Abstract
Aims
Pre-existing cardiovascular diseases (CVDs) have been proposed to identify patients at higher risk of adverse coronavirus disease 2019 (COVID-19) outcomes, but existing evidence is conflicting. Thus, it is unclear whether pre-existing CVDs are independently important predictors for severe COVID-19.
Methods and results
In a nationwide Danish cohort of hospital-screened COVID-19 patients aged ≥40, we investigated if pre-existing CVDs predict the 30-day risk of (i) composite outcome of severe COVID-19 and (ii) all-cause mortality. We estimated 30-day risks using a Cox regression model including age, sex, each CVD comorbidity, chronic obstructive pulmonary disease-asthma, diabetes, and chronic kidney disease. To illustrate CVD comorbidities’ importance, we evaluated the predicted risks of death and severe infection, for each sex, along ages 40–85. In total, 4090 COVID-19 hospital-screened patients were observed as of 26 August 2020; 22.1% had ≥1 CVD, 23.7% had severe infection within 30 days and 12.6% died. Predicted risks of both outcomes at age 75 among men with single CVD comorbidities did not differ in clinically meaningful amounts compared with men with no comorbidities risks for the composite outcome of severe infection; women with heart failure (28.2%; 95% CI 21.1–37.0%) or atrial fibrillation (30.0%; 95% CI: 24.2–36.9%) showed modest increases compared with women with no comorbidities (24.0%; 95% CI: 21.4–26.9%).
Conclusions
The results showing only modest effects of CVDs on increased risks of poor COVID-19 outcomes are important in allowing public health authorities and clinicians to provide more tailored guidance to cardiovascular patients, who have heretofore been grouped together as high risk due to their disease status.
Keywords: COVID-19, Mortality, Morbidity, Severe outcomes, Cardiovascular comorbidities, Pre-existing conditions
Introduction
Based upon preliminary data, several countries have considered people with cardiovascular diseases (CVDs) to be a high-risk group for severe outcomes following infection with SARS-CoV-2, the causative agent of coronavirus disease 2019 (COVID-19).1 Many health authorities have thus recommended stricter social distancing and/or isolation for CVD patients as compared with the baseline population.2 While these measures may reduce the risk of COVID-19 infection, they likely have negative consequences; for example, they potentially contribute to poor mental and physical health during isolation,3 and/or possibly a reduced likelihood of receiving treatment for non-COVID-19-related issues.4,5 Additionally, in-hospital decisions, such as triaging for intensive care unit (ICU) admission, are informed by the perceived likelihood of surviving. Therefore, it is imperative that clinicians and health policymakers have an accurate understanding of the interplay between CVD, COVID-19 infection, and outcomes. However, the evidence concerning the link between individual CVDs and risks of severe COVID-19 outcomes is mixed.
Several studies suggest that pre-existing CVD comorbidities are associated with an increased risk of mortality following COVID-19 infection.6–12 However, it remains unclear how COVID-19 prognosis differs between different types of CVDs. One study using a New York cohort shows that heart failure, but not coronary artery disease is associated with increased risk,12 while a study with a smaller Chinese cohort shows those with coronary heart disease were less likely to recover.10 Additionally, a smaller case-series analysis shows those with underlying CVD who do not develop myocardial injury during admission are not at increased risk.8 Moreover, one cohort study finds no independent association between CVDs and COVID-19 mortality.13
Identifying groups most likely to have severe outcomes of COVID-19 is critical in allowing the healthcare system to prioritize allocation of prevention and treatment resources where it is most needed.14,15 This will allow more tailored guidance that can minimize the burden of the epidemic without increasing the risk of poor outcomes due to COVID-19 infection. Additionally, it will be useful for improving epidemic models and projections of epidemic burden that are important for allocating appropriate resources. Lastly, clinicians can use the data to better inform their treatment and advice to patients.
Denmark has had 4090 cases among people ≥40 years of age and 516 deaths within 30 days of COVID-19 diagnosis as of 26 August 2020 as registered in the National Patient Registry that includes hospitalized cases as well as hospital outpatient screenings. In this study, we described the characteristics of all COVID-19 diagnosed cases among a primary population of hospital-screened people aged ≥40 reported to the national patient databases as of 26 August 2020, and a secondary population of all SARS-CoV-2 polymerase chain reaction (PCR) positive cases aged ≥40 in two regions in Denmark. For both populations, we assessed the importance of pre-existing cardiovascular comorbidities as predictors of severe COVID-19 infection or death. Two outcomes: (i) risk of a composite outcome of severe infection (of severe COVID-19 diagnosis, ICU admission, respirator use, or death) within 30 days of diagnosis and (ii) risk of all-cause death within 30 days.
Methods
Data sources
Data for this study were sourced from several national data registries in Denmark. All residents of Denmark are assigned a unique numeric identifier that allows easy linking between the various registries. Hospital contact information, including date and diagnosis came from the Danish National Patient Registry; diagnoses were classified according to the Danish version of the International Classification of Diseases, 10th revision (ICD-10). Polymerase chain reaction data came from the Danish Microbiology Database. Prescription drug information came from the Danish National Prescription Registry and contains data on the dispensing date, strength and quantity of all prescription drugs purchased nationally. Age, sex, and vital status (whether a person is a current resident, dead, or has emigrated, along with the date of the event) came from the Danish Civil Registration System. The completeness and quality of the Danish registries have been described and validated previously.16,17 The present study was approved by the data responsible institution Capital Region, approval number P-2019-191. Personal data from Danish registries are pseudonymized before being delivered for research purposes. No further ethical approval is required for registry studies in Denmark.
