Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a rapidly emerging virus causing the ongoing coronavirus disease 2019 (COVID-19) pandemic with no known effective prophylaxis. We investigated whether hydroxychloroquine could prevent SARS-CoV-2 in healthcare workers at high risk of exposure.
Methods
We conducted a randomized, double-blind, placebo-controlled clinical trial of healthcare workers with ongoing exposure to persons with SARS-CoV-2, including those working in emergency departments, intensive care units, COVID-19 hospital wards, and first responders. Participants across the United States and in the Canadian province of Manitoba were randomized to hydroxychloroquine loading dose then 400 mg once or twice weekly for 12 weeks. The primary endpoint was confirmed or probable COVID-19–compatible illness. We measured hydroxychloroquine whole-blood concentrations.
Results
We enrolled 1483 healthcare workers, of whom 79% reported performing aerosol-generating procedures. The incidence of COVID-19 (laboratory-confirmed or symptomatic compatible illness) was 0.27 events/person-year with once-weekly and 0.28 events/person-year with twice-weekly hydroxychloroquine compared with 0.38 events/person-year with placebo. For once-weekly hydroxychloroquine prophylaxis, the hazard ratio was .72 (95% CI, .44–1.16; P = .18) and for twice-weekly was .74 (95% CI, .46–1.19; P = .22) compared with placebo. Median hydroxychloroquine concentrations in whole blood were 98 ng/mL (IQR, 82–120) with once-weekly and 200 ng/mL (IQR, 159–258) with twice-weekly dosing. Hydroxychloroquine concentrations did not differ between participants who developed COVID-19–compatible illness (154 ng/mL) versus participants without COVID-19 (133 ng/mL; P = .08).
Conclusions
Pre-exposure prophylaxis with hydroxychloroquine once or twice weekly did not significantly reduce laboratory-confirmed COVID-19 or COVID-19–compatible illness among healthcare workers.
Clinical Trials Registration
Keywords: COVID-19, hydroxychloroquine, healthcare workers, pre-exposure prophylaxis
In this randomized clinical trial of 1483 high-risk healthcare workers, there was no significant reduction in incidence of COVID-19 with once-weekly or twice-weekly hydroxychloroquine compared with placebo.
(See the Editorial Commentary by Goldman on pages e844–7.)
Coronavirus disease 2019 (COVID-19) creates a substantial strain on the healthcare system, with frontline healthcare workers at increased risk of infection, and yet they are simultaneously essential for sustaining an adequate emergency response. Unfortunately, at present, no effective oral chemoprophylaxis or vaccination against COVID-19 exists. On 7 October 2020, the Centers for Disease Control and Prevention (CDC) reported over 173 000 cases of COVID-19 among healthcare personnel in the United States [1]. An effective pre-exposure prophylaxis medication for healthcare workers with repeated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exposure, even if only partially effective, would be a powerful public health tool to reduce transmission of SARS-CoV-2 and protect frontline workers from COVID-19 [2].
While intensive efforts are being directed towards treatment discovery and vaccine development, repurposing existing medications is a more swift and economical approach to fulfill a time-sensitive need for effective prophylaxis. Chloroquine has demonstrated in vitro activity against SARS-CoV and SARS-CoV-2 [3, 4]. Recent studies demonstrated that hydroxychloroquine, a derivative molecule of chloroquine, is also active against SARS-CoV-2 and may demonstrate greater in vitro viral inhibition [5, 6]. However, it remains unclear if in vitro activity corresponds to clinical efficacy. Randomized clinical trials in postexposure prophylaxis, early outpatient treatment, and inpatient treatment have not borne out this initial promise [7–10]. Nonetheless, some have postulated that the postexposure and early treatment trials may not have achieved therapeutic concentrations early enough to have demonstrated a benefit [7]. In India, hydroxychloroquine 400 mg weekly is recommended nationally in asymptomatic healthcare workers at high risk for COVID-19, despite no substantial evidence that it prevents COVID-19 [11].
There is ongoing interest in the concept of pre-exposure prophylaxis whereby a patient has already achieved adequate drug concentrations at the time of viral exposure. Therefore, we sought to determine the effectiveness of hydroxychloroquine as pre-exposure prophylaxis in healthcare workers at high risk of SARS-CoV-2 exposure in a randomized, placebo-controlled clinical trial setting.
METHODS
Study Design
We conducted a randomized, double-blind, placebo-controlled clinical trial (Clinicaltrials.gov NCT04328467) to evaluate whether hydroxychloroquine could prevent COVID-19 in high-risk healthcare workers across the United States and Canada. Enrollment began on 6 April 2020 and ended 26 May 2020; follow-up was completed on 13 July 2020. Participants were randomly assigned in a 2:2:1:1 ratio to receive hydroxychloroquine given as a loading dose of 400 mg (2 200-mg tablets) twice separated by 6–8 hours followed by (1) 400 mg (2 200-mg tablets) once weekly for 12 weeks or (2) 400 mg (2 200-mg tablets) twice weekly for 12 weeks, or to a placebo that was prescribed in a matched fashion including a loading dose of 2 tablets followed by 2 tablets once or twice weekly for 12 weeks.
Participants
We included healthcare workers aged 18 years and older with ongoing exposure to persons with COVID-19. A high-risk healthcare worker was defined as working in an emergency department or intensive care unit, on a dedicated COVID-19 hospital ward, as a first responder, or whose job description included regularly performing aerosol-generating procedures (eg, anesthesiologists or otolaryngologists), and included physicians, nurses, advanced-practice providers, and other personnel (eg, respiratory therapists).
