Abstract
Background
Concurrent myopericarditis and myositis can present in patients with pre-existing systemic inflammatory diseases. Here we present a case of myopericarditis and myositis associated with COVID-19, in the absence of respiratory symptoms.
Case summary
We present a middle-aged female with a history of hypertension and previous myopericarditis. The patient was admitted with symptoms of central chest pain, and ECG and echocardiographic features of myopericarditis. Her symptoms did not improve, and CT thorax suggested possible SARS-CoV-2 infection for which she tested positive, despite no respiratory symptoms. Whilst on the ward, she developed bilateral leg weakness and a raised creatine kinase (CK), and magnetic resonance imaging (MRI) of her thighs confirmed myositis. A cardiac MRI confirmed myopericarditis. She was treated with colchicine 500 μg twice daily, ibuprofen 400 mg three times day, and prednisolone 30 mg per day, and her symptoms and weakness improved.
Discussion
We describe the first reported case of concurrent myopericarditis, and myositis associated with COVID-19. Conventional therapy with colchicine, non-steroidal anti-inflammatory drugs, and glucocorticoids improved her symptoms, and reduced biochemical markers of myocardial and skeletal muscle inflammation.
Keywords: Case report, COVID-19, Myopericarditis, Myositis, SARS-CoV-2
Learning points
SARS-CoV-2 may be associated with cardiac and skeletal muscle inflammation in the absence of respiratory symptoms.
Signs and symptoms of COVID-19 associated myopericarditis and myositis may include chest pain, muscle pain and weakness.
Active myositis causes a significantly elevated CK.
Magnetic resonance imaging (MRI) is useful in confirming the diagnoses.
Colchicine, NSAIDs and glucocorticoids may be effective at relieving symptoms, improving muscle function and reducing CRP, hsTnT and CK.
Introduction
Concomitant myopericarditis and myositis is most commonly associated with endemic cardiotropic viruses,1 multisystem inflammatory diseases,2–5 immunotherapy6–8 and can occur idiopathically.9 Whilst a single case report has described this association in seasonal influenza,10 this has not been described in association with COVID-19. Here we describe the first documented case of a patient presenting with myopericarditis and myositis, associated with COVID-19.
Timeline
| Day 1 | Patient presents with chest pain. ECG demonstrates poor anterior R-wave progression (Figure 1). Blood results (Table 1) show lymphopenia, CRP of 11 and hsTnT of 77. Ibuprofen 400 mg three times daily and colchicine 500 μg twice daily were initiated. |
| Day 2 | Chest pain not improving. Prednisolone 30 mg per day was started in addition to colchicine and ibuprofen. |
| Day 3 | Chest pain symptom worsens. Chest computed tomography (CT) shows changes consistent with COVID-19 (Figure 2). Patient has a peak temperature of 37.5°C. |
| Day 4 | COVID-19 swab positive. |
| Day 5 | Patient complains of leg weakness and pain. |
| Day 6 | Lower limb weakness now affecting mobility. CK 6432, CRP 58, hsTnT 106. 0.9% sodium chloride started. |
| Day 7 | Diagnosis of likely myositis made. CK 18913 and CRP 57. |
| Day 8 | CK 32230, CRP 46. |
| Day 9 | Patient complains of worsening leg pain and weakness (power 3/5). Rheumatology advise to continue prednisolone 30 mg per day and obtain MRI of lower limbs. |
| Day 10 | Chest pain and lower limb pain/weakness improving. CK 13564, hsTnT 128 and CRP 21. |
| Day 11 | MRI confirms myositis (Figure 3). Cardiac MRI confirms myopericarditis with myocardial oedema in the basal inferoseptum and apex. CK 8255, CRP 19, hsTnT 101 (Figure 4). |
| Day 12 |
Patient’s chest pain resolves and mobility almost at baseline. CK 4922, hsTnT 65 and CRP 14. |
| Day 13 | Patient discharged with 3-month course of colchicine 500 μg twice per day, ibuprofen 400 mg three times per day and prednisolone 30 mg per day for 7 days. |
Case presentation
A 50-year-old Afro-Caribbean woman, with a history of hypertension, reactive arthritis and a previous episode of myopericarditis in 2012, presented to the emergency department with a 4-day history of central chest pain, which was made worse on lying flat and on deep inspiration. The patient had regular medications including 10 mg lisinopril and 10 mg amlodipine per day for hypertension, and 2.5 mg tibolone per day for suppression of perimenopausal symptoms. She did not complain of cough or breathlessness. She had a blood pressure of 116/74 mmHg, a pulse rate of 85 b.p.m., oxygen saturation of 97% on room air and she was afebrile. Examination findings were normal. Laboratory tests (Table 1) showed a normal haemoglobin level, white cell count and platelet count. The patient had a lymphopenia at 0.63 × 109 cells/l (normal 1.5–4.5 × 109 cells/l), her C-reactive protein (CRP) level was elevated at 11 mg/L (normal <5 mg/L) and her high-sensitivity troponin T (hsTnT) was elevated at 77 ng/L (normal <14 ng/L).
