Abstract
Background
Patients with coronavirus disease 2019 (COVID-19) experience a wide clinical spectrum, with over 2% developing fatal outcome. The prognostic factors for fatal outcome remain sparsely investigated.
Methods
A retrospective cohort study was performed in a cohort of patients with confirmed COVID-19 in one designated hospital in Wuhan, China, from 17 January–5 March 2020. The laboratory parameters and a panel of cytokines were consecutively evaluated until patients’ discharge or death. The laboratory features that could be used to predict fatal outcome were identified.
Results
Consecutively collected data on 55 laboratory parameters and cytokines from 642 patients with COVID-19 were profiled along the entire disease course, based on which 3 clinical stages (acute stage, days 1–9; critical stage, days 10–15; and convalescence stage, day 15 to observation end) were determined. Laboratory findings based on 75 deceased and 357 discharged patients revealed that, at the acute stage, fatality could be predicted by older age and abnormal lactate dehydrogenase (LDH), urea, lymphocyte count, and procalcitonin (PCT) level. At the critical stage, the fatal outcome could be predicted by age and abnormal PCT, LDH, cholinesterase, lymphocyte count, and monocyte percentage. Interleukin 6 (IL-6) was remarkably elevated, with fatal cases having a more robust production than discharged cases across the whole observation period. LDH, PCT, lymphocytes, and IL-6 were considered highly important prognostic factors for COVID-19–related death.
Conclusions
The identification of predictors that were routinely tested might allow early identification of patients at high risk of death for early aggressive intervention.
Keywords: COVID-19, SARS-CoV-2, fatal outcome, China
A full description of the laboratory abnormalities for coronavirus disease 2019 (COVID-19) is reported, based on which 3 clinical stages of the disease were defined and their application in predicting fatal COVID-19 was explored.
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, central China, in early December 2019 [1] and rapidly swept through the whole country in the following month, with thousands of cases reported in other continents by the end of February 2020. There have been more than 3 million confirmed cases around the world, with more than 200 000 deaths, and the crude case-fatality rate of COVID-19 in the world is approximately 6.4% as of 30 April 2020. According to the clinical data, COVID-19 generally causes common influenza-like illness, but those with severe pneumonia may experience acute respiratory distress syndrome (ARDS) and die of septic shock and multiorgan failure [2–6]. Recognition and treatment of these critically ill patients and those who may die from COVID-19 is becoming one of the major challenges. Older age and preexisting comorbidities were demonstrated to be consistently associated with higher risk of a fatal outcome [6–10]. The life-threatening complications that were found to predict fatal outcome included ARDS, severe pneumonia, multiple organ failure, and septic shock [6–10]. A cytokine-mediated inflammatory response might elicit immune cells to produce proinflammatory cytokines, ultimately injuring the tissue and causing multiple organ failure and even death [11–13]. However, no consensus has been made as to when these severe complications might develop and in which patients. Generally, the severity of these complications has been accompanied by abnormal levels of laboratory parameters that are commonly tested in clinical practice. The close monitoring of these laboratory parameters could help in recognizing the severe complications in the early phase. Here we performed a retrospective cohort study in a cohort of patients with confirmed COVID-19 to identify the longitudinal profile of laboratory indicators, and to explore their predictive effect in determining fatal outcome, with the aim of helping healthcare workers target limited medical resources to patients with a high risk of a fatal outcome.
METHODS
Study Sites and Patients
The retrospective cohort study was performed in a designated hospital for COVID-19 treatment in Tongji Hospital of Huazhong University of Science and Technology, Wuhan, Hubei Province. All of the patients with confirmed COVID-19 admitted to the hospital from 17 January to 14 February 2020 were enrolled into the study. All of them were admitted, confirmed, treated, and discharged according to the clinical criteria of diagnosis and discharge standards for the “Diagnosis and Treatment Scheme of New Coronavirus Infected Pneumonia” [14]. Briefly, the patients who had an epidemiological history and clinical manifestations that mimicked COVID-19 were diagnosed after examination of SARS-CoV-2 RNA by reverse transcription polymerase chain reaction (RT-PCR) [14] and chest computed tomography (CT) scanning. Serum samples were collected from all patients on admission and every 3–5 days during hospitalization for routine laboratory tests.
