Abstract
Background
The effect of immunosuppressive treatment for immune-mediated diseases on risk of the novel coronavirus disease 2019 (COVID-19) has not been established. We aimed to define the effect of targeted biologic and immunomodulator therapy on risk of COVID-19 in a multi-institutional cohort of patients with inflammatory bowel disease (IBD).
Methods
We identified patients 18 years and older who received care for IBD at Partners Healthcare between January 2019 and April 2020. The primary outcome was development of COVID-19 defined as a positive polymerase chain reaction test for severe acute respiratory syndrome coronavirus 2. Multivariable regression models were used to examine the effect of immunosuppression on risk of COVID-19 and its outcomes.
Results
In a cohort of 5302 IBD patients, 39 (0.7%) developed COVID-19. There was no difference in age, sex, or race between IBD patients with and without COVID-19. The rate of COVID-19 was similar between patients treated with immunosuppression (0.8%) compared with those who were not (0.64%; P = 0.55). After adjusting for age, sex, race, and comorbidities, use of immunosuppressive therapy was not associated with an increased risk of COVID-19 (odds ratio, 1.73; 95% confidence interval, 0.82–3.63). The presence of obesity was associated with a higher risk of COVID-19 (odds ratio, 8.29; 95% confidence interval, 3.72–18.47). There were 7 hospitalizations, 3 intensive care unit stays, and 1 death. Older age and obesity but not immunosuppressive treatment were associated with severe COVID-19 infection.
Conclusions
The use of systemic immunosuppression was not associated with an increased risk of COVID-19 in a multi-institutional cohort of patients with IBD.
Keywords: Crohn’s, immunosuppression, COVID-19, biologics
Introduction
The emergence of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) led to a global pandemic affecting over 38 million individuals worldwide and resulting in over 1.09 million deaths to date. Though the virus causes multi-organ sequelae, it most commonly manifests as a respiratory disease ranging in severity from a mild upper respiratory illness to severe pneumonia, acute respiratory distress syndrome (ARDS), multi-organ failure, and death.1, 2 Due to the recent emergence of SARS-CoV-2, risk factors for contracting the illness are poorly understood. Data from the initial wave of infections in China, Europe, and the United States suggest that older individuals and those with underlying comorbidities may be at greatest risk.1–4 Included in the latter group are patients who are immunosuppressed, either as a consequence of underlying disease or pharmacologic treatment.5, 6 Inflammatory bowel diseases, including ulcerative colitis (UC) and Crohn’s disease (CD), are debilitating immune-mediated diseases affecting nearly 7 million individuals worldwide.7 Systemic immunosuppression through use of targeted biologics or small molecules is the cornerstone of effective IBD management.8,9 Consequently, the interplay between immunosuppression and COVID-19 infection in patients with IBD is a pressing clinical question.
Several studies have now been published examining the impact of IBD diagnosis on outcomes after COVID-19 disease.10, 11 These have shown that immunosuppression is not associated with worse outcomes among those with COVID-19 disease and that outcomes in patients with IBD are comparable with those without underlying IBD. However, an important challenge in extrapolating those findings concluding that immunosuppression does not influence risk of acquisition of disease is the potential for inherent bias in the studies published thus far. Because testing for the SARS-CoV-2 virus is not universal in those with suspicious symptoms, it is plausible and indeed likely that the threshold for testing is lower in those with immunosuppression since it may more directly impact interruption of such therapy. In contrast, testing may be reserved for more severe illness in those not on immunosuppression and thus not deemed high risk. This would bias away from demonstrating harm with immunosuppression. However, one can surmise that this lower threshold for testing would introduce a bias in the opposite direction in estimating risk of disease acquisition inasmuch as the higher rate of testing in an immunosuppressed population would actually bias toward demonstrating higher risk of documented infection. Thus in the absence of universal testing, complementary studies examining impact of immunosuppression on COVID-19 risk and COVID-19 outcomes are both required to accurately impact our practice. The only prior study that has attempted to evaluate risk of disease related to drug exposure used the American Veterans affairs cohort and was limited by ability to examine only thiopurine and antitumor necrosis factor (anti-TNF) exposure but not that of other biologics or combination therapy. Furthermore, it consisted predominantly of older (mean age 63 years) male patients.12 Consequently, there is an important gap in the literature examining the effect of novel non-TNF biologics and combination therapy on risk of SARS-CoV-2 virus acquisition and COVID-19 disease.
