Abstract
Background and Aims
The COVID-19 risk and disease course in inflammatory bowel disease [IBD] patients remains uncertain. Therefore, we aimed to assess the clinical presentation, disease course, and outcomes of COVID-19 in IBD patients. Second, we determined COVID-19 incidences in IBD patients and compared this with the general population.
Methods
We conducted a multicentre, nationwide IBD cohort study in The Netherlands and identified patients with COVID-19. First, we assessed the COVID-19 disease course and outcomes. Second, we compared COVID-19 incidences between our IBD study cohort and the general Dutch population.
Results
We established an IBD cohort of 34 763 patients. COVID-19 was diagnosed in 100/34 763 patients [0.29%]; 20/100 of these patients [20%] had severe COVID-19 defined as admission to the intensive care unit, mechanical ventilation, and/or death. Hospitalisation occurred in 59/100 [59.0%] patients and 13/100 [13.0%] died. All patients who died had comorbidities and all but one were ≥65 years old. In line, we identified ≥1 comorbidity as an independent risk factor for hospitalisation (odds ratio [OR] 4.20, 95% confidence interval [CI] 1.58–11.17,; p = 0.004). Incidences of COVID-19 between the IBD study cohort and the general population were comparable (287.6 [95% CI 236.6–349.7] versus 333.0 [95% CI 329.3–336.7] per 100000 patients, respectively; p = 0.15).
Conclusions
Of 100 cases with IBD and COVID-19, 20% developed severe COVID-19, 59% were hospitalised and 13% died. A comparable COVID-19 risk was found between the IBD cohort [100/34 763 = 0.29%] and the general Dutch population. The presence of ≥1 comorbidities was an independent risk factor for hospitalisation due to COVID-19.
Keywords: Ulcerative colitis, Crohn’s disease, severe COVID-19, mortality, intensive care unit
1. Introduction
Coronavirus disease 2019 [COVID-19] is an infectious respiratory syndrome resulting in mild or severe respiratory illness. It is caused by a coronavirus [SARS-CoV-2] which enters the cells through the angiotensin-converting enzyme 2 [ACE2] receptor resulting in an inflammatory response.1 The first cases were described in December 2019 in Wuhan, China. Since then, the virus rapidly spread over the world and on March 11, 2020, the World Health Organization declared it a pandemic. COVID-19 can affect all individuals and older patients and patients with co-existing illnesses, such as diabetes and hypertension, are at increased risk.2 Mortality from COVID-19 is estimated at 1.5% to 3%.3
Inflammatory bowel diseases [IBD], including Crohn’s disease [CD] and ulcerative colitis [UC], are chronic inflammatory disorders of the gastrointestinal tract characterised by a variety of symptoms such as abdominal pain and [haemorrhagic] diarrhoea. It is thought to be the result of an overactive mucosal immune response against gut microorganisms in genetically susceptible individuals. Many patients use immune-modifying medical therapies to control IBD, but these drugs also come with an increased risk of [opportunistic] infection.4 Consequently, IBD patients on immunosuppressive medical therapy may encounter an increased COVID-19 infection risk. Moreover, the upregulation of ACE2 in IBD patients, facilitating the entry and replication of SARS-CoV-2, may further increase the COVID-19 risk.1,5 By contrast, immunosuppressive therapy may also have a potential benefit on clinical outcomes in COVID-19, since it may suppress not only mucosal inflammation but also the SARS-CoV-2-driven systemic inflammatory immune response.1
Given these anticipated effects of SARS-CoV-2 infection, IBD, and immunosuppressive medication, the COVID-19 risk and disease course in IBD patients remain uncertain. Some studies have assessed clinical characteristics and the disease course of COVID-19 in IBD patients.6–13 In general, these reports identified a similar disease course and similar risk factors associated with a worse COVID-19 outcome compared with the general population.8–11 However, most of these studies were limited by small cohorts and harbour reporting or selection bias.11,14 Moreover, incidence data are lacking in most studies.
To systematically investigate the COVID-19 risk and disease course in IBD patients, we established a nationwide IBD cohort [n = 34 763] in The Netherlands. We aimed to assess the clinical presentation, disease course, and clinical outcomes of COVID-19 in this nationwide IBD cohort. Moreover, we determined the COVID-19 incidence in IBD patients and compared this with the general Dutch population.
2. Methods
2.1. Study design
We established a multicentre, retrospective cohort of IBD patients to study the incidence, disease course, and clinical outcomes of COVID-19 in The Netherlands. To assess the disease course, we performed a nested case control study. Two academic and 18 non-academic hospitals participated in this study. Participating centres were primarily located in the central and southern provinces of The Netherlands, as these regions reported the highest incidence of COVID-19 in The Netherlands. Incidences of COVID-19 were compared between the IBD study cohort and the general Dutch population as reported by the Dutch National Institute for Public Health and the Environment [RIVM].15
2.2. Outcomes
To assess the disease course and clinical outcomes of COVID-19 in IBD, we used the primary composite outcome ‘severe COVID-19’. This was defined as either: a] admission to an intensive care unit [ICU]; and/or b] the use of mechanical ventilation; and/or c] death. Previous studies used this outcome to assess disease severity of COVID-19 or other serious infectious diseases such as H7N9 infection.2,11 Our secondary outcome included the hospital admission rate of IBD patients due to COVID-19.
To compare the COVID-19 incidence between the IBD study cohort and the general population, we determined both the incidence of all reported COVID-19 cases and the hospitalisation incidence due to COVID-19.
2.3. Patient identification
We identified patients in each participating hospital using diagnosis treatment combinations [DTC]. DTCs are based on the International Classification of Disease [ICD], 10th revision, and are the foundation of the health care payment system in The Netherlands. DTCs were previously used for validated and reliable identification of IBD patients in The Netherlands.16 First we performed, from May 2019 until June 2020 in every participating centre, a DTC search including the terms ‘Crohn’s disease’ [DTC 601] or ‘ulcerative colitis’ [DTC 602] to determine the size of the total IBD study cohort in each participating centre. Second, we combined the terms ‘Crohn’s disease’ [DTC 601] or ‘ulcerative colitis’ [DTC 602] with ‘acute respiratory disorder caused by 2019 novel coronavirus’ [ICD-10 code U07.1], ‘suspicion of acute respiratory disorder caused by 2019 novel coronavirus’ [ICD-10 code U07.2], or ‘acute respiratory disorder caused by 2019 novel coronavirus excluded’ [ICD-10 code Z03.8]17 to identify potential IBD patients diagnosed with COVID-19. All potential cases were manually verified in the electronic patient charts.
