Abstract
Background
The coronavirus disease 2019 (COVID-19) has caused a global pandemic with an unprecedented burden on health and the economy worldwide. Although it primarily involves the respiratory tract system, cardiovascular complications, particularly arterial and venous thrombosis, are frequently reported and are associated with adverse outcomes.
Case summary
We describe the case of a 57-year-old female who presented with acute hypoxic respiratory failure and shock. She was found to have left lower extremity deep vein thrombosis and a suspected pulmonary embolism. A large mobile right atrial mass was found on echocardiogram. Given the large thrombus burden that portended an extremely high risk for embolization to the pulmonary arteries, emergent percutaneous aspiration of an organized thrombus (rather than thrombolysis) was performed using the AngioVac system (Angiodynamics Inc., Latham, NY, USA) complicated by haemodynamic collapse due to acute right ventricular failure. An Impella RP (Abiomed, Danvers, MA, USA) was then placed, with rapid stabilization of haemodynamics. The patient tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). She was treated with antimicrobial and systemic anticoagulation therapy. She was successfully weaned off the Impella RP on post-operative day 4 and was extubated on day 5. She was discharged on day 16 in a stable condition.
Discussion
Incident venous thrombo-embolism is frequently encountered in COVID-19 patients. We report the first case of a large intracardiac thrombus associated with SARS-CoV-2 infection managed successfully with percutaneous thrombectomy and right ventricular mechanical circulatory support.
Keywords: Case report, COVID-19, Thrombus, Aspiration thrombectomy, Percutaneous, Impella
Learning points
Cardiovascular complications, particularly venous and arterial thrombosis, are frequently reported in coronavirus disease 2019 (COVID-19).
This is the first unusual case of a large intracardiac in situ thrombus in a COVID-19 patient.
Percutaneous mechanical thrombectomy has a potential role in emergent aspiration of large right heart thrombus.
Right ventricular mechanical circulatory support can be beneficial in management of acute decompensated right heart failure with cardiogenic shock.
Introduction
Coronavirus disease 2019 (COVID-19) is a systemic infectious disease caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It has caused a global pandemic, with >6 million infected and 360 000 deaths worldwide as of 30 May 2020.1,2 Although SARS-CoV-2 infection primarily affects the lower respiratory tract system, emerging reports suggest direct and indirect cardiovascular complications including acute myocardial injury, cardiomyopathy, arrhythmias, and acute coronary syndrome.3,4 Furthermore, severe inflammatory state, hypoxia, and immobilization in COVID-19 patients impose a high risk of venous thrombo-embolism (VTE).5–7 We report a case of a large right heart thrombus leading to right ventricular (RV) failure in a 57-year-old female with SARS-CoV-2 infection, that was successfully managed with percutaneous aspiration thrombectomy and RV mechanical circulatory support.
Timeline
| Day 0 | Patient admitted with acute hypoxic respiratory failure and shock. |
| Day 1 | Transthoracic echocardiogram showed a large mobile mass in the right atrium. Given the state of shock and the large thrombus burden, emergent aspiration of an organized thrombus was performed using an AngioVac cannula. An Impella RP was placed due to decompensated right ventricular (RV) failure post-thrombectomy. |
