Abstract
Endoscopy is an essential component in the management of inflammatory bowel disease [IBD]. There is a risk of SARS-CoV-2 transmission during endoscopic procedures. The International Organization for the study of IBD [IOIBD] has developed 11 position statements, based on an online survey, that focus on how to prioritise endoscopies in IBD patients during the COVID-19 pandemic, alternative modes for disease monitoring, and ways to triage the high number of postponed endoscopies after the pandemic. We propose to pre-screen patients for suspected or confirmed COVID-19 and test for SARS-CoV-2 before endoscopy if available. High priority endoscopies during pandemic include acute gastrointestinal bleed, acute severe ulcerative colitis, new IBD diagnosis, cholangitis in primary sclerosing cholangitis, and partial bowel obstruction. Alternative modes of monitoring using clinical symptoms, serum inflammatory markers, and faecal calprotectin should be considered during the pandemic. Prioritising access to endoscopy in the post-pandemic period should be guided by control of COVID-19 in the local community and availability of manpower and personal protective equipment. Endoscopy should be considered within 3 months after the pandemic for patients with a past history of dysplasia and endoscopic resection for dysplastic lesion. Endoscopy should be considered 3–6 months after the pandemic for assessment of postoperative recurrence or new biologic initiation. Endoscopy can be postponed until after 6 months of pandemic for routine IBD surveillance and assessment of mucosal healing.
Keywords: Endoscopy, COVID-19, inflammatory bowel disease
1. Introduction
Endoscopic assessment is essential for disease diagnosis, monitoring, and evaluation of treatment response in patients with inflammatory bowel disease [IBD]. Given that novel coronavirus SARS-CoV-2 has been isolated in gastric, duodenal, and rectal biopsies, and viral RNA is detectable in faeces of 50% of all COVID-19 patients, 1,2 there is a theoretical risk of SAR-CoV-2 transmission to health care workers during endoscopic procedures.3,4 A single virus-shedding patient with a high viral load can potentially contaminate the endoscopy unit with the virus that is viable for up to 3 days, putting both health care workers and uninfected patients at risk.5,6 Although there remains no documented case of SARS- CoV-2 transmission through endoscopy, endoscopy departments remain fertile grounds for viral spread because aerosolisation of bodily secretions occurs with active insufflation, air suctioning, and oxygen administration during endoscopy.6 These concerns have led to a high demand for personal protective equipment [PPE] and have also compelled endoscopy units to restrict their services to only essential cases during the outbreak.6 COVID-19 may also vary in its presentation, from asymptomatic states to subjects presenting with anosmia, fever, and respiratory and/or gastrointestinal [GI] symptoms.7–9 GI symptoms are described in approximately 30% of COVID-19 patients and, among them, diarrhoea has been reported in 2–49.5% of patients.2,10,11 Distinguishing GI manifestations of COVID-19 from active inflammatory bowel disease [IBD] can be challenging. Furthermore, stool samples in infected patients remained positive for SARS-CoV-2 for up to 1 month even after respiratory samples have become negative, suggesting prolonged viral shedding.1,2,12
To date, the American College of Gastroenterology, British Society of Gastroenterology, Asian Pacific Society of Digestive Endoscopy, and several national GI societies have provided general recommendations for endoscopies during COVID-19.13,14 In view of the profound impact of COVID-19 on IBD patients, the International Organization for the study of IBD [IOIBD] recently developed a task force to focus on how to prioritise endoscopies in IBD patients during the pandemic, what are the alternative modes for monitoring, and how to triage the high number of postponed endoscopies after the pandemic. Our objective is to provide recommendations to IBD clinicians, nurses, and surgeons on the best practice and management of IBD endoscopies during and after the pandemic. The health and safety of patients, their families, and health care workers are of paramount priority.
