, Shoenfeld Y. Sex-based differences in autoimmune diseases. Ann Ist Super Sanita 2016;52:205–212. [DOI] [PubMed] [Google Scholar]
- 107. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Li J, Wang Q, Zheng W, Ma J, Zhang W, Wang W, Tian X. Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine 2014;93:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Otrock ZK, Eby CS. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol 2015;90:220–224. [DOI] [PubMed] [Google Scholar]
- 110. Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, Perlman S. Dysregulated type i interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 2016;19:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wang N, Zhan Y, Zhu L, Hou Z, Liu F, Song P, Qiu F, Wang X, Zou X, Wan D, Qian X, Wang S, Guo Y, Yu H, Cui M, Tong G, Xu Y, Zheng Z, Lu Y, Hong P. Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients. Cell Host Microbe 2020;28:455–464.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. McFadyen JD, Stevens H, Peter K. The emerging threat of (micro)thrombosis in COVID-19 and its therapeutic implications. Circ Res 2020;127:571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, Galli M, Catena E, Tosoni A, Gianatti A, Nebuloni M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 2020;20:1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Akhmerov A, Marbán E. COVID-19 and the Heart. Circ Res 2020;126:1443–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Batty GD, Hamer M. Vascular risk factors, Framingham risk score, and COVID-19: community-based cohort study. Cardiovasc Res 2020;116:1664–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Perrino C, Ferdinandy P, Bøtker HE, Brundel BJJM, Collins P, Davidson SM, Ruijter HD, Engel FB, Gerdts E, Girao H, Gyöngyösi M, Hausenloy DJ, Lecour S, Madonna R, Marber M, Murphy E, Pesce M, Regitz-Zagrosek V, Sluijter JPG, Steffens S, Gollmann-Tepeköylü C, Van Laake LW, Van Linthout S, Schulz R, Ytrehus K. Improving translational research in sex-specific effects of comorbidities and risk factors in ischaemic heart disease and cardioprotection: position paper and recommendations of the ESC Working Group on Cellular Biology of the Heart. Cardiovasc Res 2020; doi: 10.1093/cvr/cvaa155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie M-A, Dubé M-P, Rhainds D, Provencher M, Blondeau L, Orfanos A, L’Allier PL, Guertin M-C, Roubille F. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 119. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 120. Tong DC, Quinn S, Nasis A, Hiew C, Roberts-Thomson P, Adams H, Sriamareswaran R, Htun NM, Wilson W, Stub D, van GW, Howes L, Collins N, Yong AS, Bhindi R, Whitbourn R, Lee A, Hengel C, Asrress K, Freeman M, Amerena J, Wilson A, Layland J. Colchicine in patients with acute coronary syndrome: the Australian COPS Randomized Clinical Trial. Circulation 2020; doi:10.1161/CIRCULATIONAHA.120.050771. [DOI] [PubMed] [Google Scholar]
- 121. Opstal TSJ, Hoogeveen RM, Fiolet ATL, Silvis MJM, Bax WA, Kleijn DD, Mosterd A, Stroes ESG, Cornel JH; The SHK. Colchicine attenuates inflammation beyond the inflammasome in chronic coronary artery disease: a LoDoCo2 proteomic substudy. Circulation 2020; doi:10.1161/CIRCULATIONAHA.120.050560. [DOI] [PubMed] [Google Scholar]
- 122. Freeman TL, Swartz TH. Targeting the NLRP3 inflammasome in severe COVID-19. Front Immunol Frontiers 2020;11: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rodrigues TS, Sa KS, Ishimoto AY, Becerra A, Oliveira S, Almeida L, Goncalves AV, Perucello DB, Andrade WA, Castro R, Veras FP, Toller-Kawahisa JE, Nascimento DC, Lima MD, Silva CM, Caetite DB, Martins RB, Castro IA, Pontelli MC, Barros FD, Amaral N D, Giannini MC, Bonjorno LP, Lopes MIF, Benatti MN, Santana RC, Vilar FC, Auxiliadora-Martins M, Luppino-Assad R, Almeida SD, de Oliveira FR, Batah SS, Siyuan L, Benatti MN, Cunha TM, Alves-Filho JC, Cunha FQ, Larissa D Cunha, Frantz FG, Kohlsdorf T, Fabro AT, Arruda E, de Oliveira RDR, Louzada-Junior P, Zamboni DS. Inflammasome activation in COVID-19 patients. medRxiv 2020; doi:10.1101/2020.08.05.20168872. [Google Scholar]