| Day 2 | Significant improvement in patient’s haemodynamics and mixed venous oxygen, with successful weaning of vasopressors. PCR for SARS-CoV2 infection came back positive and the patient started on antimicrobial therapy. |
| Day 3 | Repeat echocardiogram consistent with improvement in RV systolic function. |
| Day 4 | Patient was weaned off the Impella RP and it was removed at the bedside. |
| Day 5 | Renal parameters deteriorated with anuria. A temporary haemodialysis catheter was inserted for initiation of renal replacement therapy. |
| Day 6 | Patient was weaned off mechanical ventilation and extubated. Intermittent haemodialysis was started. |
| Day 14 | Improvement in urine output with return of renal functions to baseline. No further requirement for haemodialysis. |
| Day 23 | Patient discharged home in stable condition with home health services. |
Case presentation
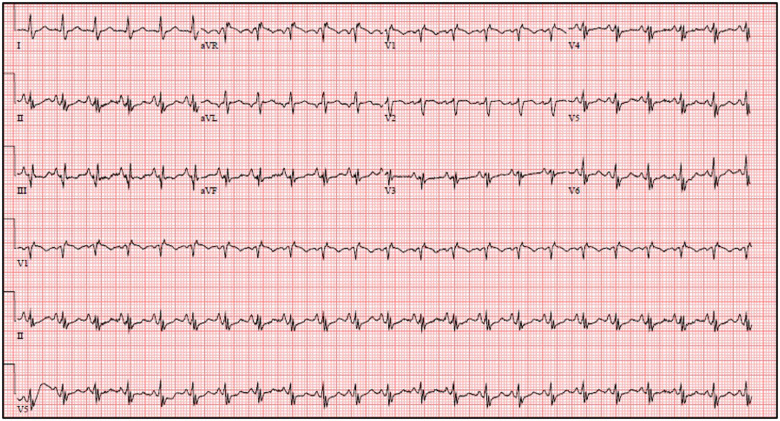
A 57-year-old female with bronchial asthma presented to the emergency department (ED) with a 1-week history of worsening shortness of breath, fever, and intermittent nausea. She also reported left lower extremity pain and swelling. Her husband had similar symptoms. On initial evaluation, she was hypoxic and tachycardic. Her blood pressure was 90/67 mmHg, heart rate 111 b.p.m., and saturation 96% on 4 L of oxygen through a nasal cannula. Physical examination was significant for clear breath sounds and left leg oedema. ECG showed sinus tachycardia, incomplete right bundle branch block, and Q waves in lead III (Figure 1). Laboratory work-up was pertinent for leukocytosis of 27.35 × 109/μL, absolute lymphocyte count of 0.27 × 109/μL, creatinine 2.2 mg/dL, lactic acid 6.8 mmol/L, troponin-T 0.21 ng/mL, probrain natriuretic peptide (pro-BNP) 8969 pg/mL, C-reactive protein (CRP) 266.9 mg/L, and ferritin 3233 ng/mL. Chest X-ray did not show any acute cardiopulmonary pathology. A rapid COVID test in the ED was negative. A venous duplex scan of the left leg revealed a large occlusive thrombus in the left femoral and popliteal veins. Transthoracic echocardiogram showed a large mobile filling defect in the right atrium (RA) partially prolapsing into a dysfunctional right ventricle with positive McConnell’s sign (Figure 2; Supplementary material online, Video S1). Left ventricular ejection fraction was preserved. The patient was started on levophed infusion. Given that the patient was in shock with an imminent risk of a catastrophic event in the case of embolization of the right heart mass, the decision was made to proceed with emergent percutaneous aspiration with the Angio-Vac system (Angiodynamics Inc., Latham, NY, USA) rather than intravenous or catheter- directed thrombolysis which would have minimal effect on a large thrombus.
Figure 1.
12-lead ECG showing sinus tachycardia, incomplete right bundle branch block, and Q waves in lead III.
Figure 2.
Apical four-chamber view of the trans-thoracic echocardiogram showing a large mobile mass (red arrow) in the right atrium partially prolapsing into the right ventricle (A). Short-axis view of the intraoperative transoesophageal echocardiogram confirmed the mass (yellow arrow) (B). Cine-angiogram showing aspiration of the mass with the Angiovac cannula (C). An organized thrombus (green arrow) extracted from the right heart (D).
