Abstract
Angiotensin-converting enzyme 2 (ACE2) has been highlighted for its role as a receptor for SARS-CoV-2, responsible for the current COVID-19 pandemic. This review summarizes current knowledge about ACE2 as a multifunctional protein, focusing on its relevance in inflammatory bowel disease (IBD). As an enzyme, ACE2 may be protective in IBD because it favors the counter-regulatory arm of the renin-angiotensin system or deleterious because it metabolizes other anti-inflammatory/repairing elements. Meanwhile, as a receptor for SARS-CoV-2, the impact of ACE2 expression/activity on infection is still under debate because no direct evidence has been reported and, again, both protective and deleterious pathways are possible. Research has shown that ACE2 regulates the expression of the neutral amino acid transporter B0AT1, controlling tryptophan-associated intestinal inflammation and nutritional status. Finally, intact membrane-bound or shed soluble ACE2 can also trigger integrin signaling, modulating the response to anti-integrin biologic drugs used to treat IBD (such as vedolizumab) and fibrosis, a long-term complication of IBD. As such, future studies on ACE2 expression/activity in IBD can improve monitoring of the disease and explore an alternative pharmacological target.
Keywords: inflammatory bowel disease, IBD, angiotensin-converting enzyme 2, SARS-CoV-2 receptor, tryptophan absorption, integrin signaling
INTRODUCTION
The COVID-19 pandemic has brought angiotensin-converting enzyme 2 (ACE2) to scientific and public discussion since it was identified as the receptor for SARS-CoV-2.1 This protein is multifunctional and has physiological and pathophysiological relevance. It is better known for its role as an enzyme of the renin-angiotensin system (RAS), although it also acts as a chaperone molecule for amino acid transport and the integrin ligand and, as noted, a receptor for SARS-CoV-2. The expression of ACE2 is prominent in the intestine, and its actions have been reported to be relevant for inflammatory status, such as that of inflammatory bowel disease (IBD).
Research has reported that IBD is highly prevalent and negatively affects patients’ quality of life because of general discomfort, urge to defecate, diarrhea (sometimes bloody), chronic abdominal pain, and weight loss.2 The disease comprises Crohn disease (CD) and ulcerative colitis (UC). In CD, the inflammation may affect any region of the GI tract, is transmural, and usually has a discontinuous pattern. On the other hand, UC is characterized by a continuous colonic distal-to-proximal inflammation but one that is limited to the colonic mucosa.2 The etiopathology of IBD remains unclear, but uncontrolled bacterial colonization, disruption of the epithelial barrier, dysregulation of the homeostatic balance, and unregulated immune response play a role.2 Actual evidence is still scarce, but it is hoped that further understanding of the role of ACE2 in IBD pathology and therapeutic response modulation will ground its use as a biomarker of disease activity and/or treatment response and will ideally contribute to refine and develop new therapeutic strategies.
ACE2 AS A REGULATORY ENZYME—ALTERED CATALYTIC ACTIVITY IN IBD?
Catalytic Activity of ACE2
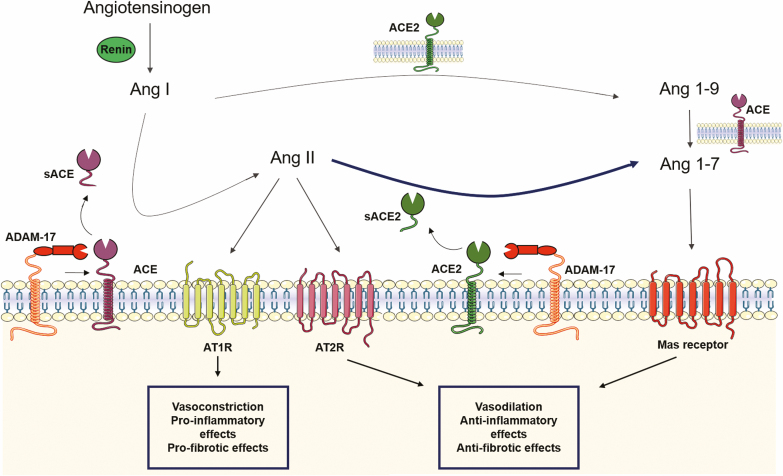
The RAS cascade (Fig. 1) starts with angiotensinogen (traditionally secreted by the liver) that is cleaved by renin (mostly derived from the kidney) into angiotensin (Ang) I, which is further cleaved by ACE (highly expressed by pulmonary endothelial cells) into Ang II, the major effector peptide of the system. Ang II can activate Ang II type 1 receptors (AT1R) and type 2 receptors (Fig. 1). Generally, Ang II type 2 receptors mediate effects that are the opposite of those mediated by AT1R. The first physiological effect attributed to the RAS is the regulation of fluid homeostasis and blood pressure, which is also its best-characterized function (Fig. 1).3 Research has shown that Ang II is a potent vasoconstrictor and promotes sodium/water reabsorption; these effects are of high clinical relevance because drugs that block the RAS (ie, ACE inhibitors [ACEi] and AT1R antagonists/blockers [ARB]) are first-line therapeutic options for the treatment of hypertension and associated organ damage.
