Abstract
Aims
The main severe complications of SARS-CoV-2 infection are pneumonia and respiratory distress syndrome. Recent studies, however, reported that cardiac injury, as assessed by troponin levels, is associated with a worse outcome in these patients. No study hitherto assessed whether the simple standard electrocardiogram (ECG) may be helpful for risk stratification in these patients.
Methods and results
We studied 324 consecutive patients admitted to our Emergency Department with a confirmed diagnosis of SARS-CoV-2 infection. Standard 12-lead ECG recorded on admission was assessed for cardiac rhythm and rate, atrioventricular and intraventricular conduction, abnormal Q/QS wave, ST segment and T wave changes, corrected QT interval, and tachyarrhythmias. At a mean follow-up of 31 ± 11 days, 44 deaths occurred (13.6%). Most ECG variables were significantly associated with mortality, including atrial fibrillation (P = 0.002), increasing heart rate (P = 0.002), presence of left bundle branch block (LBBB; P < 0.001), QRS duration (P <0 .001), a QRS duration of ≥110 ms (P < 0.001), ST segment depression (P < 0.001), abnormal Q/QS wave (P = 0.034), premature ventricular complexes (PVCs; P = 0.051), and presence of any ECG abnormality [hazard ratio (HR) 4.58; 95% confidence interval (CI) 2.40–8.76; P < 0.001]. At multivariable analysis, QRS duration (P = 0.002), QRS duration ≥110 ms (P = 0.03), LBBB (P = 0.014) and presence of any ECG abnormality (P = 0.04) maintained a significant independent association with mortality.
Conclusion
Our data show that standard ECG can be helpful for an initial risk stratification of patients admitted for SARS-CoV-2 infectious disease.
Keywords: SARS-CoV-2 infection, Electrocardiogram, Mortality
Graphical Abstract
Graphical Abstract.
What’s new
We report for the first time data on the prognostic role of standard electrocardiogram (ECG) performed on admission in hospitalized patients with a diagnosis of SARS-CoV-2 infection
Our data show that the presence of any abnormal ECG finding on standard ECG was independently associated with increased mortality [hazard ratio (HR) 2.09; 95% confidence interval (CI) 1.04–4.23; P = 0.04] at a mean follow-up of 31 ± 11 days.
Individual ECG variables independently associated with mortality included presence of left bundle branch block and prolonged QRS duration.
Introduction
A novel enveloped non-segmented RNA coronavirus, named 2019-nCoV or SARS-CoV-2, is causing a pandemic outbreak of respiratory disease.1,2 Although mild or self-limiting respiratory tract illness and bilateral interstitial pneumonia are the main clinical presentations of SARS-CoV-2 infection, up to 30% of patients may suffer from acute respiratory distress syndrome, multiorgan failure, and death.3,4 Severe complications more frequently occur in male elderly subjects, in particular in those with any comorbidity, including hypertension, diabetes, and cardiovascular disease.3–7
At present there are limited data about the effects of SARS-CoV-2 infection on the cardiovascular system, but a few studies have reported that cardiac injury, as assessed by increased serum levels of cardiac troponins, occurs in ∼20% of hospitalized patients and is associated with increased short-term mortality.8–12
The electrocardiogram (ECG) is an easy tool to identify patients with acute or chronic cardiac disease through various abnormal findings, including ST-segment and T wave changes, electrical conduction disorders, and tachyarrhythmias. Yet, to the best of our knowledge, no study has hitherto assessed the prevalence and clinical implications of ECG abnormalities in patients with SARS-CoV-2 infection.
Methods
We studied consecutive patients admitted to the Emergency Department of our University Hospital (Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy) between 1 March and 15 April 2020, who were diagnosed to have SARS-CoV-2 infection and underwent standard ECG recording on admission. Patients were excluded if they were younger than 20 years or their ECG showed a pacemaker rhythm.
All patients had been referred because of fever which was unresponsive to antipyretic drugs and had symptoms consistent with SARS-CoV-2 infection (e.g. cough, dyspnoea, tachypnoea, and sputum production).
