Abstract
Background
Although the vast majority of individuals succumbing to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are elderly, infection fatality rate (IFR) estimates for the age group ≥70 years are still scarce. To this end, we assessed SARS-CoV-2 seroprevalence among retired blood donors and combined it with national coronavirus disease 2019 (COVID-19) survey data to provide reliable population-based IFR estimates for this age group.
Methods
We identified 60 926 retired blood donors aged ≥70 years in the rosters of 3 regionwide Danish blood banks and invited them to fill in a questionnaire on COVID-19–related symptoms and behaviors. Among 24 861 (40.8%) responders, we invited a random sample of 3200 individuals for blood testing. Overall, 1201 (37.5%) individuals were tested for SARS-CoV-2 antibodies (Wantai) and compared with 1110 active blood donors aged 17–69 years. Seroprevalence 95% confidence intervals (CIs) were adjusted for assay sensitivity and specificity.
Results
Among retired (aged ≥70 years) and active (aged 17–69 years) blood donors, adjusted seroprevalences were 1.4% (95% CI, .3–2.5%) and 2.5% (95% CI, 1.3–3.8%), respectively. Using available population data on COVID-19–related fatalities, IFRs for patients aged ≥70 years and for 17–69 years were estimated at 5.4% (95% CI, 2.7–6.4%) and .083% (95% CI, .054–.18%), respectively. Only 52.4% of SARS-CoV-2–seropositive retired blood donors reported having been sick since the start of the pandemic.
Conclusions
COVID-19 IFR in the age group >69 years is estimated to be 65 times the IFR for people aged 18–69 years.
Keywords: COVID-19, infection fatality rate, epidemiology, SARS-Cov-2 antibody test, SARS-CoV-2 seroprevalence
COVID-19 infection fatality rate was 5.4% and 0.083% in the age group >69 years and 17–69 years, respectively. SARS-CoV-2 seropositives were generally less inclined to follow official recommendations against spread of disease. Only 52.4% reported to have been sick from COVID-19.
Since early in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic it has been clear that comorbidities such as diabetes mellitus, chronic cardiovascular, obstructive pulmonary, and kidney diseases are common among patients who are hospitalized for coronavirus disease 2019 (COVID-19) and among patients who succumb to the infection [1–3].
The comorbidities accumulating among deceased patients with COVID-19 are mostly prevalent in the elderly population [1]. Because of comorbidity and age-dependent frailty, the COVID-19 infection fatality rate (IFR) presumably is higher among older than among younger adults, but solid data to support this assumption are lacking.
Infection fatality rate may be approximated by relating age-specific number of COVID-19 deaths with corresponding measures of SARS-CoV-2 prevalence [4–6]. So far, only few studies have assessed how SARS-CoV-2 has spread among the elderly in the general population and with mixed results [7–10]. While a Spanish survey [8] found no strong association between age and SARS-CoV-2 seroprevalence, both a Swiss and a US investigation generally reported lower seroprevalence in the age group above 65 years than among younger adults [7, 10].
The aim of this study was to determine SARS-CoV-2 seroprevalence in an elderly retired blood-donor population in Denmark and to estimate the associated IFR for this age group. This knowledge will help tailor public health policies mitigating the impact of the pandemic.
METHODS
Study Design and Participants
We contacted 60 926 elderly retired blood donors in 3 out of the 5 Danish administrative regions (the Danish Capital Region, the Zealand Region, and the Central Denmark Region). We have previously found that these regions had the lowest (0.7%) and highest (3.0%) seroprevalence of SARS-CoV-2 antibodies among active blood donors aged 17–69 years [4].
The 60 926 retired blood donors aged 70 years or older were invited to complete a digitalized questionnaire on COVID-19 symptoms and risk factors between 16 May and 25 May 2020. Within 2 weeks of the invitation, 24 861 (40.8%) donors had filled in the questionnaire.
In order to compare the symptoms and behavior of the older retired blood donors with younger active donors, we also mailed the questionnaire electronically to 75 934 participants in the Danish Blood Donor Study from all Danish regions aged 18–69 years on 28 May 2020 [11]. A total of 24 227 (31.9%) invitees responded to this questionnaire.
