ABSTRACT
The coronavirus disease 2019 (COVID-19) pandemic created a significant impact on medically assisted reproduction (MAR) services. ESHRE decided to mobilize resources in order to collect, analyse, monitor, prepare and disseminate severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) knowledge specifically related to ART and early pregnancy. This article presents the impact of the SARS-CoV-2 pandemic focusing on reproductive healthcare. It details the rationale behind the guidance prepared to support MAR services in organizing and managing the re-start of treatments or in case of any future wave of COVID-19 disease. The guidance includes information on patient selection and informed consent, staff and patient triage and testing, adaptation of ART services, treatment planning and code of conduct. The initiatives detailed in this article are not necessarily COVID-specific and such action plans could be applied effectively to manage similar emergency situations in different areas of medicine, in the future.
Keywords: COVID-19, SARS-CoV-2, medically assisted reproduction, IVF, triage, testing, pandemic, ART services, coronavirus disease 2019, severe acute respiratory syndrome coronavirus 2
Introduction
The seriousness of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic and the unprecedented measures taken by governments to prevent the spread of disease and protect the population has impacted the way medical care is accessed and delivered. During the outbreak, medically assisted reproduction (MAR) services were curtailed or, in many cases, completely stopped to protect patients and staff and to avoid further stress for strained healthcare systems (Coronavirus COVID-19: ESHRE statement on pregnancy and conception. 19 March 2020. https://www.eshre.eu/COVID19WG). To face this new scenario, ESHRE mobilized resources in order to collect, analyse, monitor, prepare and disseminate SARS-CoV-2 knowledge specifically related to ART and early pregnancy (https://www.eshre.eu/COVID19WG). This article presents the initiatives undertaken by ESHRE and details the guidance prepared to support MAR services in organizing and managing the re-start of treatments or in case of any future wave of the disease.
SARS-CoV-2 pandemic
SARS-CoV-2 infection was declared a pandemic by the World Health Organization on the 11th March 2020, 3 months after the first accounts from the Hubei region of China. The rapid progress of the disease required the development of strategies such as isolation of individuals and extensive use of personal protective equipment (PPE) to delay the effects of SARS-CoV-2 in the general population.
In order to increase effectiveness, the list of preventative measures advanced to the lockdown of a very large proportion of economic activities such as travel, education and non-urgent services. Likewise, hospitals curtailed elective surgery and clinical throughput, and diverted their resources to the most essential care for the waves of patients suffering from coronavirus disease 2019 (COVID-19).
Despite global efforts, as of the 30th August 2020, more than 25 million individuals were confirmed to have been infected and more than 840 000 lost their lives due to the disease.
SARS-CoV-2 causes fever, dry cough, tiredness, sore throat, loss of taste or/and smell, shortness of breath, diarrhoea and conjunctivitis. Like SARS-CoV and Middle East respiratory syndrome-CoV, SARS-CoV-2 infection can lead to lethal pneumonia, yet, contrarily to previous viruses, it has a stronger human-to-human transmission (Wrapp et al., 2020; Wu, 2020). The primary route of SARS-CoV-2 transmission is through respiratory droplets from infected persons who are often asymptomatic and, as the incubation time may vary, the contagiousness of the disease is especially insidious.
For SARS-CoV-2 to mediate entry into host cells, the spike protein specifically recognizes the angiotensin-converting enzyme 2 (ACE2) as its receptor (Li et al., 2020b; Xu et al., 2020). The ACE2 receptor is present in many human tissues, including testis, ovary, vagina and uterus, although, at the moment, there is no clear evidence of its presence in gametes (Hikmet et al., 2020; Li et al., 2020b).
The lungs are the organs most affected by COVID-19, with respiratory failure developing and causing death in severe cases. Respiratory impairment can also occur if the virus affects the central nervous system (Asadi-Pooya and Simani, 2020; Li et al., 2020c).
Owing to recent identification of this disease, at present there are no established specific treatments and several potential options are being tested. Prevention is currently the most effective strategy to contain the disease.