FIGURE 1.
Current simplified view of the RAS. Ang II, the major effector peptide of this system, exerts its functions mainly via AT1R, and the opposite via the angiotensin type 2 receptor. ACE2 converts Ang II in Ang 1–7 that via the Mas receptor exerts effects opposite of those mediated by AT1R. ADAM17 cleaves both ACE and ACE2 in their soluble forms, sACE and sACE2, respectively, that maintain their catalytic activity.
This initial view of the RAS has been expanded, and it is now accepted that Ang II is also a key player in inflammation, tissue damage and immunity.3 Moreover, other identified peptides, enzymes, and receptors increase the complexity of the RAS cascade (Fig. 1). From these, the so-called counter-regulatory arm of the RAS is relevant for the current review. About 30 years ago, Chappell and colleagues4 identified the peptide Ang (1–7), with effects opposite of those caused by Ang II (Fig. 1). By the change of the century, 2 research groups5, 6 identified a homologue of ACE—ACE2, with carboxypeptidase activity, a high affinity to Ang II, and resistance to ACE inhibition. A few years later, Ang (1–7) was identified as an endogenous ligand of the Mas receptor (Fig. 1). Meanwhile, ACE2/Ang (1–7)/Mas was characterized as a counter-regulatory arm of the RAS because its activation induces effects that are the opposite of those of the classical ACE/Ang II/AT1R.3 These findings revealed a metabolic pathway that regulates the balance between Ang II (a vasoconstrictor, proinflammatory, proliferative, and pro-oxidant peptide) and Ang 1–7 (a vasodilator, anti-inflammatory, anti-proliferative, and anti-oxidant peptide).3
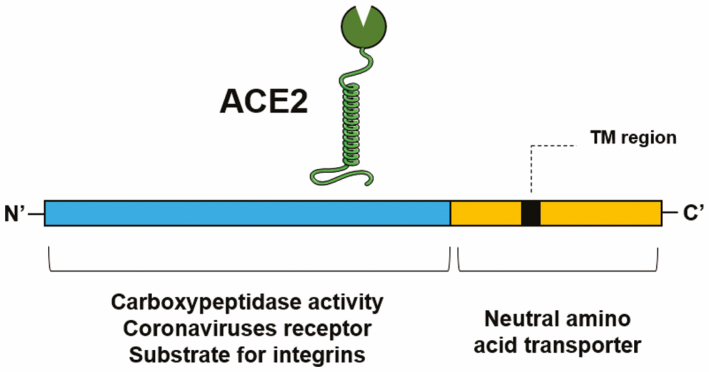
Whereas ACE2 is a type I transmembrane glycoprotein that has its catalytic active site in the extracellular amino (N’)-domain and the binding sites for SARS-CoV-2 and integrins, the binding site for the neutral amino acid transporter B0AT1 is located in the carboxy (C’)-domain (Fig. 2). Research has shown that ACE2 shares approximately 42%, 60%, and 40% of its identity with the catalytic N- and C- domains of human ACE,5, 6 respectively. This glycoprotein is a zinc carboxypeptidase; thereby, in the presence of zinc, it cleaves a single amino acid from the C-terminal of its substrate.5, 6 It cleaves Ang II into Ang 1–7 and, although it does so with less catalytic efficiency, it cleaves Ang I into Ang 1–9 that is then converted into Ang 1–7 by ACE (Fig. 1).3, 5-7
FIGURE 2.
Domain structure of ACE2. TM indicates transmembrane.