Study populations and endpoints
The primary population was defined as all people ≥40 years of age diagnosed with COVID-19 via hospital-based screenings (both inpatient and outpatient) in Denmark (ICD-10 codes: B342, B342A, B972, B972A). Patients tested outside a hospital setting are not included in the primary population. A secondary population that included where the population was defined as all people aged ≥40 who had a positive PCR test for SARS-CoV-2 from two regions in Denmark, Region Zealand and Capital Region of Denmark. This population included those tested both inside and outside of hospital settings.
There were two outcomes of interest. The first outcome was a composite outcome of severe infection, consisting of diagnosis with severe acute respiratory syndrome, care in the ICU, use of respirator, or death, including both in- and out-of-hospital deaths, within 30 days of diagnosis. The second outcome was death, both in- and out-of-hospital, within 30 days of COVID-19 diagnosis (Supplementary material online, Table S1).
Subsets of the primary and secondary populations were made based on diagnosis or test date. The first two subsets were defined from the primary population: a subset including only those with a COVID-19 diagnosis date <1 May 2020, a subset including only those with a diagnosis date ≥1 May 2020. The second set of subsets was defined from the secondary population: a subset including only those with a SARS-CoV-2 positive test date <1 May 2020, a subset including only those with a SARS-CoV-2 positive test date ≥1 May 2020. These two time periods roughly correspond to the lockdown period (<1 May), during which time COVID-19 testing was not widely used, especially for patients not considered high risk, and the post-lockdown period (≥1 May), during which time testing became more frequent nationwide.18
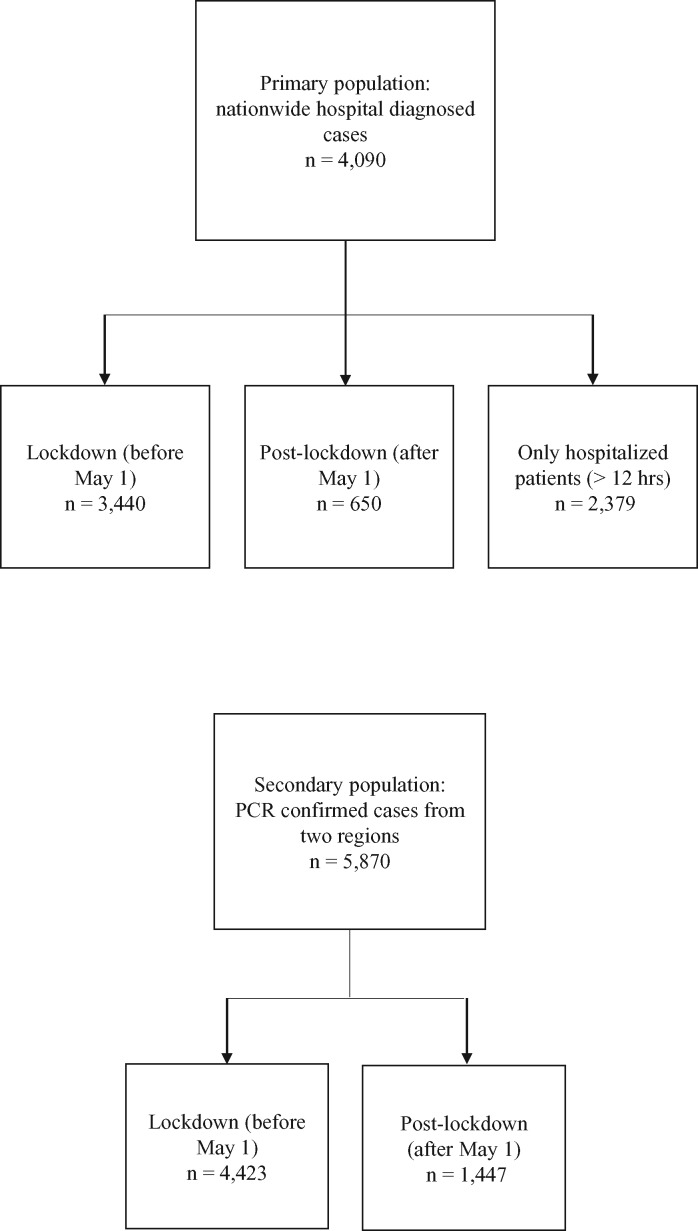
A sensitivity analysis was included on a subset of the primary population restricted to all COVID-19 patients admitted to the hospital, defined as a continuous hospital stay of ≥12 h. The outcomes and the exposures were the same as the main analysis. Figure 1 shows the definitions of each population.
Figure 1.
Definition of primary and secondary populations and sub-populations.