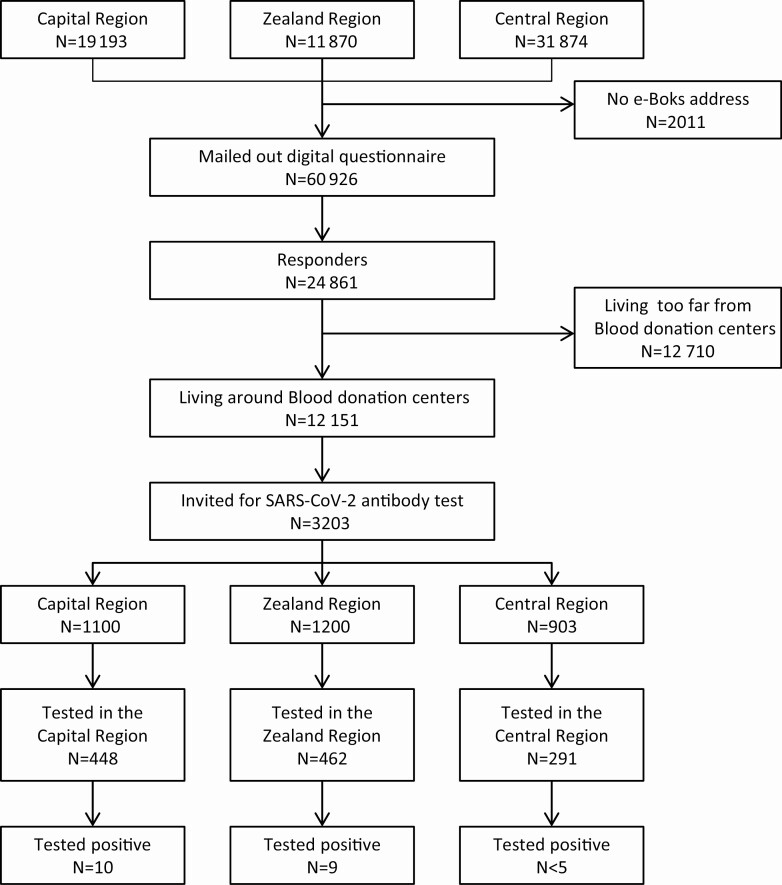
From among the retired blood donors aged 70 years or older, who completed the questionnaire, we invited a random sample of 3203 individuals living within geographical proximity of 12 bleeding sites (2 in the Central Denmark Region, 6 in the Zealand Region, and 4 in the Capital Region) for serological SARS-CoV-2 antibody measurements. The number of retired blood donors invited was determined by capacity for blood sampling in the different regions.
A total of 1201 (38%) of the invited retired blood donors showed up for testing between 2 and 19 June 2020 (Figure 1). Characteristics of the cohort in the different steps are summarized in Table 1.
Figure 1.
Patient testing flowchart. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1.
Cohort Age and Sex Distribution at Different Selection Steps
| Received Questionnaire (n = 60 926) | Responded to Questionnaire (n = 24 861) | P | Eligible for Testinga (n = 12 151) | Tested for SARS-CoV-2 Antibodies (n = 1201) | P | |
|---|---|---|---|---|---|---|
| Age, median (25th, 75th percentile), y | 75 (72,79) | 73 (71,76) | <.0001b | 73 (71,77) | 73 (71,76) | .002b |
| Women, n (%) | 26 451 (43.4) | 10 519 (42.3) | .44c | 5288 (43.5) | 517 (43.1) | .78c |
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aPersons living within a 10–20-km radius of a testing station.
bMann-Whitney U test of difference in median age.
cChi-square test of difference in percentage women.
For comparison, we also tested a random sample of 360, 250, and 500 active blood donors (aged 17–69 years) who had given blood between 1 and 12 June 2020 in the Central Denmark Region and between 22 and 26 June 2020 in the Zealand and Capital Regions, respectively. This testing was done after anonymization of samples.
All 1201 elderly participants gave informed consent to participate in the Danish Blood Donor Study before samples were taken for SARS-CoV-2 antibody measurements. The study was approved by the Zealand Regional Committee on Health Research Ethics (approval number: SJ-740) and the data protection agency of the Capital Region (P-2019-99). According to Danish legislation, analysis of anonymous material does not require consent.
Questionnaire Data
The questionnaire included the following items: symptoms of disease during the previous 3 months, including fever, nasal symptoms (sneezing, affected sense of smell and/or taste), airways (sore throat, coughing, shortness of breath), and abdominal discomfort (diarrhea, vomiting); changes in behavior including hand-washing, using a handkerchief, sneezing in the elbow, avoiding handshakes, wearing a facemask, avoiding hugging people, reduced use of public transport, avoiding crowded places, and staying at home; and history of previous polymerase chain reaction (PCR) test for COVID-19 and the test result. The questions on disease symptoms were graded on a 4-point scale ranging from no symptoms (1); yes, a few (2); yes, a lot (3); and yes, many (4). The respondents were also allowed to answer “I do not know.” For analysis, we dichotomized the scale into none (1) versus any (2–4); if the response was “I do not know” this answer was omitted from the analysis.
Laboratory Measurements
SARS-CoV-2 antibodies were measured on EDTA (ethylenediaminetetraacetic acid) plasma samples using a double-sandwich enzyme-linked immunosorbent assay (ELISA) to estimate antibodies regardless of immunoglobulin type (IgTotal) (catalog no. WS-1096; Beijing Wantai Biological Pharmacy Enterprise, Beijing, China). We have previously estimated the sensitivity and specificity of this assay to be 96.7% (95% confidence interval [CI], 92.4–98.6%) and 99.5% (95% CI, 98.7–99.8%), respectively. This estimate was obtained through a nationwide validation effort across laboratories in Denmark on 150 SARS-CoV-2 PCR-positive samples covering the disease spectrum from mild (24.6%) and moderate (50%) to severe (20.6%) symptoms and 650 control samples (L Harritshøj 2020, unpublished data). One of the 1201 ELISA measurements in the present investigation was inconclusive and omitted from analysis.