We excluded persons who reported active or prior COVID-19 (either confirmed or symptom-compatible illness), with no expected exposure to patients, or who had a contraindication to hydroxychloroquine (Supplementary Appendix, Methods S1).
Setting
We enrolled participants nationwide in the United States and the Canadian province of Manitoba. We recruited participants using social media platforms targeting healthcare providers. Participants self-enrolled via a secure internet-based survey using the Research Electronic Data Capture (REDCap) system [12]. Participants provided a digitally captured informed-consent signature after passing a comprehension assessment.
Study Assessments
Online study assessments were scheduled at enrollment, medication initiation, and weekly after enrollment. Each assessment included a report of study medication adherence, medication side effects, the number of patient-facing contact hours, contact with patients with confirmed or possible COVID-19, personal protective equipment use, COVID-19–compatible symptoms, SARS-CoV-2 testing results, and any hospitalization.
Outcomes
The primary outcome was COVID-19–free survival time by laboratory-confirmed or probable compatible illness. Confirmed COVID-19 was defined as SARS-CoV-2 polymerase chain reaction (PCR) positivity by self-report. Given the limited availability of outpatient PCR testing in many jurisdictions during our study period, particularly in April 2020, probable COVID-19 based on COVID-19–compatible symptoms was included in the composite primary endpoint. The definition of COVID-19–compatible symptoms was based on guidance from the US Council for State and Territorial Epidemiologists (Supplementary Appendix) [13]. Specifically, probable disease was defined as having cough, shortness of breath, or difficulty breathing, or 2 or more of the following symptoms: fevers, chills, rigors, myalgia, headache, sore throat, and new olfactory and taste disorders. Possible disease was defined as 1 or more COVID-19–compatible symptoms. Three blinded infectious-diseases physicians independently adjudicated cases of symptomatic participants based on the above criteria.
Secondary outcomes included incidence of confirmed SARS-CoV-2 detection, incidence of possible COVID-19, and incidence of hospitalization, death, or other adverse events. Study medication adherence and side effects were all collected through weekly self-reported surveys.
Randomization
Participants were sequentially randomized at the research pharmacies. Treatment assignments were concealed from investigators and participants. Blinded hydroxychloroquine or placebo (folic acid) was dispensed, and a 12-week supply shipped to participants by courier.
Sample Size
The trial was designed anticipating a 10% event rate of COVID-19 in high-risk healthcare workers over 12 weeks. Using log-rank testing with a 50% relative effect size to reduce new symptomatic infections, a 2-sided α of 0.025, and 80% power, an estimated 1050 participants per arm were required. The trial was powered at α = 0.025 to account for the 2 treatment dosing regimens versus placebo comparisons.
Statistical Methods
We compared the incidence of COVID-19–free survival using the log-rank test and estimated hazard ratios using a Cox proportional hazards model. We compared secondary endpoints of proportions by Fisher’s exact test. We conducted analyses with SAS software version 9.4 (SAS Institute), according to the intention-to-treat principle. Participants were right-hand censored at time of last contact for those not completing 12 weeks of follow-up. As prespecified, participants who developed COVID-19–compatible illness (ie, primary endpoint) prior to initiating the study medicine were excluded from the primary analysis.
Hydroxychloroquine Drug Concentrations
A prespecified subgroup analysis was performed to investigate whether hydroxychloroquine drug concentrations correlated with protection from COVID-19. Whole blood was self-collected from participants who consented using Neoteryx volumetric absorbed microsampling kits (Neoteryx, Torrance, CA) at least 4 weeks after study medication initiation. Hydroxychloroquine concentrations were quantified similarly to methods previously published (Supplementary Appendix) [14]. The Wilcoxon rank-sum test compared trough concentrations between participants who developed COVID-19 and those who did not.
Interim Analysis
An independent data safety and monitoring board (DSMB) reviewed the data after 25% of participants had completed 4 weeks of follow-up. Stopping guidelines were provided to the DSMB via a Lan-DeMets spending function analog of the O’Brien-Fleming boundaries for the primary outcome.
Before the first interim analysis on 21 May 2020, it became apparent that we would not meet our initial enrollment goal of 3150 participants (Supplementary Figure 2). At the first interim analysis, and without unblinding of treatment allocation, the principal investigator proposed to the DSMB stopping the enrollment due to an inability to recruit participants, with continued follow-up for those already enrolled. Enrollment was stopped on 26 May 2020, and outcomes data were collected through 13 July 2020.
