Abstract
Background
Leronlimab, a monoclonal antibody blocker of C-C chemokine receptor type 5 originally developed to treat human immunodeficiency virus infection, was administered as an open-label compassionate-use therapeutic for coronavirus disease 2019 (COVID-19).
Methods
Twenty-three hospitalized severe/critical COVID-19 patients received 700 mg leronlimab subcutaneously, repeated after 7 days in 17 of 23 patients still hospitalized. Eighteen of 23 received other experimental treatments, including convalescent plasma, hydroxychloroquine, steroids, and/or tocilizumab. Five of 23 received leronlimab after blinded, placebo-controlled trials of remdesivir, sarilumab, selinexor, or tocilizumab. Outcomes and results were extracted from medical records.
Results
Mean age was 69.5 ± 14.9 years; 20 had significant comorbidities. At baseline, 22 were receiving supplemental oxygen (3 high flow, 7 mechanical ventilation). Blood showed markedly elevated inflammatory markers (ferritin, D-dimer, C-reactive protein) and an elevated neutrophil-to-lymphocyte ratio. By day 30 after initial dosing, 17 were recovered, 2 were still hospitalized, and 4 had died. Of the 7 intubated at baseline, 4 were fully recovered off oxygen, 2 were still hospitalized, and 1 had died.
Conclusions
Leronlimab appeared safe and well tolerated. The high recovery rate suggested benefit, and those with lower inflammatory markers had better outcomes. Some, but not all, patients appeared to have dramatic clinical responses, indicating that unknown factors may determine responsiveness to leronlimab. Routine inflammatory and cell prognostic markers did not markedly change immediately after treatment, although interleukin-6 tended to fall. In some persons, C-reactive protein clearly dropped only after the second leronlimab dose, suggesting that a higher loading dose might be more effective. Future controlled trials will be informative.
Keywords: SARS-CoV-2, COVID-19, leronlimab, immunomodulatory therapy
Leronlimab is a humanized monoclonal antibody antagonist of C-C chemokine receptor type 5. We describe treatment of 23 coronavirus disease 2019 patients via open-label compassionate-use. Given the severity of disease in these patients, the overall encouraging outcomes suggest a potential benefit.
Late in 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified as the cause of an outbreak of a pneumonia syndrome (eventually termed coronavirus disease 2019 [COVID-19]) in Wuhan, China. After rapid spread of the virus across the globe, it was declared a pandemic on 11 March 2020 and is responsible for almost 8.3 million infections and 450 000 deaths as of 17 June 2020 [1]. To date, the only drug treatments with established benefit have been the polymerase inhibitor remdesivir, which has a modest impact on recovery time but no definite improvement in mortality [2], and dexamethasone, which may modestly reduce mortality in patients who require supplemental oxygen or mechanical ventilation [3]. Therefore, there has been a keen interest in developing treatments to reduce the mortality of COVID-19. Because the pathogenesis of fatal COVID-19 involves both viral infection and a hyperinflammatory state that causes end organ damage through a cytokine storm–like syndrome, therapeutic development has focused on both direct antiviral agents (such as remdesivir) and immunomodulatory agents.
Leronlimab is a humanized monoclonal antibody that binds C-C chemokine receptor type 5 (CCR5). It has undergone extensive clinical testing for the treatment of human immunodeficiency virus type 1 (HIV-1) infection [4–7]. It acts as a competitive inhibitor by binding the N-terminus and second extracellular loop and blocking CCR5-mediated HIV-1 infection of cells. CCR5 is expressed predominantly on T cells but is also found on macrophages, dendritic cells, and eosinophils to mediate chemotaxis in response to its cognate ligands that include CCL5 (RANTES), CCL3 (MIP-1α), and CCL4 (MIP-1β). These ligands are integral in the recruitment of these immune cells to inflammatory sites. Binding of leronlimab to CCR5 on cells not only blocks HIV-1 entry but also prevents CCL5-mediated calcium flux, with a 50% inhibitory concentration of about 45 µg/mL [8] and is therefore a potent inhibitor of CCR5-mediated chemotaxis.