Population Data and COVID-19 Surveillance Data
The samples for this study were collected between 2 and 19 June 2020. Public surveillance data on the number of individuals tested for SARS-CoV-2, hospitalized for COVID-19, and dying of COVID-19 are updated daily from Statens Serum Institute [12]. We retrieved population statistics from Statistics Denmark based on the Danish population in the first quarter of 2020 [13].
Statistical Analysis
Self-reported risk factors and symptoms were reported as percentages with 99.8% CIs because of multiple testing (exact CIs), and for differences between groups we reported the risk ratios (RRs). To compare age and sex compositions between groups we used Mann-Whitney U test and chi-square tests. We used the Rogan Gladen estimate to calculate the true prevalence. Confidence intervals were derived by 108-sample percentile bootstrapping independently of sampling sensitivity, specificity, and apparent prevalence using posterior binomial distributions based on the observations. Prevalences were reported as percentages with 95% CIs. The analysis was performed in RStudio 1.2 and R 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) using the EpiR package to adjust measured seroprevalence for sensitivity and specificity of the diagnostic assay as well as weighing the estimate based on population size of the bleeding sites’ recruitment areas. The weights of the geographical areas were based on the number of inhabitants in the municipalities (exact weights can be found in Supplementary Table 1).
RESULTS
The age and sex distributions of the different selection steps in the present investigation are summarized in Table 1.
A total of 24 861 (40.9%) retired blood donors aged 70 years or older and a total of 24 227 (31.9%) active blood donors aged 17–69 years answered the questionnaire on health behaviors and COVID-19 symptoms (Table 2). Adherence to official recommendations varied between 40% (staying at home) and 95% (frequent hand-washing) among the retired blood donors. For the same items, adherence among the younger active donors varied between 31% (staying at home) and 91% (frequent hand-washing), with estimates generally being lower than among the elderly retired donors (Table 2). By June 2020, use of facemasks was neither recommended nor mandatory except under certain circumstances and therefore infrequently reported by either group of blood donors (1.8%).
Table 2.
Questionnaire Data on Active Blood Donors Aged 18–69 Years and Retired Blood Donors Aged 70 Years or Older
| Responders Aged ≥70 Years | Responders Aged 18–69 Years | RRa (99.8% CI), P | |
|---|---|---|---|
| Total N | 24 861 | 24 722 | |
| Women, n (%) | 10 519 (42.3) | 13 709 (55.5) | |
| Symptoms and tests | |||
| Sick within the last 3 m | 12.7 (12.1–13.4), 24 302 | 21.9 (21.0–22.7), 24 475 | .70 (.66–.73), <.0001 |
| Previous test for SARS-CoV-2 | 17.1 (15.1–19.3), 3080 | 35.8 (33.8–37.9), 5319 | .51 (.45–.58), <.0001 |
| Previous positive SARS-CoV-2 test | 4.3 (1.9–8.0), 467 | 7.2 (5.5–9.3), 1839 | .63 (.32–1.21), .02 |
| Health behavior | |||
| Washing hands often | 94.6 (94.1–95.0), 24 861 | 90.9 (90.4–91.5), 24 722 | 1.36 (1.27–1.45), <.0001 |
| Using handkerchief | 42.7 (41.7–43.7), 24 861 | 14.7 (14.0–15.4), 24 722 | 1.85 (1.80–1.90), <.0001 |
| Sneezing in the elbow | 77.0 (76.2–77.8), 24 861 | 81.2 (80.5–82.0), 24 722 | .88 (.86–.91), <.0001 |
| Avoiding handshake | 93.5 (93.0–94.0), 24 861 | 91.7 (91.1–92.2), 24 722 | 1.15 (1.09–1.22), <.0001 |
| Using facemask | 1.8 (1.6–2.1), 24 861 | 1.8 (1.6–2.1), 24 722 | .99 (.90–1.10), .85 |
| Avoiding hugging people | 83.8 (83.0–84.5), 24 861 | 83.7 (83.0–84.5), 24 722 | 1.00 (.96–1.04), .93 |
| Reduced use of public transport | 46.3 (45.4–47.3), 24 861 | 33.9 (32.9–34.8), 24 722 | 1.29 (1.25–1.33), <.0001 |
| Avoiding crowded places | 75.5 (74.7–76.4), 24 861 | 67.4 (66.4–68.3), 24 722 | 1.23 (1.19–1.27), <.0001 |
| Staying home | 41.3 (40.3–42.3), 24 861 | 31.5 (30.6–32.4), 24 722 | 1.23 (1.20–1.26), <.0001 |
Unless otherwise stated, the data are presented as percentage that responded yes (99.8% CI), n. If n is different from the total number it is because not all responded to this item.
Abbreviations: CI, confidence interval; RR, risk ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aRR of difference in prevalence of symptoms or behavior between the 2 age groups, 99.8% CI, and P value.
Overall, 1201 (38%) elderly retired donors were tested for antibodies to SARS-CoV-2. Compared with non-invitees and nonattendees who had answered the questionnaire, those who were tested for SARS-CoV-2 antibodies were marginally more compliant with the official precautionary recommendations (Table 3). Of the 1200 elderly retired blood donors with a valid test result, 22 (1.8%) were positive for SARS-CoV-2 antibodies. The 22 SARS-CoV-2 antibody–positive individuals were more likely to report a history of symptoms compatible with COVID-19 compared with those who tested negative for SARS-CoV-2 antibodies (52.4% [99.8% CI, 20.1–83.3%] vs 15.1% [99.8% CI, 12.0–18.6%]), although individually, the distribution of specific symptoms was not statistically significantly different between the 2 groups (Table 3).