Table 1.
Clinical laboratory results
| Variable | Reference range | Hospital Day 1 | Hospital Day 2 | Hospital Day 3 | Hospital Day 4 | Hospital Day 5 | Hospital Day 6 | Hospital Day 7 | Hospital Day 8 | Hospital Day 9 | Hospital Day 10 | Hospital Day 11 | Hospital Day 12 | Hospital Day 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haemoglobin (g/L) | 115–165 | 145 | 152 | 153 | 165 | 156 | 152 | 139 | 127 | 119 | 120 | 127 | ||
| White blood cell count (109 cells/L) | 4–11 | 4.98 | 5.39 | 8.86 | 8.39 | 8.58 | 8.64 | 9.38 | 8.75 | 8.07 | 8.25 | 9.41 | ||
| Lymphocyte count (109 cells/L) | 1–4 | 0.63b | 1.64 | 1.07 | 0.75b | 0.73b | 0.96b | 1.23 | 0.88b | 0.93b | 0.99b | 1.24 | ||
| Sodium (mmol/L) | 133–146 | 141 | 136 | 131b | 129b | 128b | 128b | 134 | 139 | 139 | 140 | |||
| Potassium (mmol/L) | 3.5–5.3 | 3.6 | 3.8 | 4.6 | 4.5 | 4.5 | 3.4b | 3.4b | 3.8 | 3.9 | ||||
| Urea (mmol/L) | 2.5–7.8 | 2.8 | 5.5 | 6.1 | 4.8 | 3.9 | 4.1 | 4.1 | 4.2 | 3.9 | 5.1 | |||
| Creatinine (µmol/L) | 45–84 | 73 | 78 | 76 | 61 | 52 | 57 | 59 | 59 | 63 | 67 | |||
| Albumin (g/L) | 38–51 | 45 | 38 | 31b | 28b | 27b | 28b | 30b | ||||||
| Creatine kinase (U/L) | 26–192 | 6432a | 18 913a | 32 230a | 13 564a | 8255a | 4922a | |||||||
| Lactate dehydrogenase (U/L) | 135–214 | 625a | 314a | |||||||||||
| Ferritin (µg/L) | 15–300 | 172 | 139a | |||||||||||
| C-reactive protein (mg/L) | 0–4 | 11a | 15a | 31a | 58a | 57a | 46a | 27a | 21a | 19a | 14a | 11a | ||
| High-sensitivity troponin T (ng/L) | 0–14 | 77a | 33a | 118a | 106a | 103a | 127a | 128a | 101a | 65a | ||||
| Lupus anticoagulant | Negative | |||||||||||||
| ANA ELISA | Negative | |||||||||||||
| Complement C3 (g/L) | 0.75–1.65 | 1.03 | ||||||||||||
| Complement C4 (g/L) | 0.14–0.54 | 0.27 | ||||||||||||
| ANCA | Negative | |||||||||||||
| Myeloperoxidase Ab screen (U/mL) | 0–3.4 | <0.3 | ||||||||||||
| Proteinase 3 Ab screen (U/mL) | 0–1.9 | 0.2 | ||||||||||||
| Angiotensin-converting enzyme (U/L) | 20–70 | 21 | ||||||||||||
| HBsAg test | Negative | |||||||||||||
| Hepatitis C antibody | Negative | |||||||||||||
| HIV 1,2 Ab and p24 Ag (XL) | Negative | |||||||||||||
| SARS-CoV-2 RNA | Positive |
The value in the patient was above the normal range.
The value in the patient was below the normal range.