The effect of COVID-19 on reproductive healthcare
Reproductive medicine centres have a high concentration of staff members owing to the multidisciplinary expertise required. To optimize efficacy and efficiency, patients are treated according to tight schedules by teams of professionals that very often overlap their shifts.
In March–April 2020, during the pandemic escalation, most MAR centres around the world reduced numbers of, or even stopped, their routine activities with the aim of minimizing the risk of infection and to comply with governmental decisions to social distance. Patients’ and professionals’ anxiety was increased by the lack of specific evidence on viral aggressiveness and potential vertical transmission. It still remains unknown if the virus has any effect upon the embryo in vivo and in vitro. As regards vertical transmission, while some initial papers offered reassurance (Chen et al., 2020; Liu et al., 2020,, the case for is becoming stronger lately (Deniz and Tezer, 2020). Last but not least, in many ART units attached to hospitals, ventilators, ultrasound equipment and staff were diverted to COVID-19 wards, leaving ART units unable to deliver full service. For these reasons, the impact in the MAR field was dramatic and widespread.
ESHRE’S contribution on guidance for patients and professionals
ESHRE produced its first recommendation related to the practice of ART during the emergence of the pandemic on 19th March 2020 (Coronavirus COVID-19: ESHRE statement on pregnancy and conception. 19 March 2020. https://www.eshre.eu/COVID19WG). Subsequently, an ESHRE COVID-19 Working Group (WG) was established, tasked to monitor and inform its members, the scientific community and patients on the development of the pandemic and its impact upon ART services.
During the pandemic escalation (Phase 1), the WG collected scientific evidence, and released a recommendation to discontinue all routine MAR activities (except fertility preservation for medical reasons) (https://www.eshre.eu/COVID19WG). When the disease spread slowed, the WG disseminated the ‘ESHRE Guidance on recommencing ART treatments’, whose rationale and content are detailed below. In addition, a questionnaire was forwarded to ESHRE National Representatives to assess MAR activities in Europe, with results showing that treatments were completely or partly suspended in the majority of European countries from March to April, and resuming as of May 2020 (The ESHRE COVID-19 Working group et al., 2020). Finally, the WG solicited and promoted the reporting of cases aimed at investigating the potential effects of SARS-CoV-2 on MAR pregnancies. Clinicians and scientists are encouraged to submit their case reports through the dedicated platform at https://nl.surveymonkey.com/r/COVID19ART. This last initiative was implemented to collect evidence on the impact of SARS-CoV-2 upon the first and second trimester of pregnancy as, to date, the published data are limited to third-trimester pregnancies and/or deliveries (Rasmussen et al., 2020; Schmid et al., 2020; Schwartz, 2020; Schwartz and Graham, 2020; Liu et al., 2020).
ESHRE’s effort was not singular, as other Societies have published COVID-19 recommendations. Of relevance is that ESHRE and two other global societies, namely the American Society for Reproductive Medicine and the International Federation of Fertility Societies, jointly offered solutions to the challenges of COVID-19 impact upon the provision and access to infertility treatment (Veiga et al., 2020).
ESHRE guidance on recommencing ART treatments
While MAR services were suspended, no government or scientific society recommended postponing spontaneous pregnancies because of the COVID-19 pandemic.
Following an initial MAR activity suspension, a gradual re-start of activities occurred from May 2020. ESHRE guidance is based on the principle that maintaining safety requires careful and sustained planning. Generally speaking, ART activities are strictly regulated and subject to high standards of safety and quality (De los Santos et al., 2016). However, the SARS-CoV-2 pandemic required specific preventative measures to minimize the risk of infection during ART procedures.
Taking into consideration the local, national and governmental guidance/recommendation related to COVID-19 and the principles of good clinical and laboratory practice, a comprehensive safety plan must be implemented, based on the following pillars:
Discussion, agreement and consent to start treatment
Staff and patient triage
Access to advice and treatment
Adaptation of ART services
Treatment cycle planning
Code of Conduct for staff and patients
Discussion, agreement and consent to start treatment
Adequate patient selection means not only preventing the possibly infected and undiagnosed patients from attending the services but also advising patients with a higher risk of developing severe disease to postpone treatment. Patients suffering from systemic pathologies, such as diabetes, hypertension, heart, lung and liver or kidney disease, those using immunosuppressant therapy (i.e. post-transplant patients) should first be referred to expert healthcare professionals for assessment of their clinical situation and evaluation of the option of starting treatments at a safer time.