RESULTS
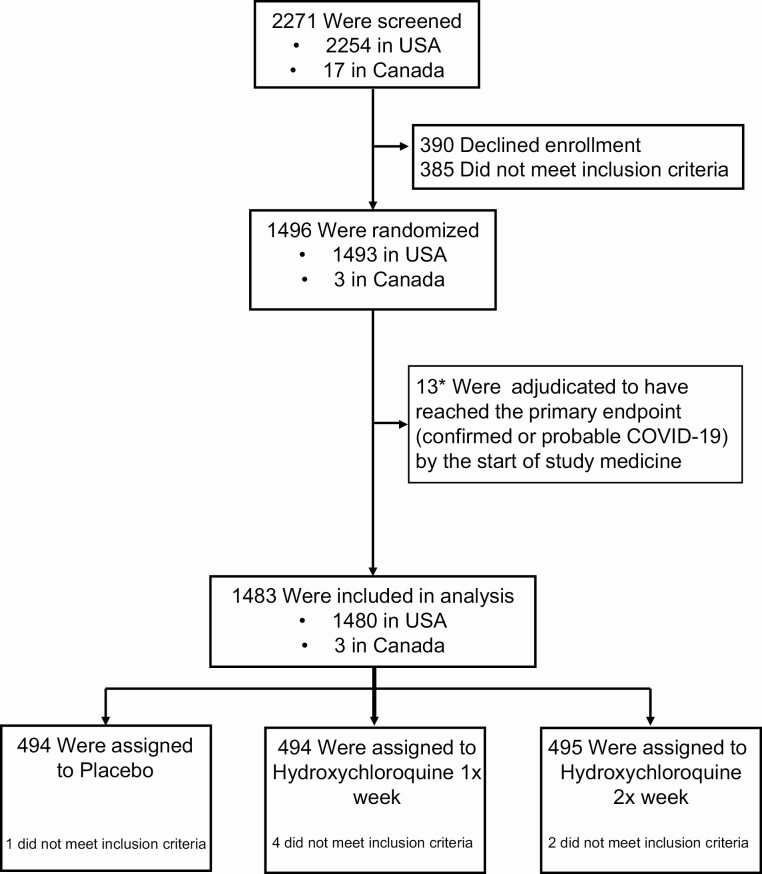
Of 2271 persons screened, 1483 high-risk healthcare workers from the United States and Canada were enrolled with 494 randomized to once-weekly hydroxychloroquine, 495 randomized to twice-weekly hydroxychloroquine, and 494 randomized to placebo (Figure 1). Participant demographics are provided in Table 1. The median age of participants was 41 years (interquartile range [IQR], 34 to 49), and 51% (760 of 1483) were women. Overall, 66% reported no chronic medical conditions (982 of 1483), while 14% (205 of 1483) reported hypertension and 10% reported asthma (150 of 1483). The primary location of work was the emergency department for 41% (607 of 1483), intensive care units for 18% (269 of 1483), operating rooms for 12% (178 of 1483), COVID-19 hospital wards for 10% (154 of 1483), and ambulance/first-response teams for 8% (118 of 1483).
Figure 1.
CONSORT diagram. Abbreviations: CONSORT, Consolidated Standards of Reporting Trials; COVID-19, coronavirus disease 2019.
Table 1.
Baseline Demographic Characteristics
| Characteristic | Placebo | Hydroxychloroquine Once Weekly | Hydroxychloroquine Twice Weekly |
|---|---|---|---|
| Number randomized | 494 | 494 | 495 |
| Age, median (IQR), y | 40 (34, 48) | 42 (35, 49) | 41 (35, 49) |
| Weight, median (IQR), kg | 80 (68, 95) | 79 (67, 93) | 82 (68, 95) |
| Female,a n (%) | 241 (48.8) | 261 (52.8) | 258 (52.1) |
| Ethnicity (all that apply), n (%) | |||
| White or Caucasian | 419 (84.8) | 431 (87.2) | 421 (85.1) |
| Black or African | 10 (2.0) | 5 (1.0) | 5 (1.0) |
| Asian | 29 (5.9) | 23 (4.7) | 23 (4.6) |
| Native Hawaiian or Pacific Islander | 1 (0.2) | 0 (0.0) | 1 (0.2) |
| Hispanic or Latino | 18 (3.6) | 18 (3.6) | 22 (4.4) |
| Native American or Alaska Native | 8 (1.6) | 4 (0.8) | 7 (1.4) |
| Middle Eastern | 4 (0.8) | 6 (1.2) | 5 (1.0) |
| South Asian | 12 (2.4) | 17 (3.4) | 18 (3.6) |
| Other | 4 (0.8) | 3 (0.6) | 1 (0.2) |
| Current smoker, n (%) | 13 (2.6) | 17 (3.4) | 21 (4.2) |
| Chronic health conditions, n (%) | |||
| High blood pressure | 60 (12.1) | 79 (16.0) | 66 (13.3) |
| Asthma | 59 (11.9) | 46 (9.3) | 45 (9.1) |
| None | 336 (68.0) | 311 (63.0) | 335 (67.7) |
| Risk factors for acquisition of SARS-CoV-2 at screening | |||
| Interacted with COVID-19 patients when not wearing a mask or face shield, n (%) | |||
| Yes | 62 (12.6) | 69 (14.1) | 85 (17.2) |
| No | 432 (87.4) | 422 (85.9) | 409 (82.8) |
| Perform aerosol-generating procedures?, n (%) | 410 (83.0) | 378 (77.0) | 377 (76.3) |
| No. of aerosol-generating procedures performed per week, mean (SD) | 10 (31.4) | 9 (12.2) | 9 (12.5) |
| Setting of occupational exposure, n (%) | |||
| Emergency department | 190 (38.5) | 210 (42.5) | 207 (40.8) |
| Intensive care unit | 85 (17.2) | 82 (16.6) | 102 (20.6) |
| Operating room | 75 (15.2) | 61 (12.3) | 42 (8.5) |
| COVID-19 ward | 56 (11.3) | 51 (10.3) | 47 (9.5) |
| Ambulance | 45 (9.1) | 40 (8.1) | 33 (6.7) |
| Congregate care setting | 20 (4.0) | 19 (3.8) | 27 (5.5) |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
aNo pregnant women were enrolled, 30 women reported breastfeeding at baseline.