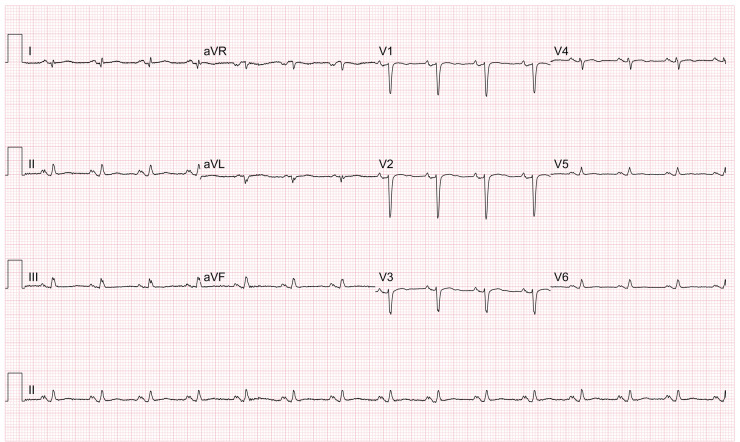
An electrocardiogram (ECG) showed poor R-wave progression and small QRS complexes (Figure 1). An echocardiogram showed a trivial anterior pericardial effusion with good biventricular function. A chest radiograph was normal. The patient was admitted under the cardiology team with a presumed diagnosis of myopericarditis based on her symptoms and laboratory results. She was started on colchicine (500 μg twice daily) and ibuprofen (400 mg three times daily) as per conventional therapy for myopericarditis.11
Figure 1.
12-lead ECG showing poor R-wave progression anteriorly and small QRS complexes.
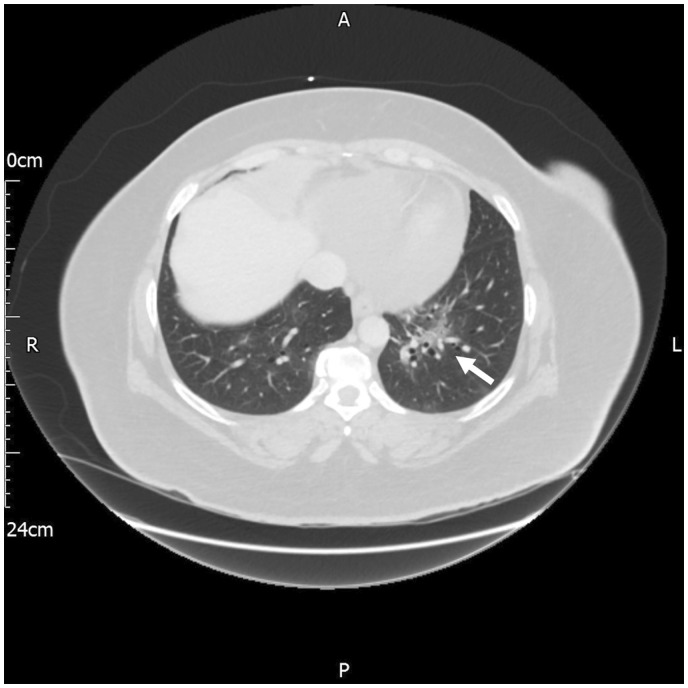
The patient continued to complain of chest pain on Day 2. Given that the symptoms had not improved on 1200 mg per day of ibuprofen, 30 mg per day of prednisolone was started as additional therapy with the aim of settling the myopericardial inflammation. By Day 3, the patient’s chest pain had not improved, and so a computed tomography (CT) scan of the patient’s thorax was obtained to exclude possible alternative diagnoses which could explain the patient’s symptoms, or to confirm the diagnosis of unresolving myopericardial inflammation and identify consistent features such as pericardial thickening and calcification. The CT thorax showed a small pericardial effusion and non-specific bronchocentric ground-glass opacities in the left lower zone which was reported as being suspicious for COVID-19 (Figure 2). At this time, the patient had a peak temperature of 37.5°C. An oropharyngeal swab tested positive for SARS-CoV-2.
Figure 2.
CT thorax showing left lower zone bronchocentric ground-glass changes consistent with COVID-19 and small pericardial effusion.