2. Results of endoscopy survey during the COVID-19 pandemic
In consecutive webinars organised by the IOIBD from March 20, 2020 to April 30, 2020, key issues relating to endoscopy during IBD was raised. The members of the IBD endoscopy taskforce [SCN, YC, CNB, MSS] and two IBD fellows [JM, LH] were appointed by the IOIBD to review current literature and develop position statements. An online survey was conducted among IOIBD members between April 3 and April 24, 2020, targeting five key components of endoscopy practice including: pre-screening, indications for endoscopy, protection of health care workers, alternative modes of monitoring, and stepwise resumption of endoscopy service after COVID-19. A total of 38 gastroenterologists from North America [n = 12], Europe [n = 18], Oceania [n = 1], Asia [n = 5], Israel [n = 1], and South America [n = 1] participated in this survey. Except for one, all were IOIBD members.
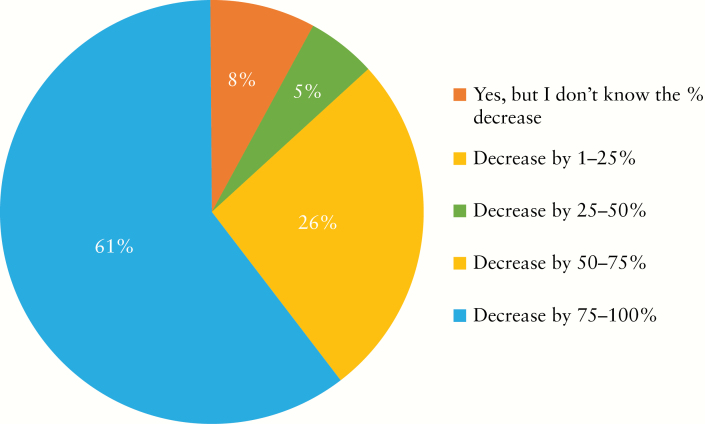
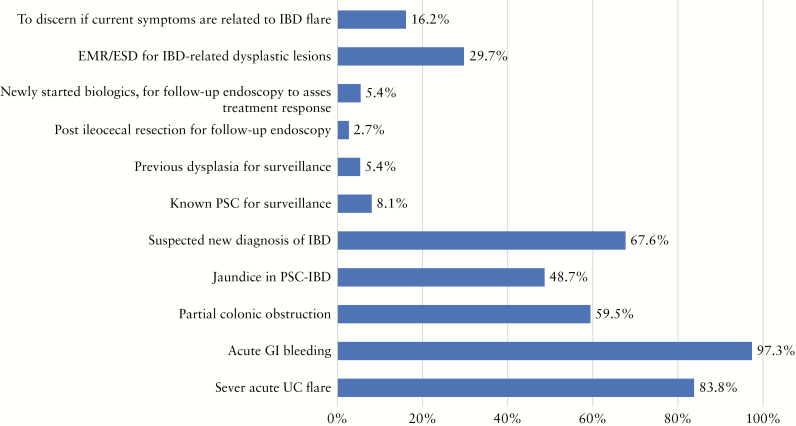
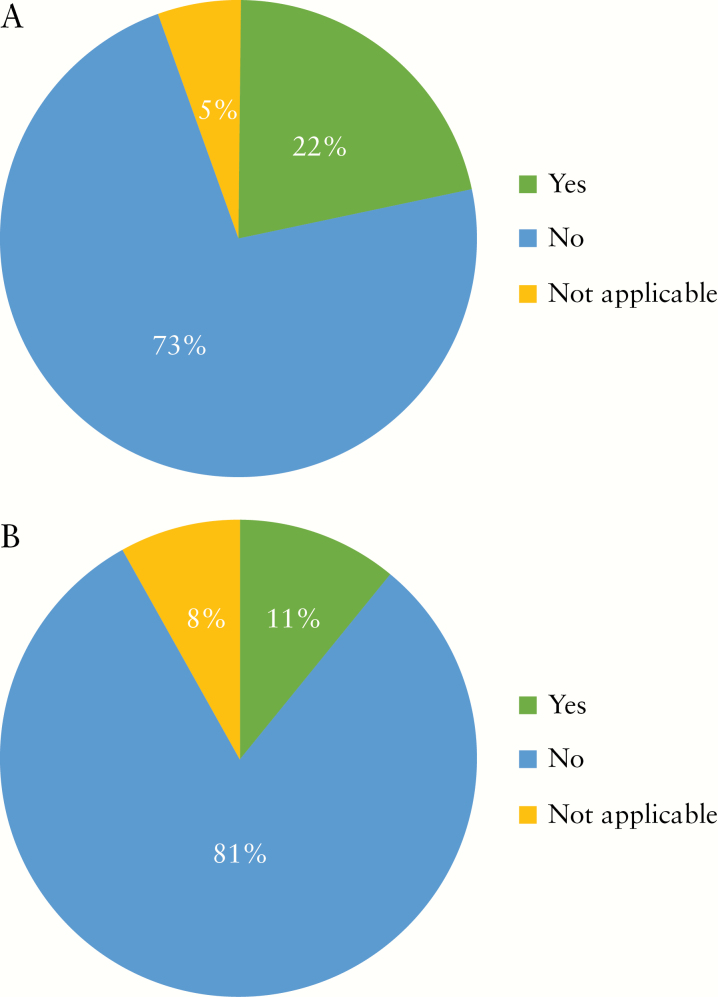
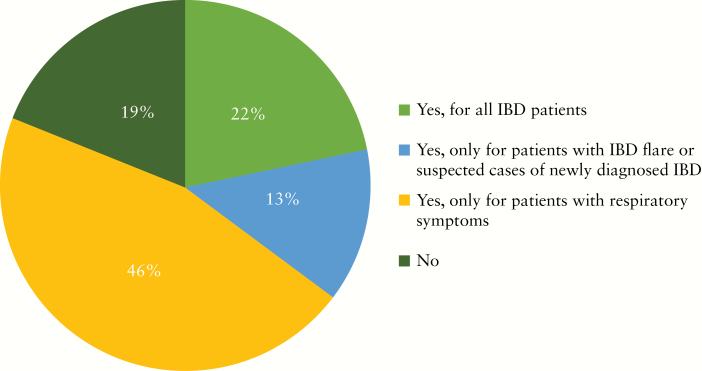
During the COVID-19 outbreak, nearly two-thirds of institutions have decreased the number of endoscopies for IBD by over 50% [Figure 1]. The majority of participants would consider the following as indications for urgent endoscopies during COVID-19 outbreak: acute GI bleeding, acute severe ulcerative colitis [UC] exacerbation, suspected new diagnosis of IBD, partial colonic obstruction, and jaundice in an IBD patient with primary sclerosing cholangitis [Figure 2]. The top three