Overall, 91% reported more than 14 hours of direct contact with patients per week (1346 of 1483), and 79% of participants reported routinely performing aerosol-generating procedures (1165 of 1483), with an average of 9 procedures performed per week. For aerosol-generating procedures, 94% (1098 of 1165) reported typically wearing an N95 respirator or powered air-purifying respirator.
Primary Outcome
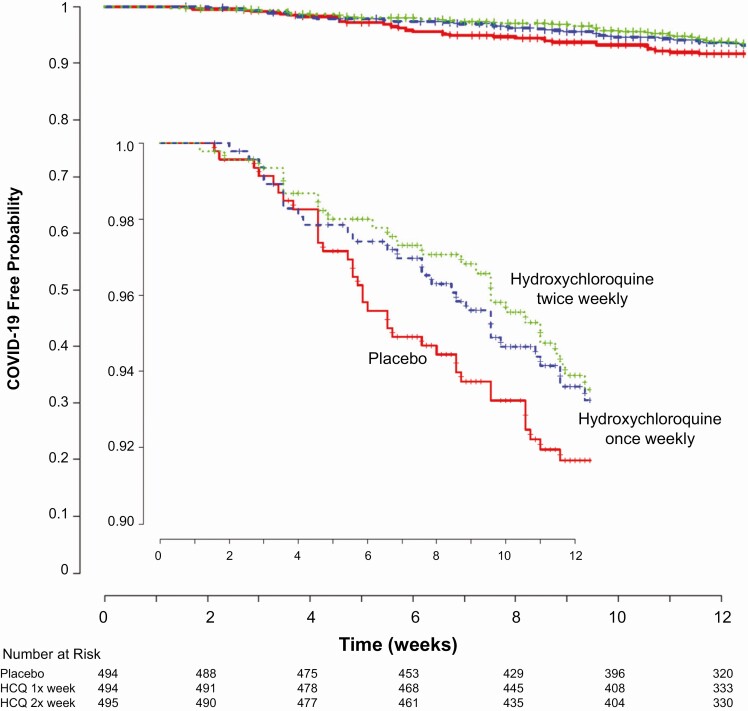
The study accrued 311 person-years of follow up, and 97 participants (6.5%) developed COVID-19 (either PCR confirmed or symptomatically compatible illness) during the trial. Overall, confirmed or probable COVID-19–compatible illness occurred in 29 (5.9%) receiving once-weekly hydroxychloroquine, 29 (5.9%) receiving twice-weekly hydroxychloroquine, and 39 (7.9%) receiving placebo. The corresponding incidence of COVID-19 or compatible illness was 0.27 and 0.28 events per person-year for those taking hydroxychloroquine once or twice weekly, respectively, as compared with 0.38 events per person-year in those receiving placebo (Table 2). Compared with placebo, the hazard ratios for COVID-19 or compatible illness were .72 (95% confidence interval [CI], .44–1.16; P = .18) with once-weekly and .74 (95% CI, .46–1.19; P = .22) with twice-weekly hydroxychloroquine, respectively (Figure 2). When hydroxychloroquine arms were combined, the hazard ratio for COVID-19 or compatible illness was .73 (95% CI, .48–1.09; P = .12) compared with placebo.
Table 2.
Incidence of COVID-19 With Hydroxychloroquine as Pre-exposure Prophylaxis
| Outcome | Placebo | Hydroxychloroquine Once Weekly | Hydroxychloroquine Twice Weekly | |||||
|---|---|---|---|---|---|---|---|---|
| No. of Infections (%) | Event Rate per Person-year (95% CI) | No. of Infections (%) | Event Rate per Person-year (95% CI) | Hazard Ratio (95% CI) | No. of Infections (%) | Event Rate per Person-year (95% CI) | Hazard Ratio (95% CI) | |
| PCR positive or probable COVID-19 | 39 (7.9) | .38 (.26–.50) | 29 (5.9) | .27 (.17–.37) | .72 (.44–1.16) | 29 (5.9) | .28 (.18–.38) | .74 (.46–1.19) |
| PCR confirmed COVID-19 | 6 (1.2) | .06 (.01–.10) | 4 (0.8) | .04 (.00–.07) | .65 (.18–2.32) | 7 (1.4) | .07 (.02–.12) | 1.18 (.40–3.51) |
| COVID-19 compatible with symptoms | 38 (7.7) | .38 (.26–.49) | 29 (5.9) | .28 (.18–.38) | .73 (.45–1.19) | 28 (5.7) | .28 (.17–.38) | .74 (.45–1.20) |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction.
Figure 2.