The immunopathogenesis of COVID-19 likely involves the excessive influx of immune cells into the lung. The original SARS caused by the closely related virus SARS-CoV-1 has clinical findings that are very similar to those of COVID-19 [9, 10], and that virus elicits high levels of CCL5 expression by airway epithelial cells and macrophages [11, 12]. In SARS-CoV-2 infection, activated macrophages in the airways express high levels of CCL3, with the highest expression seen in patients with critical COVID-19 [13]. Thus, it is likely that CCR5-mediated chemotaxis similarly contributes to the excessive lung inflammation seen in COVID-19. Leronlimab has been proposed as an immunomodulatory treatment and has been tested in a few patients with anecdotal success [14, 15]. Here, we report the outcomes of 23 COVID-19 patients who received open-label compassionate-use leronlimab at our medical center in April 2020; this is the largest reported series to date.
METHODS
Study Participants
Leronlimab was given on an open-label compassionate-use basis to patients hospitalized with polymerase chain reaction–confirmed SARS-CoV-2 infection at 2 hospitals of the University of California at Los Angeles (UCLA) academic medical center in April 2020. Each participant or his/her legally authorized representative (LAR) provided written informed consent prior to treatment. The UCLA Institutional Review Board (IRB) reviewed and approved the protocol, and each leronlimab treatment was given under an individual emergency investigational new drug approval through the US Food and Drug Administration (FDA). Infectious diseases physicians who evaluated COVID-19 patients referred them for potential leronlimab therapy when other experimental therapeutic options were contraindicated or exhausted. Patients were excluded if they were aged <18 years, pregnant or breastfeeding, or unable to provide informed consent directly or through a LAR.
Leronlimab Administration
Leronlimab 700 mg was administered via 2 abdominal subcutaneous injections of 350 mg each, with repeat dosing if they remained hospitalized after 7 days. All treatment decisions and laboratory monitoring were left to the discretion of the physicians caring for the patients.
Clinical Data Collection
Laboratory testing values, adverse events, and oxygen requirements were collected via manual chart review and electronic health records extraction, supported through an IRB- approved institutional Clinical Research COVID Registry. The fraction of inspired oxygen on patients who did not receive mechanical ventilation was estimated [16]. All patients were followed up for at least 30 days after the first administration of leronlimab. A positive clinical response for the purposes of this study was defined as survival without further need for acute hospital care at 30 days of follow-up.
Statistical Analyses
Laboratory data were compared using nonparametric Mann-Whitney tests. All graphs and statistical analyses were performed using GraphPad Prism v8.4.2.
RESULTS
Demographics of Patients Who Received Compassionate-Use Open-Label Leronlimab
Twenty-three patients received leronlimab on an open-label compassionate-use basis (Table 1, Supplementary Table 1). Reflecting our regional demographics of COVID-19 cases, these individuals were older than typical patients seen for infectious disease consultation at our institution (mean, 69.5 years; standard deviation [SD], 14.9), had a slight male predominance (57% male vs 43% female), were racially and ethnically diverse (70% White, 17% Asian, and 9% other, with 35% Latinx), and had frequent medical comorbidities (20 of 23 with significant underlying active chronic medical conditions). Few patients were underweight (2 of 23 with body mass index [BMI] <18.5 kg/m2), overweight (2 of 23 with BMI 25.0–29.9 kg/m2), or obese (3 of 23 with BMI >29.9 kg/m2). Comorbidities were wide ranging, most commonly hypertension (13 of 23), chronic kidney disease (8 of 23), and type 2 diabetes mellitus (5 of 23). Others included organ transplantation (heart, 2 of 23; liver, 2 of 23; kidney, 1 of 23), malignancy (breast, 1 of 23; lymphoma, 1 of 23), vascular disease (heart, 1 of 23; carotid, 1 of 23), chronic obstructive pulmonary disease (2 of 23), pulmonary fibrosis (1 of 23), and rheumatologic/immunologic disorders (Sweet syndrome, 1 of 23; rheumatoid arthritis, 1 of 23). Overall, this was a high-risk cohort with significant comorbidities.
Table 1.