Outside the RAS, ACE2 also cleaves the C-terminal amino acid of peptides like ghrelin, apelin-13, and apelin-367. Moreover, ACE2 circulates in the bloodstream in a soluble form (sACE2) that results from the shedding of the ectodomain of ACE2 by TNF-α convertase (also named ADAM17)8 and preserves its catalytic activity.9
Catalytic Effect of ACE2 in the Gut
Researchers’ initial view of the endocrine elements of the RAS gave place to the view that all RAS components are expressed ubiquitously, with local autocrine/paracrine tissue RAS being regulated independently from circulating RAS. Relevant for this review, there is an intestinal RAS.10 Although ACE2 expression in the lung is concentrated in a small population of type II alveolar cells,11 its expression in the gastrointestinal (GI) system seems to be higher and more diffuse,10 being expressed in the oral mucosa, esophagus, stomach, pancreas, duodenum, ileum, colon, and rectum.10 In the intestine, ACE2 is expressed in the epithelial brush border, muscularis mucosa, muscularis propria, microvascular endothelium, and vascular smooth muscle cells, with marked expression in the ileum, colon, and mesenteric microvascular endothelium (see Garg, Angus, et al10 for an extended review). In human intestinal biopsies, the catalytic activity of ACE2 is higher in the terminal ileum when compared with that in the colon.12 Most substrates of ACE2 are also present in the GI tract. Research has found Ang II in the crypt and crypt-villus junction epithelial cells of the small intestine.13 Ghrelin and apelin are more abundant in the stomach, but they also exist in the duodenum and, at lower levels, in the ileum, cecum, and colon of the rat.14, 15
In addition, ACE2 has an important role in limiting Ang II-mediated effects and favoring Ang 1–7 production and effects. In the intestine, ACE2 controls bicarbonate secretion and the absorption of electrolytes and glucose.10 In the colon, Ang II regulates motility, promoting colonic contraction.10 However, Ang 1–7 has anti-inflammatory16 and antifibrotic12 effects. By metabolizing ghrelin,7 ACE2 decreases the levels of this anti-inflammatory intestinal peptide.17 Finally, ACE2 hydrolyzes apelin7, which has proliferative effects on colonic epithelial cells.18
Catalytic Effect of ACE2 in IBD
In addition to its physiological role, ACE2 participates in pathological mechanisms in the GI tract, and there is evidence from both experimental and clinical studies of ACE2 activity having a role in the pathophysiology of IBD. This has been shown in studies with pharmacological or genetic manipulations and through the effects of Ang II and Ang (1–7) (Fig. 1).
Clinical studies evaluating ACE2 in IBD are just starting to be published. A recent proteomic analysis of intestinal tissue found no alteration in ACE2 between samples from patients with IBD or control patients, although the authors reported a higher level of ACE2 and ACE in patients with CD than in those with UC.19 Recently, Garg, Royce et al12 characterized the RAS in samples from patients with IBD. They found similar mRNA levels of ACE2 between intestinal samples from healthy control patients and patients with IBD but higher ACE2 protein levels (and the ACE2/ACE ratio) in samples from patients with IBD,12 suggesting that the inflammatory trigger differentially regulates transcription and translation. In addition, ACE2 was found to be more expressed in intestinal samples from noninflamed regions of patients with IBD than in those from healthy control patients, and this finding was associated with a higher expression of Ang (1–7).12 Moreover, whereas the intestinal expression of ACE2 inversely correlated with fibrosis, ACE and fibrosis were positively correlated.12 Finally, those authors found that plasma ACE2 activity20 of the ACE2/ACE ratio12 was increased in patients with IBD and that the ACE2/ACE activity ration was related to inflammation, because the difference from the control patients was blunted when adjusted to fecal calprotectin levels, a marker of intestinal inflammation.
Overall, although these data require more explanation, the findings of Garg et al12 suggest a reduction in the functional activity of mucosal ACE2 particularly in the areas of active inflammation, prompting a decrease in residual Ang 1–7 levels but an increase in circulating ACE2 activity. This variation may reflect the shedding of mucosal ACE2 (forming sACE2) by ADAM 17, which is highly expressed in inflammation presumably to promote resolution.12
Few studies have assessed the impact of RAS-blocking drugs (ACEi or ARB) on the outcomes of patients with IBD. One study enrolling 222 patients (minimum 6 months follow-up) showed that patients on RAS blockers had better outcomes (fewer hospitalizations, surgeries, or corticosteroid prescriptions) than patients who were not under RAS blockade therapy.21 Similarly, another study (296 patients, minimum follow-up of 2 years)12 showed that concomitant treatment with RAS blockers was associated with fewer hospitalizations—that is, less need for surgery. However, this study failed to identify differences in those outcomes when ACEi and ARB were introduced to a cohort of individuals who were followed up for 6 months.21 A study that enrolled more patients (764 patients, of whom 104 were on ACEi or ARB)22 reported a tendency toward lower rates of disease-related adverse outcomes (rate of flares; need for corticosteroid treatment, hospitalization, or surgery). Although none of these studies related ACE2 levels and/or their activity with the outcomes or use of RAS-blocking drugs, both ACEi and ARB are associated with increased ACE2 activity (lower Ang II availability). Further clinical trials are needed, particularly with more representative populations of patients with IBD, to clarify the usefulness of ACEi or ARB in IBD management and to explore the possibility of ACE2 being a prognostic marker.