The demographic characteristics (age and sex), clinical data, and laboratory findings on admission were acquired from our Institutional database. When available, cardiac troponin I (cTnI) and N-terminal probrain natriuretic peptide (NT-proBNP) serum levels were also measured. The upper normal level for cTnI for our laboratory was 39 ng/L, whereas the upper normal level for NT-proBNP was 450 pg/mL.
A history of known heart disease included any evidence of ischaemic heart disease (previous myocardial infarction, coronary revascularization, and/or documented obstructive coronary artery disease at angiography), heart failure, moderate to severe valvular heart disease, or cardiomyopathy, as documented in the patient’s clinical reports. The presence of pre-existing comorbidities, including chronic obstructive pulmonary disease (Gold stage 3–4), severe renal failure (stage 4–5), stroke in the previous 12 months, chronic inflammatory disease, and neoplasia, was also recorded.
SARS-CoV-2 testing was based on the protocol released by the World Health Organization (WHO).13 Nasopharyngeal swab specimens were collected in all patients, detecting SARS-CoV-2 RNA by reverse transcription–polymerase chain reaction (RT–PCR). Most patients underwent chest X-ray or thoracic computed tomography (CT) scan to confirm the diagnosis. The study was approved by the Ethics Committee of our Institution.
ECG analysis and definitions
Twelve-lead standard ECGs were recorded on admission with a Mortara ELI 350 ECG machine (Mortara Instrument Europe, Bologna, Italy). The ECGs were retrieved by our dedicated Institutional ECG storage server (Mortara X-scribe; Mortara S.p.A. Bologna, Italy), and independently analysed by two trained fellows in cardiology (A.D.V. and S.E.R.). Discordances were solved by consensus, with the supervision of the senior expert in electrocardiography (G.A.L.). The ECGs were analysed before proceeding with assessment of clinical outcome, that was therefore unknown to the ECG readers.
ECGs were analysed for the following parameters: rhythm, presence of atrioventricular blocks, complete right (RBBB) or left (LBBB) bundle branch block, ST-segment and/or T wave abnormalities suggesting myocardial ischaemia or injury, and abnormal Q/QS waves typical of a previous myocardial infarction. The presence of premature supraventricular (PSVCs) and ventricular (PVCs) complexes was also recorded.
Heart rate, PR and QT intervals, and QRS duration were derived from the automatic measurements by the Mortara X-Scibe software, after checking for the reliability of the measurements. Patients with LBBB were excluded from ST-segment and T wave analyses, whereas in patients with RBBB, only leads V1–V4 were excluded from ST-segment and T wave analysis. ST-segment depression (STD) was diagnosed when a horizontal or downsloping displacement of the ST segment below the isoelectric line of ≥0.5 mm, persisting at 0.08 s from the J point, was detectable in at least two contiguous leads. A separate analysis was also conducted considering as abnormal an STD of ≥1 mm. ST-segment elevation (STE) was diagnosed when the J point was elevated by ≥1 mm, the ST-segment was still elevated after 0.08 s, and morphology was judged to be compatible with an ischaemic or pericarditis origin. An abnormal T wave was diagnosed in the case of T wave inversion ≥1 mm in at least two contiguous leads (except V1 and aVR). The QT interval was corrected for heart rate (cQT) using Bazett’s formula [cQT = QT(ms)/√RR(s)]. In patients with intraventricular conduction defects (i.e. QRS duration ≥120 ms), however, the cQT was calculated using the formula validated by Rautaharju et al. [cQTRR-QRS = QT – 155 × (60/HR – 1) – 0.93 × (QRS – 139) + k], where k = –22 ms for men and –34 ms for women.14
The presence of any ECG abnormality was defined as the presence of one or more of the following abnormalities: non-sinus rhythm; ≥II degree atrioventricular block; QRS duration ≥110 ms, STD or STE, negative T wave, cQT ≥460 in women or ≥450 in men15 pathological Q/QS waves; and presence of PSVCs or PVCs.