Participant Characteristics
| Demographics | |
|---|---|
| Age, y | 68.5 ± 14.9 |
| Sex | |
| Male | 13 (57%) |
| Female | 10 (43%) |
| Race | |
| Asian | 4 (17%) |
| Black | 0 (0%) |
| White | 16 (70%) |
| Other | 2 (9%) |
| Unknown | 1 (4%) |
| Latinx status | |
| Latinx | 8 (35%) |
| Not Latinx | 14 (61%) |
| Unknown | 1 (4%) |
| BMI, kg/m2 | 24.7 ± 5.6 |
| Comorbidity | |
| Chronic cardiac disease (other than hypertension) | 7 (30%) |
| Chronic pulmonary disease (chronic obstructive pulmonary disease, asthma) | 3 (13%) |
| Hypertension | 13 (57%) |
| Chronic kidney disease | 7 (30%) |
| Chronic liver disease | 0 (0%) |
| Cancer | 2 (9%) |
| Human immunodeficiency virus | 0 (0%) |
| Diabetes | 7 (30%) |
| Organ transplant | 5 (22%) |
| Any other form of immunosuppression | 6 (26%) |
| Obesity (BMI >30 kg/m2) | 3 (13%) |
| Chronic oxygen requirement | 0 (0%) |
| None | 3 (13%) |
| Baseline characteristics/laboratory results at time of first leronlimab dose | |
| Days of symptoms | 9.7 ± 6.5 |
| Vasopressor support | 5 (22%) |
| Low-flow supplemental oxygen | 12 (52%) |
| High-flow supplemental oxygen | 3 (13%) |
| Mechanical ventilation | 7 (30%) |
| White blood cell count, 103 cells/µL | 6.74 ± 3.10 |
| Absolute neutrophil count, 103 cells/µL | 4.88 ± 2.67 |
| Absolute lymphocyte count, 103 cells/µL | 1.05 ± .59 |
| Neutrophil-to-lymphocyte ratio | 6.34 ± 5.60 |
| Lactate dehydrogenase, U/L | 366 ± 142 |
| Ferritin, µg/L | 2235 ± 2332 |
| D-Dimer, mg/L | 3018 ± 2850 |
| C-reactive protein, mg/L | 9.2 ± 7.9 |
| Other coronavirus disease 2019 therapies | |
| Hydroxychloroquine (open label) | 7 (30%) |
| Tocilizumab (open label) | 2 (9%) |
| Sarilumab clinical trial (placebo-controlled) | 1 (4%) |
| Selinexor clinical trial (placebo-controlled) | 1 (4%) |
| High-dose steroids | 3 (13%) |
| Remdesivira | 1 (4%) |
| Convalescent plasma (open label) | 10 (43%) |
| Outcomes (day 30) | |
| Recovered, no supplemental oxygen required | 16 (70%) |
| Recovered, low-flow oxygen required | 1 (4%) |
| Still hospitalized | 2 (9%) |
| Died | 4 (17%) |
Abbreviation: BMI, body mass index.
aTwo persons were enrolled in a randomized, controlled trial of remdesivir; unblinding revealed that 1 received remdesivir and 1 received placebo.
Baseline Clinical Characteristics of Individuals and Treatment With Leronlimab
The patients were admitted to the hospital an average of 5.6 days after onset of symptoms (range, 0–18 days; SD, 4.8). As summarized in Table 1, the first dose of leronlimab was administered an average of 9.7 days after symptoms onset (range, 1–25 days; SD, 6.5). At the time of dosing, 22 of 23 individuals were receiving supplemental oxygen, including 3 on high-flow oxygen and 7 on mechanical ventilation, and 5 of 23 required vasopressor support. Laboratory blood markers of disease (Figure 1) exhibited moderately increased lactate dehydrogenase (LDH; mean, 366 ± 142 U/L) but markedly elevated inflammatory markers including ferritin (mean, 2235 ± 2332 µg/L), D-dimer (mean, 3018 ± 2850 mg/L), and C-reactive protein (CRP; mean, 9.2 ± 7.9 mg/L). Examination of blood leukocytes indicated relatively normal neutrophil counts (mean, 4882 ± 2637/µL, with 4 of 23 values above the normal upper limit of 6900/µL), depressed total lymphocyte counts (mean, 1049 ± 592/µL, with 15 of 23 values below the normal lower limit of 1300/µL), and thus elevated neutrophil-to-lymphocyte ratios (mean, 6.3 ± 5.6, with 21 of 23 >2 and 18 of 23 >3). Monocyte counts were relatively normal (mean, 515 ± 227/µL, with 0 of 23 below the lower limit of 200/µL and 2 of 23 above the upper limit of 800/µL). Overall, these clinical and laboratory parameters reflected a relatively severely ill cohort of COVID-19 patients by both inflammatory markers and clinical status.