Preclinical data with RAS-blocking drugs also show improvement in macroscopic and microscopic alterations and control of inflammation, oxidative stress, and apoptosis.23 In addition, Khajah et al16 described enhanced ACE2/Ang 1–7/Mas expression in a dextran sodium sulfate (DSS)-induced experimental model of colitis and colitis improvement after Ang 1–7 administration, suggesting activation of the counter-regulatory arm of the RAS with protective effects. Furthermore, although genetic deletion of ACE2 causes minor24 or no25 morphological and ultrastructure alterations in mice, it promotes a massive inflammatory reaction when IBD is experimentally induced with DSS.25
Given all the data mentioned above, one could say that ACE2 has a protective role in clinical or experimental IBD and that its pharmacological or genetic blockade would worsen colitis. Intriguingly, one study points to the opposite conclusion. In the DSS model of experimental colitis, pharmacological inhibition of ACE2 with (S,S)-2-(1-Carboxy-2-(3-(3,5-dichlorobenzyl)-3H-imidazol-4-yl)-ethylamino)-4-methylpentanoic acid (GL1001) attenuated the severity of experimental colitis, improved the pathologic alterations, and decreased myeloperoxidase levels in a similar manner as sulfasalazine treatment.26
Other substrates of ACE2 are also associated with IBD. Ghrelin has a protective anti-inflammatory role in experimental colitis,27 and its levels are increased in patients with IBD with active disease, likely to control the TNF-α-mediated inflammatory response.28 As such, ghrelin receptor agonists are being tested as a new therapeutic approach in IBD17. In addition, apelin production is increased in experimental and clinical IBD18 and probably contributes to tissue repair because it stimulates the proliferation of colonic epithelial cells.18
Thus, considering the scarce data available to date, ACE2 may exert a dual role in IBD: a protective role in the RAS because it decreases the levels of predominantly proinflammatory Ang II, and a deleterious role in the case of ghrelin and apelin levels because ACE2 metabolizes these anti-inflammatory/repairing elements.
ACE2 AS CORONAVIRUS RECEPTOR—ARE PATIENTS WITH IBD AT INCREASED RISK?
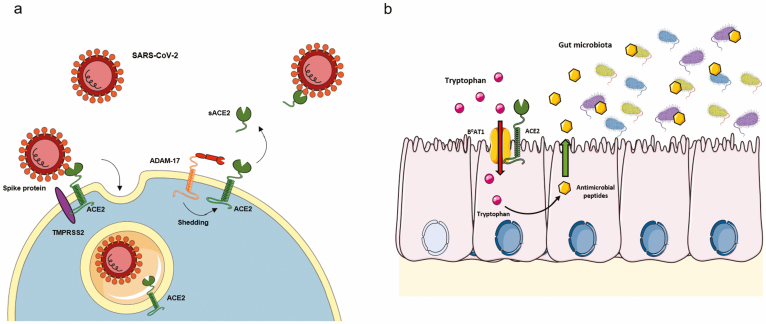
Research has shown that ACE2 is the binding receptor for coronaviruses including SARS-CoV (Fig. 3A)29 and SARS-CoV-2, currently causing the COVID-19 pandemic.1 Coronaviruses are single-strand RNA viruses with a bilayer envelope with multiple surface spike glycoproteins.30 The S1 and S2 subunits of these spike proteins are essential for host cell entry: the S1 subunit allows interactions with the host cell, and the S2 subunit promotes membrane fusion, resulting in the release of the virion into the host cell cytosol (Fig. 3A).30 Transgenic mice overexpressing human ACE2 are more susceptible to SARS-CoV infection and develop more severe disease than the correspondent wild-type controls.31 The binding site for the SARS-CoV and SARS-CoV-2 spike protein is located at the N-terminal domain of ACE2, close to the catalytic site (Fig. 3A),32 but the binding of coronaviruses to ACE2 does not affect its catalytic peptidase activity. Indeed, researchers have assessed that cells expressing catalytic inactive mutants of ACE2 can still be infected by SARS-CoV and that ACE2 substrates can still interact with the catalytic pocket of ACE2 when the spike protein of the coronaviruses is bounded, which has also been shown in the structure of SARS spike protein-bound ACE2.33 Interestingly, the interaction between SARS-CoV-2 and ACE2 is stronger than that between SARS-CoV and ACE2,34 which may explain why COVID-19 quickly affected all continents, being declared a pandemic by the World Health Organization on March 11, 2020 (https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200301-sitrep-51-COVID-19.pdf).
FIGURE 3.
Noncatalytic functions of ACE2. A, ACE2 as a receptor for SARS-CoV-2. Upon binding, both SARS-CoV-2 and ACE2 are internalized. In the shedding of membrane ACE2, producing sACE2 may be a strategy to avoid SARS-CoV-2 infecting the cell. B, ACE2 as a chaperone for the B0AT1 neutral amino acid receptor. The metabolism of tryptophan is essential in maintaining gut microbiota, controlling gut inflammation.