Table 3.
Self-reported Symptoms in Tested and Not Tested Participants Aged 70 Years or Older
| Responders Who Were Not Invited for Testing | Responders Invited for Testing But Did Not Accept Invitation | Tested Negative | Tested Positive | RRa (99.8% CI), P | |
|---|---|---|---|---|---|
| Total N | 21 658 | 2002 | 1178 | 22 | |
| Women, % (99.8% CI), n | 42.2 (41.1–43.2), 21 658 | 43.3 (39.9–46.8), 2002 | 42.9 (38.4–47.4), 1178 | 50 (19.0–81.0), 22 | 1.33 (.35–4.89), .50 |
| Symptoms and tests | |||||
| Sick within the last 3 mb | 12.6 (11.9–13.3), 21 170 | 12.1 (10.0–14.6), 1954 | 15.1 (12.0–18.6), 1156 | 52.4 (20.,1–83.3), 21 | 5.86 (1.55–22.1), <.0001 |
| Feverc | 51.9 (48.2–55.6), 1751 | 51.2 (38.9–63.4), 162 | 55.4 (41.0–69.1), 121 | 57.1 (7.7–96.8), 7 | 1.07 (.11–10.6), .93 |
| Running or blocked nosec | 72.6 (69.3–75.8), 1793 | 76.3 (64.6–85.7), 160 | 79.7 (66.6–89.4), 123 | 66.7 (16.3–97.6), 9 | .54 (.07–4.32), .35 |
| Loss of sense of smellc | 27.8 (24.2–31.7), 1379 | 27.5 (16.0–41.5), 120 | 32.7 (19.1–48.6), 98 | 66.7 (9.4–99.2), 6 | 3.78 (.28–50.8), .09 |
| Loss of sense of tastec | 26.3 (22.7–30.1), 1375 | 25.2 (14.3–38.8), 123 | 33.0 (19.3–49.0), 97 | 66.7 (9.4–99.2), 6 | 3.72 (.28–50.1), .09 |
| Sneezingc | 75.8 (72.4–78.9), 1712 | 80.5 (68.9–894), 149 | 76.7 (62.8–87.5), 116 | 50 (6.4–93.5), 8 | .33 (.04–2.69), .09 |
| Sore throatc | 58.5 (54.8–62.2), 1717 | 56.1 (43.4–68.3), 155 | 65.9 (51.6–78.3), 123 | 33.3 (2.4–83.7), 9 | .29 (.03–2.36), .05 |
| Coughingc | 77.8 (74.7–80.6), 1920 | 75.6 (64.2–85.0), 168 | 82.4 (70.2–91.2), 131 | 90 (37.6–99.9), 10 | 1.85 (.08–44.5), .54 |
| Dyspneac | 49.9 (46.1–53.7), 1643 | 44.4 (31.7–57.6), 142 | 36.1 (22.6–51.4), 108 | 37.5 (2.7–88.0), 8 | 1.05 (.12–9.32), .94 |
| Vomitingc | 8.7 (6.5–11.2), 1405 | 9.2 (3.2–19.6), 130 | 8.8 (2.5–20.8), 102 | 11.7 (0.0–71.1), 8 | 1.29 (.03–11.7), .82 |
| Diarrheac | 28.9 (25.3–32.7), 1491 | 36.7 (24.6–50.1), 139 | 27.4 (15.3–42.3), 106 | 11.1 (0.0–66.5), 9 | .35 (.01–8.79), .29 |
| Previous test for SARS-CoV-2b | 17.3 (15.2–19.6), 2660 | 14.9 (8.6–23.3), 235 | 14.4 (7.3–24.2), 174 | 18.2 (0.4–67.9), 11 | 1.30 (.13–13.3), .72 |
| Previous positive SARS-CoV-2 testb | 3.8 (1.6–7.5), 412 | 3.3 (0.0–26.9), 30 | 4.3 (0.0–33.7), 23 | 100 (3.1–100), 2 | |
| Health behavior | |||||
| Washing hands oftenb | 94.5 (94.1–95.0), 21 658 | 94.4 (92.6–95.9), 2003 | 95.6 (93.4–97.2), 1178 | 86.3 (52.7–99.1), 22 | .30 (.05–1.98), .04 |
| Using handkerchiefb | 42.7 (41.7–43.7), 21 658 | 44.2 (40.8–47.7), 2003 | 43.2 (38.8–47.7), 1178 | 40.9 (12.9–74.1), 22 | .91 (.24–3.44), .83 |
| Sneezing in the elbowb | 76.8 (76.0–77.7), 21 658 | 75.7 (72.6–78.6), 2003 | 81.1 (77.3–84.5), 1178 | 63.6 (29.7–89.8), 22 | .42 (.11–1.61), .04 |
| Avoiding handshakeb | 89.2 (88.6–89.8), 21 658 | 93.3 (91.4–94.9), 2003 | 94.4 (92.0–96.3), 1178 | 90.9 (58.5–99.8), 22 | .60 (.06–5.75), .48 |
| Using facemaskb | 1.8 (1.5–2.1), 21 658 | 2.0 (1.2–3.2), 2003 | 2.2 (1.1–3.9), 1178 | 0 (0–22.9), 22 | |
| Avoiding hugging peopleb | 83.6 (82.8–84.3), 21 658 | 84.7 (81.3–86.4), 2003 | 87.4 (84.2–90.2), 1178 | 54.5 (22.3–84.2), 22 | .18 (.05–.67), <.0001 |
| Reduced use of public transportb | 46.1 (45.1–47.1), 21 658 | 50.4 (47.0–53.9), 2003 | 52.0 (47.4–56.5), 1178 | 45.5 (15.8–77.7), 22 | .77 (.21–2.87), .55 |
| Avoiding crowded placesb | 75.5 (74.6–76.3), 21 658 | 76.3 (73.2–79.2), 2003 | 76.3 (72.3–80.0), 1178 | 68.2 (33.7–92.3), 22 | .67 (.17–2.72), .38 |
| Staying homeb | 41.5 (40.5–42.5), 21 658 | 41.9 (38.5–45.4), 2003 | 37.5 (33.2–42.0), 1178 | 45.5 (15.8–77.7), 22 | 1.38 (.37–5.11), .45 |
| Other risk factors | |||||
| Current smokingb | 8.7 (8.2–9.3), 21 557 | 8.2 (6.4–10.3), 1995 | 9.1 (6.7–11.9), 1178 | 9.1 (0.2–41.1), 22 | 1.01 (.10–9.69), 1.00 |
| Current alcohol consumption, median standard drinks/week (25%, 75%) | 5 (2,10) | 5 (2,10) | 5 (2,10) | 2 (0,5) |