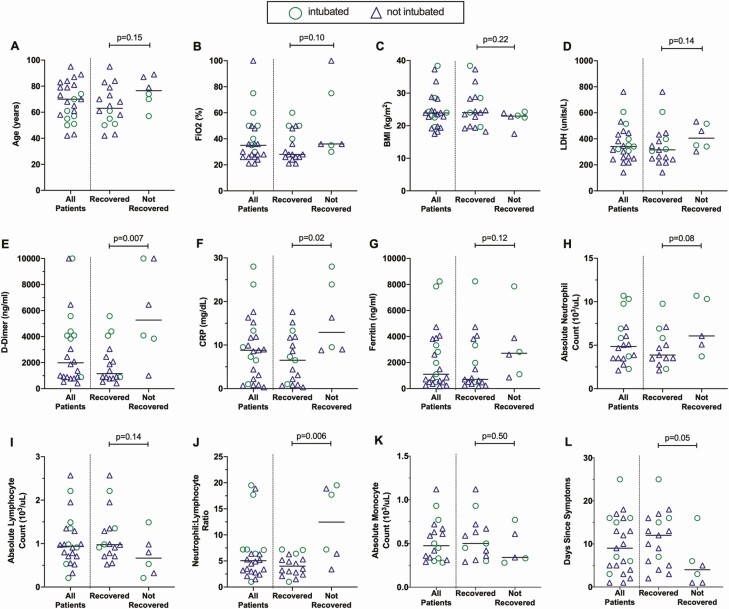
Figure 1.
Differences in blood inflammatory markers at baseline between recovered and nonrecovered patients. Baseline clinical characteristics, laboratory values, and blood counts were compared between patients treated with leronlimab who recovered (n = 17) and those who did not recover (n = 6). Patients who required mechanical ventilation are indicated by circles and those who did not are indicated by triangles. Medians for all recovered and nonrecovered persons are indicated by horizontal bars. Differences between the groups were assessed using the Mann-Whitney test and P values shown. Abbreviations: BMI, body mass index; CRP, C-reactive protein; FiO2, fraction of inspired oxygen; LDH, lactate dehydrogenase.
Safety of Leronlimab and Concurrent Treatments Given for COVID-19
Leronlimab was well tolerated with no noted adverse events with the exception of 1 person (participant F) who developed a moderate maculopapular skin rash that was likely due to concurrent cephalosporin administration. Seventeen of 23 patients received 2 doses 1 week apart. Of the 6 who received only 1 dose, the second dose was not given to 3 due to hospital discharge before the second dose, 1 due to skin rash, and 2 due to death. In addition to leronlimab, 18 of 23 patients received other experimental treatments for COVID-19 (Table 1, Supplementary Table 1, Supplementary Figure 2). Coadministered treatments included convalescent plasma (10 of 23), hydroxychloroquine (5 of 23), treatment dose steroids (4 of 23), and open-label tocilizumab (2 of 23). Five persons received leronlimab after disease progression during blinded, placebo-controlled trials of remdesivir (2 of 23), sarilumab (1 of 23), selinexor (1 of 23), or tocilizumab (1 of 23).
Clinical Outcomes After Leronlimab Treatment
The status of participants was assessed at 30 days after the first dose of leronlimab (Supplementary Table 1). Defining recovery as survival and no longer being hospitalized, 17 of 23 (74%) were recovered, of whom 1 still required supplemental oxygen (1 L/min). Two of 23 (9%) were still alive but remained hospitalized, and 4 of 23 (17%) had died. Examining the subset of 7 patients who were intubated at the time of leronlimab treatment initiation, 4 of 7 (57%) were recovered and required no supplemental oxygen, while 2 of 7 (29%) were alive but remained hospitalized and 1 of 7 (14%) had died. The 2 initially intubated patients who were still hospitalized on day 30 eventually stabilized and were discharged from the hospital breathing spontaneously.