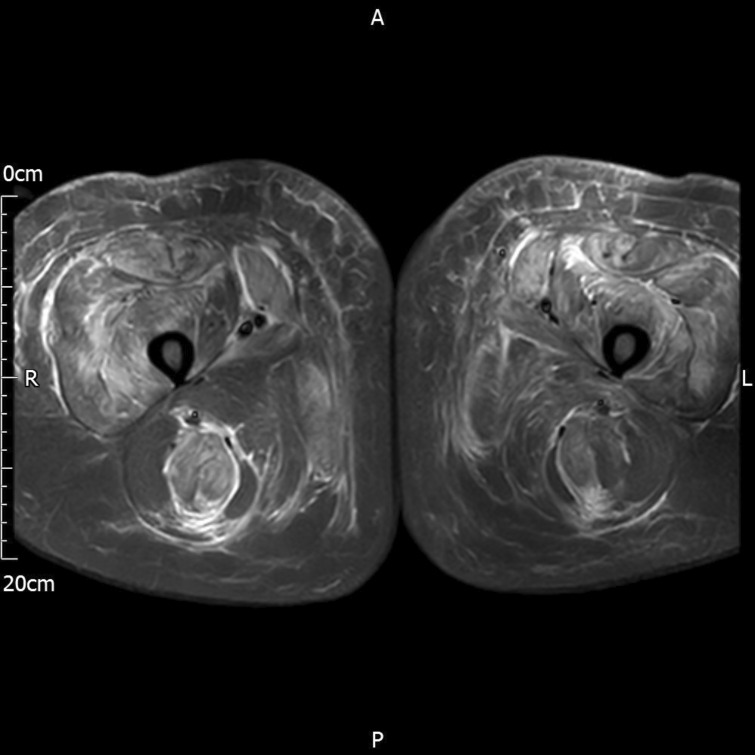
On Day 5, the patient began to experience proximal leg weakness effecting hip flexors and knee extensors, with tenderness over both quadricep muscles and associated lethargy. Oral codeine phosphate (30 mg) was started and administered four times daily for analgesia. On Day 6, a serum creatinine kinase (CK) was measured to diagnose myositis given the ongoing leg weakness, and this was found to be elevated at 6432 U/L (normal value 25–200 U/L). Intravenous 0.9% sodium chloride was initiated to avoid rhabdomyolysis-associated acute renal injury. Repeat hsTnT and CRP were measured at 106 ng/L and 58 mg/L, respectively. On physical examination, the patient had 3/5 power of both quadricep muscles. A rheumatological review was sought and myositis was clinically diagnosed. A magnetic resonance imaging (MRI) scan of both thighs was suggested. It was advised that the patient continue 30 mg prednisolone daily for the next 7 days. The patient continued to experience progressive weakness and tenderness of both quadricep muscles until day 8, at which point the CK peaked at 32230 U/L. An MRI of both lower limbs confirmed features of diffuse myositis with symmetrical appearances involving the anterior, medial and posterior muscle compartments of the thighs with subcutaneous oedema (Figure 3).
Figure 3.
MRI of both thighs demonstrating generalized subcutaneous oedema and symmetrical diffuse signal alteration in all muscle compartments consistent with myositis.
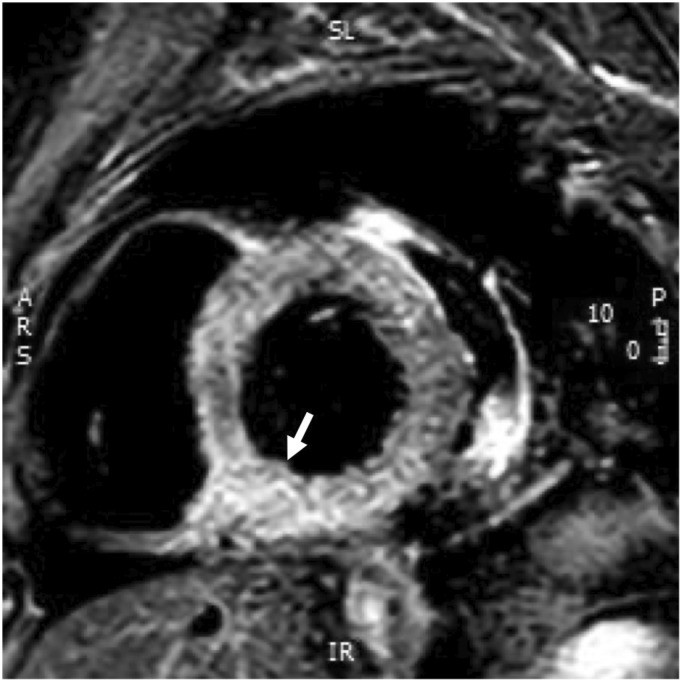
Her autoimmune screen (ANA and ANCA) and hepatitis B, C, and HIV serology were negative. There were no significant changes of either the ECG or echocardiogram when compared with those taken on admission. A cardiac MRI showed normal left and right ventricular function, with possible evidence of myocardial oedema in the basal inferoseptum, in keeping with myocarditis, as well as a 16 mm circumferential pericardial effusion (Figure 4).
Figure 4.