indications that reached the highest consensus for urgent endoscopies during the outbreak included acute GI bleeding [97%], severe acute UC flare [84%], and suspected new IBD diagnosis [68%] [Figure 2]. Around three-quarters of participants would stop performing research endoscopies in IBD as part of industry-sponsored [73%] or non-industry-sponsored [81%] clinical trials during the COVID-19 outbreak [Figure 3a, b]. Almost half [46%] reported cessation of small bowel enteroscopies during outbreak. The majority of participants [81%] were concerned about faecal shedding of SARS-CoV-2 virus during colonoscopy:22% would test for SARS-CoV-2 in all IBD patients before endoscopy, 14% would test only patients with an IBD flare or suspected cases of newly diagnosed IBD, and 46% would test only for IBD patients with respiratory symptoms [Figure 4]. If consulted on a patient with new onset GI symptoms but no respiratory symptoms or fever, 36% would recommend SARS-COV2 viral testing.
Figure 1.
Has your institution decreased the number of endoscopies for IBD during the COVID-19 outbreak?
Figure 2.
Amon the indications below for endoscopy, which do you still use as an indication for endoscopy during COVID-19 outbreak?
Figure 3.
a. Do you perform research endoscopy as part of industry-sponsored clinical trials in IBD as scheduled during the COVID-19 outbreak? b. Do you perform research endoscopy in IBD for non-industry sponsored trials as scheduled during the COVID-19 outbreak?
Figure 4.
Do you perform SARS-CoV-2 viral testing for IBD patients before endoscopy?