Definitions of exposures
The exposures of interest were pre-existing ischaemic heart disease, heart failure, and atrial fibrillation. Exposures to control for included pulmonary disease (chronic obstructive pulmonary disease or asthma), pre-existing diabetes (type I or II), and chronic kidney disease. Exposures were defined using a combination of ICD-10 codes and history of prescription medication usage (Supplementary material online, Table S2). Cardiovascular comorbidities were included if diagnosis occurred between 1 January 2000, and the day before COVID-19 diagnosis. Patients diagnosed with acute coronary syndrome in the 6 months preceding COVID-19 diagnosis were excluded, as they likely represent a qualitatively different patient group due to the recent, acute nature of their condition. Chronic obstructive pulmonary disease (COPD)-asthma was defined as the receipt of drugs for obstructive airway diseases at least twice within any 180-period after the age of 40 or diagnosis of COPD (ICD-10: J41, J43-44). Selection of exposures of interest and exposure to control for was based upon previous findings and clinical/policy relevance. Specifically, regarding the exposures of interest, there were three main considerations: (i) the three exposures used comprise conditions that are more homogenous in terms of aetiology and presentation than, for example, stroke patients. (ii) These comorbidities have high prevalence and public health importance in Denmark. (iii) Power considerations limited the number of exposures of interest that we could investigate.
Statistical analysis
Categorical and continuous variables were presented as n (%) and mean (standard deviation), respectively. The time origin for all patients was the date of COVID-19 diagnosis. Patients were followed until 26 August 2020 or death, whichever came first. Cox regression was used to predict the risk of the outcomes within 30 days. Separate models were fitted for the composite outcome of severe infection and the death-only outcome. The fitted models were used to predict the absolute risk of each outcome for each exposure of interest (ischaemic heart disease, heart failure, and atrial fibrillation), while holding all other exposures negative, across ages 40–85 for both sexes.
The Cox model included patient age at COVID-19 diagnosis/PCR test date, and interaction between sex and all exposures (ischaemic heart disease, heart failure, atrial fibrillation, COPD-asthma, and diabetes). Patients’ age was included as a continuous variable with non-linear effects by way of restricted cubic splines.19
Data management and statistical analysis was performed in R version 3.6.1.20
Results
Patient characteristics
A total of 4090 hospital-screened people aged ≥40 were diagnosed with COVID-19 and included in the primary population. A total of 904 (22.1%) had at least one CVD comorbidity, 541(13.2%) had ischaemic heart disease, 271 (6.6%) had heart failure, and 479 (11.7%) had atrial fibrillation (Table 1). The three most frequent combinations of comorbidities among those with at least one CVD were ischaemic heart disease only (n = 173, 4.2%), atrial fibrillation only (n = 150, 3.7%), and ischaemic heart disease combined with diabetes (n = 45, 1.1%) (Supplementary material online, Table S3). As of 26 August 2020, a total of 969 (23.7%) had met the definition for severe infection (severe COVID-19 diagnosis, ICU admission, respirator use, or death) within 30 days of diagnosis; of these, 191 (19.7%) had ischaemic heart disease, 119 (12.3%) had heart failure, and 204 (21.1%) had atrial fibrillation. A total of 516 (12.6%) had died within 30 days of diagnosis; of these, 115 (22.3%) had ischaemic heart disease, 90 (17.4%) had heart failure, and 150 (29.1%) had atrial fibrillation. A total of 3021 (75.3%) had neither of the outcomes of interest after 30 days of follow-up; of these, 333 (11.0%) had ischaemic heart disease, 146 (4.8%) had heart failure, and 264 (8.7%) had atrial fibrillation (Table 2). A total of 100 (2.4%) had neither of the outcomes of interest and had <30 days of follow-up by the end of data collection. Patients with continuous hospital stays >12 h accounted for 58.2% (n = 2379) of the study population.
Table 1.
Patient characteristics stratified by cardiovascular exposures of interest
| Patient characteristics | Total (n = 4090) | Ischemic heart disease (n = 541) | Heart failure (n = 271) | Atrial fibrillation (n = 479) |
|---|---|---|---|---|
| Age | 63.5 (14.9) | 73.4 (11.6) | 76.9 (11.3) | 77.6 (11.1) |
| Sex | 2043 (50.0) | 333 (61.6) | 162 (59.8) | 266 (55.5) |
| Ischaemic heart disease | 541 (13.2) | 470 (100.0) | 151 (55.7) | 163 (34.0) |
| Heart failure | 271 (6.6) | 151 (27.9) | 236 (100.0) | 149 (31.1) |
| Atrial fibrillation | 479 (11.7) | 163 (30.1) | 149 (55.0) | 401 (100.0) |
| COPDa | 627 (15.3) | 143 (26.4) | 99 (36.5) | 131 (27.3) |
| Diabetes | 598 (14.6) | 169 (31.2) | 100 (36.9) | 121 (25.3) |
| Chronic kidney disease | 293 (7.2) | 102 (18.9) | 77 (28.4) | 86 (18.0) |
| Cancer | 423 (10.3) | 73 (13.5) | 43 (15.9) | 83 (17.3) |
| Hypertension | 1281 (31.3) | 369 (68.2) | 233 (86.0) | 324 (67.6) |
| Stroke | 389 (9.5) | 100 (18.5) | 64 (23.6) | 116 (24.2) |
| Liver disease | 111 (2.7) | 15 (2.8) | 9 (3.3) | 10 (2.1) |
| Beta-blockers | 641 (15.7) | 261 (48.2) | 180 (66.4) | 264 (55.1) |
| Calcium channel Blockers | 571 (14.0) | 123 (22.7) | 32 (11.8) | 87 (18.2) |
| Ras inhibitor | 1029 (25.2) | 251 (46.4) | 147 (54.2) | 179 (37.4) |
| Loop diuretics | 486 (11.9) | 182 (33.6) | 166 (61.3) | 189 (39.5) |
| Asprin | 401 (9.8) | 208 (38.4) | 72 (26.6) | 43 (9.0) |
| Statin | 942 (23.0) | 316 (58.4) | 133 (49.1) | 190 (39.7) |
| Anticoagulation | 446 (10.9) | 146 (27.0) | 137 (50.6) | 328 (68.5) |
aCOPD, chronic obstructive pulmonary disease.