Data Collection
A medical record review was performed to collect information on demographic characteristics (age, gender, preexisting comorbidities, the date of onset of symptoms), clinical symptoms and signs, and laboratory test results (complete blood count, liver function, renal function, electrolytes test, C-reactive protein [CRP], lactate dehydrogenase [LDH], myocardial enzymes, procalcitonin) that were measured during the entire hospitalization. The outcomes that were updated to 5 March 2020 were obtained from electronic medical records for the final analysis, which included date of discharge or death and hospitalization. The information was retrieved from medical records and entered into an EpiData database (EpiData Association, Odense, Denmark) by a group of trained study staff. The study was conducted in accordance with guidelines approved by the Ethics Committees from Tongji Hospital (Wuhan, China).
Serum Cytokine Measurements
The serum samples, which were kept refrigerated at −80℃, were used for the current evaluation of serum inflammatory cytokines, including interleukin (IL) 1β (IL-1β), soluble IL-2 receptor (IL-2r), IL-6, IL-8, IL-10, and tumor necrosis factor α (TNF-α), which were measured using a solid-phase, 2-site chemiluminescence immunometric assay on an Immulite 1000 (Siemens Healthcare Diagnostics, Eschborn, Germany) according to the manufacturer’s instructions.
Statistical Analysis
Descriptive statistics were performed with continuous variables presented as medians and interquartile ranges (IQRs) and categorical variables summarized as frequencies and proportions. Categorical variables were compared with χ 2 or Fisher’s exact tests and continuous variables with 2-sample Wilcoxon rank-sum test. The repeated measurement data of the 55 laboratory parameters were retrieved from disease onset until the last observation, based on which the disease course was defined into phases by applying generalized estimating equations (GEEs). At each clinical stage, the laboratory parameters were tested to determine the predictive cutoff points for fatal outcome based on the means of the laboratory parameters by calculating the area under the receiver operating characteristic (ROC) curves. Sensitivity and specificity were calculated according to ROC curves for each parameter. Multivariate analyses were performed using a logistic regression model to adjust for the effects from age, sex, and underlying conditions. The Kaplan-Meier method was used to determine the probability of the outcome over the duration of follow-up and to generate survival curves, which were tested by the log-rank test. The hazard ratio (HR) and 95% confidence interval (CIs) were determined by a Cox regression model. All analyses were performed using STATA 14.0 (StataCorp LLP, College Station, TX), and P < .05 was considered statistically significant.
RESULTS
Demographic and Clinical Features of Patients
During 17 January to 14 February 2020, a total of 642 patients with RT-PCR–confirmed SARS-COV-2 were enrolled. The median age was 63.5 years (IQR, 52–70 years) and 324 (50.5%) were female; all patients were Wuhan residents. The median duration of hospital stay was 22 days (IQR, 15–24 days). The median length from disease onset to death was 21 days (IQR, 15–27 days) and to discharge was 31 days (IQR, 25–36 days). Of the 642 patients, 353 (55.0%) had underlying conditions on admission, including hypertension, diabetes, coronary heart disease, cerebrovascular disease, chronic lung disease, chronic kidney disease, and chronic liver disease. By 5 March 2020, 432 patients had a determined outcome, including 357 (82.6%) who recovered and were discharged, 75 (17.4%) patients who died, and the remaining 210 patients who remained hospitalized (Table 1).
Table 1.