Markers Associated With Recovery After Leronlimab Treatment
Among demographic and clinical factors, there were statistically insignificant trends for younger age (Figure 1A) and lower oxygen (Figure 1B) requirement and no significant differences in BMI (Figure 1C) or LDH level (Figure 1D) at baseline between those who had recovered by 30 days vs those who had not recovered. For inflammatory markers, baseline D-dimer (Figure 1E) and CRP (Figure 1F) were significantly higher in those who did not recover (P = .007 and P = .02, respectively), and ferritin was insignificantly higher (Figure 1G). Of blood leukocytes, neutrophil (Figure 1H) and lymphocyte (Figure 1I) counts were insignificantly higher and lower, respectively, in those who did not recover, but the ratio of neutrophils to lymphocytes (Figure 1J) was significantly higher (P = .006). Monocytes were insignificantly lower in those who did not recover (Figure 1K). Most participants did not have serial plasma interleukin-6 (IL-6) measurements, but most of those who did exhibited reductions in IL-6 temporally correlated to leronlimab administration, although several had confounding treatments (Supplementary Figure 1). Overall, these findings were consistent with previous reports of predictors of risk in COVID-19.
Changes in Inflammatory and Cell Markers After Leronlimab Treatment
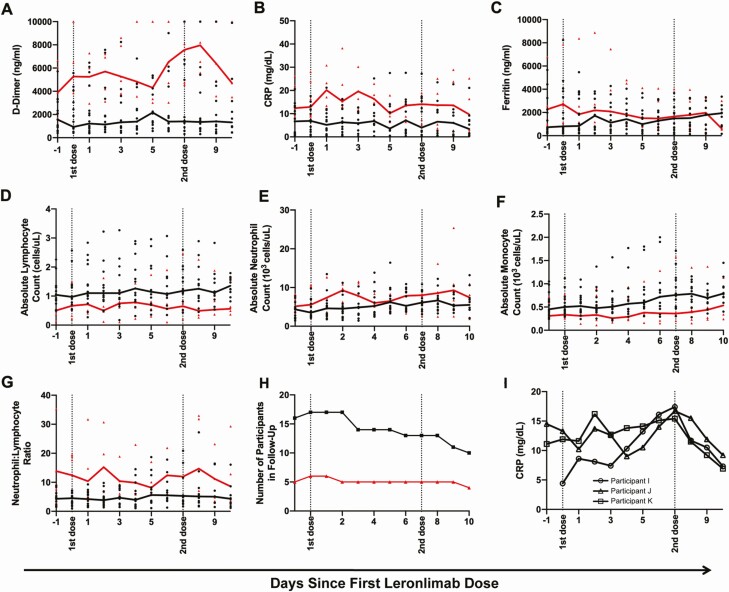
Examination of disease markers in the 10 days after treatment with leronlimab suggested some differences between those who recovered vs those who did not. D-dimer levels in both subsets remained relatively stable during dosing, remaining moderately elevated in recovered persons and markedly elevated in unrecovered persons (Figure 2A). CRP in the recovered group remained relatively stable, perhaps dropping after the second dose; in the unrecovered group, CRP seemed to remain persistently elevated, perhaps with a slight decrease after the second dose (Figure 2B). Ferritin decreased in unrecovered persons and increased slightly in the recovered group, converging at a similar level by 10 days in both groups (Figure 2C). Absolute lymphocyte counts overall appeared unchanged in both groups but persistently lower in the unrecovered group (Figure 2D). Absolute neutrophil and monocyte counts were similarly unchanged but persistently distinct between the recovered and unrecovered groups (Figure 2E and 2F). The neutrophil-to-lymphocyte ratio was unchanged in those who did recover and was elevated but appeared to decrease with each leronlimab dose in those who did not recover (Figure 2G). While these were the general patterns noted, a confounder was the dropout of patients from these analyses (Figure 2H) due to early hospital discharge in the recovered group and death in the unrecovered group. Finally, only a few patients had plasma IL-6 levels monitored serially (Supplementary Figure 1); most of these exhibited acute reductions in IL-6.
Figure 2.
Changes in blood inflammatory markers during the course of leronlimab therapy for coronavirus disease 2019. A–G, Clinical laboratory test values and blood counts are plotted over time with the x-axis showing days since leronlimab treatment in recovered vs nonrecovered participants. Dotted lines indicate timing of leronlimab doses. The lines depict the median values for each group, and individual values are shown as dots. The recovered patients (n = 17) are plotted in black and nonrecovered patients (n = 6) are plotted in red. Plotted markers include: A, D-dimer; B, CRP; C, ferritin; D, absolute lymphocyte count; E, absolute neutrophil count; F, absolute monocyte count; and G, ratio of neutrophils to lymphocytes. H, The number of patients followed in each group is plotted, where the black and red lines indicate the recovered and nonrecovered groups, respectively. I, The CRP levels for 3 participants are plotted. Note that participants I and K received no other antiviral or immunomodulatory treatments at any time, while participant J was in a remdesivir vs placebo trial from day –10 to day –1 and received treatment dose steroid from days 1 to 3. Abbreviation: CRP, C-reactive protein.