Table 2.
Outcomes of interest stratified by exposures of interest in primary population
| Exposure of interest | Death within 30 days (n = 516) | Composite outcome of severe infection within 30 days (n = 972)a | Non-severe outcome within 30 days (n = 3018)b |
|---|---|---|---|
| Atrial fibrillation | 150 (29.1) | 204 (21.0) | 264 (8.7) |
| Heart failure | 90 (17.4) | 119 (12.2) | 146 (4.8) |
| Ischaemic heart disease | 115 (22.3) | 192 (19.8) | 332 (11.0) |
Defined as diagnosis with severe acute respiratory syndrome, care in the ICU, use of respirator, or death, within 30 days of diagnosis.
Defined as people who had 30 days of follow-up with no outcome of interest.
A total of 3440 patients were diagnosed before 1 May 2020 (lockdown period), and 650 were diagnosed between 1 May and 26 August 2020 (post-lockdown). The patient characteristics of the primary population in the lockdown and post-lockdown periods are shown in Supplementary material online, Tables S4 and S5, and the outcomes of interests stratified by exposures of interest for the same populations, respectively, are shown in Supplementary material online, Tables S6 and S7.
A total of 5870 people had a PCR positive test for SARS-CoV-2 in Region Zealand or Capital Region of Denmark and were included in the secondary population. Of these, 4423 had a test date before 1 May 2020 (lockdown period), and 1447 had a test date between 1 May and 26 August (post-lockdown). The distributions of exposures in the entire secondary population, the lockdown, and the post-lockdown populations are shown in Supplementary material online, Tables S8–S10, respectively, and the distributions of the outcomes of interests are shown in Supplementary material online, Tables S11–S13.
Risk of severe infection and death
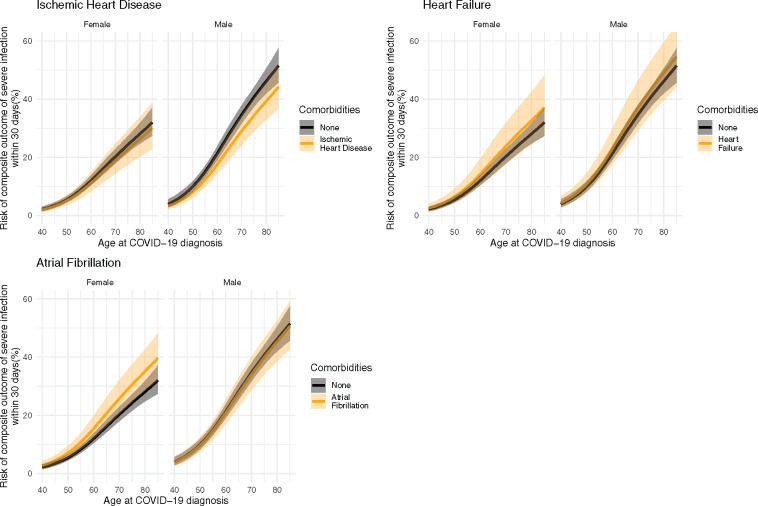
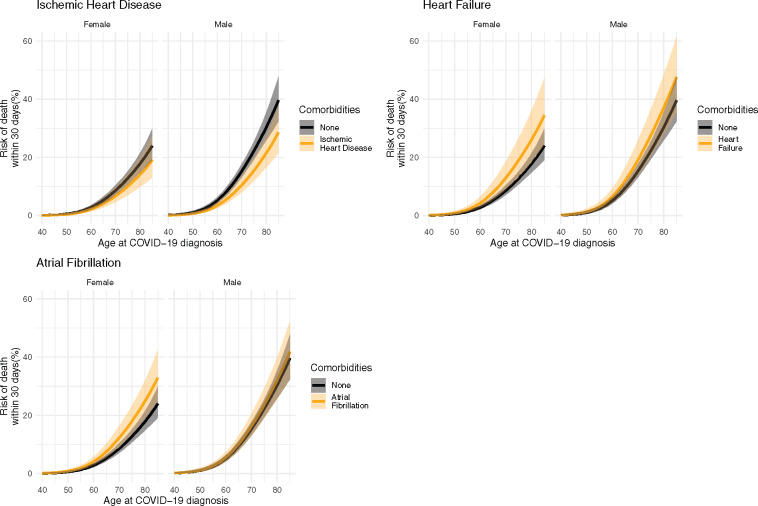
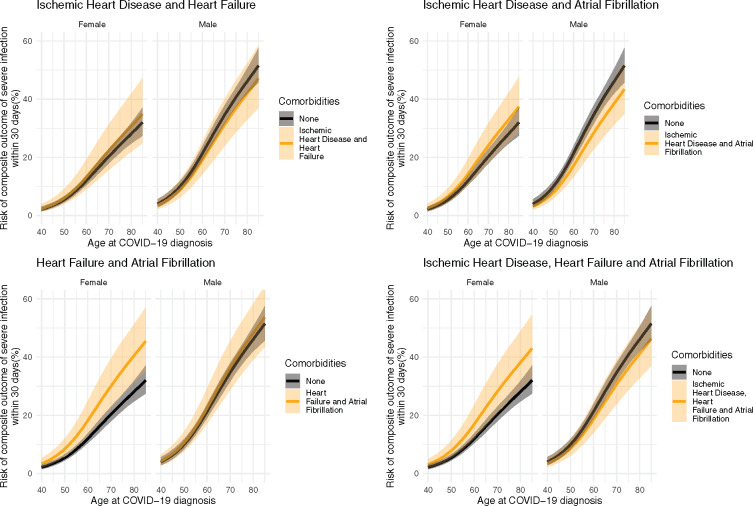
To illustrate the importance of the CVD comorbidities as risk factors, Figure 2 shows the 30-day risk of the composite outcome of severe infection (severe COVID-19 diagnosis, ICU admission, respirator use, or death) for the entire primary population and Supplementary material online, Figures S1 and S2 show the same outcome, but for the lockdown and post-lockdown populations, respectively, Supplementary material online, Figures S3–S5 show the same outcome for the entire secondary population, the lockdown subset, and the post-lockdown subset of the secondary populations, respectively. Figure 3 shows the risk of 30-day mortality across all ages by sex for single CVD comorbidities for the entire primary population, and Supplementary material online, Figures