In this study, we aimed to use data from a large, multi-institutional cohort of patients with IBD in Massachusetts, the state with the third highest number of cases of COVID-19 infections in the United States to accomplish 4 things: (1) define the risk of documented COVID-19 disease in patients on aminosalicylates, anti-TNF, and non-TNF biologics; (2) examine the impact of combination therapy on disease risk; (3) compare risk factors in an IBD cohort that underwent testing and were confirmed negative for the SARS-CoV-2 virus to account for selection bias in testing; and (4) define the impact of immunosuppression and comorbidity on disease outcomes. Defining this risk accurately is of critical relevance to the management of IBD and other immune-mediated diseases that rely on systemic immunosuppression for disease control.
METHODS
Study Population
This study included patients receiving care at 2 tertiary referral hospitals in Boston, the Massachusetts General Hospital (MGH) and Brigham and Women’s Hospital (BWH), and other participating hospitals within the Partners HealthCare system. Partners HealthCare is the largest health care provider in Massachusetts and provides primary, secondary, and tertiary referral care to residents of New England. The study population was identified using the Partners Research Patient Data Repository (RPDR) and active query of gastroenterologists at MGH and BWH to find cases. Use of RPDR has been described in prior publications.13, 14 In brief, RPDR is an automatically and continually updated data repository that warehouses information obtained from any health care encounter within the Partners HealthCare system, including all inpatient and ambulatory encounters, procedures and laboratory and radiologic tests that occur in any of the affiliated hospitals.
For this study, we identified eligible patients age 18 years and older with at least 1 International Classification of Diseases 10th edition (ICD-10) code for CD (K50.x) or UC (K51.x) between January 1, 2019, and April 25, 2020. In addition, to accurately define the denominator as patients actively receiving care for IBD, we also required at least 1 prescription for any of the following: (1) oral aminosalicylate (mesalamine, balsalazide, sulfasalazine); (2) immunomodulator (azathioprine, mercaptopurine, methotrexate); (3) biologic including tumor necrosis factor-α antagonists (anti-TNF), anti-integrins (vedolizumab), anti-interleukin 12/23 agents (ustekinumab); or (4) janus kinase inhibitor (tofacitinib) during this period. This approach of combining diagnosis codes and prescriptions demonstrated good accuracy for case ascertainment in prior studies of IBD.15
Definition of COVID-19 Infection
The primary study outcome was development of COVID-19, defined as a positive polymerase chain reaction (PCR) test for SARS-CoV-2 on nasopharyngeal swab. Each positive case was reviewed, and diagnosis was confirmed by an attending gastroenterologist. During the study period, there was no screening of asymptomatic patients. Only patients who met criteria for significant symptoms (fever and respiratory illness) were referred for SARS-CoV2 testing. For each case, we extracted information on whether the infection was severe, defined as COVID-19 resulting in hospitalization, intensive care unit (ICU) stay, or death. Medication use at the time of development of COVID-19 disease was also ascertained for each case.
Covariates
We extracted relevant covariates from the electronic medical record: age, sex, race, and type of IBD (CD or UC). Race was defined dichotomously as white or nonwhite, with the latter including those with missing or unstated race. We identified the presence of common comorbidities that could potentially influence COVID-19 outcomes, including asthma, diabetes mellitus (DM), hypertension, and obesity,1 through ICD-10 codes for these conditions. We obtained information on medication exposures since January 2019 pertinent to the management of IBD. Patients were placed into ordinal categories of mutually exclusive therapeutic regimens, including aminosalicylates, immunomodulators, anti-TNFs (infliximab, adalimumab, certolizumab pegol, golimumab), vedolizumab, ustekinumab, tofacitnib, or combination biologic-immunomodulator therapy (“combination therapy”). We also assessed use of corticosteroids. Patients sequentially on multiple biologics during the study period were assigned to the category of most recent exposure.