In addition, the mechanism associated with viral entry to the host cell may be associated with disease prognosis. Research has shown that ACE2 can be internalized together with the receptor-binding domain of the spike protein of SARS-CoV-2 (Fig. 3A).35 As a result, ACE2 is removed from the cell membrane, disrupting the balance between the classic and counter-regulating arms of the RAS in favor of the ACE/Ang II/AT1R axis.36 This process will perpetuate Ang II-mediated effects such as vasoconstriction, inflammation, and fibrosis, possibly leading to severe acute lung failure.37 Heurich et al35 observed that TMPRSS2, a transmembrane serine protease, facilitates viral internalization while cleaving ACE2 somewhere in its C’-domain, forming an intracellular fragment that signals for internalization (Fig. 3A). Meanwhile, ADAM17 (competing with TMPRSS2) can facilitate38 or cannot facilitate35 the internalization of the coronavirus, but it sheds ACE2 to the extracellular space, where ACE2 retains its catalytic activity and may function as a decoy receptor for the coronavirus.38 Therefore, it is possible that the expression of ACE2 and of TMPRSS2 and ADAM17 determines whether patients with COVID-19 will have mild disease or progress to multiorgan dysfunction. Further studies are needed to understand if the cleavage of ACE2 and its consequent downregulation upon infection may contribute to SARS pathogenesis.
The broad expression of ACE2 may also justify some less-frequent signs of COVID-19. For instance, ACE2 has already been found in the salivary glands39 and saliva,40 and SARS-CoV-2 can cause sialadenitis.35 Recently, the presence of SARS-CoV-2 mRNA has been detected in stool41 and anal/rectal swabs,42 for long periods of time.43 This finding may explain some GI symptoms reported in patients with COVID-19 such as diarrhea, vomiting, and abdominal pain.44 Moreover, it opens the door for potential fecal-oral transmission, even though this event has not yet been reported.
Risk for COVID-19 in Patients With IBD
The COVID-19 pandemic has imposed changes on the treatment and follow-up of patients with IBD to minimize the increased risk of infection associated with some IBD treatments.45 For example, in Beijing, alternative methods of communication with patients with IBD were adopted to assure proper follow-up of their disease course.46 Similarly, the IBD unit from the Centro Hospitalar São João, in Oporto, Portugal, has set up a strategy to minimize the risk of infection.47 More recently, Su et al48 specified a list of recommendations regarding the clinical management of patients with IBD during this pandemic that should be adapted and followed by physicians worldwide. A study with 522 patients with IBD from Bergamo (Italy) found no patients with COVID-19.49 Mazza et al50 reported the first COVID-19 pneumonia death in a female patient aged 80 years with left-sided UC. In addition, as of March 26, 2020, 7 patients with pediatric IBD and COVID-19 were reported by the Pediatric IBD Porto group of the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHN).51 Considering the increased expression of ACE2 in the epithelial cells of noninflamed areas of patients with IBD, 12 these patients may be more likely to be infected with SARS-CoV-2. However, in 3 mouse models of colitis, epithelial ACE2 and TMPRSS2 were found to be stable or downregulated in the colonic tissue.52 Furthermore, in colonic biopsies from patients with UC and with CD, ACE2 and TMPRSS2 levels were similar to those of control patients,52 suggesting that patients with IBD are not more susceptible than patients without IBD to SARS-CoV-2 infection.
The SECURE-IBD database facilitates the reporting and monitoring of outcomes of COVID-19 occurring in patients with IBD. Indeed, as of August 1, 2020, 1830 patients with IBD (55% with CD, 45% with UC) who were infected with SARS-CoV-2 were reported according to the database. Notably, 56% of the reported patients with SARS-CoV-2 infection were patients with IBD in remission. In addition, a recent study supported the SECURE-IBD initiative, having assessed that age, smoking, and disease status are related to ACE2 and TMPRSS2 expression and potential risk factors of susceptibility to COVID-19 in patients with IBD.53
In addition, patients with IBD patients have been, globally speaking, advised not to change their current medication. However, a recent study reported that TNF-α antagonists may have a protective effect against severe COVID-19 and that 5-aminosalicylates/sulfasalazine could be potentially harmful to patients with IBD regarding SARS-CoV-2 infection.54 However, Magro, Dias, et al55 pointed out some methodological drawbacks of that study and emphasized that data should be interpreted with care and need further studies. Indeed, the mechanism of action of 5-ASAs includes binding to peroxisome proliferator–activated receptor gamma, which is present in the intestinal epithelium and increases the expression of ACE2 but also decreases the expression of TMPRSS2.