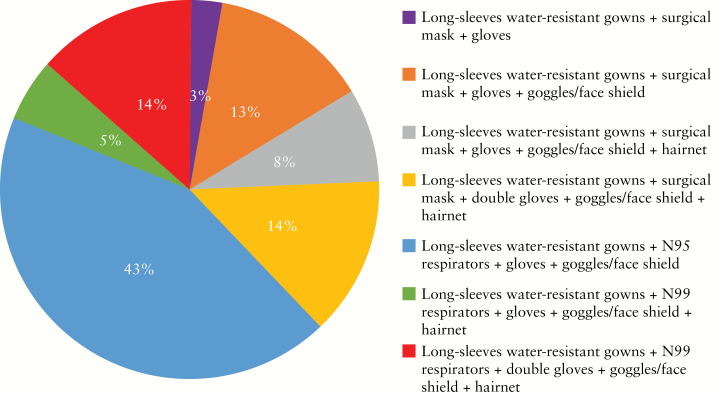
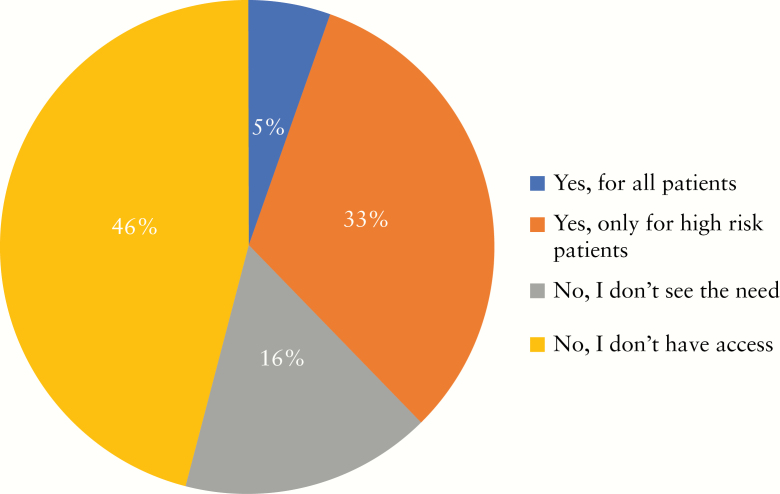
The most commonly used PPE combination [>62%] included long-sleeved water-resistant gowns with N95 respirators, gloves [single or double], goggles/face-shield, and hairnet. About 38% use surgical masks instead of N95 respirators during the outbreak [Figure 5]; 5% and 32% perform endoscopies in negative pressure rooms for all patients and for high-risk patients, respectively, and 16% did not see a need for negative pressure rooms. However, almost half [46%] of the participants reported that they had no access to negative pressure rooms [Figure 6].
Figure 5.
What kinds of personal protective equipments do you wear for IBD endoscopy during COVID-19?
Figure 6.
Do you perform IBD endoscopies in a negative pressure room?