Statistical Analysis
The study was approved by the institutional review board of Partners HealthCare. Continuous variables were expressed as means with standard deviations and compared using t tests, whereas categorical variables were defined using proportions and compared using χ2 square tests (with the Fisher modification when appropriate). First, we performed univariate logistic regression identifying factors associated with acquiring COVID-19 infection. Variables significant in the univariate analysis at P < 0.15 or those that had been previously described to modify risk for or outcomes of COVID-19 were included in multivariable regression models to identify independent predictors of infection. To ensure that the findings were not due to selection bias in referral for testing, as a sensitivity analysis we compared patients with positive SARS-CoV-2 PCR with those who tested negative. We then performed an analysis restricted to cases to identify predictors of a severe COVID-19 disease. Analyses were conducted using Stata 15.2 (StataCorp, College Station, TX).
RESULTS
Study Population
The study included a total of 5302 patients with IBD with a mean age of 46.5 years (range 18–99 years). Just under half of the cohort were men (49%), and most were white (89%). Fifty-eight percent had CD. Hypertension was the most common comorbidity (21% of the cohort), followed by diabetes (6.5%), asthma (6.5%), and obesity (6.2%). The most common immunosuppressive regimen used was TNF-antagonist monotherapy in 29.7% of the cohort. The percentage of biologic users on combination therapy ranged from 10.5% among vedolizumab users to 23.0% of TNF-antagonist users. Just over one third of the cohort (35.3%) had received a prescription for mesalamine alone. Twenty percent had received a prescription for prednisone.
A total of 39 patients (0.7%) developed COVID-19 infection. Of these, 7 resulted in severe disease (7 hospitalized, 3 ICU, 1 death). To provide context, the number of cases in Massachusetts as of May 15, 2020, was 80,497 out of an estimated population of 6.9 million (rate of infection 11 cases per 1000 individuals [1.1%]). Table 1 compares the characteristics of IBD patients who developed COVID-19 to the rest of the cohort. There was no difference in age, sex, or race between the 2 groups. There was a trend toward fewer patients with CD among the cases (44%) compared with controls (58%; P = 0.063). Among the comorbidities, obesity was much more prevalent among cases (28%) compared with controls (6%; P < 0.001), but there was no difference in prevalence of diabetes, hypertension, or asthma. Older age was not associated with higher risk of COVID-19 infection in this cohort, likely reflecting the relatively young age distribution of this population.
Table 1.
Characteristics of the Study Cohort, Stratified by COVID-19 Infection Status
| Characteristic | COVID-positive (n = 39) | COVID-negative (n = 5263) | P |
|---|---|---|---|
| Mean age (in years) (SD) | 45.6 (18.8) | 46.6 (18.3) | 0.74 |
| Sex | 0.18 | ||
| Male | 38% | 49% | |
| Female | 62% | 51% | |
| Race | 0.81 | ||
| White | 90% | 89% | |
| Nonwhite / missing | 10% | 11% | |
| IBD-type | 0.063 | ||
| Crohn’s disease | 44% | 58% | |
| Ulcerative colitis | 56% | 42% | |
| Comorbidities | |||
| Obesity | 28% | 6% | < 0.001 |
| Diabetes mellitus | 8% | 7% | 0.71 |
| Hypertension | 18% | 21% | 0.70 |
| Asthma | 10% | 6% | 0.31 |
| Medication category | 0.38 | ||
| 5-aminosalicylates | 31% | 35% | |
| Immunomodulator | 8% | 11% | |
| TNF-antagonists | 33% | 25% | |
| Vedolizumab | 18% | 11% | |
| Ustekinumab | 0% | 7% | |
| Tofacitinib | 0% | 1% | |
| Combination therapya | 10% | 10% | |
| Prednisone use | 0.95 | ||
| No | 79% | 80% | |
| Yes | 21% | 20% |
aCombination therapy refers to use of TNF-antagonists, vedolizumab, or ustekinumab in combination with an immunomodulator (azathioprine, mercaptopurine, methotrexate).