Clinical outcome
The primary endpoint of the study was total mortality. The vital status of patients was determined at 26 April 2020 by consulting the clinical database of our hospital. Discharged patients were censored at the date of discharge.
Statistical analysis
Data are reported as mean with standard deviation for continuous variables and number and proportions for discrete variables. Due to asymmetrical distribution of values, C-reactive protein, cTnI and NT-proBNP serum levels are reported as median with interquartile range. The association of individual clinical and ECG variables with death was assessed by univariable survival Cox regression analysis. Multivariable Cox regression was first performed on clinical variables, including in the model only those with a P-value ≤0.1 and leaving in the final model only those with P < 0.05. Then ECG variables with a P-value of ≤0.1 at univariable analysis were individually entered in the model to assess their independent association with outcome. When indicated, proportions were compared by Fisher exact test.
Since cTnI and NT-proBNP were available only for a minority of patients (see later), they necessarily could not be included in the main statistical analyses. However, we separately assessed whether the ECG also added prognostic information when values of these prognostic biomarkers were known.
A P < 0.05 was always required for statistical significance. Data were analysed by the SPSS 21.0 statistical software (SPSS Italia, Inc., Florence, Italy).
Results
General results
A flow chart of patients’ enrolment is shown in Figure 1. Overall, 502 patients referred to the Emergency Department of our Hospital during the period of the study had a diagnosis of SARS-CoV-2 infection. ECG was not available in 172 patients (34.3%), who were either discharged home without performing an ECG (n = 52) or were hospitalized but the ECG was not recorded on admission (n = 120). Thus, 330 patients (65.7%) with a confirmed diagnosis of SARS-CoV-2 infection who were hospitalized and had a standard ECG performed on admission were considered for the study. Six patients (1.8%) were excluded, however, because of a pacemaker rhythm, which did not allow a reliable assessment of the ECG parameters considered for the study (see below). Therefore, the study population eventually included 324 SARS-CoV-2 patients. The main clinical findings of these patients are summarized in Table 1. As shown, cTnI and NT-proBNP were available for 43 (13.3%) and 66 (20.4%) patients only. An SpO2 <90% was present in 74 patients (22.8%).
Figure 1.
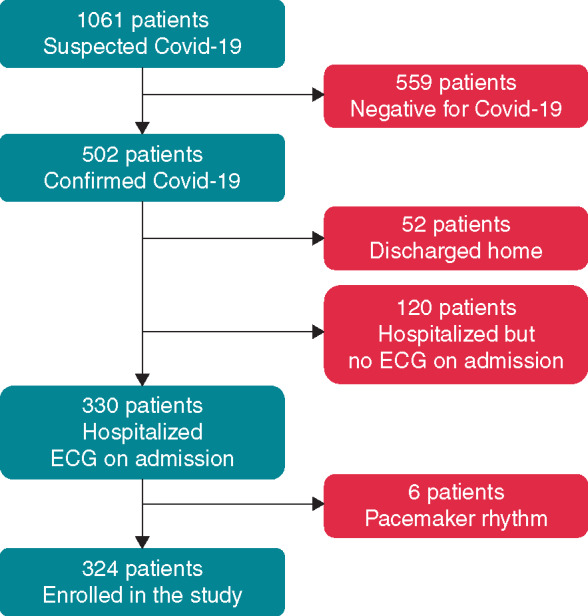
Flow chart of patients’ enrolment. Covid-19, SARS-CoV-2 disease.
Table 1.