S6 and S7 show the same outcome for the lockdown and post-lockdown populations, respectively. Supplementary material online, Figures S8–S10 show the same outcome for the entire secondary population, the lockdown subset, and the post-lockdown subset of the secondary populations, respectively. To illustrate the importance of multiple CVD comorbidities, Figures 4 and 5 show the same outcomes (composite outcome of severe infection and death, respectively) for the entire primary population, but for people with more than one pre-existing CVD comorbidity. We report below the risks for a 75-year old as an example patient using the model fitted to the entire primary population.
Figure 2.
Predicted 30-day risk of the composite outcome of severe infection (severe COVID-19 diagnosis, intensive care unit admission, respirator use, or death) among hospital-screen patients (primary population). Predictions are made based across ages for both sexes for each cardiovascular comorbidity in the absence of any other comorbidities.
Figure 3.
Predicted 30-day risk of death among hospital-screen patients (primary population). Predictions are made across ages for both sexes for each cardiovascular comorbidity in the absence of any other comorbidities.
Figure 4.
Predicted 30-day risk of the composite outcome of severe infection (severe COVID-19 diagnosis, intensive care unit admission, respirator use, or death) among hospital-screen patients (primary population). Predictions are made across ages for both sexes for each combination of cardiovascular comorbidities in the absence of any other comorbidities.
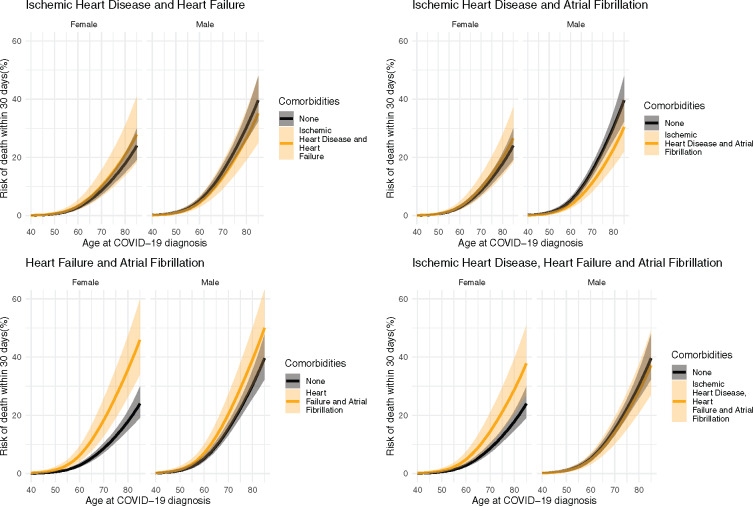
Figure 5.
Predicted 30-day risk of death among hospital-screen patients (primary population). Predictions are made across ages for both sexes for each combination of cardiovascular comorbidities in the absence of any other comorbidities.
The predicted risk of the composite outcome of severe infection for 75-year-old males with no comorbidities is 40.5% (95% CI: 37.3–43.9). The corresponding risks for 75-year-old males with single CVD comorbidities were as follows: ischaemic heart disease, 33.9% (95% CI: 28.5–40.1%); heart failure, 43.4% (95% CI: 34.8–53.1%); and atrial fibrillation, 39.4% (95% CI: 33.5–45.9%). For multiple CVD comorbidities, the corresponding risks for 75-year males were as follows: ischaemic heart disease and heart failure, 36.5% (95% CI: 28.4–46.0%); ischaemic heart disease and atrial fibrillation, 32.9% (95% CI: 26.6–40.3%); heart failure and atrial fibrillation, 42.2% (95% CI: 33.9–51.7%); and ischaemic heart disease, heart failure, and atrial fibrillation, 35.5% (95% CI: 28.0–44.2%).