Basic Characteristics of Patients With COVID-19
| Characteristics | All Patients (N = 642) | Hospitalized (n = 210) | Discharged (n = 357) | Died (n = 75) | P a |
|---|---|---|---|---|---|
| Age, median (IQR), years | 63.5 (52–70) | 66 (57–71) | 60 (46–67) | 70 (62–77) | < .001 |
| ≥65 years, n (%) | 296 (46.11) | 121 (57.62) | 124 (34.73) | 51 (68.00) | < .001 |
| <65 years, n (%) | 346 (53.89) | 89 (42.38) | 233 (65.27) | 24 (32.00) | |
| Sex, n (%) | |||||
| Male | 318 (49.53) | 101 (48.10) | 168 (47.06) | 49 (65.33) | .004 |
| Female | 324 (50.47) | 109 (51.90) | 189 (52.94) | 26 (34.67) | |
| Interval period, median (IQR), days | |||||
| Length of stay | 22 (15–24) | 24 (23–29) | 19 (14–23) | 11 (5–15) | < .001 |
| From onset to admission | 11 (7–14) | 12 (8–16) | 10 (7–14) | 10 (6–14) | .712 |
| Clinical course from onset to outcome | 33 (25–38) | 38 (33–41) | 31 (25–36) | 21 (15–27) | < .001 |
| Initial symptoms, n (%) | |||||
| Fever | 503 (78.35) | 166 (79.05) | 280 (78.43) | 57 (76.00) | .644 |
| Cough | 400 (62.31) | 146 (69.52) | 210 (58.82) | 44 (58.67) | .980 |
| Asthenia | 171 (26.64) | 56 (26.67) | 94 (26.33) | 21 (28.00) | .766 |
| Shortness of breath | 171 (26.64) | 59 (28.1) | 87 (24.37) | 25 (33.33) | .107 |
| Chest tightness | 149 (23.21) | 60 (28.57) | 69 (19.33) | 20 (26.67) | .153 |
| Diarrhea | 110 (17.13) | 31 (14.76) | 70 (19.61) | 9 (12) | .121 |
| Anorexia | 71 (11.06) | 24 (11.43) | 40 (11.2) | 7 (9.33) | .636 |
| Nausea or vomiting | 58 (9.03) | 18 (8.57) | 37 (10.36) | 3 (4.00) | .084 |
| Comorbidities, n (%) | 353 (54.98) | 130 (61.90) | 168 (47.06) | 55 (73.33) | < .001 |
| Hypertension | 226 (35.2) | 91 (43.33) | 101 (28.29) | 34 (45.33) | .004 |
| Diabetes | 97 (15.11) | 34 (16.19) | 47 (13.17) | 16 (21.33) | .068 |
| Coronary heart disease | 61 (9.5) | 22 (10.48) | 25 (7.00) | 14 (18.67) | .001 |
| Chronic lung disease | 43 (6.7) | 9 (4.29) | 26 (7.28) | 8 (10.67) | .323 |
| Chronic kidney disease | 21 (3.27) | 9 (4.29) | 9 (2.52) | 3 (4) | .479 |
| Cerebrovascular disease | 17 (2.65) | 6 (2.86) | 9 (2.52) | 2 (2.67) | 1.000 |
| Chronic liver disease | 11 (1.71) | 1 (.48) | 8 (2.24) | 2 (2.67) | .687 |
| Other chronic diseasesb | 42 (6.54) | 20 (9.52) | 16 (4.48) | 6 (8.00) | .208 |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range.
aComparison between the patients who were discharged and those who died.
bOther chronic diseases include hyperthyroidism, hypothyroidism, polycystic ovary, chronic digestive disorders, malignancy, cholecystitis, and epilepsy.
In comparison with discharged patients, those who died were older (70 [IQR, 62–77] vs 60 [IQR, 46–67] years), more likely to be male (65.3% vs 47.1%), and have a higher frequency of an underlying disorder, especially hypertension (45.3% vs 28.3%) and coronary heart disease (18.7% vs 7.0%). The interval from disease onset to hospital admission was comparable. The frequently seen symptoms at hospital admission were fever, cough, asthenia, wheeze, and chest tightness, all with similar frequencies between groups.
Laboratory Parameter Profiles and Definition of Clinical Phases
For each patient, 49 laboratory parameters and 6 cytokines that were tested at a median of 4 time points (IQR, 2–6) were used for analysis (the full list is shown in Supplementary Table 1). Briefly, 3 clinical phases were tentatively grouped in a random fashion (such as 1–6/7–12/>12, 1–7/8–13/>13, etc) and each evaluated parameter was compared between 3 phases by GEEs for deceased and discharged patients, respectively (Supplementary Table 2). When the clinical stages were grouped into 1–9, 10–15, and >15 days, 36 of the 49 parameters displayed interstage differences (P < .05) for the discharged group and 32 parameters displayed interstage significant differences for the fatal group (P < .05) separately (Supplementary Table 3). This yielded a higher number of significant indicators compared with all the other grouping methods, which was reflective of a distinct interstage dynamic pattern of the laboratory indicators. The clinical progression was accordingly divided into the acute stage (1–9 days post–symptom onset), the critical stage (10–15 days post–symptom onset), and the late stage (>15 days post–symptom onset).