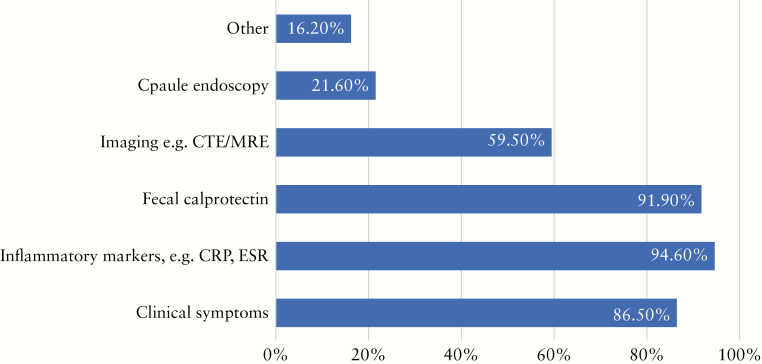
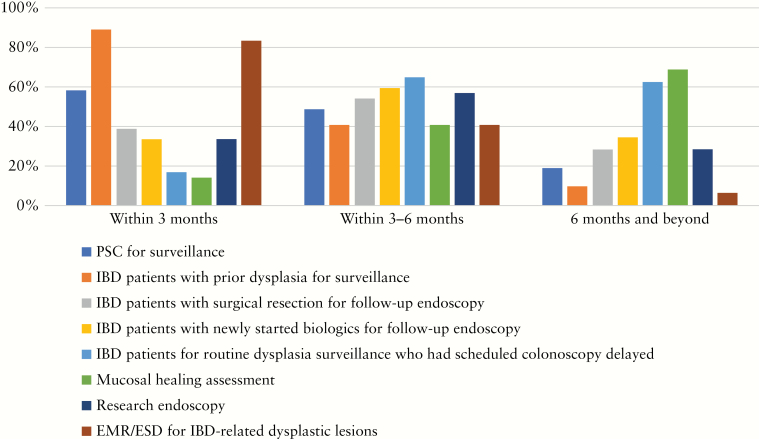
There is unanimous agreement that alternative modes of non-endoscopic based tools for disease monitoring are required during the outbreak. Participants would use the following methods of disease monitoring: blood-based inflammatory markers [95%], faecal calprotectin [92%], clinical symptoms [86%], imaging with computed tomography enterography/magnetic resonance enterography [CTE/MRE] [59%], and capsule endoscopy [22%] [Figure 7]. Over 80% considered that surveillance of IBD patients with previous dysplasia and endoscopic submucosal dissection/endoscopic mucosal resection [EMR/ESD] for IBD-related dysplastic lesions should be performed as priority within 3 months after the COVID-19 outbreak. Regarding endoscopies that should be scheduled within 3–6 months, most included follow-up endoscopies of patients with newly started biologic treatment, follow-up endoscopies after surgical resections, and routine dysplasia surveillance that had to be delayed. Most considered that routine surveillance endoscopy and procedures to assess mucosal healing can be delayed for more than 6 months [Figure 8; Table 1].
Figure 7.
Which modes of monitoring will you consider during the COVID-19 outbreak?
Figure 8.
Among the indications for endoscopy listed below, which will you plan to re-schedule as priority [within 3 months]/within 36 months/ after 6 months after the COVID-19 outbreak?
Table 1.
IOIBD Position Statements: best practice guidance for endoscopy for IBD during the COVID-19 pandemic.
| 1. Pre-screen patients for suspected or confirmed COVID-19 before endoscopy. |
| 2. Test patients for SARS-CoV-2 before endoscopy if available [and based on local outbreak situation]. |
| 3. Patients should wear surgical masks and be unaccompanied in the endoscopy suite. |
| 4. High-priority endoscopies during pandemic include acute gastrointestinal bleed, acute severe UC, new IBD diagnosis, cholangitis in PSC and IBD, and unresolved partial bowel obstruction. |
| 5. Research or clinical trial endoscopy should be scheduled based on unit’s resources and on patient care factors. |
| 6. Adequate protective gear should be provided to personnel in endoscopy suites and endoscopy should be performed in negative pressure rooms if available. |
| 7. Extra precaution is recommended during colonoscopies as prolonged faecal shedding of SARS-CoV-2 can occur. |
| 8. Combined with clinical symptoms, consider serum inflammatory markers and faecal calprotectin as alternative modes of monitoring during pandemic. |
| 9. Consider CTE, MRE, capsule endoscopy, abdominal US if readily available. |
| 10. Prioritising access to endoscopy for IBD in the post-pandemic period should be guided by control of COVID-19 in the local community and availability of manpower and PPE. |
| i] Endoscopy should be considered for subjects with a past history of dysplasia for surveillance and EMR/ESD for dysplastic lesion within 3 months after pandemic. |
| ii] Endoscopy should be considered for postoperative recurrence assessment and assessment after new biologic initiation 3–6 months after pandemic. |
| iii] Routine IBD surveillance and assessment of mucosal healing should be postponed until after 6 months. |
| 11. Provide access to helplines/follow-up appointment after endoscopy to all patients. |
UC, ulcerative colitis; IBD, inflammatory bowel disease; PSC, primary sclerosing cholangitis; CTE, computed tomography enterography; MRE, magnetic resonance enterography; US, ultrasound; PPE, personal protective equipment; EMR//ESD, endoscopic mucosal resection/endoscopic submucosal dissection.