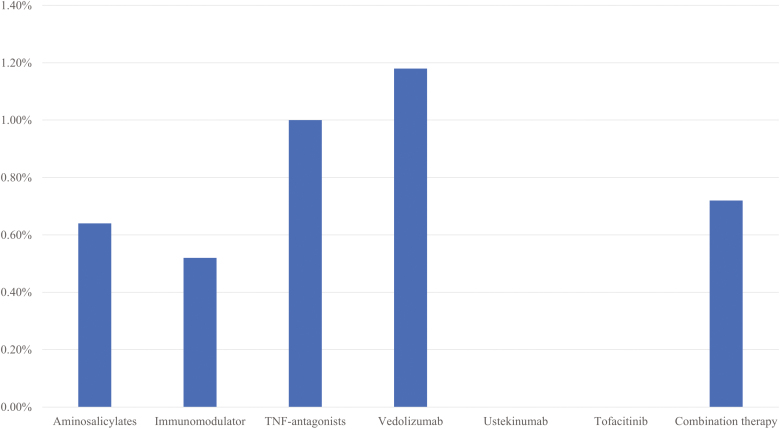
We observed no effect of medication exposure on the risk of COVID-19 infection in patients with IBD. The frequency of infection among those only on aminosalicylate therapy was 0.64%, which was similar to 0.5% on immunomodulators, 1% on TNF-antagonists, and 1.2% on vedolizumab monotherapy. Four infections occurred in those on combination biologic-immunomodulator therapy, whereas none were noted in those on either ustekinumab or tofacitinib alone (Fig. 1). Overall, the rate of COVID-19 infection was similar between those not treated with immunosuppression (0.64%) and those treated with immunosuppression (0.8%; P = 0.55). Corticosteroid use in the past year was not associated with risk of COVID-19 infection in our cohort (users 0.37% vs nonusers 0.36%; P = 0.95). On multivariable analysis adjusting for age, sex, race, IBD-type, and comorbidity, use of any immunosuppressive therapy was not associated with an increased risk of COVID-19 infection (odds ratio [OR], 1.73; 95% CI, 0.82–3.63). The only significant independent predictors were a diagnosis of CD (OR, 0.46; 95% CI, 0.23–0.91) and obesity (OR, 8.29; 95% CI, 3.72–18.47). Separately, compared with no immunosuppression, the use of conventional immunomodulators (OR, 0.89; 95% CI, 0.33–2.44), anti-TNFs (OR, 1.58; 95% CI, 0.70–3.54), or vedolizumab (OR, 1.87; 95% CI, 0.71–4.94) was not associated with an increase in risk of COVID-19 infection. Similar results were obtained when restricting the analysis to only IBD patients who had been tested for COVID-19, comparing those with positive PCR testing to those with confirmed negative tests (OR, 0.78; 95% CI, 0.28–2.17; Supplemental Table 1).
Figure 1.
Risk of COVID-19 infection by medication category.
Table 2 compares patients who developed severe COVID-19 with those with mild disease. Those who developed severe disease were likely to be older (mean age 65.4 years vs 41.2 years; P = 0.001) and female (P = 0.02). They were also more likely to be obese (71% vs 19%), have diabetes (29% vs 3%), have hypertension (57% vs 9%), or have asthma (29% vs 6%; P = 0.078). Interestingly, the proportion of patients on any immunosuppression was lower in those with severe disease (29%) compared with those with mild disease (78%; P = 0.010). However on multivariable analysis, only older age (P = 0.018) and obesity (P = 0.033) were associated with severe disease, and immunosuppression use was no longer statistically significant (P = 0.27).
Table 2.