2.4. Inclusion and exclusion criteria
All adult patients [≥18 years] with IBD, including UC, CD, or IBD-unclassified [IBD-U], with an established COVID-19 diagnosis, were eligible for inclusion as a case. A COVID-19 diagnosis was based on a positive SARS-CoV-2 polymerasechain reaction [PCR] and/or a COVID-19 Reporting and Data System [CO-RADS] category 5 on chest computed tomography [CT].18 We excluded patients who were diagnosed with IBD less than 2 weeks before COVID-19 diagnosis.
2.5. Data collection
Demographic and clinical data were anonymously extracted from the electronic patient charts. The following baseline characteristics were collected: gender, age, medical history, pharmacological therapy, body mass index [BMI], smoking status. Relevant comorbidity data were categorised into diabetes, hypertension, cardiovascular disease, asthma or chronic obstructive pulmonary disease, cerebrovascular accident, chronic renal disease, chronic liver disease, previous cancer, and solid organ transplantation. Regarding IBD, we collected information on IBD type, age at IBD diagnosis, disease extent according to the Montreal classification, and IBD activity according to the physician global assessment score. In addition, data regarding IBD-related medical therapy were collected, including the adjustments made due to COVID-19. We extracted the following variables regarding COVID-19: date of diagnosis, clinical symptoms and signs, outcome of SARS-CoV-2 PCR, outcome of the chest CT according to CO-RADS, and initiated [medical] treatment. Furthermore, we collected COVID-19 outcomes including death, [duration of] ICU admission, and ICU treatment including mechanical ventilation, renal replacement therapy, and extracorporeal membrane oxygenation.
2.6. COVID-19 incidence and mortality rate in the IBD study cohort and general population
Incidence data regarding COVID-19 in the general Dutch population were extracted from the Dutch National Institute for Public Health and the Environment [RIVM]. This institute systematically collects and analyses all reported SARS-CoV-2 infections and hospital admissions due to COVID-19 in The Netherlands.15 They report 2-weekly incidence data of each Municipal Public Health Service [GGD], which divides The Netherlands into 355 catchment areas. Every hospital in The Netherlands provides care to multiple surrounding Municipal Public Health Services areas.
First, we compared the COVID-19 incidence between the IBD study cohort and the general population during 4 months [March 2020 until June 2020] per participating centre. Per centre, incidence data were compared with the corresponding region, since COVID-19 incidences differ across The Netherlands. The corresponding region of each hospital is defined by the area of the Municipal Public Health Service in which the hospital is located and all neighbouring Municipal Public Health Services areas. Second, we compared COVID-19 incidences per 2 weeks between the IBD study cohort and the general population from the corresponding regions. Both incidences based on all reported COVID-19 cases, incidences based on hospital admission due to COVID-19, and COVID-19 related mortality rates were compared.
2.7. Statistics
All analyses were performed with IBM SPSS statistical software package version 25 [Armonk, NY]. Descriptive statistics were used to describe baseline data and variables related to IBD and COVID-19. Continuous variables are expressed as medians and interquartile range or mean and standard deviation, depending on distribution. Categorical variables were reported as frequencies with valid percentages. These are defined as the percentage of observations in a category out of the total number of non-missing responses in that category.
We performed explorative analyses with univariable and multivariable logistic regression on the primary outcome [severe COVID-19] and secondary outcome [hospitalisation due to COVID-19] in order to estimate the effects of several factors. These factors included: age [≥65 years versus <65 years], gender, BMI [≥25 kg/m2 versus <25 kg/m2], smoking [ever versus never], number of comorbidities [0 versus ≥1], disease [CD versus UC/IBD-U], disease activity [no versus mild/moderate/severe], and IBD medication. In case of a p-value of <0.2 in univariable analysis, variables were included in the multivariable analysis. A p-value of <0.05 was considered statistically significant.
To assess COVID-19 incidences in the IBD study cohort, we first computed these incidences by dividing the number of new COVID-19 cases in a specified time period by the total IBD study cohort. We computed both a COVID-19 incidence during 4 months [March 2020–June 2020] and per 2 weeks. The 95% confidence intervals for a single proportion were counted for all incidences. Second, we compared incidences between the IBD study cohort and the general population in the corresponding regions using two-by-two tables at the Open Source of Epidemiologic Statistics for public health19; χ 2 testing or Fisher’s exact test [if expected cell counts were <5] were performed to compare incidences.
2.8. Ethics
The study was approved by the Medical Ethics Review Committee region Arnhem – Nijmegen [Registration number 2020–6473]. Subsequently, local approval was obtained in each participating hospital.
3. Results
3.1. Patient identification
We established a multicentre IBD cohort of 34 763 patients [53.5% UC, 46.5% CD]. In total, 100 of 34 763 IBD patients [0.29%] were diagnosed with COVID-19 [Table 1]. COVID-19 diagnoses were made by a positive SARS-CoV-2 PCR in 96/100 patients [96.0%]. Three of 100 patients with typical complaints had a CO-RADS category 5 on chest CT without a positive SARS-CoV-2 PCR being available. In one patient with suspected COVID-19 based on symptoms, serological testing demonstrated antibodies to SARS-CoV-2 indicating a previous SARS-CoV-2 infection.
Table 1.
Baseline characteristics of IBD patients with COVID-19.