Kaplan-Meier estimates of time to COVID-19–compatible illness. The probability of SARS-CoV-2 infection over time is shown for the 3 study groups. The hazard ratio for twice-weekly hydroxychloroquine prophylaxis was .72 (95% CI, .44–1.16; P = .18) and for once-weekly was .74 (95% CI, .46–1.19; P = .22) as compared with placebo. The inset graph shows more detail. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; HCQ, hydroxychloroquine; pts, patients; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Of the 97 with COVID-19 (PCR confirmed or symptomatically compatible illness), 17 were PCR positive (18%), 42 (43%) had no PCR testing during their illness, and 38 (39%) had a negative PCR test during illness (Supplementary Table 3). Of the 38 with a negative PCR, 30 were collected within 4 days prior to symptom onset and 8 collected within 11 days after symptom onset. The hazard ratio for PCR-confirmed COVID-19 was .65 (95% CI, .18–2.32; P = .51) for once-weekly hydroxychloroquine and 1.18 (95% CI, .40–3.51; P = .77) for twice-weekly hydroxychloroquine (Table 2).
Medication Adherence and Side Effects
Self-reported adherence to the study medicine was not significantly different by treatment group (Supplementary Figure 9). Of those who reported full adherence at 80% or more of surveys, COVID-19 occurred in 8.5% (28/331) of participants assigned to placebo, 5.7% (20/351) of participants assigned to once-weekly hydroxychloroquine (hazard ratio, .66; 95% CI, .37–1.17; P = .16) and 5.7% (18/316) of participants assigned to twice-weekly hydroxychloroquine (hazard ratio, .68; 95% CI, .37–1.22; P = .19).
Side effects were reported in 21% (100 of 469) of participants assigned to placebo (Supplementary Table 4), 31% (148 of 473; P < .001) in the once-weekly hydroxychloroquine group and 36% (168 of 463; P < .001) in the twice-weekly hydroxychloroquine group. The most common side effect was stomach upset and nausea (placebo, 12.2%; hydroxychloroquine once-weekly, 17.5%; and hydroxychloroquine twice-weekly, 19.4%), followed by gastrointestinal disturbance and diarrhea (placebo, 7.5%; hydroxychloroquine once-weekly, 12.9%; and hydroxychloroquine twice-weekly, 17.1%).
Other Secondary Outcomes
Twenty hospitalizations occurred during the study: 9 in the placebo arm, 3 in the hydroxychloroquine once-weekly arm, and 8 in the hydroxychloroquine twice-weekly arm. Reasons for hospitalization are summarized in the Supplementary Appendix. Two hospitalizations were related to COVID-19 (1 placebo group, 1 twice-weekly group). One person in the placebo group was hospitalized twice for new atrial fibrillation, and 1 person in the hydroxychloroquine twice-weekly arm was hospitalized for syncope and new supraventricular tachycardia—a possible hydroxychloroquine-related serious adverse event. No intensive care unit stays or deaths occurred.
In prespecified subgroup analyses, there were no significant differences in treatment efficacy (Supplementary Appendix).
Hydroxychloroquine Drug Concentrations
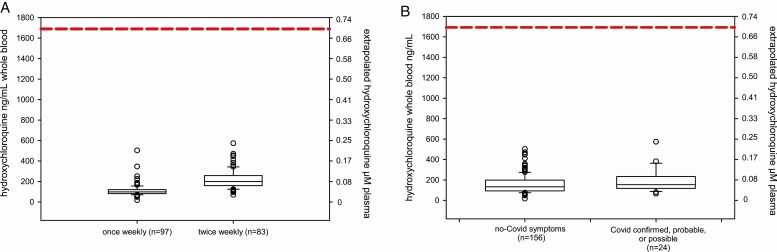
Hydroxychloroquine concentrations were measured in dried whole blood from 180 participants in the hydroxychloroquine groups, of whom 18 were confirmed or probable COVID-19 and 6 were considered possible COVID-19 (Supplementary Table 7). Hydroxychloroquine was detectable in all samples from participants assigned to hydroxychloroquine. Median (IQR) concentrations were higher in the twice-weekly dosing group (200 ng/mL; IQR, 159–258 ng/mL) compared with the once-weekly dosing group (98 ng/mL; IQR, 82–120 ng/mL) (P < .0001). Median concentrations did not differ between COVID-19–confirmed, –probable, or –possible cases (154 ng/mL; IQR, 119–231 ng/mL) compared with participants without COVID-19 (133 ng/mL; IQR, 93–198 ng/mL) (P = .08) (Figure 3). To exclude surreptitious crossovers, we measured hydroxychloroquine concentrations in 49 participants (10%) randomized to placebo, and all were below the limit of quantification of 50 ng/mL.
Figure 3.
Hydroxychloroquine drug concentrations. Right-side axes indicate extrapolated plasma concentrations assuming a blood to plasma ratio of 7.2 and hydroxychloroquine molecular weight of 336 g/mol. A, Trough drug concentrations in participants taking once-weekly compared with twice-weekly hydroxychloroquine. All participants had detectable hydroxychloroquine in whole-blood samples. B, Drug concentrations in participants from both hydroxychloroquine arms with and without COVID-19–compatible illness. Participants with COVID-19–compatible illness had median concentrations of 154 ng/mL compared with 133 ng/mL among those without symptomatic illness (P = .08). Dashed lines indicate extrapolated EC50 target assuming a blood to plasma ratio of 7.2, target EC50 of 0.7 µm = 235 ng/mL plasma = 1690 ng/mL whole blood. Abbreviations: COVID-19, coronavirus disease 2019; EC50 = half maximal effective concentration.
DISCUSSION
In this randomized, double-blind, placebo-controlled trial evaluating hydroxychloroquine as pre-exposure prophylaxis for COVID-19 in high-risk healthcare workers, we found no statistically significant reduction in COVID-19 incidence in those receiving 400 mg weekly or twice-weekly hydroxychloroquine when compared with placebo. Reasons for no effect observed may be due to hydroxychloroquine concentrations being too low or because hydroxychloroquine is ineffective against COVID-19 in vivo [15].