- 124. Cavalli G, Luca GD, Campochiaro C, Della-Torre E, Ripa M, Canetti D, Oltolini C, Castiglioni B, Din CT, Boffini N, Tomelleri A, Farina N, Ruggeri A, Rovere-Querini P, Lucca GD, Martinenghi S, Scotti R, Tresoldi M, Ciceri F, Landoni G, Zangrillo A, Scarpellini P, Dagna L. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol 2020;2:e325–e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Cauchois R, Koubi M, Delarbre D, Manet C, Carvelli J, Blasco VB, Jean R, Fouche L, Bornet C, Pauly V, Mazodier K, Pestre V, Jarrot P-A, Dinarello CA, Kaplanski G. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci USA 2020;117:18951–18953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Campochiaro C, Dagna L. The conundrum of interleukin-6 blockade in COVID-19. Lancet Rheumatol 2020;2:e579–e580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Biran N, Ip A, Ahn J, Go RC, Wang S, Mathura S, Sinclaire BA, Bednarz U, Marafelias M, Hansen E, Siegel DS, Goy AH, Pecora AL, Sawczuk IS, Koniaris LS, Simwenyi M, Varga DW, Tank LK, Stein AA, Allusson V, Lin GS, Oser WF, Tuma RA, Reichman J, Brusco L, Carpenter KL, Costanzo EJ, Vivona V, Goldberg SL. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol 2020;2:e603–e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol 2019;19:517–532. [DOI] [PubMed] [Google Scholar]
- 129. Mikolajczyk TP, Guzik TJ. Adaptive immunity in hypertension. Curr Hypertens Rep 2019;21:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Krishnan SM, Ling YH, Huuskes BM, Ferens DM, Saini N, Chan CT, Diep H, Kett MM, Samuel CS, Kemp-Harper BK, Robertson AAB, Cooper MA, Peter K, Latz E, Mansell AS, Sobey CG, Drummond GR, Vinh A. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc Res 2019;115:776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Gao C, Cai Y, Zhang K, Zhou L, Zhang Y, Zhang X, Li Q, Li W, Yang S, Zhao X, Zhao Y, Wang H, Liu Y, Yin Z, Zhang R, Wang R, Yang M, Hui C, Wijns W, McEvoy JW, Soliman O, Onuma Y, Serruys PW, Tao L, Li F. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J 2020;41:2058–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zaldivia MTK, Rivera J, Hering D, Marusic P, Sata Y, Lim B, Eikelis N, Lee R, Lambert GW, Esler MD, Htun NM, Duval J, Hammond L, Eisenhardt SU, Flierl U, Schlaich MP, Peter K. Renal denervation reduces monocyte activation and monocyte-platelet aggregate formation: an anti-inflammatory effect relevant for cardiovascular risk. Hypertension 2017;69:323–331. [DOI] [PubMed] [Google Scholar]
- 133. Zaldivia MTK, Hering D, Marusic P, Sata Y, Lee R, Esler MD, Htun NM, Duval J, Hammond L, Flierl U, Wang X, Drummond GR, Walton A, Gardiner EE, Andrews RK, Schlaich MP, Peter K. Successful renal denervation decreases the platelet activation status in hypertensive patients. Cardiovasc Res 2020;116:202–210. [DOI] [PubMed] [Google Scholar]
- 134. Youn J-C, Yu HT, Lim BJ, Koh MJ, Lee J, Chang D-Y, Choi YS, Lee S-H, Kang S-M, Jang Y, Yoo OJ, Shin E-C, Park S. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 2013;62:126–133. [DOI] [PubMed] [Google Scholar]
- 135. Mathew D, Giles JR, Baxter AE, Greenplate AR, Wu JE, Alanio C, Oldridge DA, Kuri-Cervantes L, Pampena MB, D’Andrea K, Manne S, Chen Z, Huang YJ, Reilly JP, Weisman AR, Ittner CAG, Kuthuru O, Dougherty J, Nzingha K, Han N, Kim J, Pattekar A, Goodwin EC, Anderson EM, Weirick ME, Gouma S, Arevalo CP, Bolton MJ, Chen F, Lacey SF, Hensley SE, Apostolidis S, Huang AC, Vella LA, The UPenn COVID Processing Unit, Betts MR, Meyer NJ, Wherry EJet al. Deep immune profiling of COVID-19 patients reveals patient heterogeneity and distinct immunotypes with implications for therapeutic interventions. BioRxiv Prepr Serv Biol 2020; doi:10.1101/2020.05.20.106401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sattar N, McInnes IB, McMurray JJV. Obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation 2020;142:4–6. [DOI] [PubMed] [Google Scholar]
- 137. Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, Labreuche J, Mathieu D, Pattou F, Jourdain M, Caizzo R, Caplan M, Cousin N, Duburcq T, Durand A, El Kalioubie A, Favory R, Garcia B, Girardie P, Goutay J, Houard M, Jaillette E, Kostuj N, Ledoux G, Mathieu D, Moreau AS, Niles C, Nseir S, Onimus T, Parmentier E, Préau S, Robriquet L, Rouze A, Six S, Verkindt H; The LICORN and the Lille COVID‐19 and Obesity study group. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obes Silver Spring Md 2020;28:1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, Stachel A. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis 2020;71:896–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011;121:2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol 2015;402:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol 2020;20:442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]