Abstract
Patients with inflammatory bowel diseases [IBD] are frequently treated with immunosuppressant medications. During the coronavirus disease 2019 [COVID-19] pandemic, recommendations for IBD management have included that patients should stay on their immunosuppressant medications if they are not infected with the severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2], but to temporarily hold these medications if symptomatic with COVID-19 or asymptomatic but have tested positive for SARS-CoV-2. As more IBD patients are infected globally, it is important to also understand how to manage IBD medications during convalescence while an individual with IBD is recovering from COVID-19. In this review, we address the differences between a test-based versus a symptoms-based strategy as related to COVID-19, and offer recommendations on when it is appropriate to consider restarting IBD therapy in patients testing positive for SARS-CoV-2 or with clinical symptoms consistent with COVID-19. In general, we recommend a symptoms-based approach, due to the current lack of confidence in the accuracy of available testing and the clinical significance of prolonged detection of virus via molecular testing.
Keywords: IBD, Crohn’s, de-escalation, immunomodulator, biologic
1. Introduction
Many patients with inflammatory bowel disease [IBD] require medications that affect the immune system. Although effective in treating the inflammation associated with Crohn’s disease [CD] and ulcerative colitis [UC], these medications can also increase the risk and severity of infection.1 As the severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2], causing coronavirus disease 2019 [COVID-19], spread from late 2019 to early 2020 and became pandemic, many patients with IBD and their providers were forced to consider how to manage IBD given the potential effect of immunosuppression on the risk of developing COVID-19 and its associated complications.
A number of consensus statements provide initial guidance on how to manage IBD patients in the setting of COVID-19.2–7 Most of the recommendations are related to infection prevention, responding to patients who have a positive test for SARS-CoV-2 but who are free of COVID-19 symptoms, and managing those with symptomatic COVID-19. The general guidance states that immunosuppressant medications should not be stopped prophylactically in asymptomatic and uninfected patients, but should be withheld in those who either test positive for SARS-CoV-2 without symptoms or develop clinical COVID-19.2,5,7 As more IBD patients are infected globally, it is important to also understand how to manage IBD medications during convalescence while an individual with IBD is recovering from COVID-19. We aimed to review factors contributing to the decision making around medication use during this time interval, and offer guidance on the timing of restarting IBD therapy in patients with IBD. Because there are currently inadequate evidence-based data, the following recommendations are based on a consensus of a group of international IBD and infectious disease experts convened by the International Organization for the Study of Inflammatory Bowel Disease [IOIBD].
2. Concepts Driving the Recommendations
2.1. Safety of IBD medications in patients with COVID-19
Data are accumulating on the outcomes of patients with IBD who have had COVID-19. The Surveillance Epidemiology of Coronavirus [COVID-19] Under Research Exclusion [SECURE-IBD] database is a global database intended to evaluate the outcomes of IBD patients infected with COVID-19.8 Their composite endpoint is the proportion of patients who require an admission to the intensive care unit [ICU], use of a ventilator, or death. From the data on close to 1000 patients reported to date, the medication class with the highest proportion of patients experiencing this endpoint is corticosteroids. The largest group represented in the database are patients taking anti-tumour necrosis factor [TNF] therapy, and these patients appear to be at low risk of developing any of the serious outcomes. This is consistent for drugs that inhibit interleukin [IL]-12/23 and anti-integrin molecules. The risk is a little higher with thiopurines, janus kinase [JAK] inhibitors and patients on combination therapy with an anti-TNF and a thiopurine.
Regional and individual hospital reports from hot spots such as Italy9 and New York City10 find a similar absence of an association between COVID-19 disease severity and IBD therapeutics, and the paediatric experience has also been reassuring.6 Overall, other than corticosteroids, it does not appear that the cornerstone therapies for IBD which suppress the immune system are leading to a substantial increase in adverse events related to COVID-19. It appears also that the same risk factors for the general population [ie, advanced age and comorbidities including hypertension, obesity, and diabetes] are important in the outcome of IBD patients with COVID-19.
This is reassuring, particularly in light of the fact that most of the immunosuppressing IBD medications have a long half-life and therefore are not immediately cleared after discontinuation. Nevertheless, as is standard with other serious infections, recommendations are to hold these treatments until a patient recovers and is considered to be in the convalescent stage.11 Therefore, guidance on the timing of when to recommence these medical therapies is the next important question for the clinician.