Nonetheless, we observed no difference in hydroxychloroquine concentrations between those who reported COVID-19 symptomatically compatible illness and those who did not in a subsample of trial participants. Similarly, an animal model of macaques showed that hydroxychloroquine offered no protection against SARS-CoV-2 acquisition when given as pre-exposure prophylaxis [15]. While there are no validated therapeutic target concentrations of hydroxychloroquine for protection against COVID-19, we chose dosing regimens predicted to achieve plasma concentrations above the in vitro Half maximal effective concentration (EC50) [16]. Assuming blood concentrations are 7-fold higher than plasma [17], no participants had plasma troughs higher than reported in vitro EC50. Plasma concentrations of 235 ng/mL (~0.7 µM) would extrapolate to a whole-blood target greater than 1600 ng/mL, significantly higher than troughs achieved in our study. The discrepancy between our simulated and observed concentrations is consistent with a recent analysis [18], which suggested that, due to sequestering of drug in whole-blood leukocytes and platelets not adequately removed during processing, the pharmacokinetic parameters upon which we based our simulations may have overestimated plasma concentrations [19]. This finding is likely applicable to all hydroxychloroquine trials. Notably, our whole-blood troughs suggest that, even with daily dosing, extrapolated plasma trough concentrations above EC50 are unlikely. Ongoing trials investigating the efficacy of daily dosing should consider obtaining plasma concentrations to further decipher whether daily dosing is adequate and, in the context of appropriate dosing, if hydroxychloroquine is effective at preventing SARS-CoV-2 infection. In 1 randomized trial of 125 participants, daily dosing of 600 mg hydroxychloroquine did not reduce PCR-confirmed SARS-CoV-2 infection [2]. Our results suggest that prophylaxis with 400 mg hydroxychloroquine weekly is ineffective, and recommendations for prophylactic use, such as those for healthcare workers in India, should be reconsidered.
When justifying widespread implementation of a prophylactic intervention, it is paramount to consider and predefine a required minimum efficacy. With the Food and Drug Administration’s (FDA’s) suggestion that a minimum efficacy of 50% was required for a COVID-19 vaccine to be approved, we hypothesized that a 50% relative risk reduction in confirmed or probable COVID-19 would be clinically meaningful and powered the study design as such. Our estimates of incidence of COVID-19 (confirmed or symptomatic) will be valuable for future studies of chemoprophylaxis and vaccine trials.
Enrolling participants was a challenge. We enrolled 84% of all participants (1250 of 1483) in the first 2 weeks of the trial. During 21–24 April 2020, a series of small or retrospective studies highlighted safety concerns of hydroxychloroquine [20, 21], which resulted in a warning from the FDA regarding arrhythmias and QT prolongation [22]. Thereafter, our enrollment precipitously declined. An additional study in May, which is now retracted [23], further discouraged enrollment. Enrollment was stopped on 26 May 2020 due to futility in ongoing participant recruitment. Enrollment in other North American randomized clinical trials of hydroxychloroquine was also impeded (Dee Dee Wang, personal communication, 2020). As a result of premature enrollment termination and inadequate power, it is difficult to estimate the potential societal benefit, if any, in widespread implementation.
The major limitation of this trial relates to the inherent challenges with PCR testing that have been well described—both the lack of US availability and moderate reported sensitivity early in illness. The false-negative rate of PCR testing has been reported to be 38% (range, 18–65%) on the first day of symptoms, gradually decreasing thereafter [24]. In our study, 39% (38/97) had COVID-19–compatible symptoms with a negative PCR test; however, 30 of those PCR tests were performed before symptoms began, when false negatives can be expected [24]. To address this, we included healthcare workers with symptomatic COVID-19–compatible illness despite negative PCR but separately reported this group. Further supporting this decision, COVID-19–compatible symptoms warrant self-isolation from work for 14 days for healthcare providers and reporting to occupational health, per CDC guidelines, even if PCR testing is negative [25]. However, it is unknown what proportion of persons with symptomatically compatible disease truly have SARS-CoV-2 infection, which remains a shared limitation to all outpatient COVID-19 trials in the absence of a diagnostic test with improved sensitivity. Hypothetically, if reported symptoms were due to another respiratory illness, such as influenza, they should have been evenly distributed between groups due to randomization. If one compares only PCR-confirmed disease, there was no statistical difference between groups. Second, our trial was limited by weekly self-report of outcomes, which is subject to recall bias. As mentioned previously, insufficient dosing of hydroxychloroquine remains a limitation of this study. Finally, our trial was left underpowered due to impediments to participant recruitment. With the actual sample size accrued, more rapid initial pace of accrual (85% recruited in the first 2 weeks), lower loss-to-follow-up rate in the control group, and control group event rate of 7.9%, there was 80% power with an α of 0.05 to detect a 59% relative effect per arm and a 53% relative effect (ie, hazard ratio of 0.47) when pooling the 2 hydroxychloroquine arms together. Nevertheless, the effect size estimates derived from our data will inform current policy and aid in the design of future clinical trials testing prophylaxis or vaccines.