The risk reduction and treatment information offered to patients must be fully delivered and understood, including the balance between risks and benefits if the treatment is postponed. A specific code of conduct including the moral obligation to immediately inform the treating staff if the information previously provided is no longer valid, or if symptoms arise, should be clearly detailed and agreed upon. This document should also contain a section aimed at raising awareness for relatives and friends about how and why infection could jeopardize a couple’s treatment.
Worthy a special mention are patients who developed the severe form of COVID-19 disease and required ventilation. Such patients should be considered a high-risk group as severe and permanent lung impairment occurs (Salehi et al., 2020). To avoid potential complications during anaesthesia and pregnancy, assessment of respiratory function should be carried out before proceeding with ART.
Last but not least, patients wishing to postpone the treatment for personal reasons or owing to safety concerns should be supported in their decision. All ART treatments are at patient request.
Staff and patient triage
All individuals planning to enter the MAR centre (irrespective of being staff, patients or accompanying persons, cleaners and auxiliary services, including contractors) are potentially susceptible of carrying the SARS-CoV-2 virus and may spread the disease. The incubation time for COVID-19 is variable (Sethuraman et al., 2020) and asymptomatic individuals could pass on the virus to patients or staff members. For this reason, triage regarding health status, symptoms and lifestyle of patients, staff members and individuals living in the same household should be routine before attending the MAR centre.
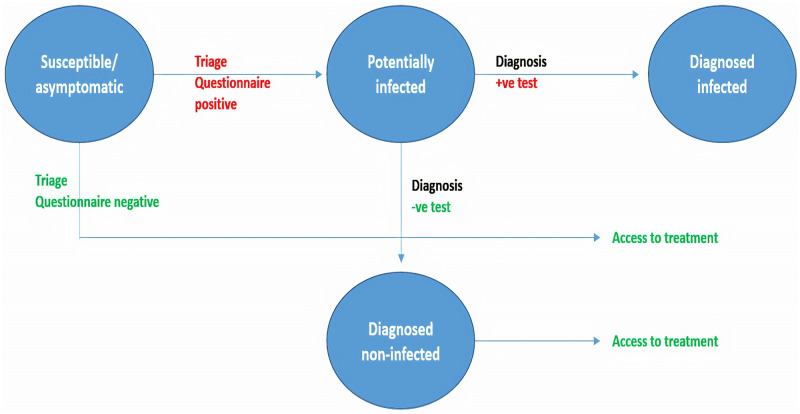
Triage is a useful tool for early detection of people at risk of infection or of potentially infected individuals, facilitating referral for further diagnostic assessment. For this reason, the triage questionnaire should be clear and detailed, and it should be updated based on evolving evidence. Patient triage is an essential step in management, as few cases are diagnosed positive after a negative triage questionnaire (González-Ravina et al., 2020). The triage principle is summarized in Fig. 1.
Figure 1.
Triage system aimed at identifying potentially infected/infected patients in order to manage subsequent access to medically ART. Adapted from Giordano et al. (2020).
Triage of staff
All staff members should fill in the triage questionnaire and preferably be SARS-CoV-2 tested 15 days before activities re-start. Re-triage should occur on a regular basis according to local prevalence of disease, prevalence of triage positivity and test availability. Any staff member that suspects she/he might be infected or is symptomatic should immediately notify the centre and undergo SARS-CoV-2 testing. A second confirmation test might be required. Staff members who test positive for IgM antibodies and/or by PCR (irrespective of symptoms) are potentially infectious and should receive health advice while undergoing self-quarantine. Documented clearance of the infection by RT-PCR or other test (IgG-IgM) is required to resume work.
Triage of patients
All patients planning to start an ART treatment should undergo triage, aiming to identify individuals with a high risk of being affected and spreading the SARS-CoV-2 infection.
Both partners should fill in the questionnaire 2 weeks before the treatment start date and every 2 weeks during treatment. Any changes in the information provided in the questionnaire should be immediately notified to the MAR centre.