| Variable | IBD patients with COVID-19 [n = 100] | Missing values |
|---|---|---|
| Male sex, n [%] | 46 [46.0] | 0 |
| Comorbidities, n [%] | ||
| Any comorbidity | 59 [59.0] | 0 |
| Diabetes | 20 [20.0] | 0 |
| Hypertension | 33 [33.0] | 0 |
| Cardiovascular disease | 32 [32.7] | 2 |
| Asthma or chronic obstructive pulmonary disease | 21 [21.9] | 4 |
| Cerebrovascular accident | 8 [8.1] | 1 |
| Chronic renal disease | 6 [6.0] | 0 |
| Chronic liver disease | 5 [5.1] | 1 |
| Previous cancer | 16 [16.0] | 0 |
| Solid organ transplantation | 1 [1.0] | 0 |
| BMI [kg/m2], median [IQR] | 26.2 [6.97] | 21 |
| Ever smoked, n [%] | 38 [46.9] | 19 |
| Age at COVID-19 diagnosis [years], median [IQR] | 62.5 [23.0] | 0 |
| Age at IBD diagnosis [years], median [IQR] | 48.5 [31.0] | 0 |
| IBD duration [years], median [IQR] | 10.3 [12] | 0 |
| IBD type | 0 | |
| Ulcerative colitis, n [%] | 59 [59.0] | |
| Crohn’s disease, n [%] | 36 [36.0] | |
| IBD-unclassified, n [%] | 5 [5.0] | |
| Ulcerative colitis extent | 0 | |
| Proctitis [Montreal E1], n [%] | 13 [20.6] | |
| Left-sided colitis [Montreal E2], n [%] | 30 [47.6] | |
| Extended colitis [Montreal E3], n [%] | 20 [31.7] | |
| Crohn’s disease extent | 0 | |
| Ileal [Montreal L1], n [%] | 17 [43.6] | |
| Colonic [Montreal L2], n [%] | 10 [25.6] | |
| Ileocolonic [Montreal L3], n [%] | 12 [30.8] | |
| Upper gastrointestinal disease [Montreal L4], n [%] | 2 [5.7] | |
| Perianal disease activity, n [%] | 6 [15.8] | |
| Crohn’s disease phenotype | 6 | |
| Non-stricturing, non- penetrating [Montreal B1], n [%] | 26 [76.5] | |
| Stricturing [Montreal B2], n [%] | 5 [14.7] | |
| Penetrating [Montreal B3], n [%] | 3 [8.8] | |
| IBD activity by physician global assessment | 4 | |
| Remission, n [%] | 70 [72.9] | |
| Mild, n [%] | 16 [16.7] | |
| Moderate, n [%] | 8 [8.3] | |
| Severe, n [%] | 2 [2.1] | |
| Concomitant IBD-related medical therapy, n [%] | ||
| None | 23 [23.0] | 0 |
| Systemic corticosteroids | 22 [22.2] | 1 |
| 5-aminosalicylates | 56 [56.0] | 0 |
| Thiopurines | 24 [24.0] | 0 |
| Methotrexate | 2 [2.0] | 0 |
| Calcineurin inhibitors | 0 [0] | 0 |
| Anti-TNF | 13 [13.1] | 1 |
| Vedolizumab | 1 [1.0] | 0 |
| Ustekinumab | 1 [1.0] | 0 |
| Tofacitinib | 1 [1.0] | 0 |
n, number; IBD, inflammatory bowel disease; TNF, tumour necrosis factor; IQR, interquartile range; BMI, body mass index.
3.2. Patient characteristics
Baseline characteristics of the 100 IBD patients with COVID-19 are shown in Table 1. Median age at COVID-19 diagnosis was 62.5 years [interquartile range 23.0] and there was a slight predominance of females [54.0%]. At least one comorbidity was reported for 59 of 100 [59.0%] patients and 46.9% were [ex]smokers.
We included 59 patients with UC [59.0%], 36 patients with CD [36.0%], and five patients with IBD-U [5.0%]. In most patients, IBD was in remission [72.9%]. Most patients with COVID-19 did not use any IBD-related medical therapy [no IBD medication: 23/100, 23.0%] or were on 5-aminosalicylates [56/100, 56.0%]. Systemic corticosteroids were used by 22 of 100 patients [13 prednisone, nine budesonide]. After a diagnosis of COVID-19, accelerated IBD steroid tapering took place in 13.6% of patients [3/22]. In addition, discontinuation of IBD therapy was reported for 13 of 24 patients [54.2%] on thiopurines, in four of 13 patients [30.8%] on anti-tumour necrosis factor [TNF], in both patients on methotrexate, and in the single patient on tofacitinib.
3.3. COVID-19 clinical characteristics and outcomes
The most common symptoms of COVID-19 infection were cough [80.2%], fatigue [78.5%], shortness of breath [72.6%], fever [61.0%], and myalgia [41.4%], as shown in Supplementary Table 1, available as Supplementary data at ECCO-JCC online. In addition, 38.6% of patients had diarrhoea. Other gastrointestinal complaints such as nausea and vomiting were reported in respectively 26.6% and 8.9% of patients.
Of 100 IBD patients, 59 [59.0%] required hospitalisation due to COVID-19, and 10 [10.0%] were admitted to the ICU. During ICU admission, seven patients required mechanical ventilation, but data on mechanical ventilation were lacking for the other three patients due to relocation to other hospitals. Finally, 13 out of 100 patients [13.0%] died. A summary of characteristics of deceased patients is shown in Table 2. The median age of COVID-19 diagnosis in the deceased patients was 77 years [interquartile range 13.5]. Ten of 13 patients [76.9%] had more than one comorbidity. In 10/13 patients, IBD was in remission. One patient was not admitted to the hospital and died at home. Only three out of 13 patients [23.1%] who died were admitted to the ICU.
Table 2.
Overview of all IBD patients with COVID-19 who died.
| Case number | Age | Gender | Comorbidity | IBD type | IBD duration | Disease activity | IBD medication | Symptoms | GI symptoms | COVID-19 treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 80 | Male | CVD | UC | 45 y | Remission | No | Fever | No | No | Hospital admission, died after 10 days |
| COPD | Headache | ||||||||||
| Lymphoma | Cough | ||||||||||
| Sore throat | |||||||||||
| Fatigue | |||||||||||
| 2 | 86 | Male | Diabetes | CD | 12 y | Remission | Aminosalicylates | Unknown | Unknown | Unknown | No hospital admission, died at home |
| Hypertension | Azathioprine | ||||||||||
| CVD | |||||||||||
| CVA | |||||||||||
| NASH | |||||||||||
| 3 | 76 | Female | Diabetes | UC | 1 m | Unknown | Aminosalicylates | Fever with chills | Diarrhoea | Hydroxy-chloroquine | Hospital admission, died after 11 days |