An effective means of prophylaxis for high-risk healthcare workers remains a critical need in the context of a growing and relentless pandemic. The COVID PREP study evaluated the effectiveness of once-weekly and twice-weekly hydroxychloroquine to prevent COVID-19 in high-risk healthcare workers across the United States and Canada. There was no statistically significant reduction in the incidence of COVID-19 in our trial. However, investigation into more frequent dosing may be warranted. Prior to embarking on further clinical trials, and for current studies to complete enrollment, the perception of equipoise in the medical community and the public will need to change dramatically.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. R. R. and D. R. B. conceived of the trial. R. R. wrote the clinical protocol with the assistance of S. M. L., C. P. S., D. R. B., M. R. N., J. M. B., B. I. R., P. L., and I. A. M., and statistical input from A. S. B., N. W. E., and K. H. H. L. J. M. collaborated and adapted the study for Canadian sites with input from T. C. L. K. H. H., A. S. B., and N. W. E. conducted the statistical analyses, with the analysis being guaranteed by K. H. H. A. S. B. developed the REDCap database with help from M. F. P., K. A. P., S. M. L., and C. P. S. A. S. B. maintained the database. T. C. L. and E. G. M. adapted the database in Canada. M. L. A., C. P. S., A. A. N., M. F. P., K. A. P., E. C. O., D. A. W., L. J. M., and R. R. conducted participant follow-up. Advertising and outreach were conducted by S. J. D., D. R. B., R. R., S. M. L., C. P. S., M. F. P., A. S. B., A. A. N., E. C. O., P. L., D. A. W., and L. J. M., R. R., C. P. S., and S. M. L. conducted case adjudication. The hydroxychloroquine drug levels substudy was conceived by M. R. N., R. R., D. K., and R. Z., and levels were analyzed by M. R. N. R. R. wrote the first draft of the manuscript and is the overall study guarantor with help from S. M. L., D. R. B., C. P. S., M. F. P., K. A. P., E. C. O., A. A. N., and D. A. W. All authors reviewed and revised and approved the final version of the manuscript. R. R. is the FDA Investigational New Drug sponsor.
Acknowledgments. The authors thank the healthcare workers around North America who volunteered to participate in this trial in order to obtain knowledge for society. We thank the following DSMB members for their thoughtful service: Drs Mark Siedner, Lynn Matthews, Jeff Klausner, Bozena Morawski, and Tom Chiller. We thank Drs Jakub Tolar, Peter Igarashi, Brad Benson, and Tim Schacker for institutional support.
COVID PREP Team Members (listed alphabetically) . Mahsa Abassi (University of Minnesota, Minneapolis, Minnesota, USA); Andrew Balster (Oregon Health and Science University, Portland, Oregon, USA); Lindsey B. Collins (University of Minnesota, Minneapolis, Minnesota, USA); Glen Drobot (University of Manitoba, Winnipeg, Manitoba, Canada); Douglas S. Krakower (Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA); Sylvain A. Lother (University of Manitoba, Winnipeg, Manitoba, Canada); Dylan S. MacKay (University of Manitoba, Winnipeg, Manitoba, Canada); Cameron Meyer-Mueller (University of Minnesota, Minneapolis, Minnesota, USA); Stephen Selinsky (University of Minnesota, Minneapolis, Minnesota, USA); Dayna Solvason (The George and Fay Yee Centre for Healthcare Innovation, Winnipeg, Manitoba, Canada); Ryan Zarychanski (University of Manitoba, Winnipeg, Manitoba, Canada); Rebecca Zash (Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA).
Collaborators . Drug assay development and performance: James Fisher; Concept advisors: Archana Bhaskaran; Logistical support: Kristen Moran, Alek Lefevbre, Izabella Supel, Carmen Tse, Hongru Ren, Fiona Vickers, Jason Zou: Pharmacy support. Darlette Luke, PRISM Research Inc, Halyna Ferens, Beata Kozak.
Disclaimer. The funders did not contribute to the design, collection, management, analysis, interpretation of data, writing of the report, or the decision to submit the report for publication.
Financial support. This work was supported by Jan and David Baszucki, Steve Kirsch, the Rainwater Charitable Foundation, the Alliance of Minnesota Chinese Organizations, the Minnesota Chinese Chamber of Commerce, and the University of Minnesota Foundation. M. R. N., R. R., and M. F. P. are supported by the National Institute of Allergy and Infectious Diseases (grant numbers K08AI134262, K23AI138851, T32AI055433). S. M. L. is supported by the National Institute of Mental Health (grant number K23MH121220). C. P. S. is supported by a combined Fogarty International Center Global Fellows Scholarship/National Institute of Neurological Disorders and Stroke grant (D43TW009345). K. A. P. and E. C. O. were supported through the Doris Duke Charitable Foundation through a grant supporting the Doris Duke International Clinical Research Fellows Program at the University of Minnesota. M. L. A. is supported by National Institutes of Health grants T32GM007347 and F30CA236157. T. C. L. and E. G. M. receive research salary support from the Fonds de Recherche du Québec–Santé. In Manitoba, research support was received from the Manitoba Medical Service Foundation and Research Manitoba. Rising Pharmaceuticals in the United States provided a donation of the hydroxychloroquine tablets. The REDCap software was supported by the National Institutes of Health’s National Center for Advancing Translational Sciences (grant number UL1TR002494).