Characteristics of Patients with Mild or Severe COVID-19 Infection
| Characteristic | Severe COVID-19 (n = 7) | Mild COVID-19 (n = 32) | P |
|---|---|---|---|
| Mean age (in years) (SD) | 65.4 (17.4) | 41.3 (16.4) | 0.001 |
| Sex | 0.02 | ||
| Male | 0% | 47% | |
| Female | 100% | 53% | |
| Race | 0.078 | ||
| White | 71% | 94% | |
| Nonwhite / missing | 29% | 6% | |
| IBD-type | 0.38 | ||
| Crohn’s disease | 29% | 47% | |
| Ulcerative colitis | 71% | 53% | |
| Comorbidities | |||
| Obesity | 71% | 19% | 0.005 |
| Diabetes mellitus | 29% | 3% | 0.02 |
| Hypertension | 57% | 9% | 0.003 |
| Asthma | 29% | 6% | 0.078 |
| Medication category | 0.05 | ||
| 5-aminosalicylates | 71% | 22% | |
| Immunomodulator | 0% | 9% | |
| TNF-antagonists | 0% | 41% | |
| Vedolizumab | 29% | 16% | |
| Ustekinumab | 0% | 0% | |
| Tofacitinib | 0% | 0% | |
| Combination therapya | 0% | 13% | |
| Prednisone use | 0.83 | ||
| No | 75% | 80% | |
| Yes | 25% | 20% |
aCombination therapy refers to use of TNF-antagonists, vedolizumab, or ustekinumab in combination with an immunomodulator (azathioprine, mercaptopurine, methotrexate).
Discussion
In the 6 months since COVID-19 was declared a global pandemic, much remains unknown about risk factors for disease. Although initial data suggested immunosuppression increases risk, this was based on small numbers of patients receiving systemic chemotherapy for malignancy and immunosuppression for organ transplantation.5, 6 Those receiving targeted therapy for autoimmune disease were insufficiently represented in these cohorts to determine risk. The absence of robust data describing the risk of COVID-19 infections in this population and a body of evidence linking IBD treatments to increased risk of serious infections16–20 have introduced substantial uncertainty into the management of patients on long-term immunosuppression. In this large cohort, we reassuringly identified no excess risk of COVID-19 infection among those on various systemic and gut-targeted immunosuppressive therapies for IBD when compared with those not on immunosuppression. We also identified no effect of immunosuppression on severity of COVID-19, including need for hospitalization and mortality. In contrast, other recognized comorbidities, particularly obesity,1 increased risk of development of COVID-19 infection and severe disease in this population, consistent with prior studies.21
Our findings expand upon the experience described in few published case series and cohort studies of patients with IBD. Regarding risk of acquiring COVID-19, a retrospective review of IBD patients tested for COVID-19 within a northern California health system demonstrated no association between immunosuppressive medication use and odds of a positive test result, but this analysis included only 5 positive patients.22 Similarly, a combined analysis of French and Italian cohorts revealed no increase in risk of COVID-19 in IBD patients when compared with the estimated rate in the general population. However, this study lacked a clearly defined at-risk population to estimate association with different treatments.23 Most other published studies of COVID-19 in IBD have been case series of affected patients, attempting to define predictors of severe disease. An Italian prospective observational cohort of 79 patients with a diagnosis of IBD and COVID-19 found no association between corticosteroid (OR, 4.94; 95% CI, 0.95–25.55), thiopurine (OR, 1.21; 95% CI, 0.22–6.40), anti-TNF (OR, 1.18; 95% CI, 0.47–2.97), or vedolizumab (OR, 0.53; 95% CI, 0.16–1.73) use and risk of COVID-related pneumonia.24 There was also no association between corticosteroid or anti-TNF use and death.24 Similarly, data from the international SECURE-IBD registry for COVID-19 infections reported that only 19% of patients on antitumor necrosis factor monotherapy required hospitalization and 1% died, rates which were lower than those observed for patients on aminosalicylate therapy (46% hospitalized, 7% died).25 These findings may, in part, be a selection bias due to use of immunosuppression only in individuals considered “fitter.” Though consistent with the SECURE-IBD registry, use of immunosuppression was more frequent among those with milder disease in our cohort; upon adjusted analysis, only age and obesity were independently predictive of disease severity. In contrast to their study, we did not find an association with corticosteroid use. However, this may be a reflection of the relative inaccuracy of prescription dates to define active steroid use, given its sporadic intermittent use.