Based on the answers provided, triage procedures can result in the following scenarios (Fig. 2):
Figure 2.
Summary of potential scenarios after triage and of subsequent approaches to be adopted. COVID-19: coronavirus disease 2019.
-
Scenario 1
If both patients are asymptomatic and triaged as low risk (negative clinical history, lifestyle and no contact with potentially infected people), the couple should be accepted for treatment.
-
Scenario 2
This scenario applies to patients who recovered from a previous COVID-19 infection and to those who develop symptoms before starting or during the treatment. Patients who suffered with and recovered from COVID-19 should provide evidence of clearance and undergo IgM/IgG testing in order to be considered for treatment. In this case, additional tests or specific medical assessment could be requested to evaluate potential consequences of the disease as a further risk factor for the patient.
If one or both partners develop symptoms before starting the treatment, triage should be repeated at the beginning of ovarian stimulation. If this triage is negative, patients can proceed with the treatment. If symptoms persist, diagnostic testing should be performed. Based on the result it will be decided whether to continue treatment (negative test) or to postpone it (positive test).
If one or both partners develop symptoms during ovarian stimulation, testing should be performed. Based on the result it will be decided whether to continue treatment (negative test) or to postpone it (positive test).
-
Scenario 3
If one or both partners are symptomatic, triaged as high risk, and/or tested SARS-CoV-2 IgM or RT-PCR positive, treatment should be postponed.
If patients test positive (IgM or RT-PCR), irrespective of the scenario, test performed or symptoms, they should be regarded as infectious and referred to dedicated services in compliance with local and national guidance.
Good practice dictates that patients should be followed-up 3 weeks after oocyte retrieval (OR)/embryo transfer (ET), even if a pregnancy was not achieved, to check the state of their health and identify potential positive cases. Contact tracing measures will be activated where a post-treatment positive case is diagnosed.
Testing
RT-PCR: presence of virus
The identification of the SARS-CoV-2 virus presented the initial challenge during the pandemic, echoing the alarms that Dawson raised only 6 months previously (Dawson et al., 2019). RT-PCR is the most common method for SARS-CoV-2 detection. Both oropharyngeal (OP) and nasopharyngeal (NP) swabs have been most frequently used for diagnosis (Wang et al., 2020a).
Lately, evidence shows NP swabs to be the preferred sampling methodology (Wang et al., 2020b): the authors reviewed the results of simultaneous, separate NP (single nostril) and OP (both throat sides) swabbing from 353 patients presenting with symptoms. They showed that OP swabs on their own have a much lower detection rate compared to NP swabs and that 73.1% of NP positive cases were negative by OP swab, indicating false-negative results may occur using an OP swab only approach. They concluded that OP swabs may result in a worryingly high false-negative rate.
Multiple RT-PCR protocols have been proposed. Initial protocols used were based on the E gene and RdRp gene (Corman et al., 2020), yet some authors pointed out the limitations of the existing E gene assay, which showed high background levels hampering a clear evaluation of the assays (Konrad et al., 2020). The Centers for Disease Control and Prevention recommends targeting the N gene (Centers for Disease Control and Prevention, 2020).
As regards sensitivity and specificity of RT-PCR, a recently published paper (van Kasteren et al., 2020) showed the positive identification rate to fluctuate from 10/13 to 13/13 and that specificity appears to be 100%, all kits showing no cross-reactivity with other Coronaviruses. An important question nevertheless remains: when, during the early days of infection, can we optimally detect the virus? Kucirka et al. (2020) showed that during the 4 days of asymptomatic infection, the probability of a false-negative result decreased from 100% on Day 1 to 68% on Day 4. On the day of symptoms onset, the median false-negative rate was 38%. This decreased to 20% on Day 8 (3 days after symptom onset) then began to increase again, from 21% on Day 9 to 66% on Day 21. The false-negative rate was minimized 3 days after the onset of symptoms, on average. As such, this may be the optimal time for testing if the goal is to minimize false-negative results (Kucirka et al., 2020).