| Hypertension | Prednisolone | Headache | Antibiotics | ||||||||
| CVD | 6-mercaptopurine | Cough | |||||||||
| CVA | Sore throat | ||||||||||
| Vascular renal disease | Fatigue | ||||||||||
| Shortness of breath | |||||||||||
| 4 | 83 | Female | Hypertension | UC | 22 y | Remission | Aminosalicylates | Fever | Nausea | Hydroxy-chloroquine | Hospital admission, died after 16 days |
| CVD | Cough | Antibiotics | |||||||||
| Sputum production | |||||||||||
| Sore throat | |||||||||||
| Fatigue | |||||||||||
| Shortness of breath | |||||||||||
| 5 | 69 | Female | Hypertension | CD | 2 m | Remission | Prednisolone | Fever | Diarrhoea | Lopinavir | Hospital admission, died after 9 days |
| Azathioprine | Fatigue | Antibiotics | |||||||||
| Shortness of breath | |||||||||||
| 6 | 72 | Male | Diabetes | UC | 10 y | Mild | Aminosalicylates | Unknown | Diarrhoea | Antibiotics | Admission to intensive care unit, mechanical ventilation, died after 1 day |
| Hypertension | Prednisolone | ||||||||||
| CVD | Azathioprine | ||||||||||
| 7 | 77 | Male | CVD | UC | 9 y | Remission | Aminosalicylates | Fever | Diarrhoea | Chloroquine | Hospital admission, died after 5 days |
| Azathioprine | Cough | Antibiotics | |||||||||
| Sputum production | |||||||||||
| Fatigue | |||||||||||
| Shortness of breath | |||||||||||
| 8 | 64 | Female | Cancer | UC | 3 y | Moderate | Prednisolone | Fever | Diarrhoea | No | Hospital admission, died |
| Fatigue | |||||||||||
| Shortness of breath | |||||||||||
| 9 | 68 | Male | Diabetes | UC | 6 y | Remission | No | Cough | Diarrhoea | Chloroquine | Admission to intensive care unit, mechanical ventilation, died after 1 day |
| Hypertension | Sputum production | Antibiotics | |||||||||
| CVD | Fatigue | ||||||||||
| Asthma | Shortness of breath | ||||||||||
| 10 | 75 | Male | Diabetes | UC | 2 y | Remission | No | Chills | Chloroquine | Admission to intensive care unit, mechanical ventilation, died after 3 days | |
| CVD | Cough | ||||||||||
| Asthma | Shortness of breath | ||||||||||
| Prostate carcinoma | |||||||||||
| Renal insufficiency resulting in kidney transplant | |||||||||||
| 11 | 78 | Female | CVD | UC | 16 y | Remission | Aminosalicylates | Cough | Diarrhoea | No | Hospital admission, died after 3 days |
| Asthma/COPD | Sputum production | ||||||||||
| Mammary carcinoma | Shortness of breath | ||||||||||
| 12 | 86 | Female | Hypertension | UC | 56 y | Remission | Aminosalicylates | Cough | No | Unknown | Hospital admission, died after 14 days |
| CVD | Fatigue | ||||||||||
| CVA | Shortness of breath | ||||||||||
| 13 | 85 | Male | Prostate carcinoma | UC | 51 y | Remission | Budesonide | Fever | No | No | Hospital admission, died after 18 days |
| Chronic liver disease | Fatigue |
CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; NASH, non-alcoholic steatosis hepatis; GI, gastrointestinal; y, year; m, month; BMI, body mass index; IBD, inflammatory bowel disease; IQR, interquartile range; TNF, tumour necrosis factor; UC, ulcerative colitis; CD, Crohn’s disease.
Approximately half of all patients [48.9%] received medical treatment for COVID-19. Most of these patients received antibiotic therapy [35.5%]. In the minority of cases, medical therapies with potential antiviral effects were used such as chloroquine [17.2%], hydroxychloroquine [6.5%], and lopinavir [2.2%].
3.4. Risk factors for severe COVID-19 and hospitalisation
The primary outcome, severe COVID-19 [ICU/mechanical ventilation/death], was reported in 20 out of 100 patients [20.0%]. Univariable analyses showed a statistically significant association between older age [≥65 years] and severe COVID-19 (odds atio [OR] 2.94, 95% confidence interval [CI] 1.06–8.17; p = 0.039; Supplementary Table 2A, available as Supplementary data at ECCO-JCC online). However, this potential risk factor was not confirmed in multivariable analyses. By contrast, the multivariable analyses did identify UC as an independent risk factor to develop severe COVID-19 [OR 5.46, 95% confidence interval 1.03–28.88; p = 0.046].
The secondary outcome, hospitalisation due to COVID-19, was observed in 59 out of 100 patients [59.0%]. We found a significant association in the univariable analyses between hospitalisation and the potential risk factors age ≥65 years [OR 2.86, 95% confidence interval 1.23–6.67; p = 0.015], male sex [OR 2.29, 95% confidence interval 1.00–5.21; p = 0.049], [ex]smoking [OR 2.93, 95% confidence interval 1.15–7.49; p = 0.024], and ≥1 comorbidities [OR 5.08, 95% confidence interval 2.14‐12.07; p <0.001; Supplementary Table 2B, available as Supplementary data at ECCO-JCC online]. In the multivariable analyses ≥1 comorbidities was an independent risk factor for hospitalisation [OR 4.20, 95% confidence interval 1.58–11.17; p = 0.004].
3.5. COVID-19 incidence and mortality rate in the IBD study cohort versus the general population
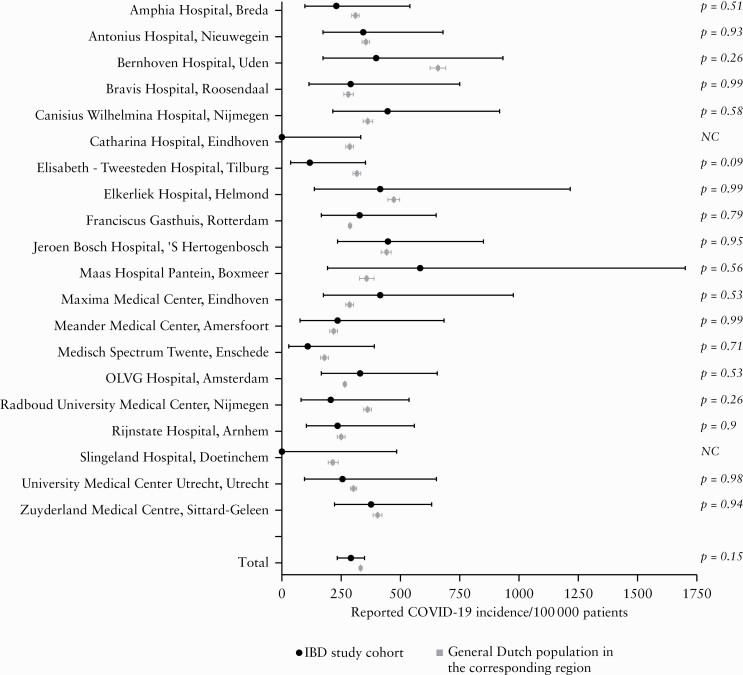
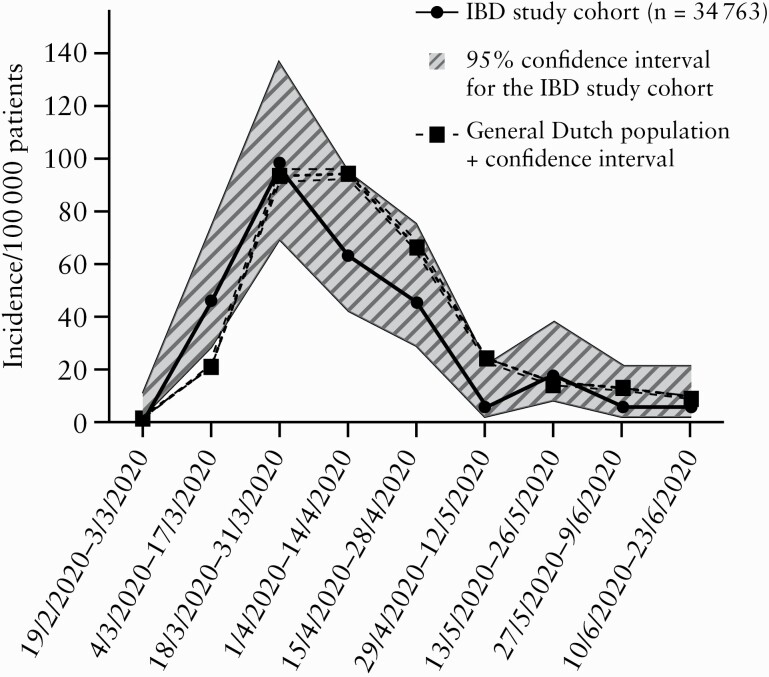
Table 3 and Figure 1 show the 4-month COVID-19 incidence per 100 000 individuals for both the IBD study cohort and the general population, stratified per participating centre. The incidence of reported COVID-19 was comparable between the IBD study cohort [287.6 per 100 000 patients; 95% confidence interval 236.6–349.7] and the general population [333.0 per 100 000 subjects; 95% confidence interval 329.3–336.7; p = 0.15]. In line, incidences for all reported COVID-19 cases per 2 weeks in the IBD study cohort followed a similar course as in the general population, with overlapping 95% confidence intervals [Figure 2].