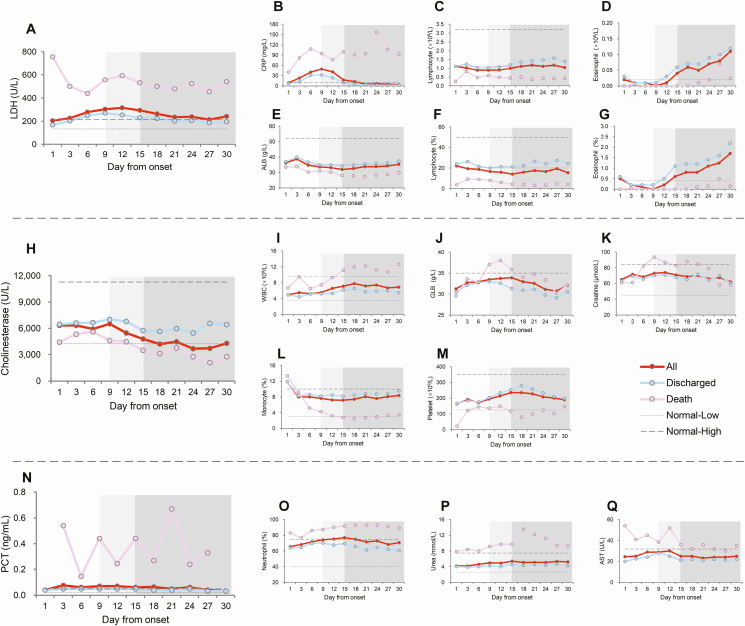
Seventeen of the 49 laboratory parameters were obviously deviated from normal values, which were grouped into 3 clusters based on their dynamic patterns (Figure 1 and Supplementary Figure 1). For the first cluster of 7 laboratory indicators (CRP, LDH, albumin [ALB], lymphocyte count [LYM], lymphocyte percentage [LYM%], eosinophil count, and eosinophil percentage), a similar trend was observed in the 2 groups, in that the results started to deviate slightly from normal value ranges at the acute stage, maintaining the increasing trend and attaining the peak or nadir level at the critical stage, and especially to a more significant extent in the fatal cases. At the third stage, these measurements reverted to their physiological ranges for those who were discharged but stayed at abnormal ranges for the fatal cases (Figure 1A).
Figure 1.
A–Q, Dynamic profiles of 17 laboratory parameters in 642 patients with COVID-19. Mean values according to days from disease onset are shown. The acute, critical, and late stages of clinical progression are marked by the no-, light-, and dark grayshaded areas in the columns. Abbreviations: ALB, albumin; AST, aspartate amino transferase; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; GLB, globulin; LDH, lactate dehydrogenase; PCT, procalcitonin; WBC, white blood cells.
For the second cluster of 4 laboratory indicators—PCT, amino transferase (AST), urea, and neutrophil percentage (NEUT%)—the abnormal results were clearly observed in fatal cases throughout the clinical course, but for the discharged group the mean values largely remained within the normal range (Figure 1B).
For the third cluster of 6 laboratory indicators, the abnormal results were seen only in fatal cases, and only started at the critical stage for white blood cell (WBC) count, globulin, creatine, and cholinesterase or started at the third stage for platelet count and monocyte percentage (MONO%), while keeping within the normal range for those who were discharged (Figure 1C). No abnormal result or only a slight deviation from normal was observed for the other 32 indicators, which were excluded from further analysis (Supplementary Figure 1).