Inflammatory Marker CRP in Some Individuals Appeared Not to Drop Until the Second Dose of Leronlimab
Across groups, leronlimab treatment did not demonstrate marked effects on disease markers in the 10-day time span following the first dose (Figure 2A–2H). However, in several persons, the marker CRP appeared to show no decrease after the first dose but a dramatic drop after the second dose of leronlimab (Figure 2I). While many individuals had confounding treatments, these specific examples suggested a temporal relationship of CRP reduction associated with the second dose.
DISCUSSION
This series is the largest reported cohort of leronlimab-treated COVID-19 patients to date. Given that leronlimab has shown a highly favorable safety profile in more than 800 patients treated in FDA approval trials for the treatment of HIV-1 [4–7], we administered it to several individuals on an open-label compassionate-use basis when therapeutic and clinical trials options were relatively limited. Leronlimab appeared well tolerated and safe in our population, with only 1 possible adverse event being a maculopapular rash that was attributable to concurrent use of a cephalosporin antibiotic.
Limited anecdotal evidence from 2 small studies has suggested that leronlimab may improve outcomes in COVID-19 infection. Patterson et al examined outcomes in 10 “terminally ill” patients [14], of whom 7 were on mechanical ventilation, 1 was on high-flow oxygen, and 2 were on low-flow oxygen. Six of 10 survived 14 days after treatment with leronlimab, with 2 able to be successfully removed from mechanical ventilation. Similar to our study, there were no clear changes in general clinical markers (or several cytokines not monitored in our study) with the exception of a consistent drop in IL-6 in most persons. Akalin et al reported a small series of renal transplantation patients with COVID-19, of whom 6 received leronlimab [15], and also observed a rapid drop in IL-6. Both studies also found changes in T cells, with normalization of CD4+ and CD8+ subsets amounts and ratios (particularly an increase in the CD8+ T-cell subset).
Interestingly, our data suggest that the current leronlimab dosing regimen may be suboptimal, since several patients exhibited rapid drops in the inflammatory marker CRP only after the second dose. Given its estimated half-life of about 10 days, the second dose after 7 days should achieve a higher peak than the first dose, and the maximal effect would not be achieved until after the second dose. This is consistent with the receptor occupancy data presented by Patterson et al [14], showing maximal effect after the second dose of the same regimen. Also, our data do not appear to demonstrate a relationship between outcome after leronlimab and the duration of disease at the time of dosing (Supplementary Table 1).
Because this was not a controlled trial, the impact of leronlimab is not directly ascertainable. However, the data provide anecdotal evidence for a benefit from treatment. Most of our patients had significant risk factors for severe disease, including age >60 years and comorbidities associated with poor outcome [17, 18]. Of the 23, 7 required mechanical ventilation at the time of leronlimab dosing. Of these critically ill patients, 6 of 7 were alive after more than 70 days, a substantially higher survival rate than other reports of critically ill COVID-19 patients [19–22], which range from 42% to 61% in cohorts followed about 30 days after hospitalization. Of the 15 with milder (but still “severe” as defined by supplemental oxygen requirement) illness, 13 no longer required further acute hospital care by 30 days. Thus, the overall outcomes for this high-risk group of patients were better than historically observed in multiple reports from the same time frame.
It was also notable that clinical responsiveness to treatment was highly variable; some patients appeared to have a rapid dramatic response to treatment (eg, participants A and F who were rapidly extubated after being on ventilators and participants B, C, and D who were weaned off supplemental oxygen and discharged home within 3 days), while others seemed to have less effect. Mechanistic studies may uncover determinants and markers for leronlimab responsiveness, such as CCR5 occupancy [14]. Controlled trials will be required to assess the benefit of treatment.