Potential conflicts of interest. J. M. B. reports research support from Genentech, BMS, and Incyte and consulting fees from Novartis, outside the submitted work. B. I. R. reports grants and personal fees from Merck, Pfizer, BMS, Genentech, Aveo, and Arrowhead; personal fees from Aravive and GSK; grants from AstraZeneca; and nonfinancial support from Merck, outside the submitted work. I. A. M. reports advisory board fees from Novartis, Pfizer, Genentech, Lilly, Puma, Abbvie, Immunomedics, Macrogenics, Seattle Genetics, AstraZeneca, and GSK and research support from Pfizer and Genentech, outside the submitted work. D. R. B. has provided free advice regarding clinical trial design and implementation to more than 100 citizens, investigators, institutions, or corporations as asked since 17 March 2020. No reimbursement for providing clinical trial design advice has been requested. There is a relationship with Gilead, which makes remdesivir, which is an intravenous medicine used for COVID-19 treatment in hospitalized patients, and has provided grants and Ambisome antifungal medication to the Infectious Disease Institute in Uganda and Meningitis Foundation for cryptococcal meningitis–related research. Remdesivir is not directly relevant to pre-exposure prophylaxis (or postexposure prophylaxis or outpatient oral therapy) for COVID-19, but this is in the realm of treatment of COVID-19. D. R. B. has received $17.79 worth of food/beverage on 23 April 2018 at a medical conference on World Health Organization Essential Diagnostics, which received funding by Gilead. D. R. B. collaborates with multiple pharmaceutical companies making novel antifungal medicines for cryptococcal meningitis in public–private research partnerships, without any financial interests or payments from these companies. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
COVID PREP team:
Mahsa Abassi, Andrew Balster, Lindsey B Collins, Glen Drobot, Douglas S Krakower, Sylvain A Lother, Dylan S MacKay, Cameron Meyer-Mueller, Stephen Selinsky, Dayna Solvason, Ryan Zarychanski, and Rebecca Zash
References
- 1. National Center for Immunization and Respiratory Diseases (NCIRD) Division of Viral Diseases. Coronavirus disease 2019 (COVID-19): cases in the U.S. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 14 July 2020.
- 2. Abella BS, Jolkovsky EL, Biney BT, et al. Efficacy and safety of hydroxychloroquine vs placebo for pre-exposure SARS-CoV-2 prophylaxis among health care workers: a randomized clinical trial. JAMA Intern Me d 2020. In Press. Online Sept 30, 2020 doi: 10.1001/jamainternmed.2020.6319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2005; 2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020; 30:269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 2020; 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020; 71:732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med 2020; 383:517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med 2020; 173:623–31. doi: 10.7326/M20-4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med 2020; [Preprint] October 8, 2020. doi: 10.1056/NEJMoa2022926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Solidarity trial. Q&A: hydroxychloroquine and COVID-19. Available at: https://www.who.int/news-room/q-a-detail/q-a-hydroxychloroquine-and-covid-19. Accessed 19 June 2020.
- 11. Rathi S, Ish P, Kalantri A, Kalantri S. Hydroxychloroquine prophylaxis for COVID-19 contacts in India. Lancet Infect Dis 2020; 20:1118–19. doi: 10.1016/S1473-3099(20)30313-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Council of State and Territorial Epidemiologists. Interim-20-ID-01: standardized surveillance case definition and national notification for 2019 novel coronavirus disease (COVID-19). Available at: https://www.cste.org/resource/resmgr/2020ps/Interim-20-ID-01_COVID-19.pdf. Accessed 5 April 2020. [Google Scholar]
- 14. Qu Y, Brady K, Apilado R, et al. Capillary blood collected on volumetric absorptive microsampling (VAMS) device for monitoring hydroxychloroquine in rheumatoid arthritis patients. J Pharm Biomed Anal 2017; 140:334–41. [DOI] [PubMed] [Google Scholar]
- 15. Maisonnasse P, Guedj J, Contreras V, et al. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature 2020; 585:584–7. doi: 10.1038/s41586-020-2558-4 [DOI] [PubMed] [Google Scholar]
- 16. Al-Kofahi M, Jacobson P, Boulware DR, et al. Finding the dose for hydroxychloroquine prophylaxis for COVID-19: the desperate search for effectiveness. Clin Pharmacol Ther 2020; 108:766–9. doi: 10.1002/cpt.1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tett SE, Cutler DJ, Day RO, Brown KF. A dose-ranging study of the pharmacokinetics of hydroxy-chloroquine following intravenous administration to healthy volunteers. Br J Clin Pharmacol 1988; 26:303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fan J, Zhang X, Liu J, et al. Connecting hydroxychloroquine in vitro antiviral activity to in vivo concentration for prediction of antiviral effect: a critical step in treating COVID-19 patients. Clin Infect Dis [Preprint] May 21, 2020. doi: 10.1093/cid/ciaa623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lim HS, Im JS, Cho JY, et al. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by Plasmodium vivax. Antimicrob Agents Chemother 2009; 53:1468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open 2020; 3:e208857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv [Preprint] June 5, 2020. doi: 10.1016/j.medj.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. US Food and Drug Administration. FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or. Accessed 24 April 2020. [Google Scholar]
- 23. The Lancet Editors. Expression of concern: hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet 2020; 395:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med 2020; 173:262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention. Interim U.S. guidance for risk assessment and work restrictions for healthcare personnel with potential exposure to COVID-19. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed 29 May 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.