The lack of an effect of immunosuppression on COVID-19 risk or severity may have several explanations. First, SARS-CoV-2 infects the human host by binding the angiotensin 1 converting enzyme 2 (ACE2) receptor, which is then cleaved by transmembrane serine protease 2 (TMPRSS2) to induce viral entry into the cell.26–28 The ACE2 receptor is expressed in many organs, including type 2 surfactant-secreting alveolar cells of the lungs and gastrointestinal epithelial cells, with highest concentrations in the duodenum, terminal ileum, and colon.29, 30 Burgueño et al recently demonstrated no significant difference in ACE2 or TMPRSS2 expression in colonic organoids derived from patients with ulcerative colitis as compared with controls, supporting that ulcerative colitis alone may not impact risk of COVID-19 infection.31 However, expression of ACE2 was lower in patients on antitumor necrosis factor drugs, vedolizumab, ustekinumab, and steroids as compared with patients on no immunosuppression,31 which may limit viral entry and subsequent severity. Second, by virtue of being grouped together in the high-risk category, it is plausible that patients on systemic immunosuppression may have practiced stricter quarantine measures and self-isolated more rigorously than those not on such treatments, decreasing their risk of viral infection. Third, COVID-19 has been associated with a cytokine storm, and patients with severe disease often demonstrate markedly elevated inflammatory cytokines such as interleukin (IL)-6 on presentation.32 Indeed, in addition to antiviral therapy, one of the avenues being explored for COVID-19 treatment is targeted immunosuppression, including IL-6 antagonists,33, 34 with some even proposing a role for steroids or TNF-antagonists.35
There are several implications to our results and strengths to our study. To our knowledge, the present study is one of the largest cohorts to date examining the impact of immunosuppression on risk of COVID-19 infection. Our findings suggest that those on immunosuppression for IBD are not at higher risk for COVID-19, nor are they at risk for more severe disease. Given the reliance on immunosuppression for effective management of several immune-mediated diseases, our findings may provide broad reassurance to providers treating patients with these diseases and support existing professional society recommendations to continue immunosuppression in such patients during the COVID-19 pandemic.36–40 However, it should also be acknowledged that our study period comprised primarily a time where most may have been self-isolating, and thus continued study is necessary once societies reopen and risk of exposure is higher.
We readily acknowledge the limitations to our study. Due to lack of widespread availability of testing, it is possible that patients with mild symptoms may not have been tested and thus not captured as cases in our analysis. Second, we did not have granular information on dose of medications and relied on the presence of a prescription for the relevant drug in the past year. Patients who had self-discontinued treatment, particularly early in the pandemic, would not have been identified as nonusers. Third, we did not have the ability to examine disease-related factors, such as active inflammation, on COVID-19 risk. Finally, though we supplemented case ascertainment by actively querying treating providers, it is possible that some patients may have been tested outside our health system and their providers not notified.
In conclusion, immunosuppressive treatment for management of IBD was not associated with an increase in risk of COVID-19 infection in a large, multi-institution cohort. Though our findings suggest that cautiously continuing such treatments for IBD is warranted, further study is necessary during the next phase of the pandemic with re-integration of society in the setting of ongoing exposure risk.
Supplementary Material
Author Contribution: KEB, BK, and ANA contributed to study concept and design. KEB, BK, JRA, RWW, MJH, WWC, and ANA contributed to acquisition of data. ANA contributed to statistical analysis. KEB, BK, and ANA drafted the manuscript. All authors contributed to interpretation of data and critically revised the manuscript for important intellectual content.
Conflicts of Interest: AA has served as a scientific advisory board member for Abbvie, Gilead, and Kyn Therapeutics and received research grants from Pfizer. JA has consulted for Pfizer, Takeda, Janssen, Finch Therapeutics, Pandion, Servatus, Artugen, and Iterative Scopes and received research support from Merck. RW has consulted for Abbvie and Nestle. BK, KB, PL, HK, FC, MH, and WC have no relevant conflicts.