The patient was intubated prior to the procedure. Intra-operative transoesophageal echocardiogram (TOE) confirmed a large mobile mass in the RA (Figure 2). The right internal jugular (IJ) vein and common femoral vein (CFV) were cannulated under ultrasound guidance. After performing right heart catheterization and pulmonary angiography (Supplementary material online, Video S2), a 24 Fr dry-seal sheath was advanced into the RA through the right IJ vein for introduction of a suction cannula. A 19 Fr extracorporeal membrane oxygenation (ECMO) cannula was placed in the upper inferior vena cava through the right CFV for reinfusion of blood. The AngioVac catheter was then advanced through the right IJ sheath and multiple runs of aspiration were performed with extraction of a large organized thrombus (Figure 2; Supplementary material online, Video S3). TOE showed resolution of the thrombus; however, embolization of small thrombi was noted in the right ventricle and pulmonary artery. Post-thrombectomy, the patient further decompensated, with systolic blood pressure dropping to 50 mmHg and heart rate to ∼40 b.p.m. CPR was initiated and maintained for 5 min with the standard ACLS protocol. Repeat haemodynamics showed elevated RA pressures of 20 mmHg (baseline 12 mmHg) and mixed venous oxygen (MVO2) of 42% along with an akinetic right ventricle on TOE, findings consistent with acute RV failure. At this time, the 19 Fr cannula in the right CFV was upgraded to a 24 Fr sheath, and an Impella RP (Abiomed, Danvers, MA, USA) was inserted in the pulmonary artery for RV support (Figure 2). The arterial blood pressure waveform showed an immediate increase in systolic blood pressure from <50 mmHg to >120 mmHg after Impella placement, as demonstrated in Supplementary material online, Video S4. Also, there was significant improvement in MVO2. The right IJ cannula was removed and the patient was transferred to the intensive care unit (ICU) in stable condition.
COVID PCR was reported positive the following day, and the patient was started on hydroxychloroquine and azithromycin. She was anticoagulated with intravenous heparin. Further hypercoagulability and malignancy work-up was planned. She remained haemodynamically stable with consistent improvement in her MVO2 and RV function on post-operative days 1 and 2. Repeat echocardiogram showed resolution of the thrombus and improvement in RV contractility (Figure 3). Her further ICU course was essentially uncomplicated besides acute renal failure requiring temporary haemodialysis. She was successfully weaned off the Impella and it was subsequently removed on post-operative day 4. The patient was extubated on day 5 and transferred to a routine ward. She was eventually discharged in a stable condition with home care on day 23 of hospitalization.
Figure 3.
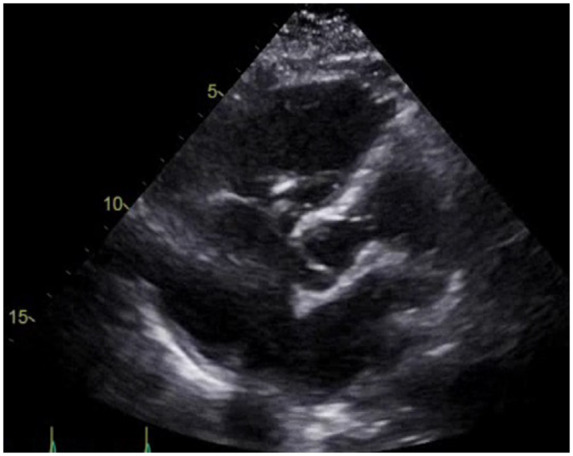
Apical five-chamber view of the post-operative transthoracic echocardiogram showing resolution of the thrombus in the right atrium.