Much controversy has been raised around ACEi and ARB and their putative detrimental or beneficial effect in the setting of COVID-19.9 Some animal studies reported an upregulation of ACE2 after treatment with ACEi or ARB, justifying the concern that taking such medications could promote viral entrance, leading to exacerbated viral load in cells and higher susceptibility to COVID-19.56 Animal studies are predominantly directed to quantifying tissue expression of ACE2 and not as much the circulating/soluble form of ACE2, which despite being cleaved from the cell surface preserves its protective catalytic activity.9 Nevertheless, the downregulation of ACE2 induced by SARS-CoV-2 infection can be very detrimental because it can compromise the generation of beneficial peptides, such as Ang (1–7). However, there are no targeted studies in humans to confirm or reject this hypothesis. An Italian study with 6272 patients revealed that the use of ACEi or ARB was more frequent among participants with COVID-19 than in noninfected matched controls, but there was no associated risk with COVID-19 disease.57 Considering the existing evidence, cardiology and hypertension societies worldwide have supported the maintenance of previously prescribed ACEi and ARB, unless there is an alternative clinical reason for suspension.58
There is accumulating evidence that RAS dysfunction may have a central role in the pathophysiology of COVID-19, with Ang II contributing to organ injury.59 Indeed, the outcomes of COVID-19 have been reportedly worse in patients with diabetes, hypertension, and cardiovascular disease,11 conditions associated with reduced baseline levels of ACE2 expression.59 Research has shown that Ang II promotes pulmonary vasoconstriction and inflammatory organ damage, ultimately progressing to acute lung injury.60 In addition, in vitro studies have revealed that sACE2 may be a competitive interceptor of SARS-CoV-2, thereby preventing the binding of the viral particle to the mucosal full-length ACE2, contributing to decreasing the probability of infection (Fig. 3A).61 This condition would also preserve membrane-bounded ACE2 and its associated beneficial actions. The administration of recombinant sACE2 reduced Ang II levels in a phase 2 trial enrolling patients with acute respiratory distress syndrome62 and reversed lung-injury processes in preclinical models of other viral infections.63 The downregulated ACE2 may also decrease cardioprotection, favoring COVID-19-associated myocardial injury.64 Therefore, ACE2 can be protective in patients with COVID-19,65 particularly in those patients with severe lung injury. In this context, the results of ongoing clinical trials assessing the safety and efficacy of RAS modulators (including ACEi, ARB, and recombinant human sACE2) in the setting of COVID-19 are eagerly awaited.
Overall, the current evidence suggests that high levels of ACE2 may increase SARS-CoV-2 infection, but on the other hand they may benefit the course of the disease by limiting the deleterious effects of Ang II. As noted earlier, the balance between the expression of ADAM17 and TMPRSS2 is also a key point in this puzzle.
ACE2 AS A REGULATOR OF AMINO ACID TRANSPORT—A BASE FOR MALNUTRITION IN IBD?
Part of the amino acids needed for normal functions comes from the diet and is absorbed and metabolized by enterocytes of the small intestine.66 The gut epithelial barrier is the first line of defense against harmful pathogens producing antimicrobial proteins,66 for example, and is important for protein metabolism and host immune responses. As such, malnutrition represents a risk for developing uncontrolled immune responses and, possibly, immune disorders of the GI tract, as in IBD.
Tryptophan is an essential neutral amino acid that is mainly derived from the diet, and its metabolism is crucial for maintenance of gut microbiota, gut-brain axis functionality, and immune response.67 Relevant to this process are probiotics, which exert important beneficial effects on tryptophan metabolism because they directly convert tryptophan in serotonin.68
The question of how ACE2 relates to these conditions remains. Several studies using different approaches have support the notion that ACE2 is an auxiliary protein for the expression of B0AT1, a neutral amino acid transporter (Fig. 3B).69 Kowalczuk et al69 showed that ACE2 markedly increases intestinal B0AT1 activity, trafficking it to the apical surface, where both proteins collaborate to digest peptides and transport the resultant amino acids. Furthermore, it has been possible to identify ACE2 as the specific auxiliary protein of B0AT1 in the small intestine of the mouse using immunofluorescence, co-immunoprecipitation, and functional data.70 In ACE2-knockout mice, the expression of B0AT1 mRNA was not affected but protein expression was decreased, as were serum levels of neutral amino acids.25 In these mice, the induction of colitis with DSS was associated with a more severe disease and tryptophan administration was able to improve the colitis.25 Notably, treatment with sACE2 did not reduce the severity of colitis, suggesting that anchorage to the cell membrane (and the intracellular domain) are crucial for tryptophan absorption.25 Finally, Hashimoto et al25 described that susceptibility to colitis increased in mice subjected to a tryptophan-free diet and that administration of Glycyl-tryptophan (to bypass the loss of B0AT1) restored serum tryptophan levels and reduced colitis susceptibility.
Thus, evidence suggests that ACE2 plays a key role in the expression of B0AT1 and, therefore, in tryptophan absorption and intestinal inflammation. Regarding IBD, alterations in tryptophan metabolism have been associated with its pathogenesis.68 In fact, fecal tryptophan levels are reduced in patients with IBD and negatively correlated with disease activity.71
ACE2 AS A LIGAND FOR INTEGRINS—A MECHANISM OF FIBROSIS IN IBD?