Table 3.
Overview of the reported and hospitalised COVID-19 incidence during 4 months [March–June] in both the IBD Study cohort and general Dutch population.
| Centre, city | IBD population | General population in the corresponding region | |||||
|---|---|---|---|---|---|---|---|
| Total IBD population [n] | Reported COVID-19 cases [n] | Hospitalised COVID-19 cases [n] | Reported COVID-19 incidence during 4 months [n/100,000, 95% CI] | Hospitalised COVID-19 incidence during 4 months [n/100,000; 95% CI] | Reported COVID-19 incidence during 4 months [n/100 000; 95% CI] |
Hospitalised COVID-19 incidence during 4 months [n/100 000; 95% CI] |
|
| Amphia Hospital, Breda | 2152 | 5 | 3 | 232.3 [99.9–542.8] | 139.4 [47.4–409.1] | 310.9 [294.4–328.3] | 73.3 [65.7–81.7] |
| Antonius Hospital, Nieuwegein | 2316 | 8 | 5 | 345.4 [175.1–680.2] | 215.8 [92.2–504.4] | 356.3 [340.9–372.3] | 67.8 [61.4–75.0] |
| Bernhoven Hospital, Uden | 1246 | 5 | 3 | 401.2 [171.5–935.9] | 240.7 [81.9–705.5] | 658.3 [625.7–692.7] | 182.3 [166.3–199.8] |
| Bravis Hospital, Roosendaal | 1363 | 4 | 3 | 293.4 [114.2–752.2] | 220.1 [74.9–645.1] | 282.2 [261.7–304.3] | 35.3 [28.5–43.7] |
| Canisius Wilhemina Hospital, Nijmegen | 1573 | 7 | 3 | 445.0 [215.8–915.8] | 190.7 [64.9–559.3] | 362.0 [343.5–381.4] | 87.4 [78.3–97.5] |
| Catharina Hospital, Eindhoven | 1142 | 0 | 0 | 0 [0–335.3] | 0 [0–335.3] | 286.6 [270.5–303.7] | 86.4 [77.7–96.0] |
| Elisabeth – Tweesteden Hospital, Tilburg | 2500 | 3 | 3 | 120.0 [40.8–352.2] | 120.0 [40.8–352.2] | 316.6 [300.6–333.4] | 84.7 [76.7–93.5] |
| Elkerliek Hospital, Helmond | 721 | 3 | 2 | 416 [141.6–1216.2] | 277.3 [76.1–1005.7] | 472.3 [447.2–498.8] | 139.0 [125.5–153.8] |
| Franciscus Gasthuis, Rotterdam | 2412 | 8 | 4 | 331.6 [168.2–653.2] | 165.8 [64.5 – 425.7] | 288.3 [280.5–296.2] | 57.8 [54.4–61.5] |
| Jeroen Bosch Hospital, ‘s Hertogenbosch | 2006 | 9 | 6 | 448.6 [236.2–850.5] | 299.1 [137.1–651.1] | 439.9 [419.5–461.3] | 126.6 [115.5–138.7] |
| Maas Hospital Pantein, Boxmeer | 515 | 3 | 1 | 582.5 [198.3–1698.5] | 194.1 [34.3–1091.6] | 357.5 [326.9–390.8] | 74.4 [61.2–90.3] |
| Maxima Medical Centre, Eindhoven | 1200 | 5 | 2 | 416.6 [178.1–971.7] | 166.6 [45.7–605.7] | 286.6 [270.5–303.7] | 86.4 [77.7–96.0] |
| Meander Medical Centre, Amersfoort | 1282 | 3 | 1 | 234 [79.6–685.8] | 78 [13.8–440.5] | 217. [202.5–233.3] | 43.6 [37.5–50.8] |
| Medical Spectrum Twente, Enschede | 1853 | 2 | 1 | 107.9 [29.6–392.7] | 53.9 [9.5–305.1] | 179.4 [165.2–194.7] | 42.4 [35.5–50.5] |
| OLGV Hospital, Amsterdam | 2405 | 8 | 6 | 332.6 [168.6–655.1] | 249.4 [114.4–543.3] | 266.5 [258.3–275.0] | 60.9 [57.0–65.1] |
| Radboud University Medical Centre, Nijmegen | 1923 | 4 | 1 | 208 [80.9–533.6] | 52 [9.2–294.0] | 362.0 [343.5–381.4] | 87.4 [78.3–97.5] |
| Rijnstate Hospital, Arnhem | 2100 | 5 | 3 | 238 [101.7–556.2] | 142.8 [48.6–419.2] | 252.1 [238.0–267.1] | 66.0 [58.5–74.4] |
| Slingeland Hospital, Doetinchem | 787 | 0 | 0 | 0 [0–485.8] | 0 [0–485.8] | 216.0 [194.8–239.6] | 46.0 [36.8–57.5] |
| University Medical Centre Utrecht, Utrecht | 1563 | 4 | 0 | 255.9 [99.6–656.2] | 0 [0–245.2] | 303.0 [290.9–315.5] | 64.9 [59.3–70.9] |
| Zuyderland Hospital, Sittard / Geleen | 3704 | 14 | 13 | 377.9 [225.3–633.5] | 350.9 [205.2–599.6] | 403.8 [385.5–423.0] | 140.6 [129.6–152.5] |
| Total | 34763 | 100 | 40 | 287.6 [236.6–349.7] | 172.5 [134.1–222.1] | 333.0 [329.3–336.7] | 84.5 [82.6–86.5] |
IBD, inflammatory bowel disease; CI, confidence interval.