The predicted risk of the composite outcome of severe infection for 75-year-old females with no comorbidities is 24.0% (95% CI: 21.4–26.9%). The corresponding risk for 75-year-old females with single CVD comorbidities were as follows: ischaemic heart disease, 22.6% (95% CI: 17.3–29.1%); heart failure, 28.2% (95% CI: 21.1–37.0%); and atrial fibrillation, 30.0% (95% CI: 24.2–37.0%). For multiple CVD comorbidities, the corresponding risks for 75-year females were as follows: ischaemic heart disease and heart failure, 26.6% (95% CI: 18.9–36.6%); ischaemic heart disease and atrial fibrillation, 28.3% (95% CI: 21.5–36.7%); heart failure and atrial fibrillation 35.0% (95% CI: 26.6–45.1%); and ischaemic heart disease, heart failure and atrial fibrillation, 33.1% (95% CI: 24.9–43.2%).
The predicted risk of death for 75-year-old males with no comorbidities is 22.3% (95% CI: 19.4–25.5%). The corresponding risks for 75-year-old males with single CVD comorbidities were as follows: ischaemic heart disease, 15.5% (95% CI: 12.1–19.8%); heart failure, 27.6% (20.5–36. 5%); and atrial fibrillation, 23.7% (19.0–29.3%). For multiple CVD comorbidities, the corresponding risks for 75-year males were as follows: ischaemic heart disease and heart failure, 19.4% (95% CI: 13.9–26.7%); ischaemic heart disease and atrial fibrillation, 16.5% (95% CI: 12.3–22.1%); heart failure and atrial fibrillation 29.3% (95% CI: 22.2–38.0%); and ischaemic heart disease, heart failure, and atrial fibrillation, 20.7% (95% CI: 15.2–27.7%).
The predicted risk of death for 75-year-old females with no comorbidities is 12.8% (95% CI: 10.8–15.2%). The corresponding risks for 75-year-old females with single CVD comorbidities were as follows: ischaemic heart disease, 10.1% (95% CI: 7.0–14.2%); heart failure, 19.1% (13.4–26.7%); and atrial fibrillation, 18.1% (13.8–23.4%). For multiple CVD comorbidities, the corresponding risks for 75-year males were as follows: ischaemic heart disease and heart failure, 15.1% (95% CI: 9.9–22.6%); ischaemic heart disease and atrial fibrillation, 14.3% (95% CI: 9.9–20.3%); heart failure and atrial fibrillation 26.5% (95% CI: 19.1–35.9%); and ischaemic heart disease, heart failure, and atrial fibrillation, 21.1% (95% CI: 14.8–29.5%).
The predicted risks of the composite outcome of severe infection across all combinations of included risk factors for a 75-year-old male and female can be found in Supplementary material online, Figures S11 and S12, respectively, and the corresponding predicted risks of death for a 75-year-old male and female can be found in Supplementary material online, Figures S13 and S14.
A sensitivity analysis performed using only hospital admitted patients from the primary population (n = 2380) showed similar results of predicted risks of death or severe infection (Supplementary material online, Figures S15 and S16).
Discussion
We examined if pre-existing ischaemic heart disease, heart failure, or atrial fibrillation were predictors of worse infection among hospital-screened COVID-19 patients. The major novel findings in this study include establishing that, among men with single CVD comorbidities, neither ischaemic heart disease, heart failure, nor atrial fibrillation predicted a higher risk of severe COVID-19 outcomes as compared with baseline risk of someone with no comorbidities, while among women, heart failure and atrial fibrillation each modestly increased risk. However, when restricting the analysis to the period during which COVID-19 testing was widely available (the post-lockdown period), the increased risk among women with heart failure or atrial fibrillation disappeared. The same trends appeared in the analyses of both the primary (hospital diagnosed) and the secondary (PCR positive) populations. Additionally, while women who have both heart failure and atrial fibrillation had a substantially higher predicted risk of death or severe infection when including the entire primary population, when restricting the data to the post-lockdown this increased risk also disappeared.
Our study adds important nuances to previous findings that pre-existing CVDs are risk factors for poor COVID-19 outcomes.8–12 While others have reported on the increased risk due to heart failure,12 previous investigations of atrial fibrillation included cohorts with too few patients with the comorbidity to meaningfully assess the importance on COVID-19 outcomes.10 Additionally, we showed for the first time that sex may play a role in determining the effect that pre-existing CVDs have on the risk of poor COVID-19 outcomes.
While we found that men in general had a higher risk of severe infection and death, pre-existing CVD seemed to predict a higher risk of poor COVID-19 outcomes only in women. However, the role of sex changed when restricting the analysis to the post-lockdown population, where heart failure showed a protective effect on risk of poor COVID-19 outcomes. The reason for this sex discrepancy is unknown, though female sex has been linked to lower Angiotensin-converting enzyme-2 receptor expression, which has been proposed to play a role in COVID-19 and cardiac disease.21 Our findings are speculative, however, and further studies are needed to explore whether the sex differences arise from biological mechanisms or from the way in which our study population was selected.