Main clinical findings of SARS-CoV-2 patients included in the study
| n = 324 | |
|---|---|
| Age (years) | 65.9 ± 15.2 |
| Male sex | 214 (66%) |
| Known heart disease | 67 (20.7%) |
| Comorbidity | 102 (31.5%) |
| Hypertension | 169 (52.2%) |
| Diabetes mellitus | 37 (11.4%) |
| Dyslipidaemia | 67 (20.7%) |
| Smoking | 86 (26.8%) |
| Haemoglobin (g/dL) | 13.8 ± 1.7 |
| Creatinine (mg/dL) | 1.21 ± 1.33 |
| C-reactive protein (mg/L)a | 77 (26–152) |
| NT-proBNP (pg/mL)a,b | 799 (258–1760) |
| Elevated NT-proBNPb | 34 (51.5%) |
| Cardiac troponin I (ng/L)a,c | 9 (3–34) |
| Elevated cardiac troponin Ic | 9 (20.9%) |
| Systolic blood pressure (mmHg) | 127.1 ± 21.3 |
| Diastolic blood pressure (mmHg) | 76.3 ± 13.1 |
| SpO2 (%) | 91.9 ± 7 |
NT-proBNP, N-terminal probrain natriuretic peptide; SpO2, peripheral capillary oxygen saturation.
Values are reported as median (interquartile range).
Data are available for 66 patients only.
Data are available for 43 patients only.
ECG findings
The main ECG findings of patients are summarized in Table 2. A normal sinus rhythm was present in 93.8% of patients, whereas atrial fibrillation was detected in 6.2%. No major atrioventricular blocks were detected. LBBB and RBBB were present in 6 (1.9%) and 25 (7.7%) patients, respectively. A significantly lengthened cQT was found in 18 patients only (5.6%). The prevalence of tachyarrhythmias was also low, as PSVCs were detected in 3.6% of patients only and PVCs in 4%. STD (≥0.5 mm) and T wave inversion (≥1 mm) were found in 17 (5.3%) and 13 (4.1%) patients, respectively, among 318 patients without LBBB. No patient had pathological STE. Any abnormal finding on the ECG was found in 120 patients (37%).
Table 2.
Main electrocardiographic findings of SARS-CoV-2 patients.
| n = 324 | |
|---|---|
| Rhythm | |
| Sinus rhythm | 304 (93.8%) |
| Atrial fibrillation/flutter | 20 (6.2%) |
| Heart rate, b.pm. | 82.4 ± 18 |
| cQT interval, ms | 407 ± 29 |
| Prolonged cQT interval | 18 (5.6%) |
| AV conduction | |
| First-degree AV block | 21 (6.9%) |
| Intraventricular conduction | |
| Left bundle branch block | 6 (1.9%) |
| Right bundle branch block | 25 (7.7%) |
| Premature supraventricular complexes | 11 (3.6) |
| Premature ventricular complexes | 13 (4%) |
| ST-segment/T wave abnormalities | |
| ST-segment depression ≥0.5 mm | 17 (5.3%) |
| ST-segment depression ≥1 mm | 4 (1.3%) |
| T-wave inversion ≥1 mm | 13 (4.1%) |
| Abnormal Q/QS waves | 14 (4.4%) |
| Any ECG abnormality | 120 (37%) |
AV, atrioventricular; cQT, corrected QT.
Clinical outcome
Overall, 44 deaths (13.6%) occurred during a follow-up of 31 ± 11 days (range 2–52). At the date of follow-up, only 61 patients (18.8%) were still hospitalized, whereas 219 (67.6%) had been discharged. Deaths occurred more frequently in patients subsequently admitted to the intensive care unit (ICU; 27 out of 79 patients, or 34.2%) compared with those not admitted to the ICU (17 out of 245 patients, or 6.9%; P < 0.001).
Clinical and ECG data of dead vs. alive patients, with results of univariable Cox regression analysis, are summarized in Table 3. Clinical variables associated with death included age, previous heart disease, comorbidity, hypertension, hypercholesterolaemia, serum creatinine, C-reactive protein, lower blood pressure values, and peripheral capillary oxygen saturation (SpO2).
Table 3.