Integrins are heterodimeric transmembrane receptors that bind to extracellular matrix, cell-surface, or soluble ligands and signal to and from the cell cytoplasm.72 They are very important in cell processes such as inflammation because they play a role in gene expression and cell adhesion, proliferation, differentiation, migration, and apoptosis.72 In 2004, it was reported for the first time that ACE2 binds integrins. Indeed, Lin et al73 described the interaction between β1 and ACE2. The authors showed that ACE2 forms a complex with integrin β1 and indicated that the catalytic activity of ACE2 is preserved while in the complex. In a later study, Clarke et al74 confirmed the binding of ACE2 to integrin β1, unraveling an ACE2-integrin α5 binding. Moreover, they clarified that these interactions are independent of an RGD motif because ACE (which does not have an RGD motif) also binds to integrin α5. They further investigated whether membrane-bound ACE2 could act as a ligand for cell adhesion and observed that indeed, Hu7 cells adhere to cells overexpressing ACE2 more than to their mock transfected controls.74 Moreover, the authors observed that sACE2 significantly reduced the phosphorylation levels of FAK (a kinase involved in the primary steps of cell adhesion), and increased Akt expression levels, a prosurvival pro-proliferative protein.74 The study confirmed that ACE2 is a ligand for integrins capable of inducing integrin signaling, thus regulating cell adhesion.74 However, further studies are needed to clarify this regulatory effect and the particular role for ACE2 and sACE2.
Integrins are present in the human large and small intestine.75 Interactions between integrin integrin alpha 4 beta 7 and the microvascular cell-adhesion molecule MAdCAM-1 or between integrin integrin alpha 4 beta 1 and the microvascular cell-adhesion molecule VCAM-1 can induce chronic inflammation in the gut, leading to the migration of T-lymphocytes and the production of inflammatory mediators, such as interleukin-17 or interferon-γ.76 In patients with IBD, there is an upregulation of the microvascular cell-adhesion molecules ICAM-1, VCAM-1, and MadCAM-177, leading to an increased recruitment of α4 integrin-expressing leukocytes. On the other hand, in a recent study using a mouse model of IBD, the genetic deletion of the β7 integrin or the antibody blockade of an α4β7-MAdCAM-1 interaction worsened colitis.78
Given their relevance in intestinal inflammation, integrins have become a target for IBD therapy. The first drug targeting integrins approved for IBD was vedolizumab, a monoclonal antibody directed against the α4β7 heterodimer, which shows clinical efficacy and effectiveness comparable to anti-TNF-α antibodies.79 Currently, several other molecules targeting α4 or β7 integrin or the α4β7 heterodimer are under clinical development or have been recently approved. Research has reported that sACE2 suppressed integrin signaling by lowering the proportion of phosphorylated Akt,74 which is usually increased in the intestinal mucosa of patients with UC.80 This finding seems to suggest that ACE2, through the modulation of integrin pathways, may have a role in the treatment of IBD, but further studies are required to explore this hypothesis.
CONCLUSIONS
Knowledge of the physiologic and pathophysiologic roles of ACE2 is still scarce. Its catalytic activity is well characterized in the cardiovascular and renal systems, but little information exists regarding other organ systems, such as the GI tract. In addition, an understanding of the role of the ACE2/Ang 1–7/Mas axis in pathologic conditions was classically limited to cardiovascular diseases. Notwithstanding this research, the view for ACE2 as a multifunctional protein has achieved importance recently. The current COVID-19 pandemic has highlighted the role of ACE2 as a receptor for coronaviruses, but research is needed to understand whether ACE2 levels contribute to the pathogenesis of COVID-19 or could benefit the course of the disease by decreasing the deleterious effects of Ang II. In addition, the association between ACE2, intestinal amino acid transport, and IBD deserves further attention with the expectation of better understanding the malnutrition that is common in patients with IBD. Finally, the binding of ACE2 to integrins raises concerns and hopes, although by the time of this review there were only 2 published articles on the subject.
In conclusion, exploring the multifunctional roles of ACE2 in IBD (by characterizing its expression/activity in the blood, gut, and/or feces of patients with IBD and healthy control patients) will deepen the knowledge about the pathophysiology of this disease. In line with this goal, the identification of a new biomarker of disease activity, treatment response, and new drug target, thereby triggering the development of new therapeutic options, is expected to impact patient care.
ACKNOWLEDGMENTS
The authors thank Rita Faria for the precious contribution with scientific design.
Supported by: This work was supported by Fundação para a Ciência e a Tecnologia, under the Partnership Agreement (UIDB 50006/2020 and SFRH/BD/145654/2019 to MFD). Also, MM thanks Grupo de Estudos da Doença Inflamatória Intestinal for funding.
Conflicts of interest: FM served as speaker and received honoraria from AbbVie, Biogen, Falk, Ferring, Hospira, Janssen, Laboratorios Vitoria, Merck Sharp & Dohme, Pfizer, Takeda, Sandoz, and UCB. The other authors have no competing interests to declare.