The Discriminative Power of Laboratory Parameters in Determining Fatal Outcome
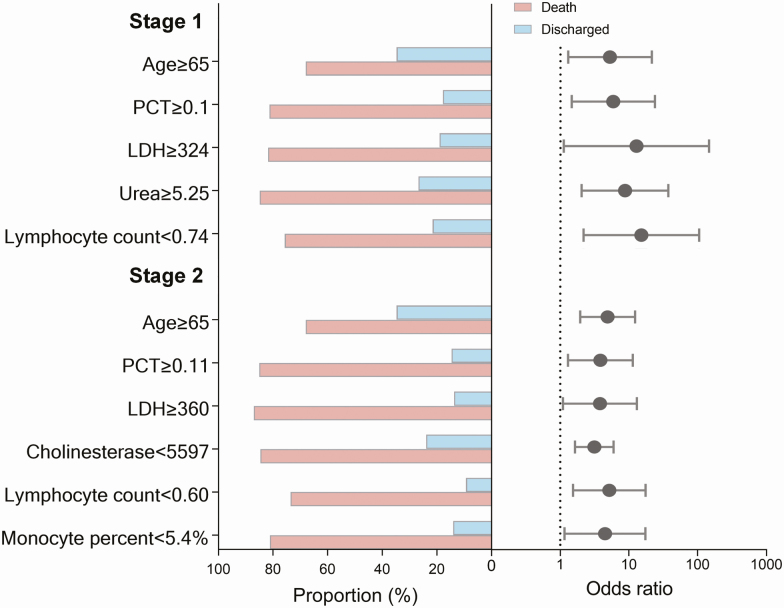
Using the ROC curve method, of the 17 laboratory findings that displayed a statistically significant difference on univariate analysis, the optimum diagnostic cutoff points of the fatal versus discharged groups were obtained for the acute stage and critical stages separately (Supplementary Table 4). For the acute stage evaluation, 7 indicators had an area under the curve (AUC) >0.8, from which the LYM% was removed due to its high correlation with other indicators (Supplementary Figure 2); thereafter, the remaining 6 indicators, including CRP, PCT, LDH, urea, LYM, and NEUT%, were entered into a multivariate logistic regression model, together with age, sex, and preexisting hypertension and coronary heart disease. Finally, age, LDH, urea, LYM, and PCT were determined to be independently predictive of a fatal outcome. The odds ratios (ORs) for a fatal outcome were 3.136, 5.179, 3.792, 3.848, and 4.490 in the presence of age ≥65 years old, LDH ≥324 U/L, urea ≥5.25 mmol/L, LYM <0.74 × 109/L, and PCT‖≥0.1 ng/mL, respectively (Figure 2 and Supplementary Table 5). These indicators collectively could discriminate 87% of the patients who might progress to a fatal outcome (data not shown).
Figure 2.
The proportion of deaths and adjusted ORs for deaths of patients with COVID-19 by age and laboratory signs. The black points show the adjusted ORs for death and the error bars represent the 95% CIs. ORs were adjusted for age, sex, and preexisting conditions. The dotted line indicates an adjusted OR of 1. Abbreviations: CI, confidence interval; LDH, lactate dehydrogenase; OR, odds ratio; PCT, procalcitonin.
For the critical stage evaluation, 12 indicators had an AUC >0.8, from which the LYM% and NEUT% were removed due to their high correlation with other indicators (Supplementary Figure 2); thereafter, the remaining 10 (ie, CRP, PCT, LDH, cholinesterase, ALB, urea, LYM, NEUT, MONO%, and WBCs) were entered into a multivariate logistic regression model, together with age, sex, and preexisting hypertension and coronary heart disease. Finally, age, PCT, LDH, cholinesterase, LYM, and MONO% were determined to be independently associated with fatal outcome. The ORs for a fatal outcome were 4.903, 15.059, 8.702, 12.764, 5.884, and 5.261 in the presence of age ≥65 years, PCT ≥0.11 ng/mL, LDH ≥360 U/L, cholinesterase <5597 U/L, LYM <0.60 × 109/L, and MONO% <5.4%, respectively (Figure 2 and Supplementary Table 5). These indicators collectively could discriminate 92% of the patients who might progress to a fatal outcome (data not shown).