Main clinical and ECG findings of patients who were still alive or had died
| Dead (n = 44) | Alive (n = 280) | HR | 95% CI | P-value | |
|---|---|---|---|---|---|
| Clinical findings | |||||
| Age, years | 77.8 ± 9 | 64.1 ± 15 | 1.08 | 1.05–1.10 | <0.001 |
| Male sex (%) | 34 (77.3) | 180 (64.3) | 1.79 | 0.89–3.63 | 0.11 |
| Known heart disease (%) | 17 (38.6) | 50 (17.9) | 2.67 | 1.46–4.91 | 0.002 |
| Comorbidity (%) | 27 (61.4) | 75 (26.8) | 4.07 | 2.22–7.48 | <0.001 |
| Hypertension (%) | 36 (81.8) | 133 (47.5) | 4.60 | 2.14–9.89 | <0.001 |
| Diabetes mellitus (%) | 5 (11.4) | 32 (11.4) | 1.01 | 0.40–2.57 | 0.98 |
| Dyslipidaemia (%) | 16 (36.4) | 51 (18.2) | 2.51 | 1.36–4.64 | 0.003 |
| Smoking (%) | 12 (27.3) | 74 (26.7) | 1.00 | 0.52–1.95 | 0.99 |
| Haemoglobin, g/dL | 13.2 ± 2.1 | 13.9 ± 1.6 | 0.81 | 0.69–0.96 | 0.013 |
| Creatinine, mg/dL | 1.84 ± 1.5 | 1.09 ± 1.3 | 1.17 | 1.06–1.28 | 0.001 |
| C-reactive protein, mg/La | 153.1 (90–189) | 62.5 (23–139) | 1.01 | 1.00–1.01 | <0.001 |
| SBP, mmHg | 119.1 ± 20 | 128.4 ± 21 | 0.98 | 0.96–0.99 | 0.008 |
| DBP, mmHg | 72.3 ± 15 | 77 ± 13 | 0.97 | 0.95–1.00 | 0.023 |
| SpO2, % | 85.8 ± 9.6 | 92.9 ± 5.9 | 0.93 | 0.91–0.95 | <0.001 |
| cTnI, ng/La,b | 56 (22–319) | 7.5 (3–17) | 1.01 | 1.00–1.01 | 0.010 |
| Elevated cTnIb | 4 (57.1) | 5 (13.9) | 6.89 | 1.53–31.0 | 0.012 |
| NT-proBNP, pg/mLa,c | 1470 (730–5150) | 535 (99–1213) | 1.00 | 1.00–1.00 | <0.001 |
| Elevated NT-proBNPc | 21 (91.3) | 23 (53.5) | 6.98 | 1.63–29.8 | 0.009 |
| ECG findings | |||||
| Heart rate, b.p.m. | 89.6 ± 25 | 81.3 ± 17 | 1.02 | 1.01–1.04 | 0.002 |
| Atrial fibrillation (%) | 7 (15.9) | 13 (4.6) | 3.51 | 1.57–7.88 | 0.002 |
| PR interval, ms | 165 ± 28 | 159.4 ± 31 | 1.00 | 1.00–1.01 | 0.33 |
| LBBB (%) | 4 (9.1) | 2 (0.7) | 9.48 | 3.37–26.6 | <0.001 |
| RBBB (%) | 6 (13.6) | 19 (6.8) | 2.02 | 0.85–4.78 | 0.11 |
| QRS, ms | 110.5 ± 29 | 97.3 ± 15 | 1.03 | 1.02–1.04 | <0.001 |
| QRS ≥110 ms (%) | 17 (38.6) | 41 (14.6) | 3.23 | 1.76–5.92 | <0.001 |
| cQT interval | 408 ± 36 | 407 ± 28 | 1.00 | 0.99–1.01 | 0.89 |
| Abnormal cQT (%) | 4 (9.1) | 14 (5) | 1.78 | 0.64–4.97 | 0.27 |
| PSVCs (%) | 2 (5.4) | 9 (3.4) | 1.64 | 0.39–6.80 | 0.50 |
| PVCs (%) | 4 (9.1) | 9 (3.2) | 2.79 | 1.00–7.79 | 0.051 |
| STD ≥0.5 mm | 7 (17.5) | 10 (3.6) | 4.66 | 2.06–10.6 | <0.001 |
| T-wave inversion ≥1 mm | 3 (7.5) | 10 (3.6) | 2.20 | 0.68–7.14 | 0.19 |
| ST/T abnormalities | 10 (25) | 18 (6.5) | 2.94 | 1.15–7.51 | 0.024 |
| Abnormal Q/QS wave | 4 (10) | 10 (3.6) | 3.05 | 1.09–8.58 | 0.034 |
| Any ECG abnormality | 31 (70.5) | 89 (31.8) | 4.58 | 2.40–8.76 | <0.001 |
cTnI, cardiac troponin I; NT-proBNP, N-terminal probrain natriuretic peptide; SBP, systolic blood pressure; DBP, diastolic blood pressure; SpO2, peripheral capillary oxygen saturation; LBBB, left bundle branch block; RBBB, right bundle branch block; cQT, corrected QT interval; PSVCs, premature supraventricular complexes; PVCs, premature ventricular complexes; STD, ST-segment depression.