Basal T2 STIR with increased pixel intensity of the inferobasal slice compared with the skeletal muscle, indicating myocardial oedema consistent with myocarditis.
On day 12, a CK was measured at 4922 U/L and a CRP at 14 mg/L. The patient’s symptoms of leg pain and weakness improved. On day 13, the patient’s leg and chest pain had resolved. The lower limb weakness was still improving, and the patient was discharged with a 3-month course of 500 μg twice-daily colchicine and 400 mg three times daily ibuprofen, and 7 days of 30 mg once-daily prednisolone. A telephone follow-up was carried out 2 weeks after discharge which confirmed that the patient had remained symptom free. The lower limb weakness normalized within 48 hours of discharge. The patient will have a repeat cardiac MRI 4 weeks after discharge to assess resolution of myocardial oedema and ensure there has been no reduction in cardiac function.
Discussion
This case describes a patient with COVID-19 presenting with myopericarditis and myositis in the absence of respiratory symptoms. COVID-19 has been found to be associated with a spectrum of cardiovascular sequelae, including acute coronary syndrome,12 atrial fibrillation,13 and heart muscle disease,14 all triggered in at least some part by elevated systemic inflammation. Interestingly, those patients who suffer cytokine storm are at greater risk of severe heart muscle dysfunction15 and cardiac death,16 which suggests a strong inflammatory component to the virus disease pathophysiology and subsequent interaction with cardiac myocytes. Here we have identified that COVID-19 can be associated with clinically significant inflammation of both cardiac and skeletal muscle, whilst not causing conventional symptoms of cough or breathlessness. In this case, we demonstrate that therapy with non-steroidal anti-inflammatory drugs (NSAIDs), colchicine, and a glucocorticoid can be effective in providing symptomatic relief, improving muscle function, and reducing inflammation in COVID-19-associated muscle inflammation.
The sequence of events in this case suggests that COVID-19 can be associated with simultaneous cardiac and skeletal muscle inflammation. Whilst myopericarditis is a recognized complication of COVID-19,17 this case describes sequential and indiscriminate cardiac and skeletal muscle inflammation, which is novel. The exact mechanism causing this is not yet known but may be from a combination of direct damage from the virus, cytokine interactions, microangiopathy and hypoxia. While myalgia is a common symptom, clinicians should be aware of the possibility of fulminant myositis.18
Despite the unique healthcare challenges associated with COVID-19, specifically the limited access to non-emergency inpatient radiology imaging, we secured the diagnosis with rapid inpatient MRI of the heart and proximal lower limb muscles. Whilst a tissue diagnosis to confirm myositis and myocardial inflammation would be interesting, the radiological signs of muscle inflammation are pathognomonic with magnetic resonance. Hence, to limit unnecessary patient and staff exposure, and to maintain reasonable profession distancing where possible, we believe that the MRI of both the heart and lower limb muscles together with the biochemistry secure the diagnosis. Whilst this patient had a history of myopericarditis, which may be an important factor in the development of her symptoms with COVID-19, no association linking SARS-CoV-2 with flares in patients with known inflammatory serositis has yet been identified. This would, however, be an interesting area for future research.
Conventional treatment for myopericarditis and myositis includes the use of ibuprofen and colchicine (ESC class Ia recommendation) and, if associated with an autoimmune disease, a low-dose steroid can be used (ESC class IIa recommendation).11 Given the rapid onset of symptoms, we elected not to increase the dose of NSAIDs, rather we added in glucocorticoid as an additional therapy as we felt this would provide a greater anti-inflammatory effect and lead to more rapid symptomatic relief. Whilst there are no treatment guidelines for the management of myositis, it is widely accepted that glucocorticoids are the first-line therapy.19 In this case, we demonstrate that glucocorticoids, in combination with NSAIDs and colchicine, can be effective in relieving painful symptoms, improving muscle function, and reducing biochemical markers of inflammation in COVID-19 associated myopericarditis and myositis.
Lead author biography

Asad Shabbir is an honorary Cardiology Registrar at the Royal Berkshire Hospital, Reading, UK. He is undertaking a PhD at Queen Mary University of London in the Centre for Cardiovascular Medicine and Devices. He has an interest in interventional cardiology, general internal medicine, and vascular inflammation basic science.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: C.F.C. is the Assistant Editor for European Heart Journal - Case Reports and A.S. is an Associate Editor for European Heart Journal - Case Reports. Neither author was involved with the editing or reviewing of this article.