Figure 1.
Forest plot comparing the reported COVID-19 incidence during 4 months between the IBD Study cohort and general Dutch population. NC, not computable.
Figure 2.
Reported COVID-19 incidence per 100 000 patients per 2 weeks for both the IBD Study cohort and the general Dutch population in the corresponding region.
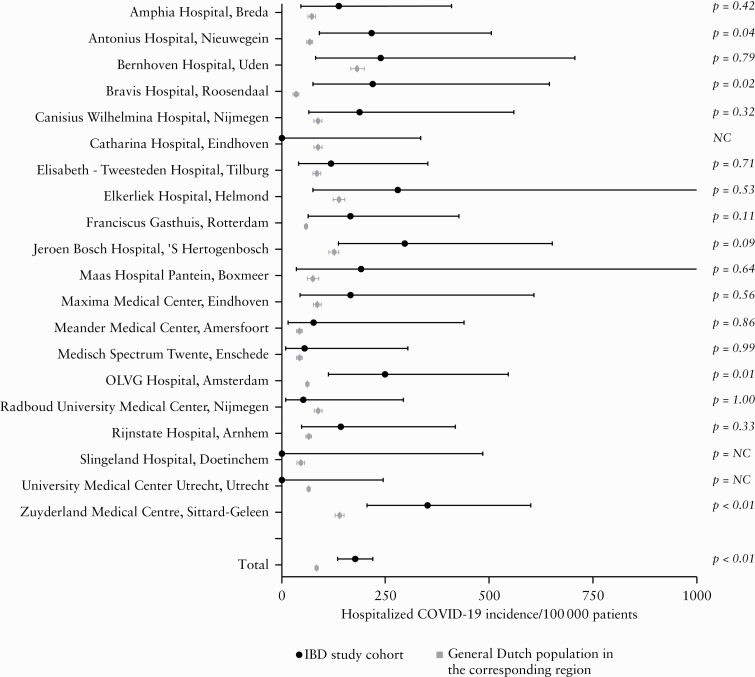
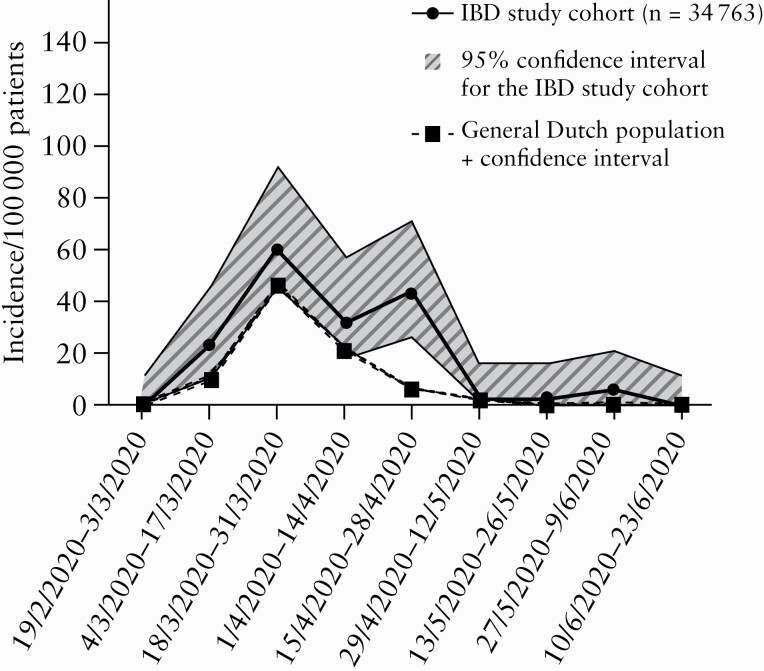
By contrast, we found higher 4-month incidence rates for hospital admissions due to COVID-19 in the IBD study cohort [177.2 per 100 000 patients; 95% confidence interval 137.7–228.1] compared with the general population (84.5 per 100 000 patients, 95% confidence interval [82.6–86.5]; p <0.01; Figure 3). Similarly, higher 2-weekly hospitalisation incidence rates were found in the IBD study cohort at specific time points [March 4, 2020–March 17, 2020; p = 0.01, April 15, 2020 –April 28, 2020,]; p <0.01, May 27, 2020–June 9, 2020; p =0.01; Figure 4].
Figure 3.
Forest plot comparing the hospitalised COVID-19 incidence during 4 months between the IBD Study cohort and general Dutch population. NC, not computable.
Figure 4.
Hospitalised COVID-19 incidence per 100 000 patients per 2 weeks for both the IBD Study cohort and the general Dutch population in the corresponding region.
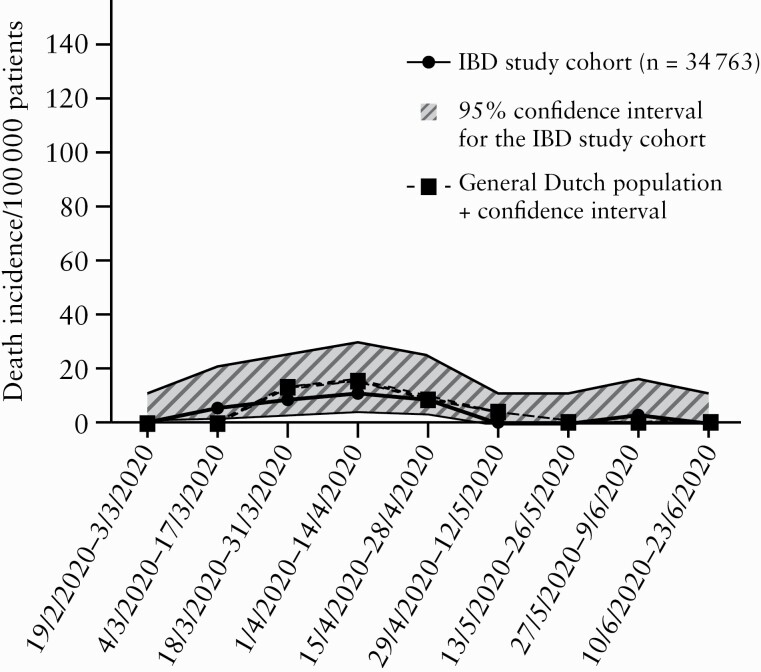
In line with the reported COVID-19 incidence, we found comparable 4-month COVID-19 related mortality rates between the IBD study cohort [37.3 per 100 000 patients; 95% confidence interval 21.9–64.0] and the general population [44.9 per 100 000 patients; 95% confidence interval 43.5–46.3; p = 0.51]. Mortality rates per 2 weeks were comparable as well, with overlapping confidence intervals [Figure 5].