Median (interquartile range).
Data are available for 7 dead and 36 living patients, respectively.
Data are available for 23 dead and 43 living patients, respectively.
Most ECG variables were also associated with death, including atrial fibrillation (P = 0.002), increasing heart rate (P = 0.002), presence of LBBB (P < 0.001), QRS duration (P < 0.001), a QRS duration ≥110 ms (including patients with LBBB or RBBB; P < 0.001), STD (P < 0.001), abnormal Q/QS wave (P = 0.034), and PVCs (P = 0.051). STD ≥1 mm was also associated with death (P = 0.031), but it was found in only 4 patients (1.26%), 2 in those still alive (0.7%) and 2 in patients who had died (5%), and was not considered in further analyses. Finally, the detection of any ECG abnormality was also significantly associated with death (P < 0.001).
Multivariable analysis
Clinical and laboratory variables associated with death at multivariable survival Cox regression analysis included age (HR 1.08; 95% CI 1.05–1.11; P < 0.001), SpO2 (HR 0.95; 95% CI 0.92–0.98; P < 0.001), creatinine (HR 1.27; 95% CI 1.10–1.47; P = 0.001), C-reactive protein (HR 1.01; 95% CI 1.00–1.01; P = 0.004), and comorbidity (HR 1.92; 95% CI 0.89–4.14; P = 0.02).
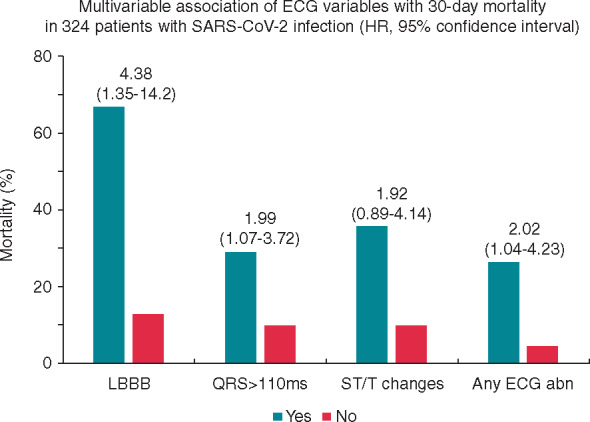
When added to these variables, QRS duration as a continuous variable (HR 1.02; 95% CI 1.01–1.03; P = 0.002), QRS duration ≥110 ms (HR 1.99; 95% CI 1.07–3.72; P = 0.03), and LBBB (HR 4.38; 95% CI 1.35–14.2; P = 0.014) maintained independent association with mortality, with STD/T wave inversion being of borderline statistical significance (HR 1.92; 95% CI 0.89–4.14; P = 0.09). Furthermore, the presence of any ECG abnormality was also independently associated with fatal outcome (HR 2.09; 95% CI 1.04–4.23; P = 0.04) (Figure 2).
Figure 2.