Most of these patients did receive additional possibly confounding treatments for COVID-19. Although several received hydroxychloroquine (including participant V who had been chronically ill with rheumatoid arthritis), the effect of this treatment was unlikely to be significant given the results of randomized, controlled trials demonstrating no benefit [23, 24]. IL-6 receptor blocking antibodies were given to 2 participants as open-label treatments, and 2 others were in placebo-controlled trials of these agents. A recent press release regarding a controlled trial , Evaluating Minority Patients with Actemra (EMPACTA) suggested that tocilizumab may modestly reduce mortality (from 19% to 12%) in severely ill patients [25], and a trial of sarilumab was halted for futility [26]. Thus, the influence of these agents should be minimal in our cohort. While remdesivir has been shown to be helpful, particularly in persons who require no more than low-flow oxygen supplementation [2], only 1 participant (on a ventilator) received this treatment. Several participants received convalescent plasma, but this intervention appears to have only a small effect on 30-day mortality, particularly when given within 3 days of diagnosis [27]. Most of our participants received plasma much later, so it would seem unlikely that plasma was a significant contributor to our survival rate 30 days after leronlimab. Finally, 3 participants received high-dose steroids, the intervention shown to have the greatest impact on survival [3]. However, the reported survival benefit of steroids is greatest for intubated patients (29% vs 41% mortality) and minimal for patients who require only noninvasive oxygen supplementation (22% vs 25%); only 1 of our participants who received steroids was intubated. Thus, it is unlikely that steroids and other interventions aimed at treating COVID-19 markedly affected survival in our cohort overall.
The prognostic value of some biomarkers was confirmed in our cohort, and these markers also reflected the severity of disease in our participants. In particular, higher CRP [17] and D-dimer [18, 28] blood levels predicted worse outcome, which is in agreement with prior analyses of COVID-19 in general. Also, an increased ratio of blood neutrophils to lymphocytes, another previously reported marker of disease severity, was highly significant [29, 30]. The increased ratio appeared to be a combination of increased neutrophils and decreased lymphocytes, with low lymphocytes being a previously reported poor prognostic factor [17, 31], although neither was statistically significant individually, perhaps due to limited sample size. Similarly, a trend for higher ferritin [32] in those who did not meet recovery criteria was present but not statistically significant. As opposed to prior suggestions that blood monocyte elevation is a prognostic indicator of poor outcome [33, 34], our recovered patients had slightly higher (but not statistically significant) monocyte levels than those who did not recover. Baseline LDH, also a previously reported factor [17, 31], was also not significantly associated with outcome in our cohort. Some of these discrepancies were likely due to our limited sample size. Too few patients had serial measurements of IL-6 [32, 35] to assess this marker reliably. The relationship of inflammatory markers in response to leronlimab treatment is unclear. It is possible that persons with less inflammation had better outcomes after leronlimab treatment simply because they were less ill at baseline. Alternatively, it can be hypothesized that leronlimab could be more effective earlier in the inflammatory response by preventing chemotaxis of immune cells to effector sites, such as the lung, and less effective later because those sites are already maximally infiltrated with those cells.
Overall, our findings suggest a benefit of leronlimab in the treatment of severe COVID-19, including for persons who require mechanical ventilation. Some routine clinical markers of disease severity (blood CRP, D-dimer, and neutrophil-to-lymphocyte ratio) were associated with outcome after leronlimab treatment while others were not (ferritin, lymphocyte count, monocyte count, LDH). Whether this is due to our small cohort size or differences between leronlimab treated vs untreated patients is unclear. Randomized, placebo-controlled trials are now underway and should help provide more helpful data to clarify efficacy and predictors of response to leronlimab when treating COVID-19.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the patients and their families for their participation.
Disclaimer. The funder had no role in the study design, data collection, analysis of data, interpretation of data, writing of the manuscript, or decision to submit the manuscript for publication.
Financial support. This work was supported by the Clinical and Translational Science Institute Office of Clinical Research funded by a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health (UL1TR001881).
Potential conflicts of interest. J. B. S. is a paid consultant for CytoDyn Inc. O. O. Y. is on the executive board of directors of Applied Medical Inc and holds stock ownership. O. O. Y. is a founder and holds stock ownership in CDR3 Therapeutics Inc. A. N. is a founder and holds stock ownership in InVista Health Inc. K. D. is employed by Amarex Clinical Research, which is contracted by CytoDyn to manage the clinical development of leronlimab for the treatment of coronavirus disease 2019. D. G. M. reports research grants from Gilead outside the submitted work. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Johns Hopkins University. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html. Accessed June 17, 2020.