2.2. Ability of testing to identify those with active SARS-CoV-2 infection
Sampling of the nasopharynx for SARS-CoV-2 RNA polymerase chain reaction [PCR] is the clinical gold standard for the diagnosis of COVID-19. Whereas the loss of detectable viral RNA coupled with clinical recovery would be a reasonable indicator for the safe re-start of IBD therapy, numerous studies have demonstrated persistent RNA detection in nasopharyngeal [NP] and oropharyngeal [OP] swabs, as well as saliva, for 28 days or more in a substantial proportion of patients without IBD.12 Whether the RNA detected represents viable virus is unknown.13,14 Reports of prolonged shedding of SARS-CoV-2 RNA has also been noted in immunocompromised patients and animals with other coronaviruses.15,16 In contrast, it is known that the NP swab for SARS-CoV-2 RNA detection can yield false-negative results, possibly due to sampling error or the shift of the virus from the upper to the lower airway during disease progression. Therefore, reliance on NP swabs alone to guide the restart of IBD medication is not recommended.
Serological [antibody] testing also has significant limitations. Antibodies to SARS-CoV-2 become reliably detectable 14 or more days after symptom onset; earlier in the course of infection, serological testing is more likely to be negative. There is also considerable variability in the performance of SARS-CoV-2 serological assays, including those that have received Emergency Use Authorization [EUA] from the US Food and Drug Administration [FDA]. The Infectious Diseases Society of America [IDSA] notes that false-negatives are an issue, especially when the prevalence of true previous infections is less than 5%. False-positives are also possible and can be misleading due to potential cross-reactivity with other common cold coronaviruses.17 Importantly, there is no evidence to date demonstrating that SARS-CoV-2 seropositivity protects against re-infection or is durable. The IDSA concludes that at the current time, antibody testing may be helpful to measure exposure prevalence at the population level, but has less utility in individual patient assessments. In the future, such testing may have a role in evaluating individual immunity.
2.3. Test- and non test-based approaches for determining transmission risk
Although the issues of when to discontinue infection control measures and return an infected health care provider to work are distinct from that of the re-initiation of IBD medications, there are a number of shared considerations. In issuing guidance for both situations, the Centers of Disease Control and Prevention [CDC] have offered a test-based and a symptoms-based approach.14,18 The test-based approach calls for resolution of fever and symptoms plus a pair of consecutive respiratory specimens with undetectable SARS-CoV-2 RNA results. The symptom-based strategy uses times from symptoms onset [≥10 days] and improvement including resolution of fever without antipyretics [≥3 days] as criteria. This strategy reflects the low likelihood of transmission from clinically recovering individuals over time.
The approach to determining return to work and discontinuation of transmission-based precautions for persons with COVID-19 can inform decision making regarding the timing of re-initiation of IBD medications during COVID-19 convalescence, as these situations share a primary concern for the presence of viable virus.
3. Recommendations
These recommendations take into consideration what is known about the safety of IBD therapies with COVID-19 and other viral infections, the limitations of current testing modalities, and practical considerations focused on getting patients back on the medications they need to control their IBD while reducing the risk of a flare or the development of antidrug antibodies. A ‘test-based’ strategy may be required in some scenarios; however, for most patients, we recommend a ‘symptoms-based strategy’.
3.1. Symptoms-based strategy
For patients with IBD who have had their immunosuppressant therapy held due to COVID-19, the decision of when to re-start therapy should follow a symptoms-based strategy.
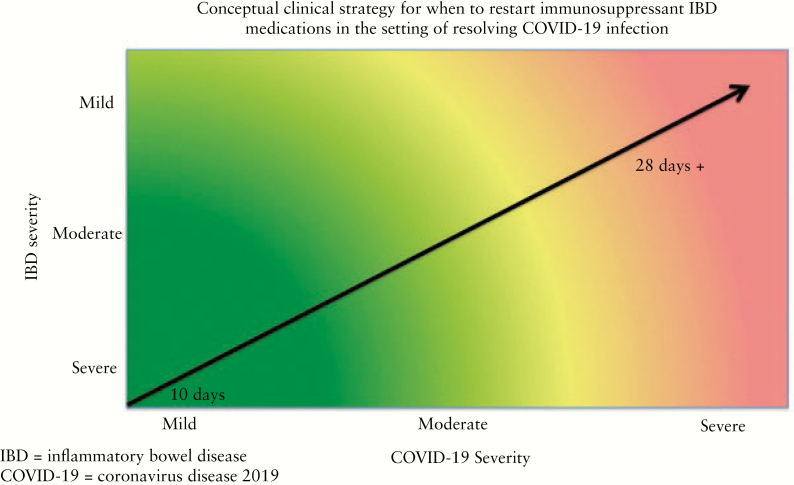
The timing of re-starting IBD immunosuppressant therapy should be influenced by the clinical severity of both IBD and COVID-19. Figure 1 shows a conceptual framework for how to consider the timing for re-starting. For those with mild COVID-19 and a history of difficult to control IBD, earlier re-start of IBD therapy may be considered, whereas for those with more severe COVID-19 and well-controlled IBD, a delay in IBD therapy re-start may be desired.