Figure 5.
COVID-19 mortality per 100 000 patients per 2 weeks for both the IBD Study cohort and the general Dutch population in the corresponding region.
4. Discussion
This retrospective study performed in 20 Dutch hospitals describes the impact of the COVID-19 pandemic on the Dutch IBD population. We identified 100 IBD patients with COVID-19, of whom 20% developed a severe disease course. The mortality and hospitalisation rates were 13% and 59%, respectively. A similar incidence of COVID-19 was found between the IBD cohort [n = 34 763] and the general population (287.6 [95% confidence interval 236.6–349.7] versus 333.0 [95% confidence interval 329.3–336.7] per 100 000 patients, respectively; p = 0.15). The presence of ≥1 comorbidities was an independent risk factor for hospitalisation of IBD patients with COVID-19, whereas immunosuppressive or biological medication did not emerge as a risk factor.
Severe COVID-19 [ICU admission, mechanical ventilation, and/or death] was reported in 20% of our IBD cases with COVID-19; 59% were hospitalised and 13% died. In line, one previous meta-analysis regarding IBD patients with COVID-19 reported a hospitalisation rate of 20–67% and a mortality rate of 0–20%.14 However, lower rates of severe COVID-19 and hospitalisation due to COVID-19 are reported as well. As such one Chinese study, including 1099 patients with COVID-19, reported severe COVID-19 in 6.1%.2 Compared with our findings, this difference might be explained by a lower comorbidity rate in the Chinese cohort [75% versus 58.2%, respectively]. Another Italian study including 79 patients with IBD and COVID-19 reported lower hospitalisation and mortality rates compared with our cohort as well [28% versus 59% and 8% versus 13%, respectively].8 Furthermore, a multicentre research network study found that IBD patients were not at increased risk for hospitalisation or COVID-19 related death compared with non-IBD patients.13 Our relatively high hospitalisation and severe COVID-19 rates could be the result of the restrictive testing policy in The Netherland. Due to limited testing capacity, especially in the early phase of the pandemic, SARS-CoV-2 PCRs were only performed if clinical consequences were expected such as hospital admission. Thus, if patients did not qualify for an emergency room visit or hospital admission, no SARS-CoV-2 PCR would be performed. This could have biased our results by only including patients with severe illness and could have led to the high rates of hospitalisation and severe COVID-19. The relatively high hospitalisation rate in The Netherlands compared with other countries in Europe, as reported by the European Centre for Disease Prevention and Control, further supports this theory.20
We identified UC as single risk factor for the development of severe COVID-19, and ≥1 comorbidity as an independent risk factor for hospitalisation. Given the limited number of cases that developed severe COVID-19 [n = 20] we need to interpret the risk factor UC with caution. Similarly to our results, previous studies identified the presence of comorbidities as a risk factor for severe COVID-19 and death.8,11 In contrast, the SECURE-IBD study additionally identified use of either systemic corticosteroid or 5-ASA/sulphasalazine as risk factors for severe COVID-19.11 This study was criticised in view of the reported high rate of anti-TNF use [43.4%], thereby potentially overestimating the effect of corticosteroids and 5-ASA treatment on COVID-19 severity.21 In contrast, only 13.1% of IBD patients included in our study were treated with anti-TNF monotherapy or combination therapy during COVID-19, and they potentially reflect a more representative cohort of IBD patients. As a result, this could explain why we did not identify use of any IBD-related medical therapy as a risk factor for severe COVID-19 or hospitalisation.
The incidences of COVID-19 in our IBD study cohort [n = 34 763] and the general Dutch population in the corresponding regions were comparable (287.6 [95% confidence interval 236.6–349.7] versus 333.0 [95% confidence interval 329.3–336.7] per 100 000 patients, respectively). In addition, we found a comparable mortality rate between groups [p = 0.51]. These results are in line with the findings of a French/Italian cohort study, which reported a comparable cumulative COVID-19 incidence between the IBD and general population [0.0025 versus 0.0017, respectively].22 Similarly, a single-centre Spanish case series reported a cumulative incidence of COVID-19 infection of 630 per 100 000 IBD patients [12 out of 1912 IBD patients], compared with 660 per 100 000 for the total population of Madrid [43 877 cases in 6.7 million inhabitants]. The authors found a comparable mortality ratio [OR 0.95, 95% CI: 0.84–1.06; p = 0.36] as well.10 Another retrospective study in Northern California in the USA showed that the prevalence of COVID-19 among IBD patients was similar to the prevalence in the general population in that region [3.0% versus 2.8%, respectively].23 The authors of the latter papers suggested that IBD patients are not facing an increased risk for COVID-19 despite the use of immunosuppressive drugs, which was relatively high [average 37%] in both studies.24 It should be noted that our cohort had a relatively low use of biologics [16%] and none of the patients with severe COVID-19 used anti-TNF. These data support the hypothesis that IBD patients, despite immune modulating therapies, do not have an increased risk of COVID-19. Several factors may contribute to this observation, including awareness among physicians of potential COVID-19 risk during immunosuppression, and increased caution among IBD patients in isolating themselves from others in fear of contracting COVID-19.
Strengths of this study include its nationwide coverage by the large number of hospitals which cover 125 of the 355 municipalities [35.2%] of the country, including over 34 000 IBD patients. The participating centres were primarily located in the regions reporting the highest incidence of COVID-19. This large coverage, including academic but mainly non-academic hospitals, allowed us to build a cohort that approximates the national IBD population, limiting selection bias. In addition, the most affected parts of the country were included, ensuring sufficient COVID-19 cases for statistical analysis. Finally, we were able to systematically identify COVID-19 patients as well as the total number of IBD patients for the participating hospitals, allowing the calculation of COVID-19 incidences.
This study is limited by the following factors. First, the restrictive testing policy in The Netherlands may have led to a significant under-reporting of COVID-19 cases. However, this limitation applies to both the IBD study cohort and the general population, allowing us to compare COVID-19 incidences between groups. In addition, this under-reporting especially applies to patients with a predicted mild COVID-19 course, since an expected severe course would qualify for testing. Consequently, this may have resulted in overestimated severe COVID-19 and hospitalisation rates. Second, the reporting criteria, identification, and data collection of COVID-19 differed between the IBD study cohort and general population, which inevitably affects the comparison between groups. For example, we retrospectively identified IBD cases with COVID-19 whereas data from the general population came from the Dutch National Institute for Public Health and the Environment [RIVM]. Due to our retrospective search, we could have missed IBD cases with COVID-19 [especially cases with a predicted mild COVID-19 course without any hospital-related contact and thus no DTC]. Moreover, we had no access to the individual patient data of the multicentre IBD study cohort of 34 763 patients, which hampered calculating and comparing standardised incidence ratios. Finally, due to the limited number of cases with COVID-19 and IBD, our multivariable analyses are at risk of being underpowered.