Abbreviations: CI, confidence interval; RR, risk ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aRR of difference in prevalence of symptoms and behavior between those who tested positive and those who tested negative for SARS-CoV-2, 99.8% CI, and P value.
bPresented data are percentage “yes” (99.8% CI), n. When n does not equal the number in the group it is because not all responded to the item.
cData are percentage “more than a little” (99.8% CI), n. When n does not equal the number in the group it is because not all responded to the item.
For 4 out of 5 officially recommended precautions against spread of disease, self-reported compliance was lower among the elderly blood donors who tested positive for SARS-CoV-2 antibodies than among those who did not have antibodies, although this difference was statistically significant only for 1 recommendation (avoiding hugging or kissing on the cheeks) (54.5% [99.8% CI, 22.3–84.2%] vs 87.4% [99.8% CI, 84.2–90.2%]) (Table 3). With regard to staying at home as much as possible, compliance was higher among SARS-CoV-2–positive participants than among SARS-CoV-2–negative participants (Table 3).
The adjusted SARS-CoV-2 seropositivity prevalences for men and women combined among those 70 years or older were 2.2% (95% CI, .6–4.1%), 1.9% (95% CI, .4–3.7%), and .7% (95% CI, −.4–3.0%) in the Capital, Zealand, and Central Regions, respectively (Table 4). In these 3 regions we also tested for SARS-CoV-2 antibodies in an anonymized sample of active blood donors aged 17–69 years, yielding corresponding adjusted prevalences of 5.3% (95% CI, 2.3–9.2%), 2.5% (95% CI, .6–5.3%), and 1.1% (95% CI, −.3–2.8%), respectively.
Table 4.
SARS-CoV-2 Seropositivity in Retired Blood Donors Aged 70 Years or Older and Active Blood Donors Aged 17–69 Years
| Region | ||||
|---|---|---|---|---|
| Capital | Zealand | Central Denmark | Total | |
| Tested, ≥70 y, n | ||||
| Nonreactive, n | 439 | 452 | 288 | 1179 |
| Reactive, n | 10 | 9 | <5 | 22 |
| Total, n | 449 | 461 | 291 | 1201 |
| ≥70 years, seroprevalence | ||||
| Unadjusted, % | 2.2 (1.1–4.1) | 2.0 (0.9–3.7) | 1.0 (0.2–3.0) | 1.8 (1.2–2.8) |
| Adjusted, % | 2.2 (0.6–4.1) | 1.9 (0.4–3.7) | 0.7 (−0.4–3.0) | 1.4 (0.3–2.5) |
| Citizens, ≥70 y, n | 231 731 | 139 827 | 184 324 | 555 882 |
| Expected seropositives, n | 5098 (1390–9501) | 2656 (559–5174) | 1290 (0–5529) | 7782 (1668–13 897) |
| Confirmed cases, n | 1194 | 394 | 243 | 1831 |
| Ratio of confirmed cases to expected seropositives, % | 23.4 (12.6–85.9) | 14.8 (7.6–70.5) | 18.8 (4.4–100) | 23.5 (13.2–100) |
| Confirmed deaths, n | 311 | 107 | 60 | 478 |
| IFR, % | 6.1 (3.3–22.4) | 4.0 (2.1–19.1) | 4.7 (1.1–100) | 6.1 (3.4–28.6) |
| Tested, 17–69 y, n | ||||
| Nonreactive, n | 479 | 243 | 355 | 1077 |
| Reactive, n | 21 | 7 | 5 | 33 |
| Total, n | 500 | 250 | 360 | 1110 |
| 17–69 y, seroprevalence | ||||
| Unadjusted, % | 4.2 (2.6–6.3) | 2.8 (1.1–5.7) | 1.4 (0.5–3.2) | 3.0 (2.1–4.1) |
| Adjusted, % | 3.9 (2.1–6.0) | 2.5 (0.6–5.3) | 1.1 (−0.3–2.8) | 2.5 (1.3–3.8) |
| Citizens, 17–69 y, n | 1 268 536 | 544 560 | 887 939 | 2 701 035 |
| Expected seropositives, n | 49 472 (26 639–76 112) | 13 614 (3267–28 861) | 9767 (0–24 862) | 67 525 (35 113–102 639) |
| Confirmed cases, n | 5575 | 1543 | 1320 | 8438 |
| Ratio of confirmed cases to expected seropositives, % | 11.3 (7.3–20.9) | 11.3 (5.3–47.2) | 13.5 (5.3–100) | 12.5 (8.2–24.0) |
| Confirmed deaths, n | 45 | 17 | 9 | 71 |
| IFR, % | 0.09 (0.06 –0.17) | 0.12 (0.06–0.52) | 0.09 (0.04–100) | 0.11 (0.07–0.2) |
Abbreviations: IFR, infection fatality rate; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
These 3 regions represent the entire spectrum of regional-level COVID-19 seroprevalences detected in Denmark so far [4, 12]. Thus, according to official statistics, the 2 administrative regions not included in this investigation (the North and South Denmark Regions) have a slightly lower prevalence of patients treated for COVID-19 than the Central Region [12].