Based on our survey, we have developed 11 position statements to guide IBD-related endoscopies during the outbreak [Box 1]. Our task force emphasises that risk management protocols should be developed according to health care priorities and available resources of individual centres. These statements should be considered as a framework for good clinical practice.
3. Who gets scoped now and who can wait
Decreasing the endoscopy service by >50% during an initial outbreak can help to preserve the surge capacity for hospitals to manage a massive number of suspected or confirmed cases of COVID-19, safeguard the potential transmission between patients and health care providers, and allow time for health care providers to receive appropriate education and training on infection control measures during endoscopy. In all other situations apart from those listed as priority, endoscopy can be postponed or replaced with the use of non-invasive biomarkers, cross-sectional imaging such as ultrasonography, or video capsule enteroscopy during the pandemic. A research endoscopy service can be provided if the individual centre has available resources and manpower, but this has been undertaken in only a minority of centres.
4. How to prepare IBD patients for endoscopy?
In all IBD patients, careful evaluation for symptoms, a temperature check, and exposure history to COVID-19 is recommended before entry to the endoscopic unit. If resources are available, IBD patients should be tested for COVID-19 [on the basis of SARS-CoV-2 RT-PCR testing of a nasopharyngeal swab] before undergoing endoscopy, to minimise potential risk [Table 1]. Negative swab tests for patients with a high index of suspicion for COVID-19 may need to be retested before admittance, depending on local guidance. Testing in low-risk populations should be guided by the pre-test probability and sensitivity of the test assay used within the institution, and in some centres may not be warranted, depending on the local situation of outbreak. When IBD patients arrive at the endoscopy suite, they should be wearing surgical masks and come unaccompanied.
5. How to protect health care professionals during endoscopy?
Adequate protective gear should be provided to personnel in endoscopy suites and endoscopy should be performed in negative pressure rooms if available. Health care workers at endoscopy centres are at increased risk of infection of COVID-19 by inhalation of airborne droplets, conjunctival contact with splash, and faecal contamination. It has been reported that the SARS-CoV-2 virus could remain viable in aerosols for up to 3 h and could be detected on different surfaces for up to 3 days, indicating that aerosol transmission of SARS-CoV-2 is plausible.6 The IOIBD considers colonoscopy a potentially high-risk procedure [Table 1]. Meta-analysis demonstrated that 70.3% of COVID-19 patients still tested positive for SARS-CoV-2 RNA in stool after negative respiratory tract samples.2 SARS-CoV-2 RNA could be detected in the upper GI tract and rectum in severely ill patients.15 Live SARS-CoV-2 was also detected in stool samples from patients who did not have diarrhoea.16 Positive insufflation during colonoscopy could also pose a risk of generating aerosol and increase the risk of SARS-CoV-2 transmission. The majority of the endoscopy societies recommend wearing at least surgical masks, gowns, gloves, and goggles or face shields for endoscopy and N95 respirators or FFP2/3 masks for highly suspected or confirmed cases of COVID-19.14 Several societies in North America, Europe, Canada, and Brazil recommended double gloving.14 We recommend the use of long-sleeved water resistant gowns, N95 respirators, gloves, goggles or face shields, plus hairnets during endoscopy. Only essential personnel should be present in the endoscopy room. Involved health care personnel should receive adequate training on donning and doffing personal protective equipment.