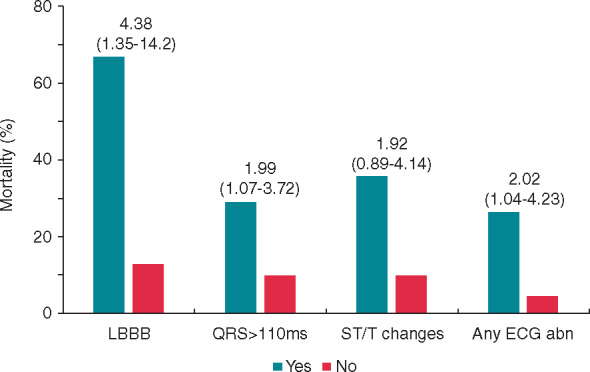
The graph shows 30-day mortality of patients with (Yes) or without (No) some dichotomous abnormalities detected at standard electrocardiogram (ECG) on admission. Multivariable association of ECG variables with mortality (hazard ratio with 95% confidence interval) is reported above the columns. Variables used in the multivariable model included age, comorbidities, SpO2, C-reactive protein, and creatinine levels. Abn, abnormality; LBBB, left bundle branch block.
ECG findings and biomarkers
Among 43 patients with cTnI measurement on admission, 7 deaths were recorded (16.3%). cTnI levels were significantly associated with death (Table 3). Both QRS duration (HR 1.05; 95% CI 1.01–1.08; P = 0.013) and QRS duration ≥110 ms (HR 7.18; 95% CI 1.44–35.9; P = 0.016) still showed significant association with death when adjusted for cTnI levels in this small group of patients.
Among 66 patients with available NT-proBNP levels, 23 deaths occurred (34.8%). Levels of NT-proBNP were significantly associated with death (Table 3). QRS duration (HR 1.03; 95% CI 1.01–1.05; P < 0.001), QRS duration ≥110 ms (HR 3.24; 95% CI 1.40–7.51; P = 0.006), and LBBB (HR 3.62; 95% CI 1.07–12.3; P = 0.039) still showed significant association with death when adjusted for NT-proBNP levels also in this small subgroup of patients.
Discussion
Our data show that the simple standard ECG, easily obtainable on admission, can be helpful to identify patients with a worse short-term clinical outcome among those admitted to the emergency room for a SARS-CoV-2 infection. Most ECG abnormalities were significantly associated with death at univariable analysis and, most importantly, some ECG findings maintained an independent association with outcome in multivariable analysis including independent significant clinical and laboratory variables.
Specifically, ECG variables independently associated with outcome were those indicating a delay in ventricular depolarization, i.e. QRS lengthening and, in particular, the presence of LBBB. Patients with LBBB, in fact, were the group with the highest mortality (67%) among our patients, with a more than four-fold increase of the risk despite the fact that it was present in only six patients (1.85%). Importantly, ECG variables maintained an independent association with death even after correction for cTnI and NT-proBNP in the small subgroups of patients in which these biomarkers of myocardial involvement were obtained.
The dramatic pandemic SARS-CoV-2 infection has resulted in a rather high mortality, in particular in some regions of the world (e.g. northern Italy, the USA, and Spain)16 and some subgroups of patients (e.g. the elderly, with cardiovascular disease or other relevant comorbidity).5,6,17,18 Interstitial pneumonia and acute respiratory distress syndrome are the most ominous complications in these patients.3,4 Some studies, however, have suggested that a sizeable number of SARS-CoV-2 patients have evidence of cardiac injury, as suggested by increased levels of troponin, and this is associated with a relevant increase of fatal events.9–11
No previous study, however, assessed whether the simple recording of standard ECG might also be helpful for risk stratification of these patients, despite the fact that ECG can be obtained rapidly and is known to give relevant information about the presence of cardiac abnormalities. Of note, in a study reporting on the prognostic value of myocardial injury in SARS-CoV-2 infection, the few patients with a troponin increase who also underwent ECG recording all showed significant ST-segment/T wave changes that already suggested a cardiac involvement.9
Importantly, the ECG may help stratify patients not only by revealing acute changes, such as ST-segment/T wave abnormalities or possible new arrhythmias or conduction disorders, but also by showing chronic abnormalities suggesting an underlying cardiac disease, which has also been reported to predict a negative outcome in these patients.3–7 Our study revealed a rather low prevalence of individual ECG abnormalities in SARS-CoV-2 patients, at least at the early assessment in the emergency room, although, on the whole, 37% of patients showed some abnormal ECG finding. The meaning of the abnormalities detected in our patients could not be ascertained as we did not have previous ECGs for comparison. Accordingly, ECG abnormalities might have been pre-existing prior to admission, and, therefore, be a clue to pre-existing cardiac disease, or may have occurred as a result of the acute infective status, either as a direct cardiac involvement in the infective/inflammatory process or as a consequence of the severe respiratory and stressful conditions of patients, particularly in those with underlying heart disease.