Supplementary Material
References
- 1. Tschope C, Cooper LT, Torre-Amione G, Van Linthout S.. Management of myocarditis-related cardiomyopathy in adults. Circ Res 2019;124:1568–1583. [DOI] [PubMed] [Google Scholar]
- 2. West S, Killian P, Lawless O.. Association of myositis and myopericarditis in progressive systemic sclerosis. Arthritis Rheum 1981;24:662–667. [DOI] [PubMed] [Google Scholar]
- 3. Zhang L, Wang GC, Ma L, Zu N.. Cardiac involvement in adult polymyositis or dermatomyositis: a systematic review. Clin Cardiol 2012;35:686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kennedy L, Mitchinson MJ.. Giant cell arteritis with myositis and myocarditis. Calif Med 1971;115:84–87. [PMC free article] [PubMed] [Google Scholar]
- 5. Garg V, Tan W, Ardehali R, Shah J, Huynh T, Aksoy O.. Giant cell myocarditis masquerading as orbital myositis with a rapid, fulminant course necessitating mechanical support and heart transplantation. ESC Heart Fail 2017;4:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xing Q, Zhang ZW, Lin QH, Shen LH, Wang PM, Zhang S, Fan M, Zhu B.. Myositis–myasthenia gravis overlap syndrome complicated with myasthenia crisis and myocarditis associated with anti-programmed cell death-1 (sintilimab) therapy for lung adenocarcinoma. Ann Transl Med 2020;8:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lie G, Weickhardt A, Kearney L, Lam Q, John T, Liew D, Arulananda S.. Nivolumab resulting in persistently elevated troponin levels despite clinical remission of myocarditis and myositis in a patient with malignant pleural mesothelioma: case report. Transl Lung Cancer Res 2020;9:360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asawaeer M, Barton D, Radio S, Chatzizisis YS.. Tyrosine kinase inhibitor-induced acute myocarditis, myositis, and cardiogenic shock. Methodist DeBakey Cardiovasc J 2018;14:e5–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ibrahim A, Meagher E, Fraser A, Kiernan TJ.. A young male with severe myocarditis and skeletal muscle myositis. Case Rep Cardiol 2018;2018:5698739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kumar K, Guirgis M, Zieroth S, Lo E, Menkis AH, Arora RC, Freed DH.. Influenza myocarditis and myositis: case presentation and review of the literature. Can J Cardiol 2011;27:514–522. [DOI] [PubMed] [Google Scholar]
- 11. Adler Y, Charron P, Imazio M, Badano L, Baron-Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C, Maisch B, Mayosi B, Pavie A, Ristic AD, Sabaté Tenas M, Seferovic P, Swedberg K, Tomkowski W; ESC Scientific Document Group. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC). Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fischer Q, Darmon A, Ducrocq G, Feldman L.. Case report of anterior ST-elevation myocardial infarction in a patient with coronavirus disease-2019. Eur Heart J Case Rep 2020;doi: 10.1093/ehjcr/ytaa131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schnaubelt S, Breyer M-K, Siller-Matula J, Domanovits H.. Atrial fibrillation: a risk factor for unfavourable outcome in COVID-19? A case report. Eur Heart J Case Rep 2020;doi.org/10.1093/ehjcr/ytaa166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Demertzis ZD, Dagher C, Fadel RA, Malette KM, Bradley PB, Brar I, Rabbani BT, Suleyman G.. Cardiac sequelae of novel coronavirus disease 2019 (COVID-19): a clinical case series. Eur Heart J Case Rep 2020;doi: 10.1093/ehjcr/ytaa179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E.. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020;22:911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baldi E, Sechi GM, Mare C, Canevari F, Brancaglione A, Primi R, Klersy C, Palo A, Contri E, Ronchi V, Beretta G, Reali F, Parogni P, Facchin F, Bua D, Rizzi U, Bussi D, Ruggeri S, Oltrona Visconti L, Savastano S; Lombardia CARe Researchers. Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy. N Engl J Med 2020;383:496–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ventura Perez B, Soler JA, Dominguez Mafe E, Monmeney JV, Solsona Caravaca J, Broseta Torres R, García-Gonzalez P, Higueras Ortega L, Lopez-Lereu MP, Maceira AM.. Subacute perimyocarditis in a young patient with COVID-19 infection. Eur Heart J Case Rep 2020;doi: 10.1093/ehjcr/ytaa157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G, Winterton D.. Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. J Card Fail 2020;26:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oddis CV. Update on the pharmacological treatment of adult myositis. J Intern Med 2016;280:63–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.