Assuming that the SARS-CoV-2 seroprevalence observed for the Central Region in the present investigation also applies to the North and South Regions, and that blood donors are representative of the general population, we estimate an adjusted nationwide SARS-CoV-2 seroprevalence of 2.2% (95% CI, 1.0–3.4%) in the age group of 17–69 years and of 1.2% (95% CI, .1–2.4%) among individuals aged 70 years or older in the Danish population. For the entire age group aged 17 years or older the adjusted seroprevalence was 1.6% (95% CI, .58–2.5%).
On 22 June 2020, the official nationwide number of individuals aged 70 years or older registered with SARS-CoV-2 since the beginning of the pandemic was 2180. The seroprevalence estimated in the present investigation suggests that this number corresponds to only 21.6% (95% CI, 10.8–28.9%) of all COVID-19 cases in the age group at the time.
As of 22 June, the total number of registered deaths in Denmark from COVID-19 was 71 and 542 for the age group below and above 70 years, respectively. Given the estimated seroprevalences, these numbers correspond to IFRs of .083% (95% CI, .054–.18%) and 5.4% (95% CI, 2.7–64.0%) among people aged 17–69 years and aged 70 years or older, respectively. Thus, the IFR among people aged 70 years or older is 65 (95% CI, 40–356) times the IFR among people aged 17–69 years. The IFR for the adult Danish population aged 17 years or older was .81% (95% CI, .52–2.2%).
DISCUSSION
This Danish study on health behavior, COVID-19 symptoms, and SARS-CoV-2 antibody prevalence among retired and active blood donors suggests that older adults (here, those aged 70 years or older) in general are more likely to adhere to official recommendations to reduce SARS-CoV-2 transmission than younger adults (here, those aged 17–69 years). Consistent with this age-dependent difference in guideline adherence, our study also indicated that the SARS-CoV-2 seroprevalence in Denmark is lower (1.2%) among older than among younger (2.2%) adults, even though this difference was not statistically significant. In support of the suspected underlying mechanism, questionnaire data showed that older retired blood donors who tested positive for SARS-CoV-2 antibodies were less likely to have adhered to official recommendations to prevent COVID-19 than older retired blood donors testing negative for antibodies. Overall, our data indicate that the IFR for COVID-19 among individuals aged 70 years or older is 5.4% (95% CI, 2.7–64%)—that is, 65 times that estimated for younger adults aged 17–69 years.
In our study, only about half of the older retired blood donors who tested positive for SARS-CoV-2 antibodies recollected feeling sick with COVID-19 symptoms since the start of the pandemic. This proportion of apparently asymptomatic infections with SARS-CoV-2 is in the same range as in previous reports from both the United States and South America [14, 15]. Although based on a small number of SARS-CoV-2–seropositive participants and even disregarding potential underreporting of symptoms in our investigation, our findings show that, also among the elderly, the course of COVID-19 is highly variable and that silent sero-converters may not be a negligible source of infection.
Only a small minority of participants with self-reported COVID-19 symptoms reported having had a PCR test for SARS-CoV-2 and even fewer had had a positive PCR test. This could be the result of the early Danish recommendation of staying home and not consulting one’s general practitioner if sick to avoid virus transmission to frail patients. Regardless, our findings suggest that individuals with COVID-19 may previously have escaped detection by the testing efforts implemented by Danish health authorities. This notion is further corroborated by the seemingly small fraction (21.6%) of SARS-CoV-2 infections among the elderly recognized by the health authorities as estimated from the present study.