6. What are alternative models of monitoring?
In order to monitor patients without endoscopy during the active phase of the pandemic, we recommend continuing clinical service remotely by phone or video conference whenever possible. Patients should be provided with access to helplines in case of emergency, on top of regularly scheduled follow-up appointments. We recommend using serum and faecal inflammatory markers such as C-reactive protein [CRP], erythrocyte sedimentation rate [ESR], and faecal calprotectin to improve diagnostic accuracy [Table 1].17,18 Access to faecal calprotectin might be limited in certain centres due to concerns regarding faecal shedding. If available, point of care faecal calprotectin should be considered, as it has been shown to have accuracy similar to ELISA-based testing [enzyme-linked immunosorbent assay].19,20 This can be especially useful in patients with presumed high-risk features for COVID 19 disease [elderly >65 years, male gender, smoking, cardiovascular and pulmonary disease, steroid use] in whom unnecessary exposure or visits to blood test laboratories or hospitals should be avoided.7, 8,21–23
We also recommend assessment of patients with suspected active IBD or IBD-related complication with CT enterography or MR enterography, capsule endoscopy, and abdominal ultrasound, based on local availability. Moreover, this should be considered when endoscopy is unlikely to provide full disease extent and where additional imaging is likely to be necessary. The modality of imaging studies availability varies among regions, sites, and hospitals and should be considered case by case and based on local resources and guidelines. For detailed information, IOIBD has issued a specific statement on managing IBD outpatients during the pandemic.
7. How to return to normal service after the pandemic?
We have proposed an endoscopy plan for gradual return to normal service after thepandemic. Stepwise resumption of endoscopy service sshould be guided by the control of COVID-19 in the local community. As the severity of outbreak and health care resources vary among different regions and sites, the time point of returning to normal services will be individual.24 Different approaches are based on factors such as new infection rates, local resources of PPE, and accumulated volume of postponed cases/indication of postponed endoscopies.13,25 It is important that centres have a system of tracking postponed and cancelled endoscopies, as it will be unlikely that full service can be resumed immediately; postponed endoscopies will be additional to the normal workload. We recommend re-scheduling endoscopies according to the urgency of their indication, in three time categories. First, we recommend performing endoscopies for surveillance of patients who had previous dysplasia as well as polypectomy/EMR/ESD for dysplastic IBD-associated lesions within 3 months. Second, we recommend performing endoscopies for assessment of postoperative disease recurrence in Crohn’s disease as well as follow-up endoscopies after newly started biologic therapy within 3–6 months after resuming services. Third, we recommend that endoscopies for routine surveillance and assessment of mucosal healing can be delayed to beyond 6 months [Table 1].
8. Research gaps and outlook
The COVID-19 pandemic is still ongoing in many countries, causing significant disruption and an enormous burden to our health care systems and care of patients with chronic illnesses such as IBD. Currently, it is uncertain how long this pandemic will continue, and when it will be before endoscopic units return to functioning at normal capacity. When the pandemic is over, we will face two major challenges: how to prevent new outbreaks, and how to prioritise waiting lists due to cancellations of endoscopic procedures during the pandemic. A prolonged period without endoscopy could have long-term implications for patients, including risk of suboptimal management and cancer development. Patients with IBD have a chronic disease, with periods of relapse and remission requiring endoscopy monitoring of the effectiveness of medical therapy and also screening for dysplasia and colorectal cancer. In the era of treat to target, with the goal of mucosal healing, using alternative modes to monitor disease outcome may be the new norm and the cost-effectiveness and sensitivity of such measures need evaluation. Further research should also focus on carriage of virus in the gut epithelium or stool and the possibility of faecal-oral transmission of SARS-CoV-2 virus, the potential of endoscopic transmission of SARS-CoV-2 virus, and the accuracy and feasibility of rapid testing for SARS-CoV-2 before endoscopy in all patients. Ultimately, data collection after the pandemic on the consequences of deferred endoscopy in the management of IBD patients is essential.
Funding
This paper was published as part of a supplement financially supported by ECCO and IOIBD.