Notably, our data confirm that elderly patients with worse clinical conditions, as suggested by the presence of known heart disease, comorbidity, and hypertension, are at higher risk of death. In our population, however, age, impaired peripheral oxygenation, impaired renal function, grade of the inflammatory reaction (as assessed by C-reactive protein serum levels), and presence of comorbidity were the clinical variables independently associated with outcome. Furthermore, in agreement with previous reports,9,10 our data also show that higher levels of cTnI and NT-proBNP are predictive of a negative outcome. However, due to our hospital policy to measure these markers only in selected patients, according to clinical indications, they were available in only a minority of patients, and, therefore, they could not be adequately assessed in our study.
Limitations of the study
Some limitations of our study should be acknowledged. First, several single ECG abnormalities were detected in small numbers of patients and, therefore, the estimate of their predictive value should be verified in larger populations. Secondly, our population only included hospitalized patients with SARS-CoV-2 infection who underwent ECG recording (see Figure 1); accordingly, our sample may not be representative of the whole population of patients. Thirdly, as observed above, we had only a minority of patients with troponin and NT-proBNP measurements, as the decision to measure the levels of these biomarkers was at the discretion of the attending physician in the emergency room; therefore, the exact role of biomarkers and ECG findings and their relationship in predicting outcome should also be assessed in further studies. Fourthly, no assessment of cardiac function by echocardiography was done in our patients, unless it was crucial for the patient’s management; this approach, however, was chosen to avoid unnecessary exposure of operators to the risk of infection, in line with specifically developed recommendations.19 Fifthly, the follow-up of patients was short, and a subset of patients (18.8%) were still hospitalized at the time of follow-up; thus, it is not possible to exclude that our results might change by following patients for a longer period of time. Finally, we could not establish whether the ECG abnormalities detected in our patients were pre-existing or represented new findings as a result of the SARS-CoV-2 infection; however, our objective was to investigate the prognostic role of admission ECG as a clue to cardiac issues or involvement.
Conclusions
Our data show that standard ECG may be helpful for an initial risk stratification of patients admitted because of SARS-CoV-2 disease. The detection of delayed intraventricular conduction, in particular an LBBB, was the ECG abnormality most significantly associated with outcome, suggesting that careful attention should be paid in following these patients during SARS-CoV-2 infection.
Conflicts of interest: none declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Hui DS, Azhar EI, Madani TA, Ntoumi F, Kock R, Dar O . et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 2020;91:264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 8. Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A. et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation 2020;141:1648–1655. [DOI] [PubMed] [Google Scholar]
- 9. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F. et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T. et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020;17:259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020;5:831–840. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novelcoronavirus-(ncov)-infection-is-suspected
- 14. Rautaharju PM, Zhang ZM, Prineas R, Heiss G. Assessment of prolonged QT and JT intervals in ventricular conduction defects. Am J Cardiol 2004;93:1017–1021. [DOI] [PubMed] [Google Scholar]
- 15. Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ. et al. ; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009;53:982–991. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization. WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int
- 17. Wang L, He W, Yu X, Hu D, Bao M, Liu H. et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect 2020;80:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Banerjee A, Pasea L, Harris S, Gonzalez-Izquierdo A, Torralbo A, Shallcross L. et al. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet 2020;395:1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Skulstad H, Cosyns B, Popescu BA, Galderisi M, Di Salvo G, Donal E. et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging 2020;21:592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