Although we found that several CVD comorbidities are not linked with worse COVID-19 outcomes, especially among men, our data supported previous findings indicating a higher overall comorbidity burden coincides in a higher risk of worse outcomes. 22 It is thus possible that CVD comorbidities not associated with clinically significant increases in risk of poor COVID-19 outcomes in our study still present a real increase in risk; the effect size, however, may be too small to be illuminated by our sample. Additionally, patients with multiple pre-existing CVD comorbidities, including comorbidities not investigated in our study, likely represent a higher risk of poor outcomes. This would be consistent with previous work showing that pre-existing CVD comorbidities impact both the clinical course and outcomes of CVD patients.23
Our results support the utility of more granular COVID-19 recommendations for CVD patients. Given the potential longevity of the pandemic, it is imperative for the mental and physical well-being of CVD patients that restrictions on social and physical activity are as minimal as necessary. Tailoring recommendations for CVD patients not at higher risk from COVID-19 infections would be an important dimension for improving quality of life and reducing collateral damage for these patients. However, although our findings suggest that ischaemic heart disease, heart failure, or atrial fibrillation are not important predictors of severe outcomes of COVID-19, especially among men, many patients with these highly prevalent cardiovascular comorbidities will be at increased risk of severe COVID-19 due to age alone.
Strength and limitations
A major strength of the present study was our access to complete medical histories of all hospital-screened COVID-19 patients. This allowed us to investigate the importance of pre-existing CVD comorbidities on the absolute risk of severe COVID-19 outcomes in a nationwide cohort of hospital-screened COVID-19 patients in a public healthcare system controlling for age, sex, and other comorbidities with multivariate analysis. Additionally, we expect to receive monthly data updates from the Danish health authorities.
There were, however, some data limitations to our study. The study population during the lockdown period was likely a highly selected population, as testing rates were low, test-positivity rates high. Additionally, the primary population only included those who have had contact with the Danish hospital system (both inpatient and outpatient contacts). This suggests our estimates of absolute risk were likely biased upwards during the lockdown period and thus likely represent more severe cases as compared with the entirety of the true SARS-CoV-2 positive population. Moreover, if COVID-19 patients who had no CVD history had less severe infections, and were less likely to be in contact with the hospital system and enter our primary population, our estimates of the effect of CVD diseases would be biased towards the null. Conversely, there is evidence of a reduction in healthcare-seeking behaviour during the pandemic among those with a history of CVD, or those experiencing CVD-related symptoms.24–29 If those with pre-existing CVD comorbidities were more likely to avoid contact with the hospital system than their CVD-free peers, unless their condition deteriorated to the point requiring intervention, then our estimates would be biased in the opposite direction, away from the null. Indeed, the analysis using only the post-lockdown data from both the primary (hospital contacts) and secondary populations (PCR positive cases from Region Zealand and Capital Region of Denmark) suggests data from during the lockdown period was biased away from the null. Taken together, this suggests our conclusions of minimal increases in absolute risk due to pre-existing CVD comorbidities were robust.
Our dataset was likely missing ICU admission data for those patients who were not yet discharged from hospital as of 26 August 2020, and 2.4% of the primary study population had not yet reached an endpoint, either 30-day event free, severe infection, or death, by the date of our data capture. Another data limitation arose from lack of data concerning potential confounding variables, such as body mass index. Previous work has demonstrated that individuals with obesity had higher odds of poor COVID-19 outcomes.30 The interactions between CVD comorbidities, obesity, and COVID-19 outcomes deserve further investigation. Yet despite these limitations, our analysis allowed us to bracket the potential effect size of the increased absolute risk of severe outcomes predicted by pre-existing CVD status.
Finally, the generalizability of our findings may present another limitation. The epidemiology of the COVID-19 outbreak varies across countries, as different countries employed different management and control approaches, which may affect the apparent impact of CVD comorbidities. The Danish authorities enacted strict lockdowns earlier in the epidemic as compared with some countries where the epidemic was seeded earlier in the calendar year. The resulting epidemic peak in Denmark, and the corresponding peak burden on the healthcare system, was lower than many countries and regions.18 In some regions where healthcare systems were more stressed, and/or the ICU capacity exceeded during the epidemic peak, patients with pre-existing comorbidities may have received substandard care These patient groups in these regions may have thus been at higher risk of poor outcomes, independent of any causal relationship between CVD comorbidities and SARS-CoV-2 infection.31,32 In contrast, Danish ICU capacity was not exceeded during the study period.33 Additionally, Denmark has an advanced healthcare system with universal healthcare for all residents and a high quality of cardiac care.34 Thus, patients in the present study with stable CVD may have represented a relatively well-treated population. Our findings may not be generalizable to countries with poorer cardiac care or instances where healthcare systems are overburdened by the COVID-19 epidemic.
Conclusion
Pre-existing ischaemic heart disease, heart failure, or atrial fibrillation in a population of hospital-screened COVID-19 patients were not important predictors for risk of severe infection among men, while heart failure and atrial fibrillation were both potentially a predictor of modest increased risk of poor outcomes among women, but only early in the epidemic when COVID-19 testing rates were low. Our findings show the need for more bespoke public health and clinical recommendations concerning cardiovascular patients.
Data availability
The data underlying this article cannot be shared publicly due to the privacy of individuals who participated in the study.
Supplementary material
Supplementary material is available at European Heart Journal – Quality of Care and Clinical Outcomes online.