We surveyed health-related behavior among the participants in our study. The official Danish recommendations are as follows: (1) stay home if you are sick, (2) clean your hands regularly, (3) sneeze in your elbow, (4) avoid hugging or close contact, and (5) avoid crowded places. The Danish political interventions towards the COVID-19 pandemic are summarized in Supplementary Table 2. In our investigation, retired blood donors aged 70 years or older who tested positive for SARS-CoV-2 antibodies had been less inclined (54.5–90.9%) to follow official recommendations than those who tested negative (76.6–95.6%), with the exception of staying at home more. Whether this tendency to stay at home more has resulted in a more relaxed attitude towards other precautions cannot be ruled out. This finding may also be an example of reverse causality—that is, individuals who experience symptoms may be more likely to report that they stayed at home. Regardless, although based on small numbers, the association between taking SARS-CoV-2 precautions and testing negative for antibodies is reassuring in terms of their effectiveness.
As of now there are only few studies on health behavior and compliance with recommendations and their impact on COVID-19. A previous questionnaire-based study from 27 different countries including Denmark reported that elderly people aged 70 years or older were less compliant than people aged 60–70 years [16]. We cannot confirm this finding in our study; rather, older retired donors tended to adhere more strongly to official guidelines than younger donors.
Compared with retired blood donors aged 70 years or older, active blood donors aged 17–69 years twice as often reported having been sick with COVID-19 symptoms (21.9% vs 12.7%), having been tested for SARS-CoV-2 (35.8% vs 17.1% of those reporting sickness), and having had a positive SARS-CoV-2 test (7.2% vs 4.3% of those reporting test results). These differences in both COVID-19 prevalence and in test seeking may reflect age-dependent variations in mobility and in physical distancing, also apparent in the present study. Whereas precautions to limit the spread of SARS-CoV-2 with regard to hand-washing, hugging, and handshaking were followed by large proportions of both active and retired donors, active donors aged 18–69 years did not stay home or avoid public transport or crowded places to the same extent as the retired blood donors aged 70 years or older. It is not surprising that individuals of working age and with children at home face problems isolating themselves from other people. This could expose them to more infectious agents and explain why the active donors report much higher disease rates than the retired donors.
Based on official Danish reports on COVID-19–related deaths, we estimated the IFR to be 5.4% in the age group of 70 years or older. This was 65 times the IFR among active blood donors aged 17–69 years. Our IFR estimate among older individuals was only slightly lower than the 6.9% recently reported from rural settings in Latin America [17]. These estimates for the elderly population are, as expected, much higher than previously reported estimates for all ages from Greece [18], China [19], and cruise ships [20] as well as the estimate we reported in the younger donor population [4].
The strength of the present study is that we have combined questionnaire data and serological SARS-CoV-2 measurements on a large population of retired Danish blood donors aged 70 years or older. In particular, blood donors are more inclined to participate in public health studies than other Danes (T. Brodersen, 2019, unpublished results) and are also familiar with the process of blood sampling, both factors adding to the participation rate. Geographically, we carried out the investigation in 3 of the 5 Danish administrative regions to ensure that regional variation in COVID-19 prevalence was represented. Indeed, we found the same geographical variation in SARS-CoV-2 seroprevalence in the older age group as that we have reported among Danish blood donors aged 17–69 years [4], which also mirrors the official incidence of COVID-19 across Denmark. Active blood donors resemble the average general population, with the exception of people with low income, people who are marginalized, and men who have had sex with men [21]. We also know that the mortality among blood donors relative quickly approaches that of the general population after cessation of the donation activity (T. Brodersen, 2019, unpublished results).
While we assume that the same participation bias applied to active and retired blood donors concerning the questionnaire part of the study, low participation rates warrant caution in the interpretation of our results. With regard to the serological survey, participants invited to blood sampling were required to be free of symptoms of infectious disease and to be able to meet for testing at a bleeding site. Diseased and immobile elderly individuals are therefore likely to be underrepresented in our study population of older retired blood donors. There have been outbreaks at Danish nursing homes that will not be reflected by this study. It is therefore unknown which way this selection has biased the estimate. However, given that the proportion of individuals above the age of 70 years who live at nursing homes is less than 5%, we assume that this bias will have limited influence on the overall result. Another possible bias is the inadequate antibody responses that are frequently seen among elderly individuals. This phenomenon has primarily been reported in studies on vaccine responses [22]. Whether the antibody response to SARS-CoV-2 infection is as inadequate as has been reported from vaccine studies is uncertain and, thus, it is not possible to quantify the impact of this bias. However, if applicable to SARS-CoV-2 infection and to the present study, we may have underestimated the SARS-CoV-2 seroprevalence among the elderly retired blood donors and consequently overestimated the corresponding IFR for this age segment.