Discussion
Patients with COVID-19 and cardiovascular complications have been shown to have worse outcomes.8,9 Incident VTE is frequently encountered in these patients. In a multicentre study from the Netherlands, the incidence of VTE was reported to be in 31% of COVID-19 patients admitted to the ICU.6 In another study conducted in Wuhan, China, VTE was reported in 25%.10 The proposed mechanism of accelerated thrombosis is hypercoagulability induced by severe inflammation, endothelial dysfunction, and stasis.11,12 Several laboratory parameters support this hypothesis characterized by elevations in fibrinogen and D-dimer.13 Some patients with severe COVID-19 infection can develop thrombosis from disseminated intravascular coagulation (DIC). Interleukin-6 (IL-6) levels may also correlate with disease severity and a procoagulant profile.14
Large vessel and intracardiac thrombus have not yet been reported in a COVID-19 patient. Our patient had a large occlusive deep vein thrombosis (DVT) and right atrial thrombosis. She was in clinical shock with acute respiratory failure; thus, CT angiography of the chest could not be performed. Intra-operative TOE and pulmonary angiogram did show evidence of small thrombi in the main pulmonary artery. We decided to proceed with percutaneous thrombectomy as there was imminent risk of a catastrophic outcome without any intervention. The Angiovac system which was used is a vacuum-based device, FDA-approved in 2014 for percutaneous drainage of undesirable material such as fresh thrombi or emboli. It is composed of a venous drainage and a reinfusion cannula connected to an extracorporeal circuit and bypass pump. When the pump is started, suction force is created, facilitating aspiration of thrombotic material. Our patient had profound hypotension and bradycardia post-aspiration thrombectomy. Distal embolization of the thrombus to the pulmonary vasculature causing acute RV failure is a likely explanation. Similarly, intra-operative TOE confirmed severely reduced RV systolic function. Although there is a paucity of data regarding use of the Impella RP in cardiogenic shock and acute RV failure from pulmonary thrombo-embolism, we decided to place RV mechanical support due to acute decompensation post-thrombectomy. The early initiation of support helped in rapid stabilization of the haemodynamics as well as in preventing irreversible RV damage.
Herein, we report the first case of a large intracardiac in situ thrombus associated with SARS-CoV-2 infection. Our case illustrated not only the potential value of the Angiovac device in the management of this case, but also the potential of mechanical RV support in the management of associated right heart decompensation and cardiogenic shock.
Lead author biography

Amir Kaki, MD is an Interventional Cardiologist and Director of Mechanical Circulatory Support/High risk coronary interventions at Ascension St John Hospital and Medical Center in Detroit, Michigan, USA. His areas of interest include cardiogenic shock and large bore vascular access management.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: A.K. is a speaker and proctor for Abiomed. The other authors have no financial or proprietary interest in the subject matter of this article.
Supplementary Material
References
- 1.The Center for Systems Science and Engineering (CSSE) at John Hopkins University. https://coronavirus.jhu.edu/map.html
- 2.COVID-19 information dashboard from World Health Organization (WHO). www.who.int/news-room/feature-stories/detail/who-updates-covid-19-dashboard-with-better-data-visualization
- 3. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B.. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y.. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 2020;109:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.,Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis T, Politou M, Psaltopoulou T, Gerotziafas G, Dimopoulos MA.. Hematological findings and complications of COVID-19. Am J Hematol 2020;95:834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klok F, Kruip M, van der Meer N, Arbous M, Gommers D, Kant K, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H.. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A.. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 2020;18:1738–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng YY, Ma YT, Zhang JY, Xie X.. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020;17:259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Driggin E, Madhavan M, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, Brown TS, Der Nigoghossian C, Zidar DA, Haythe J, Brodie D, Beckman JA, Kirtane AJ, Stone GW, Krumholz HM, Parikh SA.. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol 2020;75:2352–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cui S, Chen S, Li X, Liu S, Wang F.. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020;18:1421–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marongiu F, Mameli A, Grandone E, Barcellona D.. Pulmonary thrombosis: a clinical pathological entity distinct from pulmonary thrombosis? Semin Thromb Hemost 2019;45:778–783. [DOI] [PubMed] [Google Scholar]
- 12. Libby P, Simon DI.. Inflammation and thrombosis: the clot thickens. Circulation 2001;103:1718–1720. [DOI] [PubMed] [Google Scholar]
- 13. Lippi G, Plebani M.. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med 2020;58:1131–1134. [DOI] [PubMed] [Google Scholar]
- 14. Han H, Yang L, Liu R, Liu F, Wu K, Li J, Liu XH, Zhu CL.. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med 2020;58:1116–1120. [DOI] [PubMed] [Google Scholar]
- 15. Starck C, Dreizler T, Falk V.. The AngioVac system as a bail-out option in infective valve endocarditis. Ann Cardiothorac Surg 2019;8:675–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.