-
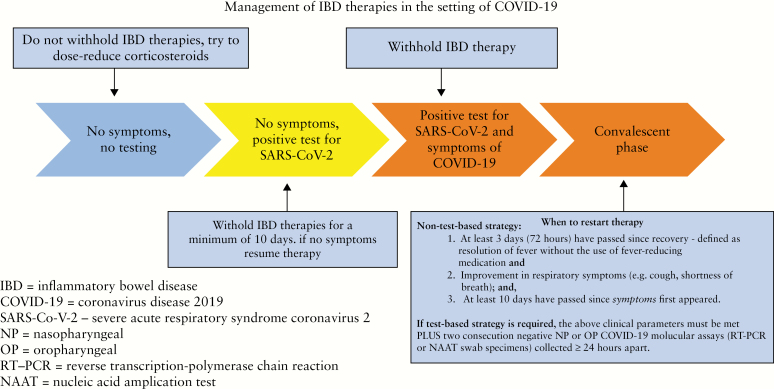
For a symptom-based strategy there are several requirements that need to be met before resuming immunosuppressant therapy14,19 [Figure 2]:
at least 3 days [72 h] since recovery defined as resolution of fever without the use of fever-reducing medications; and
clinically meaningful improvement in respiratory symptoms [eg, cough, shortness of breath]; and
At least 10 days have passed since symptoms first appeared.
<<Figs 1 and 2 near here>>
Figure 1.
Conceptual clinical strategy for when to restart immunosuppressant inflammatory bowel disease [IBD] medications in the setting of resolving COVID-19 infection.
Figure 2.
Management of inflammatory bowel disease [IBD therapies in the setting of COVID-19.
3.2. Test-based strategy
The above clinical parameters must be met plus two consecutive negative NP or OP COVID-19 molecular assays (RT-PCR or nucleic acid amplification test [NAAT] swab specimens) collected ≥24 h apart.
In those who tested positive for SARS-CoV-2 but were asymptomatic, medication can resume at least 10 days since date of the first positive COVID-19 diagnostic test assuming they have not subsequently developed symptoms since the positive test.
3.3. General recommendations
When patients meet the above criteria [test-based or symptom-based] to resume therapy, they are also considered safe to return to a medical infusion unit to receive intravenous medications. If patients have persistent respiratory symptoms [eg, cough] they should ideally be in a private room with a surgical mask and providers should wear the personal protective equipment [PPE] that is standard in their medical setting.14
If recommencement of immunosuppressant therapy is deemed to be necessary on clinical grounds and a test has been performed and is positive for SARS-CoV-2 despite meeting the clinical criteria above, we recommend moving ahead with the scheduled treatment either at home or in an infusion unit with specific infection control measures in place.
4. Research Gaps and Next Steps
As the COVID-19 pandemic evolves, more information comes to light on almost a daily basis. A critical research gap exists in the understanding of the characteristics of the various tests available and how these change during the different phases of the illness. If we can gain confidence in the accuracy of testing [PCR and/or antibody testing] and the meaning of prolonged detection of RNA via molecular testing, this ultimately can better guide the timing of restarting medications. Emerging literature is beginning to characterise the short-term COVID-19 related outcomes among IBD patients, but longer-term natural history studies are needed to further identify adverse events associated with withholding and with restarting therapy.
Funding
This project did not receive any funding. CAS and GJM are active members of the International Organization for the Study of Inflammatory Bowel Diseases [IOIBD]. This paper was published as part of a supplement financially supported by ECCO and IOIBD.