References
- 1. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu L, Gong N, Liu B, et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77:: 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collaborators GBDIBD. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 9. Feuerstein JD, Isaacs KL, Schneider Y, et al. ; AGA Institute Clinical Guidelines Committee AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158:1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lukin DJ, Kumar A, Hajifathalian K, et al. Baseline disease activity and steroid therapy stratify risk of COVID-19 in patients with inflammatory bowel disease. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481–491.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khan N, Patel D, Xie D, et al. Impact of Anti-TNF and Thiopurines medications on the development of COVID-19 in patients with inflammatory bowel disease: a Nationwide VA cohort study. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta V, Rodrigues R, Nguyen D, et al. Adjuvant use of antibiotics with corticosteroids in inflammatory bowel disease exacerbations requiring hospitalisation: a retrospective cohort study and meta-analysis. Aliment Pharmacol Ther. 2016;43:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Desai A, Zator ZA, de Silva P, et al. Older age is associated with higher rate of discontinuation of anti-TNF therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology. 2008;135:1907–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gagniere C, Bourrier A, Seksik P, et al. Risk of serious infection in healthcare workers with inflammatory bowel disease: a case-control study of the Groupe d’Etude Therapeutique des Affections Inflammatoires du tube Digestif (GETAID). Aliment Pharmacol Ther. 2018;48:713– 722. [DOI] [PubMed] [Google Scholar]
- 17. Romano C, Sinagra E, Criscuoli V, et al. Increased risk of pneumonia among patients with inflammatory bowel disease: a comparison between patients treated with biologic therapies and with conventional drugs. J Crohns Colitis. 2013;7:e405–e406. [DOI] [PubMed] [Google Scholar]
- 18. Long MD, Martin C, Sandler RS, et al. Increased risk of pneumonia among patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gregory MH, Ciorba MA, Wiitala WL, et al. The association of medications and vaccination with risk of pneumonia in inflammatory bowel disease. Inflamm Bowel Dis. 2020;26:919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tinsley A, Navabi S, Williams ED, et al. Increased risk of influenza and influenza-related complications among 140,480 patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:369–376. [DOI] [PubMed] [Google Scholar]
- 21. Stefan N, Birkenfeld AL, Schulze MB, et al. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16:341–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gubatan J, Levitte S, Balabanis T, et al. SARS-CoV-2 testing, prevalence, and predictors of COVID-19 in patients with inflammatory bowel disease in Northern California. Gastroenterology. 2020;159:1141–1144.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allocca M, Fiorino G, Zallot C, et al. Incidence and patterns of COVID-19 among inflammatory bowel disease patients from the Nancy and Milan Cohorts. Clin Gastroenterol Hepatol. 2020;18:2134–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bezzio C, Saibeni S, Variola A, et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020;69:1213–1217. [DOI] [PubMed] [Google Scholar]
- 25. Brenner EJ, Ungaro R, Colombel JF, et al. SECURE-IBD Database Public Data Update covidibd.org. 2020.
- 26. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harmer D, Gilbert M, Borman R, et al. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. [DOI] [PubMed] [Google Scholar]
- 30. Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burgueño JF, Reich A, Hazime H, et al. Expression of SARS-CoV-2 entry molecules ACE2 and TMPRSS2 in the gut of patients with IBD. Inflamm Bowel Dis. 2020;26:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen R, Sang L, Jiang M, et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J Allergy Clin Immunol. 2020;146:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang C, Wu Z, Li JW, et al. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feldmann M, Maini RN, Woody JN, et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395: 1407–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kennedy NA, Jones GR, Lamb CA, et al. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut. 2020;69:984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. COVID-19 ECCO TaskForce. 1st Interview COVID-19 ECCO Taskforce https://ecco-ibd.eu/images/6_Publication/6_8_Surveys/1st_interview_COVID-19%20ECCOTaskforce_published.pdf. 2020.
- 38. Rubin DT, Feuerstein JD, Wang AY, et al. AGA clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: expert commentary. Gastroenterology. 2020;159:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hanzel J, Ma C, Marshall JK, et al. Managing inflammatory bowel disease during COVID-19: summary of recommendations from gastrointestinal societies. Clin Gastroenterol Hepatol. 2020;18:2143–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rubin DT, Abreu MT, Rai V, et al. Management of patients with Crohn’s disease and ulcerative colitis during the COVID-19 pandemic: results of an international meeting. Gastroenterology. 2020;159:6–13.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.