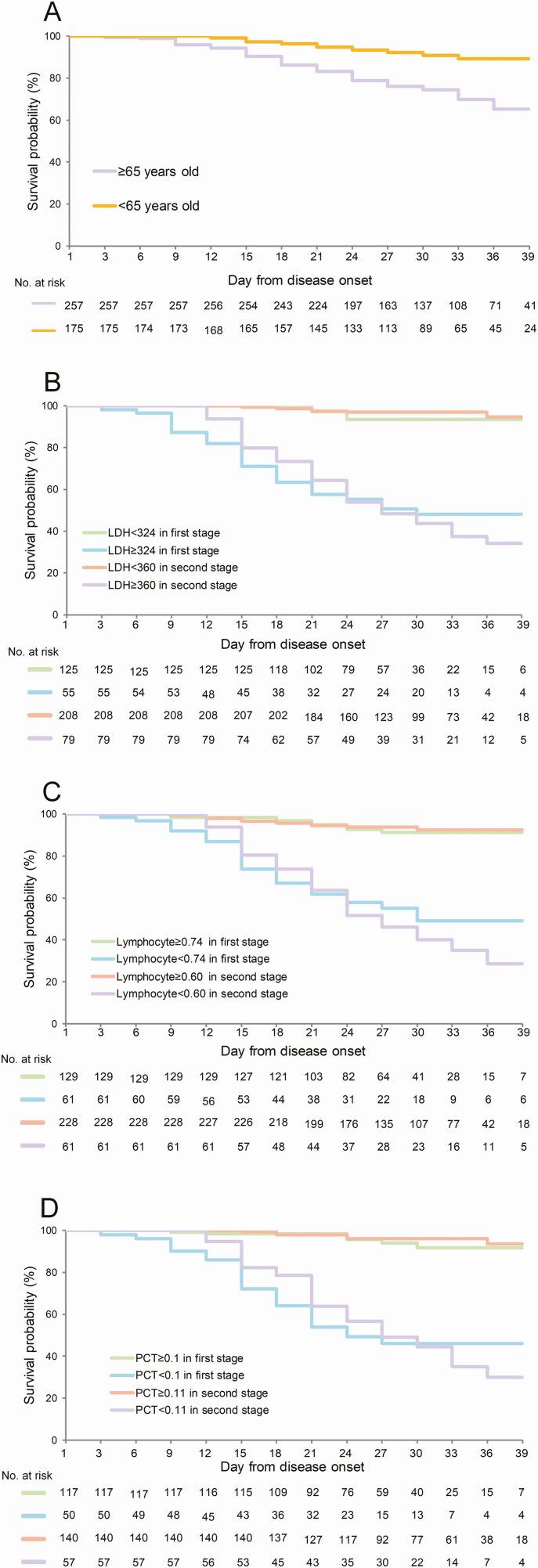
It is noteworthy that age, LDH, PCT, and lymphocytes were predictive of a fatal outcome at both stages. The survival analysis revealed an accelerated death in patients with older age, with higher levels of LDH and PCT, and lower levels of lymphocytes than the cutoff values (Figure 3. The HRs and 95% CIs are shown in Supplementary Table 6).
Figure 3.
Analysis of effect on probability of survival according to age (A), LDH (B), lymphocytes (C), and PCT (D) at 2 clinical stages. The Kaplan-Meier method was used to analyze time-to-event data. Abbreviations: LDH, lactate dehydrogenase; PCT, procalcitonin.
Serum Cytokine Profiles and Their Correlations With Laboratory Parameters
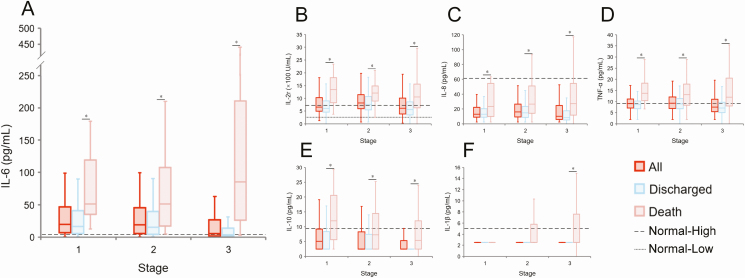
The production of IL-1β, IL-6, IL-8, IL-10, TNF-α, and IL-2r was described along the clinical course. IL-6 was elevated in both groups at the 2 early stages, with fatal cases having a more robust production than discharged cases across the whole observation period when analyzed by using GEEs (P < .001) (Figure 4 and Supplementary Table 7). In contrast, the cytokines TNF-α and IL-2r showed a persistent elevation in fatal cases at all 3 stages, but for the discharged cases these were only slightly increased at the critical stage. For IL-8, IL-10, and IL-1β, the production in all phases for both fatal and discharged cases was largely within physiological ranges, although the levels in the fatal cases were significantly higher.
Figure 4.
Dynamic profiles of 6 cytokines in the patients with COVID-19. The box plots were delineated according to the acute, critical, and late stage of clinical progression. A, IL-6; B, IL-2r; C, IL-8; D, TNF-α; E, IL-10; F, IL-1β. The data from healthy adults who underwent a health examination in a previous test were used as a normal reference to perform the analysis. *Means P < .05 between the two groups. Abbreviations: COVID-19, coronavirus disease 2019; IL, interleukin; TNF-α, tumor necrosis factor α.
We analyzed correlations between these cytokines and key clinical parameters that were measured from the same patient at the same time point. The correlation analyses showed that serum IL-6 was positively correlated with serum levels of CRP, TNF-α, PCT, NEUT%, and IL-8, but were negatively correlated with LYM% and cholinesterase levels (Supplementary Figure 2). TNF-α was positively correlated with IL-2r, IL-8, IL-6, and PCT. IL-2r was positively correlated with TNF-α, IL-8, PCT, and CRP.