Conflict of Interest
SCN has received research funds from Fering and Abbvie, and speaker’s honoraria from Ferring, Abbvie, Takeda, Pfizer, Olympus, Tillotts, Menarini, Janssen. JWYM has received research funds from Janssen. YC has received grants from AbbVie and Takeda and advisory fees from Takeda, Ferring, Celltrion, and Eli Lilly. CNB is supported in part by the Bingham Chair in Gastroenterology; he has been on the advisory boards of Abbvie Canada, Janssen Canada, Pfizer Canada, Takeda Canada, Roche Canada, consulted for Takeda and Mylan Pharmaceuticals; been on the speaker’s bureau for Abbvie Canada, Janssen Canada, Takeda Canada, and Medtronic Canada; received unrestricted educational grants from Abbvie Canada, Janssen Canada, Pfizer Canada, and Takeda Canada; and has done contract research with Abbvie, Janssen, Pfizer, Celgene, Boeringher Ingelheim, and Roche. MSS has received research support from Abbvie, Takeda, Janssen, Pfizer, Prometheus; consultant fees from Abbvie, Takeda, Janssen, Pfizer, Gilead, Ferring, Merck, Amgen; and speaker’s fees from Abbvie, Takeda, Janssen, Pfizer. LH reported no conflicts of interest.
Author Contributions
SCN: study and survey design, overseeing study and manuscript writing; JWYM and LH: survey design and analysis, and writing first draft of manuscript; YC, CNB, MSS: study design and revision of final manuscript.
References
- 1. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheung KS, Hung IFN, Chan PPYP, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology 2020. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ng SC, Tilg H. COVID-19 and the gastrointestinal tract: more than meets the eye. Gut 2020;69:973–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lamers MM, Beumer J, van der Vaart J, et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ong SWX, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] from a symptomatic patient. JAMA 2020. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 2020;382:1564–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guan WJ, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 2020;94:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther 2020. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Redd WD, Zhou JC, Hathorn KE, et al. Prevalence and characteristics of gastrointestinal symptoms in patients with SARS-CoV-2 infection in the United States: a multicentre cohort study. Gastroenterology 2020. doi: 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu Y, Guo C, Tang L, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol 2020. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiu PWY, Ng SC, Inoue H, et al. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy [APSDE-COVID statements]. Gut 2020;69:991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Castro Filho EC, Castro R, Fernandes FF, Pereira G, Perazzo H. Gastrointestinal endoscopy during COVID-19 pandemic: an updated review of guidelines and statements from international and national societies. Gastrointest Endosc 2020. doi: 10.1016/j.gie.2020.03.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin L, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 16. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin JF, Chen JM, Zuo JH, et al. Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis 2014;20:1407–15. [DOI] [PubMed] [Google Scholar]
- 18. Brand EC, Elias SG, Minderhoud IM, et al. Systematic review and external validation of prediction models based on symptoms and biomarkers for identifying endoscopic activity in Crohn’s disease. Clin Gastroenterol Hepatol 2019. doi: 10.1016/j.cgh.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 19. Heida A, Knol M, Kobold AM, Bootsman J, Dijkstra G, van Rheenen PF. Agreement between home-based measurement of stool calprotectin and ELISA results for monitoring inflammatory bowel disease activity. Clin Gastroenterol Hepatol 2017;15:1742–9.e2. [DOI] [PubMed] [Google Scholar]
- 20. Moore AC, Huang VW, Bourdages R, et al. IBDoc canadian user performance evaluation. Inflamm Bowel Dis 2019;25:1107–14. [DOI] [PubMed] [Google Scholar]
- 21. Naganuma M, Kunisaki R, Yoshimura N, Takeuchi Y, Watanabe M. A prospective analysis of the incidence of and risk factors for opportunistic infections in patients with inflammatory bowel disease. J Gastroenterol 2013;48:595–600. [DOI] [PubMed] [Google Scholar]
- 22. Fardet L, Petersen I, Nazareth I. Common infections in patients prescribed systemic glucocorticoids in primary care: a population-based cohort study. PLoS Med 2016;13:e1002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vardavas CI, Nikitara K. COVID-19 and smoking: A systematic review of the evidence. Tob Induc Dis 2020;18:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Danese S, Cecconi M, Spinelli A. Management of IBD during the COVID-19 outbreak: resetting clinical priorities. Nat Rev Gastroenterol Hepatol 2020. doi: 10.1038/s41575-020-0294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iacucci M, Cannatelli R, Labarile N, et al. Endoscopy in inflammatory bowel diseases during the COVID-19 pandemic and post-pandemic period. Lancet Gastroenterol Hepatol 2020;5:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]