At this moment, it is clear that RT-PCR testing is not 100% accurate and that risk factors, clinical presentation and symptoms must all be considered, particularly where the triage is positive, and the testing is negative (Bullis et al., 2020; Feng et al., 2020).
IgM/IgG: immune response of a person exposed
IgM/IgG presence reflects an individual’s immune response to viral exposure, and such testing should be considered complementary to triage.
Two recent papers provided an insight into the anti-SARS-CoV-2 antibody behaviour. Long et al. (2020) reported that 100% of patients tested positive for antiviral IgG within 19 days after symptoms onset and that seroconversion for IgG and IgM occurred simultaneously or sequentially. Sethuraman et al. (2020) observed that IgM and IgG seroconversion occurred in all patients between the third and fourth week of clinical illness. IgM begins to decline and almost disappears by week 7, while IgG persists beyond 7 weeks.
Considering the rate of RT-PCR false-negative results, particularly in the early stages of the disease (first 4 days of infection) (Kucirka et al., 2020), the use of serological testing to complement swabs might increase detection rates, as antibodies show up as early as Days 2–4 after symptom onset (Long et al., 2020). In the case of negative RT-PCR testing, the use of IgG and IgM may facilitate a better understanding of the diagnosis (the status) and appropriate allocation to proceed with or postpone treatment. Ultimately, local, regional and national guidelines on testing should be observed.
Access to advice and treatment
Patient and staff information and education on COVID-19 is a necessary requirement for admission for treatment and work attendance, respectively. More specifically, both groups should be instructed on the use of PPE, on the importance of social distancing, and on other preventative measures before, during and after treatment. Information on COVID-19 symptoms, SARS-CoV-2 transmission and implications should also be provided in a clear and comprehensive way. Finally, patients should acknowledge that treatment will be discontinued if their status changes from low to high risk.
Adaptation of ART services
ART units should be re-organized to ensure social distancing is respected. Routine sanitation of all areas should be performed, and specific COVID-19 sanitation procedures should be implemented in case of positive patients and/or staff members. Patient management and staff work shifts should be re-thought and adapted to the high-risk pandemic situation. Specific COVID-19 training and procedures should be put in place, and staff working patterns and planning should consider the creation of mini teams.
Also, remote access procedures should be encouraged. Whenever possible, telemedicine should be used to reduce physical contact between staff and patients. The number of people simultaneously present in the centre should be limited by managing appointments according to specific timetables and by restricting access for accompanying persons (including partner if her/his presence is not strictly necessary). Waiting areas should be re-designed to guarantee social distancing and protective screens for administrative staff installed. PPE and sanitation devices should be at disposal of patients and staff. In order to avoid discontinuation of treatments, agreements between MAR centres for the management of potential emergency closures due to COVID-19 cases are strongly recommended.
Treatment cycle planning
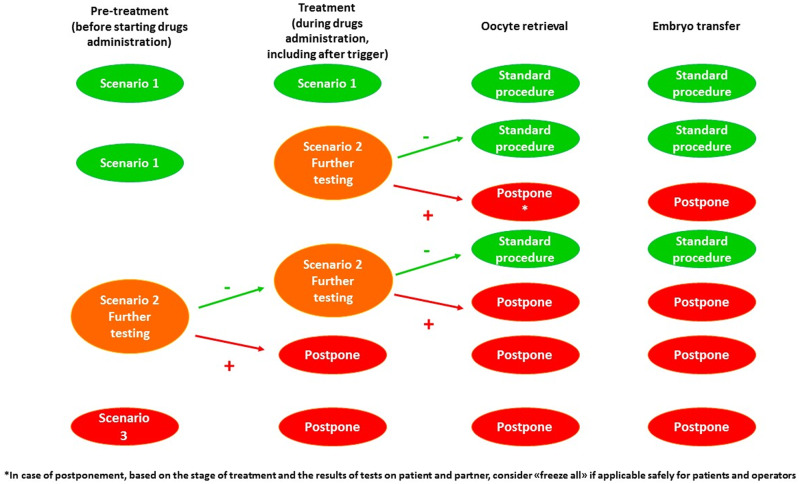
Staff members should use appropriate PPE at all times. When showing signs of infection, individuals should be isolated and tested, while patients should be carefully monitored to detect symptoms. In this case, specific action (further testing, postponement of treatment, etc.) should be taken accordingly. Procedures to be adopted should be based on triage results (Fig. 3).