The viral positive rate (calculated as the total positive sample number divided by the total detected sample number at each stage) was the highest at the acute stage (56.6%, 60/106), decreased to 30.5% (72/236) in the critical stage, and was maintained at 14.5% (153/1057) at the third stage (P < .001 at any 2 stages) (Supplementary Figure 3). The fatal cases had higher detection rates than the discharged group at all 3 stages (60.0% vs 52.9% at the acute stage, 41.7% vs 28.0% at the critical stage, and 20.8% vs 10.3% at the third stage). These differences, however, were not significant (all P > .05).
DISCUSSION
To our knowledge, this is the first study focusing on the clinical progression of COVID-19 according to results of laboratory tests. We provided a complete description of the laboratory abnormalities for COVID-19, and accordingly defined 3 distinct clinical stages. The acute stage spanned from 1 to 9 days after onset of symptoms, which corresponded to the early hospitalization and was characterized by already slightly deviated indicators of liver damage, renal damage, and impaired immunity. This suggested a common but not serious COVID-19—related organ injury, and was primarily observed among patients with a fatal outcome. This second phase (ie, 10–15 days after disease onset) was considered to be a critical stage when the intergroup differences in the important indicators were further widened, which corresponded to the time of intensive care unit (ICU) admission (duration from onset of symptoms as 9.5 days, as previously described in Yang et al [15]). The convalescence stage begins in survivors approximately 2 weeks after disease onset, with clinical symptoms beginning to resolve and laboratory measurements gradually reverting to normal, whereas thrombocytopenia and decreases in monocytes were exclusively observed in fatal cases. Using ROC, the significant predictors of progression to fatal disease were obtained for the early 2 stages, respectively. For patients who had the laboratory test performed within 9 days post–symptom onset, older age of ≥65 years, laboratory tests of lymphocyte counts below a certain level, and higher LDH, urea, and PCT over a certain level collectively could be used to discriminate 87% of the patients who might progress to a fatal outcome. For patients who had the laboratory test performed within at 10–15 days post–symptom onset, older age of ≥65 years; high serum concentrations of LDH, cholinesterase, and PCT; lower monocyte percentage; and lymphocyte count that exceeded or was below a certain cutoff value collectively could discriminate 92% of the patients who might progress to a fatal outcome.
The determinants for an adverse prognosis in COVID-19 have been explored in previous studies, with ICU admission, severe pneumonia, or death used as outcomes [7–9, 16, 17], and with associated factors differing across studies. The incongruent conclusions obtained were probably due to the limited sample sizes and the lack of adjustment for potential confounding effects by multivariate analysis. In our study, almost all the aforementioned indicators identified previously were likewise found to differ between patients who survived and those with a fatal outcome by univariate analysis; however, half of the effects disappeared when applying multivariate analysis. Moreover, the effective predictors of death differed across clinical stages, further justifying the necessity of performing a multistage study. For the first time, serum cholinesterase was found to be an important prognostic factor at the critical stage. Cholinesterase activity had been associated with the risk of fatal outcome for patients with acute myocardial injury, liver damage, respiratory failure, and shock [18–21]. On the other hand, acute cardiac injury determined by elevated troponin levels, as well as ARDS, was also strongly associated with mortality [22]. Cholinesterase activity, which was reflective of the complication of heart failure and septic shock, might be used as a useful measurement in the prediction of fatal COVID-19.
Similar to previous studies, our data clearly showed the eliciting of a cytokine storm during the acute phase of COVID-19, and at a more severe magnitude among fatal cases. An especially high expression of IL-6 was observed in all patients with COVID-19, and to a greater extent in fatal cases across the whole clinical course. A significant association between IL-6 and multiple abnormal laboratory values indicative of heart, kidney, and liver damage were observed, suggestive of an inflammatory response in eliciting tissue damage either in the lung or other target organ, ultimately causing death. The evaluation of IL-6 could be used as a prognostic factor for fatal COVID-19 at any stage of disease, which should be included in the list of routine tests for SARS-CoV-2 infection as well. Its role in defining disease prognosis as well as implications for potential therapeutic interventions require further study.