In conclusion, of 100 cases with IBD and COVID-19, 20% developed severe COVID-19 [ICU admission, mechanical ventilation, and/or death], 59% were hospitalised, and 13% died. A comparable COVID-19 risk was found between the IBD cohort [100/34 763 = 0.29%] and the general Dutch population [p = 0.15]. IBD patients were at increased risk to be admitted to the hospital if they had ≥1 comorbidities.
Funding
This study was not supported by any company or grants. The costs were borne by the authors’ institutions.
Conflict of Interest
RLW has participated in advisory boards or as a speaker or consultant for the following companies: Abbvie, Janssen, Pfizer. FH has served on advisory boards or as speaker for Abbvie, Janssen-Cilag, MSD, Takeda, Celltrion, Teva, Sandoz, Dr Falk; has received funding [grants/honoraria] from Dr Falk, Janssen-Cilag, Abbvie, Takeda; and has received consulting fees from Celgene, Janssen-Cilag.
Author Contributions
No additional writing assistance was used for this manuscript. LD, ML, and FH contributed to the design of the study. LD, ML, DJ, WD, RC, TR, JJ, NM, RW, AT, AB, MG, PB, EH, CH, MD, MH, LE, BJ, ML, MR, LG, SN, and AvB collected data. LD and ML analysed the data. LD and ML drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors approved the final version of this manuscript.
Supplementary Material
References
- 1.Monteleone G, Ardizzone S. Are patients with inflammatory bowel disease at increased risk for Covid-19 infection? J Crohns Colitis 2020;14:1334–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan Wj, Ni Zy, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubin DT, Abreu MT, Rai V, Siegel CA; International Organization for the Study of Inflammatory Bowel Disease . Management of patients with Crohn’s disease and ulcerative colitis during the coronavirus disease-2019 pandemic: results of an International Meeting. Gastroenterology 2020;159:6–13.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahier JF, Magro F, Abreu C, et al. ; European Crohn’s and Colitis Organisation [ECCO]. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2014;8:443–68. [DOI] [PubMed] [Google Scholar]
- 5.Neurath MF. Covid-19 and immunomodulation in IBD. Gut 2020. doi:gutjnl-2020–321269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodríguez-Lago I, Ramírez de la Piscina P, Elorza A, et al. Characteristics and prognosis of patients with inflammatory bowel disease during the SARS-CoV-2 pandemic in the Basque Country [Spain]. Gastroenterology 2020;159:781–3. doi: 10.1053/j.gastro.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tursi A, Angarano G, Monno L, et al. COVID-19 infection in Crohn’s disease under treatment with adalimumab. Gut 2020;69:1364–5. [DOI] [PubMed] [Google Scholar]
- 8.Bezzio C, Saibeni S, Variola A, et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut 2020. doi: gutjnl-2020–321411. [DOI] [PubMed] [Google Scholar]
- 9.Allocca M, Fiorino G, Zallot C, et al. Incidence and patterns of COVID-19 among inflammatory bowel disease patients from the Nancy and Milan cohorts. Clin Gastroenterol Hepatol 2020;18:2134–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taxonera C, Sagastagoitia I, Alba C, Mañas N, Olivares D, Rey E. 2019 novel coronavirus disease [COVID-19] in patients with inflammatory bowel diseases. Aliment Pharmacol Ther 2020;52:276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an International Registry. Gastroenterology 2020;159:P481–491.e3. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukin DJ, Kumar A, Hajifathalian K, et al. Baseline disease activity and steroid therapy stratify risk of COVID-19 in patients with inflammatory bowel disease. Gastroenterology 2020;159:1541–1544.e2. doi: 10.1053/j.gastro.2020.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S, Khan A, Chowdhry M, et al. Risk of severe COVID-19 in patients with inflammatory bowel disease in United States. a multicentre research network study. Gastroenterology 2020;159:1575–1578.e4. doi: 10.1053/j.gastro.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aziz M, Fatima R, Haghbin H, Lee-Smith W, Nawras A. The incidence and outcomes of COVID-19 in IBD patients: a rapid review and meta-analysis. Inflamm Bowel Dis 2020;26:e132–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institute for Public Health and the Environment. Current information about COVID-19 (novel coronavirus). 2020. https://www.rivm.nl/en/novel-coronavirus-covid-19/current-information. Accessed July 6 2020.
- 16.van der Valk ME, Mangen MJ, Leenders M, et al. ; COIN study group and the Dutch Initiative on Crohn and Colitis. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut 2014;63:72–9. [DOI] [PubMed] [Google Scholar]
- 17.Expertgroep ICD-10. Codeadviezen expertgroep ICD-10. 2020. https://www.dhd.nl/producten-diensten/icd10/Documents/Codeadviezen%20Expertgroep%20ICD-10%20%2001-05-2020.pdf. Accessed September 15, 2020.
- 18.Prokop M, Everdingen Wv, Vellinga TvR, et al. CO-RADS – a categorical CT assessment scheme for patients with suspected COVID-19: definition and evaluation. Radiology 2020;296:E97–E104. doi: 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dean AG, Sullivan KM, Soe MM. Open source epidemiologic statistics for public health: two by two table. 2013. https://www.openepi.com/Menu. Accessed September 15, 2020.
- 20.European Centre for Disease Prevention and Control. Data on hospital and ICU admission rates and current occupancy for COVID-19. 2020. https://www.ecdc.europa.eu/en/publications-data/download-datahospital-and-icu-admission-rates-and-current-occupancy-covid-19. Accessed September 15, 2020.
- 21.Cappello M, Busacca A, Guida L. The course of Covid 19 in inflammatory bowel disease: protective role of TNF antagonists. Response to: Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an International Registry. Gastroenterology 2020;S0016-5085(20)34920-9. [Epub aahead of print]. doi: 10.1053/j.gastro.2020.06.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allocca M, Fiorino G, Zallot C, et al. Incidence and patterns of COVID-19 among inflammatory bowel disease patients from the Nancy and Milan cohorts. Clin Gastroenterol Hepatol 2020;18:2134–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gubatan J, Levitte S, Balabanis T, Patel A, Sharma A, Habtezion A. SARS-CoV-2 testing, prevalence, and predictors of COVID-19 in patients with inflammatory bowel disease in Northern California. Gastroenterology 2020;159:1141–4.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taxonera C, Alba C, Olivares D. What is the incidence of COVID-19 in patients with IBD in western countries? Gastroenterology 2020. [Epub ahead of print]. doi: 10.1053/j.gastro.2020.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.