Figure 3.
Recommended management of patients based on three scenarios following triage.
Ovarian stimulation monitoring
Each centre should adapt its activity to physical capacity and staff availability aiming to ensure social distancing and respect for national guidance on reducing risk of COVID-19. It is recommended to start small and increase the numbers only when the professionals become confident that measures in place are working. Further adaptation includes streamlining appointments to facilitate social distancing, reducing waiting time, scanning and blood testing and facilitating a very quick turnaround time. Ultrasounds and blood tests could be limited to two or three tests (depending on protocol used), respectively, at down-regulation, during stimulation and on the day of trigger, unless clinical reasons dictate otherwise. Specific attention should be paid to hygiene of ultrasound equipment, all medical devices used and consultation rooms.
All follow-up advice and information can be given via phone or video conferencing.
Oocyte retrieval
If both partners are asymptomatic and triaged negative (Scenario 1), standard procedures should be adopted, unless symptoms arise between ovulation trigger and OR.
If the re-triage is positive for one or both partners (Scenario 2) further testing is recommended (IgM/IgG or RT-PCR). Whether to continue or to postpone treatment will be decided based on the results.
If one or both partners are triaged or tested positive (Scenario 3) before ovulation triggering or embryo thawing, the treatment should be immediately postponed, and the positive patient should be isolated and referred to dedicated services.
Exceptional measures should be applied in case of positive patients who cannot postpone treatment (high risk of ovarian hyperstimulation syndrome, fertility preservation for medical reasons, etc.). In these cases, OR can be performed by adopting procedures that reduce the risk of transmission to staff members and other patients. Great attention must be paid to PPE correct use. The operating theatre and the lab should be accurately disinfected and sanitized immediately after the procedure. Considering the risks of horizontal transmission, switching from aerosol generating general anaesthesia to i.v. sedation or local anaesthesia may also be considered.
Laboratory practice, ET and cryopreservation
The ART laboratory should adhere to established good laboratory practice guidelines (De los Santos et al., 2016) to ensure safety and quality of tissues and cells. In the circumstances of the COVID-19 pandemic, extra caution is required to avoid potential SARS-CoV-2 infections through processing of follicular fluid and semen samples (Li et al., 2020a). Native follicular fluid and seminal plasma should be diluted and safely disposed of, in individual closed containers.
Consideration must be also given to gamete and tissue cryopreservation during the COVID-19 pandemic as the stored material could potentially be contaminated with the virus (Yakass and Woodward, 2020).
If a patient becomes positive after OR, all oocytes/embryos should be cryopreserved using high security straws and/or vapour phase storage tanks. ET should be performed only in case of low-risk and asymptomatic couples. Partners should not be allowed in the operating theatre during ET.
With reference to work organization, staff should be sub-divided in mini teams to guarantee continuity of treatment and reduce unnecessary exposure. The size of the team and the timetable (daily, three daily or weekly rotation) is dependent on the number of patients treated and the number of staff in the centre. Each centre must perform due diligence by involving all staff in this decision process. The principle is that only the minimum number of staff needed should be present at any one time.
Code of Conduct for staff and patients
A Code of Conduct is a new concept in a crisis situation like the COVID-19 pandemic. It is underpinned by the ‘all for one, one for all’ principle. Only through collaboration and trust can a safe continuation or restart of activities be achieved. Collaboration and trust between patients and healthcare professionals remain essential elements of any medical treatment. An individual sense of responsibility is of utmost importance to prevent potential cases of infection for both patients and MAR centre staff members.