The study had a major limitation in that the analysis was restricted to laboratory parameters. The respiratory parameters, such as partial pressure of oxygen, oxygen saturation, and ratio of arterial oxygen partial pressure to fractional inspired oxygen were not included for analysis; however, the study was designed to provide prognostic markers that can be routinely tested even at emergency rooms and outpatient departments before these advanced respiratory indicators can be measured. The viral load was not routinely evaluated in clinical practice, and thus cannot be used in a timely fashion for the early prediction of disease. Coagulation markers such as D-dimer, which has been shown to be predictive of poor outcome in other studies, were not used in the study due to the small number tested. In the studied hospital, a highly comparable treatment regimen was administered according to the standard guideline; therefore, we believe the effect from treatment was minimized.
In summary, by exploring the longitudinal profile of laboratory parameters of patients with COVID-19 who were routinely tested in clinical practice, we consider LDH, PCT, lymphocyte count, and IL-6 to be highly important prognostic markers, which should be monitored in order to identify patients who may progress to a severe course at an early phase of the disease, and to target such patients for enrollment in clinical trials of putative therapeutic agents aimed at reducing the risk of clinical disease progression.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. H.-L. Z., Q. Y., D.-Y. Y., X. W., and H.-J. L. collected the epidemiological and clinical data. Q.-B. L., L.-K. Z. H.L. and W.L. summarized all data. H-L.Z. and Q-B.L., drafted the manuscript. W.L. and H-J.L. revised the final manuscript.
Acknowledgments. The authors thank all the medical care workers who participated in the sample collection. The study was conducted in accordance with guidelines approved by the Ethics Committees from Tongji Hospital (Wuhan, China) (TJ-IRB20200102). The Research Ethics Committee waived the requirement of informed consent before the study started because of the urgent need to collect epidemiological and clinical data. All the data were analyzed anonymously. After publication, the data will be made available to others on reasonable request to the corresponding author. A proposal with a detailed description of study objectives and statistical analysis plan will be needed for the evaluation of the reasonability of requests. Additional materials might also be required during the process of evaluation. Deidentified participant data will be provided after approval from the corresponding author and Wuhan Tongji Hospital.
Financial support. This work was supported by the National Science Fund for Distinguished Young Scholars (grant number 81825019) and China Mega-Project on Infectious Disease Prevention (grant numbers 2018ZX10713002, 2018ZX10101003, 2017ZX10103004).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020; 382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fang Y, Zhang H, Xu Y, Xie J, Pang P, Ji W. CT Manifestations of two cases of 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 2020; 295:208–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020; 63:364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu C, Chen X, Cai Y, et al. risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020: e200994. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061-1069. pii:2761044. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang B, Zhou X, Qiu Y, et al. Clinic al characteristics of 82 death cases with COVID-19. medRxiv 2020: 2020.02.26.20028191. doi: 10.1101/2020.02.26.20028191 [DOI] [Google Scholar]
- 12. The novel coronavirus outbreak: what we know and what we don’t. Cell 2020; 180:1034–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sohrabi C, Alsafi Z, O’Neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg 2020; 76:71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Health Commission of the People’s Republic of China. Diagnosis and treatment scheme of new coronavirus infected pneumonia. 7th ed Available at: http://www.nhc.gov.cn/xcs/zhengcwj/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. Accessed 4 March 2020.
- 15. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8:475–81. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46:846–8. doi: 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18:844–847. doi: 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seo M, Yamada T, Tamaki S, et al. Prognostic significance of serum cholinesterase in patients with acute decompensated heart failure: a prospective comparative study with other nutritional indices. Am J Clin Nutr 2019; 110:330–9. [DOI] [PubMed] [Google Scholar]
- 19. Li M, Chen Y, Zhang Y, et al. Admission serum cholinesterase concentration for prediction of in-hospital mortality in very elderly patients with acute ischemic stroke: a retrospective study. Aging Clin Exp Res 2020. pii:10.1007/s40520-020-01498-z. doi: 10.1007/s40520-020-01498-z [DOI] [PubMed] [Google Scholar]
- 20. Liu J-H, Chang Y-F, Wang J-Y, Qin Z, Ma Y-F. Levels of serum cholinesterase and prealbumin in patients with chronic obstructive pulmonary disease with respiratory failure and their relationships with prognosis. J Med Res 2019; 48:158–61. [Google Scholar]
- 21. Kong J, Xiang X-X. Value of serum cholinesterase in diagnosis /treatment and prognostic evaluation of liver diseases. J Clin Hepatol 2017; 33:1806–9. [Google Scholar]
- 22. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020. pii: 2763846. doi: 10.1001/jamacardio.2020.1286 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.