In conclusion, this cross-sectional study on seroprevalence, self-reported disease, and compliance indicates that social distancing reduces the risk of SARS-CoV-2. In the present study, retired blood donors aged 70 years or older were able to self-isolate to a higher extent than the younger age groups, which was associated with a lower seroprevalence in the elderly population. However, the IFR among individuals aged 70 years or older is 65 times that of younger individuals, which underscores the need for continuous precautions to avoid the general spread of SARS-CoV-2.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. O. B. P. contributed to the funding, study design, planning, data collection, data analysis, data interpretation, and writing of the manuscript. H. H. contributed to the study design, planning, data collection, data analysis, data interpretation, and writing of the manuscript. H. U., L. S. V., S. S., T. B., K. R., K. M., R. L. S., and C. E. all contributed to the study design, planning, data interpretation, and writing of the manuscript. J. N., M. S., M. D., and K. A. K. all contributed to the planning, data collection, data analysis, data interpretation, and writing of the manuscript. K. M. D., B. G.-S., I. W. P., and N. L. S. F. all contributed to the planning, data collection, data interpretation, and writing of the manuscript. J. K. B. contributed to the data analysis, data interpretation, and writing of the manuscript. L. W. T., J. D., E. S., and M. A. H. L. all contributed to the data collection, data interpretation, and writing of the manuscript.
Financial support. This work was supported by The Danish Council for Independent Research, Denmark (grant number 0214-00127B).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Iaccarino G, Grassi G, Borghi C, Ferri C, Salvetti M, Volpe M; SARS-RAS Investigators . Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS study of the Italian society of hypertension. Hypertension 2020; 76:366–72. [DOI] [PubMed] [Google Scholar]
- 2. Bajgain KT, Badal S, Bajgain BB, Santana MJ. Prevalence of comorbidities among individuals with COVID-19: a rapid review of current literature. Am J Infect Control 2020:10. doi: 10.1016/j.ajic.2020.06.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pachiega J, Afonso AJDS, Sinhorin GT, et al. . Chronic heart diseases as the most prevalent comorbidities among deaths by COVID-19 in Brazil. Rev Inst Med Trop Sao Paulo 2020; 62:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Erikstrup C, Hother CE, Pedersen OBV, et al. . Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basu A. Estimating the infection fatality rate among symptomatic COVID-19 cases in the United States. Health Aff (Millwood) 2020; 39:1229–36. [DOI] [PubMed] [Google Scholar]
- 6. Kenyon C. COVID-19 infection fatality rate associated with incidence-a population-level analysis of 19 Spanish autonomous communities. Biology 2020; 9:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stringhini S, Wisniak A, Piumatti G, et al. . Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet 2020; 396:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. . Prevalence of SARS- CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020; 396:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silveira MF, Barros AJD, Horta BL, et al. . Population-based surveys of antibodies against SARS-CoV-2 in Southern Brazil. Nat Med 2020; 26:1196–9. [DOI] [PubMed] [Google Scholar]
- 10. Havers FP, Reed C, Lim T, et al. . Seroprevalence of antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May 12, 2020. JAMA Intern Med 2020; 10 Jul 21. doi: 10.1001/jamainternmed.2020.4130 [DOI] [PubMed] [Google Scholar]
- 11. Pedersen OB, Erikstrup C, Kotzé SR, et al. . The Danish Blood Donor Study: a large, prospective cohort and biobank for medical research. Vox Sang 2012; 102:271. [DOI] [PubMed] [Google Scholar]
- 12. Danish data on surveillance o f COVID-19, Statens Serum Institut. Available at: https://experiencearcgiscom/experience/aa41b29149f24e20a4007a0c4e13db1d. Accessed 14 August 2020.
- 13. Statistics Denmark—population data. Available at: https://wwwstatistikbankendk/statbank5a/defaultasp?w=1440. Accessed 14 August 2020.
- 14. Del Brutto OH, Costa AF, Mera RM, Recalde BY, Bustos JA, Garcia HH. SARS-CoV-2 in rural Latin America: a population-based study in coastal Ecuador. Clin Infect Dis 2020 Jul 27: doi: 10.1093/cid/ciaa1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Biggs HM, Harris JB, Breakwell L, et al. ; CDC Field Surveyor Team . Estimated community seroprevalence of SARS-CoV-2 antibodies—two Georgia Counties, April 28-May 3, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daoust JF. Elderly people and responses to COVID-19 in 27 countries. PLoS One 2020; 15:e0235590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Del Brutto OH, Costa AF, Mera RM, Recalde BY, Bustos JA, García HH. SARS-CoV-2-related mortality in a rural Latin American population. Int J Infect Dis 2020; 99:226–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bogogiannidou Z, Vontas A, Dadouli K, et al. . Repeated leftover serosurvey of SARS-CoV-2 IgG antibodies, Greece, March and April 2020. Euro Surveill 2020; 25:2001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liang XH, Tang X, Luo YT, Zhang M, Feng ZP. Effects of policies and containment measures on control of COVID-19 epidemic in Chongqing. World J Clin Cases 2020; 8:2959–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bar-On YM, Flamholz A, Phillips R, Milo R. SARS-CoV-2 (COVID-19) by the numbers. Elife 2020; 9:e57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burgdorf KS, Simonsen J, Sundby A, et al. . Socio-demographic characteristics of Danish blood donors. PLoS One 2017; 12:e0169112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev 2019; 32:e00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.