The first step in establishing the Code is educating all staff and patients in relation to SARS-CoV-2. This is followed by specific recommendations in relation to engaging with the outside world, such as hygiene, awareness of risk and self-imposed measures, to reduce the risk of infection. Ideally, each centre details what restrictions they wish their staff and patients to follow. Understandably, this document would need to be in line with national recommendations, proportionate to the impact of COVID-19 in a certain region and in line with cultural norms. Therefore, the Code of Conduct should be written within the context of the centre, the patients, the staff, and the evolution of the pandemic in a specific location. Being extra cautious and meticulous in social distancing and hand hygiene is certainly advised during therapy. Both staff and patients should adapt their lifestyle in order to limit the exposure to risk and they should also inform and ask their friends and family to respect their shared responsibility in remaining healthy. The Code of Conduct is, ultimately, an agreement to being and practicing together in a safe environment.
Acceptance from all parties and continuation of such an agreement are key to safe provision of care in the MAR centre. Finally, the Code of Conduct must make it clear that it is a matter of all becoming affected by the consequences of one participant breaking the Code.
Patients and staff members will be asked to fill in and sign regularly a specific form stating that they are well and that they have complied with the provisions of the Code of Conduct in the best interest of all subjects involved. The Code is a matter for each centre to agree with their staff and their patients.
Conclusions and applicability of the model to other pandemics
This article deals with the most unusual scenario encountered in living memory: the halt of global activities due to a severe SARS-CoV-2 pandemic with enormous potential for harm. It details the role of ESHRE as a Scientific Society in the field of Reproductive Medicine and Science in supporting services and patients during such unprecedented times and the impact of the pandemic upon ART services in Europe. A working group was invited to meet weekly in order to offer swift and timely reactions to the constant evolution of the pandemic and the related scientific evidence. Actions were taken to collect reliable data and information and to disseminate them in a clear and comprehensive way. Furthermore, the group created a model of adaption of ART practice to the reality of the pandemic and proposed an original model for dealing with an emergency situation that has the potential to paralyse practice globally.
Based on these data and information, recommendations applicable to different clinical (teaching hospitals, private practices, free-standing units) and national settings were issued at different time points, at the beginning and during the pandemic, aiming to support professionals wishing to re-start activities in a safe manner, once allowed or deemed appropriate to do so.
The initiatives detailed in this article, while COVID-19 specific, are applicable to managing similar emergency situations in different areas of medicine in the future.
In particular, considering the current pandemic trend and the decreasing average age of COVID-19 patients, it is reasonable to expect that increasing numbers of patients undergoing ART are likely to be infected. Furthermore, detecting them might be challenging because a high percentage might be asymptomatic. For this reason, MAR Centres should be especially careful with triage and testing procedures in the coming months. It is also possible that triage questionnaires might have to be adjusted accordingly in the near future.
COVID-19 is a systemic disorder affecting many organs. The long-term multiorgan consequences of an acute SARS-CoV-2 infection should not be underestimated. In fact, long-term follow-up of SARS-CoV-2 infected individuals should be a health priority and the question of a previous COVID-19 event should be part of the history taking in all chronic diseases. As ACE2 receptors are present in the reproductive tract, long-term follow-up of patients that developed COVID-19 could potentially identify new causes of infertility, SARS-CoV-2 related. Awareness of asking questions related to this infectious agent could become a part of the investigation of the infertile couple in the future. Through recently established research grants dedicated to SARS-CoV-2 and reproduction, ESHRE is promoting long-term research related to this virus and its impact upon women and men of reproductive age.
Acknowledgements
The authors acknowledge the ESHRE Executive Committee for supporting the working group and Serena Sgargi for support in preparing the manuscript.
Authors’ roles
L.G. and E.M. contributed equally in conceptualization, execution and manuscript drafting. The other authors participated in revision and critical discussion. All authors approved the final version.
Funding
There was no funding for the current paper, apart from technical support from ESHRE.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci 2020;413:116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullis SSM, Crothers JW, Wayne S, Hale AJ. A cautionary tale of false-negative nasopharyngeal COVID-19 testing. IDCases 2020;20:e00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Real-time RT-PCR panel for detection 2019-Novel Coronavirus. 2020. https://www.cdc.gov/coronavirus/2019-ncov/downloads/rt-pcr-panel-for-detection-instructions.pdf (date last accessed, 20 October 2020).
- Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q. et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records [published correction appears in Lancet 2020;395(10229):1038] [published correction appears in Lancet 2020;395(10229):1038]. Lancet 2020;395:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML. et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P, Malik MR, Parvez F, Morse SS. What have we learned about Middle East respiratory syndrome coronavirus emergence in humans? A systematic literature review. Vector Borne Zoonotic Dis 2019;19:174–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De los Santos MJ, Apter S, Coticchio G, Debrock S, Lundin K, Plancha CE, Prados F, Rienzi L, Verheyen G, Woodward B. et al. ; ESHRE Guideline Group on Good Practice in IVF Labs. Revised guidelines for good practice in IVF laboratories (2015). Hum Reprod 2016;31:685–686. [DOI] [PubMed] [Google Scholar]
- Deniz M, Tezer H. Vertical transmission of SARS CoV-2: a systematic review [published online ahead of print, 2020 Jul 21]. J Matern Fetal Neonatal Med 2020;doi:10.1080/14767058.2020.1793322. [DOI] [PubMed] [Google Scholar]
- Feng H, Liu Y, Lv M, Zhong J. A case report of COVID-19 with false negative RT-PCR test: necessity of chest CT. Jpn J Radiol 2020;38:409–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G, Blanchini F, Bruno R, Colaneri P, Di Filippo A, Di Matteo A, Colaneri M. Modelling the COVID-19 epidemic and implementation of population-wide interventions in Italy. Nat Med 2020;26:855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Ravina C, Vergara V, Cruz M, Prados NAR. Is COVID-19 symptomatic triage enough? The limited value of serological testing. Hum Reprod 2020;35(Suppl):O-146. [Google Scholar]
- Hikmet F, Mear L, Uhlen M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol 2020;16:e9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad R Eberle U Dangel A Treis B Berger A Bengs K Fingerle V Liebl B Ackermann N Sing A. Rapid establishment of laboratory diagnostics for the novel coronavirus SARS-CoV-2 in Bavaria, Germany, February 2020. Euro Surveill 2020;25:2000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med 2020;173(4):262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open 2020. a;3:e208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020. b;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 2020. c;92:552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W Wang J Li W Zhou Z Liu S Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med 2020;14:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, Liao P, Qiu JF, Lin Y, Cai XF. et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020;26:845–848. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol 2020;222:415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi S, Reddy S, Gholamrezanezhad A. Long-term pulmonary consequences of coronavirus disease 2019 (COVID-19): what we know and what to expect. J Thorac Imaging 2020;35:W87-W89. [DOI] [PubMed] [Google Scholar]
- Schmid MB, Fontijn J, Ochsenbein-Kölble N, Berger C, Bassler D. COVID-19 in pregnant women. Lancet Infect Dis 2020;20:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses 2020;12:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med 2020;144:799–805. [DOI] [PubMed] [Google Scholar]
- Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA 2020;323:2249. [DOI] [PubMed] [Google Scholar]
- The ESHRE COVID-19 Working group Vermeulen N, Ata B, Gianaroli L, Mocanu E, Tapanainen T, Lundin K, Rautakallio-Hokkanen S. A. V. A picture of medically assisted reproduction activities during the COVID-19 pandemic in Europe. Hum Reprod Open 2020;2020:hoaa035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kasteren PB, van der Veer B, van den Brink S, Wijsman L, de Jonge J, van den Brandt A, Molenkamp R, Reusken C, Meijer A. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. J Clin Virol 2020;128:104412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga A, Gianaroli L, Ory S, Horton M, Feinberg E, Penzias A. Assisted reproduction and COVID-19: a joint statement of ASRM, ESHRE and IFFS [published online ahead of print, 2020 Jul 10]. Fertil Steril 2020;114:484–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020. a;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tan L, Wang X, Liu W, Lu Y, Cheng L, Sun Z. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int J Infect Dis 2020. b;94:107–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020;367:1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. Compensation of ACE2 Function for Possible Clinical Management of 2019-nCoV-Induced Acute Lung Injury. Virol Sin 2020;35:256–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020;63:457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakass MB, Woodward B. COVID-19: should we continue to cryopreserve sperm during the pandemic? Reprod Biomed Online 2020;40:905. [DOI] [PMC free article] [PubMed] [Google Scholar]