- 2. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19—preliminary report. Reply. N Engl J Med 2020; 383:994. [DOI] [PubMed] [Google Scholar]
- 3. Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv 2020; doi:2020.06.22.20137273 [Google Scholar]
- 4. Dhody K, Pourhassan N, Kazempour K, et al. PRO 140, a monoclonal antibody targeting CCR5, as a long-acting, single-agent maintenance therapy for HIV-1 infection. HIV Clin Trials 2018; 19:85–93. [DOI] [PubMed] [Google Scholar]
- 5. Jacobson JM, Lalezari JP, Thompson MA, et al. Phase 2a study of the CCR5 monoclonal antibody PRO 140 administered intravenously to HIV-infected adults. Antimicrob Agents Chemother 2010; 54:4137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobson JM, Saag MS, Thompson MA, et al. Antiviral activity of single-dose PRO 140, a CCR5 monoclonal antibody, in HIV-infected adults. J Infect Dis 2008; 198:1345–52. [DOI] [PubMed] [Google Scholar]
- 7. Jacobson JM, Thompson MA, Lalezari JP, et al. Anti-HIV-1 activity of weekly or biweekly treatment with subcutaneous PRO 140, a CCR5 monoclonal antibody. J Infect Dis 2010; 201:1481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olson WC, Rabut GE, Nagashima KA, et al. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J Virol 1999; 73:4145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017; 39:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nicholls JM, Poon LL, Lee KC, et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet 2003; 361:1773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Law HK, Cheung CY, Ng HY, et al. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood 2005; 106:2366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yen YT, Liao F, Hsiao CH, Kao CL, Chen YC, Wu-Hsieh BA. Modeling the early events of severe acute respiratory syndrome coronavirus infection in vitro. J Virol 2006; 80:2684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chua RL, Lukassen S, Trump S, et al. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nat Biotechnol 2020; 38:970–9. [DOI] [PubMed] [Google Scholar]
- 14. Patterson BK, Seethamraju H, Dhody K, et al. Disruption of the CCL5/RANTES-CCR5 pathway restores immune homeostasis and reduces plasma viral load in critical COVID-19. medRxiv 2020. [Google Scholar]
- 15. Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med 2020; 382:2475–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wettstein RB, Shelledy DC, Peters JI. Delivered oxygen concentrations using low-flow and high-flow nasal cannulas. Respir Care 2005; 50:604–9. [PubMed] [Google Scholar]
- 17. Li LQ, Huang T, Wang YQ, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol 2020; 92:577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020; 395:1763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180:934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horby P, Mafham M, Linsell L, et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. medRxiv 2020; doi:2020.07.15.20151852 [Google Scholar]
- 24. Casey JD, Johnson NJ, Semler MW, et al. Rationale and design of ORCHID: a randomized placebo-controlled clinical trial of hydroxychloroquine for adults hospitalized with COVID-19. Ann Am Thorac Soc 2020; 17:1144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roche. Roche’s phase III EMPACTA study showed Actemra/RoActemra reduced the likelihood of needing mechanical ventilation in hospitalised patients with COVID-19 associated pneumonia. Available at: https://www.roche.com/media/releases/med-cor-2020-09-18.htm. Accessed September 23, 2020.
- 26. Sanofi. Sanofi and Regeneron provide update on Kevzara® (sarilumab) phase 3 U.S. trial in COVID-19 patients. Available at: https://www.sanofi.com/en/media-room/press-releases/2020/2020-07-02-22-30-00. Accessed September 23, 2020.
- 27. Joyner MJ, Senefeld JW, Klassen SA, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. medRxiv 2020; doi:2020.08.12.20169359 [Google Scholar]
- 28. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18:844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu J, Liu Y, Xiang P, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med 2020; 18:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Y, Du X, Chen J, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect 2020; 81:e6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou Y, Fu B, Zheng X, et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev 2020; 7:998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang D, Guo R, Lei L, et al. COVID-19 infection induces readily detectable morphological and inflammation-related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome. medRxiv 2020; doi:2020.03.24.20042655 [Google Scholar]
- 35. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020; 71:769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.