Conflict of interest: C.T.-P. reports grants from Bayer and Novo Nordisk, outside the submitted work; L.K. reports personal fees from speakers honorarium from Novartis, AstrZeneca, and Boehringer, outside the submitted work; T.B.-S. reports grants from Sanofi Pasteur and GE Healthcare, other from Sanofi Pasteur, Novartis, Amgen, Sanofi Pasteur, and Amgen, outside the submitted work; all other authors reported no conflicts of interest.
Supplementary Material
References
- 1.ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance (24 April 2020).
- 2.Sundhedsstyrelsen. Coronavirus advice for people belonging to a risk group. Sundhedsstyrelsen. 2020. https://www.sst.dk/-/media/Udgivelser/2020/Corona/Infokampagne-s%C3%A5rbare/Sprogversioner/SST_Coronavirus_Saarbare_Folder_UK_digital.ashx? la=da&hash=2B08F1DFEDE059580C811FA3C2C06CDDA11F8099 (20 April 2020).
- 3. Galea S, Merchant RM, Lurie N. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention. JAMA Intern Med 2020;180:817. [DOI] [PubMed] [Google Scholar]
- 4. Walker PGT, White MT, Griffin JT, Reynolds A, Ferguson NM, Ghani AC. Malaria morbidity and mortality in Ebola-affected countries caused by decreased health-care capacity, and the potential effect of mitigation strategies: a modelling analysis. Lancet Infect Dis 2015;15:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schull MJ, Stukel TA, Vermeulen MJ, Zwarenstein M, Alter DA, Manuel DG et al. Effect of widespread restrictions on the use of hospital services during an outbreak of severe acute respiratory syndrome. CMAJ 2007;176:1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Du R-H, Liang L-R, Yang C-Q, Wang W, Cao T-Z, Li M et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J 2020;55:2000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:1294–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L et al. ; ISARIC4C Investigators. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang J, Lu S, Wang X, Jia X, Li J, Lei H et al. Do underlying cardiovascular diseases have any impact on hospitalised patients with COVID-19? Heart 2020;106:1148–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020;395:1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19—studies needed. N Engl J Med 2020;382:1194–1196. [DOI] [PubMed] [Google Scholar]
- 15. Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ 2020;368:m1198. [DOI] [PubMed] [Google Scholar]
- 16. Schmidt M, Schmidt SAJ, Adelborg K, Sundbøll J, Laugesen K, Ehrenstein V et al. The Danish health care system and epidemiological research: from health care contacts to database records. CLEP 2019;Volume 11:563–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sundbøll J, Adelborg K, Munch T, Frøslev T, Sørensen HT, Bøtker HE et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open 2016;6:e012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ECDC. Weekly COVID-19 Country Overview, Week 36, 2020. ECDC. 2020. https://covid19-country-overviews.ecdc.europa.eu/ (15 September 2020).
- 19. Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York: Springer; 2015.
- 20.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 21. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ 2020;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christensen DM, Strange JE, Gislason G, Torp-Pedersen C, Gerds T, Fosbøl E et al. Charlson comorbidity index score and risk of severe outcome and death in Danish COVID-19 patients. J Gen Intern Med 2020;35:2801–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sabatino J, De Rosa S, Di Salvo G, Indolfi C. Impact of cardiovascular risk profile on COVID-19 outcome. A meta-analysis. PLoS One 2020;15:e0237131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Rosa S, Spaccarotella C, Basso C, Calabrò MP, Curcio A, Filardi PP et al. ; Società Italiana di Cardiologia and the CCU Academy Investigators Group. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J 2020;41:2083–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer FA, Dixon S et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol 2020;75:2871–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodríguez-Leor O, Cid-Álvarez B, Ojeda S, Martín-Moreiras J, Rumoroso JR, López-Palop R et al. Impacto de la pandemia de COVID-19 sobre la actividad asistencial en cardiología intervencionista en España. REC Interv Cardiol 2020;2:82–89. [Google Scholar]
- 27. Tam CCF, Cheung KS, Lam S, Wong A. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment–elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes 2020;13:e006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andersson C, Gerds T, Fosbøl E, Phelps M, Andersen J, Lamberts M et al. Incidence of new-onset and worsening heart failure before and after the COVID-19 epidemic lockdown in Denmark: a nationwide cohort study. Circ Heart Fail 2020;13:e007274. [DOI] [PubMed] [Google Scholar]
- 29. Holt A, Gislason GH, Schou M, Zareini B, Biering-Sørensen T, Phelps M et al. New-onset atrial fibrillation: incidence, characteristics, and related events following a national COVID-19 lockdown of 5.6 million people. Eur Heart J 2020;41:3072–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH et al. Individuals with obesity and COVID‐19: a global perspective on the epidemiology and biological relationships. Obes Rev 2020;56:e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boccia S, Ricciardi W, Ioannidis JPA. What other countries can learn from Italy during the COVID-19 pandemic. JAMA Intern Med 2020;180:927. [DOI] [PubMed] [Google Scholar]
- 32. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A et al. ; for the COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020;323:1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Statens Serum Institut. COVID-19 in Denmark Epidemiological Surveillance Report (Danish). 2020. https://files.ssi.dk/COVID19-overvaagningsrapport-24042020-ds65 (24 April 2020).
- 34. Schmidt M, Jacobsen JB, Lash TL, Bøtker HE, Sørensen HT. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ 2012;344:e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals who participated in the study.