Conflict of Interest
CAS: consultant/advisory board for Abbvie, Amgen, BMS, Celgene, Lilly, Janssen, Sandoz, Pfizer, Prometheus, Sebela, Takeda; speaker for CME activities for Abbvie, Celgene, Janssen, Pfizer, Takeda; grant support from the Crohn’s and Colitis Foundation, AHRQ [1R01HS021747-01], Broad Medical Research Program, Abbvie, Janssen, Pfizer, Takeda, MiTest Health, LLC, which has a patent pending for a ‘System and Method of Communicating Predicted Medical Outcomes’, filed 3/34/10. BC has received consultant/advisory board fees from Gilead, Novartis, Sandoz; research support from Janssen, Pfizer, Ferring; speaker fees for education activities from AbbVie, Janssen, Takeda, Ferring, Pfizer. AK: consultant/advisory board for Abbvie, Janssen, Pfizer, Merck, BMS, Prometheus, Takeda, Salix; speaker’s bureau for Janssen, Takeda, Abbvie, Pfizer, Merck, Prometheus; research support from Abbvie, Janssen, Pfizer, Salix, Takeda, Celgene. JR has grant/research funding from Abbvie and Janssen and has consulted for BMS, Celgene, Janssen, Lilly, Pfizer. MK has consulted for Abbvie, Janssen, and Takeda, is a shareholder in Johnson & Johnson, and has received research support from Abbvie and Janssen. RU has served as an advisory board member or consultant for Eli Lilly, Janssen, Pfizer, and Takeda; and received research support from AbbVie, Boehringer Ingelheim, and Pfizer. RCU is supported by an NIH K23 Career Development Award [K23KD111995-01A1]. DJ is a speaker for CME activities for Pfizer. SC has nothing to disclose. DW has nothing to disclose. GJM has served as advisory board member for AbbVie, Celgene, Celtrion, Ferrirng, Genesis, Hospira, Janssen, MSD, Mylan, Pharmacosmos, Pfizer, Takeda, VIANEX; as speaker for AbbVie, Angelini, Falk Pharma, Ferring, Galenica, Cenesis, Hospira, Janssen, MSD, Omega Pharma, Takeda, VIANEX; and as consultant for MSD and Takeda; and received research support from AbbVie, Galenica, Genesis, Menarini Group, MSD, Pharmathen.
Author Contributions
CAS: manuscript development and writing. BC: manuscript development, review, and editing. AK: manuscript development, review, and editing. JRR: manuscript development, review, and editing. MDK: manuscript development, review, and editing. RCU: manuscript development, review, and editing. DFJ: manuscript development, review, and editing. SC: manuscript development, review, and editing. DAW: manuscript development, review, and editing. GJM: manuscript development, review, and editing.
References
- 1. Holmer A, Singh S. Overall and comparative safety of biologic and immunosuppressive therapy in inflammatory bowel diseases. Expert Rev Clin Immunol 2019;15:969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al-Ani AH, Prentice RE, Rentsch CA, et al. Review article: prevention, diagnosis and management of COVID-19 in the IBD patient. Aliment Pharmacol Ther 2020;52:54–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Danese S, Cecconi M, Spinelli A. Management of IBD during the COVID-19 outbreak: resetting clinical priorities. Nat Rev Gastroenterol Hepatol 2020;17:253–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kennedy NA, Jones GR, Lamb CA, et al. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut 2020;69:984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rubin DT, Feuerstein JD, Wang AY, et al. AGA clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: expert commentary. Gastroenterology 2020. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turner D, Huang Y, Martín-de-Carpi J, et al. ; Paediatric IBD Porto group of ESPGHAN Corona virus disease 2019 and paediatric inflammatory bowel diseases: global experience and provisional guidance [March 2020] from the Paediatric IBD Porto Group of the European Society of Paediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2020;70:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rubin DT, Abreu MT, Rai V, et al. Management of patients with Crohn’s disease and ulcerative colitis during the COVID-19 pandemic: results of an international meeting. Gastroenterology 2020. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brenner EJ, Ungaro RC, Colombel JF, et al. Secure-IBD Database Public Data Update. covidibd.org. Accessed May 3, 2020.
- 9. Bezzio C, Saibeni S, Variola A, et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut 2020; 69: 1213– 17. [DOI] [PubMed] [Google Scholar]
- 10. Haberman R, Axelrad J, Chen A, et al. Covid-19 in immune-mediated inflammatory diseases ‐ case series from New York. N Engl J Med 2020; 383: 85– 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beaugerie L, Rahier JF, Kirchgesner J. Predicting, preventing, and managing treatment-related complications in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2020;18:1324–35.e2. [DOI] [PubMed] [Google Scholar]
- 12. https://http://www.medrxiv.org/content/10.1101/2020.04.30.20085613v1. Accessed May 12, 2020.
- 13. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. http://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html. Acceessed on May 9, 2020.
- 15. Prescott J, Falzarano D, de Wit E, et al. Pathogenicity and viral shedding of MERS-CoV in immunocompromised rhesus macaques. Front Immunol 2018;9:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eichenberger EM, Soave R, Zappetti D, et al. Incidence, significance, and persistence of human coronavirus infection in hematopoietic stem cell transplant recipients. Bone Marrow Transplant 2019;54:1058–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. http://www.idsociety.org/globalassets/idsa/public-health/covid-19/idsa-covid-19-antibody-testing-primer.pdf. Accessed May 9, 2020.
- 18. https://http://www.cdc.gov/coronavirus/2019-ncov/hcp/return-to-work.html?CDC_AA_refVal&=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhealthcare-facilities%2Fhcp-return-work.html. Accessed May 9, 2020.
- 19. https://http://www.ecdc.europa.eu/sites/default/files/documents/covid-19-guidance-discharge-and-ending-isolation-first update.pdf. Accessed May 9, 2020.