REFERENCES
- 1. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crowley SD, Coffman TM. Recent advances involving the renin-angiotensin system. Exp Cell Res. 2012;318:1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chappell MC, Brosnihan KB, Diz DI, et al. Identification of angiotensin-(1-7) in rat brain. Evidence for differential processing of angiotensin peptides. J Biol Chem. 1989;264:16518–16523. [PubMed] [Google Scholar]
- 5. Tipnis SR, Hooper NM, Hyde R, et al. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. [DOI] [PubMed] [Google Scholar]
- 6. Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–E9. [DOI] [PubMed] [Google Scholar]
- 7. Vickers C, Hales P, Kaushik V, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. [DOI] [PubMed] [Google Scholar]
- 8. Lambert DW, Yarski M, Warner FJ, et al. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem. 2005;280:30113–30119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murray E, Tomaszewski M, Guzik TJ. Binding of SARS-CoV-2 and angiotensin-converting enzyme 2: clinical implications. Cardiovasc Res. 2020;116: e87–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garg M, Angus PW, Burrell LM, et al. Review article: the pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment Pharmacol Ther. 2012;35:414–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garg M, Royce SG, Tikellis C, et al. Imbalance of the renin-angiotensin system may contribute to inflammation and fibrosis in IBD: a novel therapeutic target? Gut. 2020;69:841–851. [DOI] [PubMed] [Google Scholar]
- 13. Shorning BY, Jardé T, McCarthy A, et al. Intestinal renin-angiotensin system is stimulated after deletion of Lkb1. Gut. 2012;61:202–213. [DOI] [PubMed] [Google Scholar]
- 14. Zhao Z, Sakai T. Characteristic features of ghrelin cells in the gastrointestinal tract and the regulation of stomach ghrelin expression and production. World J Gastroenterol. 2008;14:6306–6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang Z, Luo X, Liu M, Chen L. Function and regulation of apelin/APJ system in digestive physiology and pathology. J Cell Physiol. 2019;234:7796–7810. [DOI] [PubMed] [Google Scholar]
- 16. Khajah MA, Fateel MM, Ananthalakshmi KV, et al. Anti-inflammatory action of angiotensin 1-7 in experimental colitis. PLoS One. 2016;11:e0150861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanger GJ. Motilin, ghrelin and related neuropeptides as targets for the treatment of GI diseases. Drug Discov Today. 2008;13:234–239. [DOI] [PubMed] [Google Scholar]
- 18. Han S, Wang G, Qiu S, et al. Increased colonic apelin production in rodents with experimental colitis and in humans with IBD. Regul Pept. 2007;142:131–137. [DOI] [PubMed] [Google Scholar]
- 19. Ning L, Shan G, Sun Z, et al. Quantitative proteomic analysis reveals the deregulation of nicotinamide adenine dinucleotide metabolism and CD38 in inflammatory bowel disease. Biomed Res Int. 2019;2019:3950628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garg M, Burrell LM, Velkoska E, et al. Upregulation of circulating components of the alternative renin-angiotensin system in inflammatory bowel disease: a pilot study. J Renin Angiotensin Aldosterone Syst. 2015;16:559–569. [DOI] [PubMed] [Google Scholar]
- 21. Jacobs JD, Wagner T, Gulotta G, et al. Impact of angiotensin II signaling blockade on clinical outcomes in patients with inflammatory bowel disease. Dig Dis Sci. 2019;64:1938–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fairbrass KM, Hoshen D, Gracie DJ, et al. Effect of ACE inhibitors and angiotensin II receptor blockers on disease outcomes in inflammatory bowel disease. Gut. 2020:gutjnl-2020-321186. [Online ahead of print] doi: 10.1136/gutjnl-2020-321186. [DOI] [PubMed] [Google Scholar]
- 23. Arab HH, Al-Shorbagy MY, Abdallah DM, et al. Telmisartan attenuates colon inflammation, oxidative perturbations and apoptosis in a rat model of experimental inflammatory bowel disease. PLoS One. 2014;9:e97193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu C, Xiao L, Li F, et al. Generation of outbred Ace2 knockout mice by RNA transfection of TALENs displaying colitis reminiscent pathophysiology and inflammation. Transgenic Res. 2015;24:433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hashimoto T, Perlot T, Rehman A, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Byrnes JJ, Gross S, Ellard C, et al. Effects of the ACE2 inhibitor GL1001 on acute dextran sodium sulfate-induced colitis in mice. Inflamm Res. 2009;58:819–827. [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130:1707–1720. [DOI] [PubMed] [Google Scholar]
- 28. Peracchi M, Bardella MT, Caprioli F, et al. Circulating ghrelin levels in patients with inflammatory bowel disease. Gut. 2006;55:432–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li W, Moore MJ, Vasilieva N, et al. ACE2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Docea AO, Tsatsakis A, Albulescu D, et al. A new threat from an old enemy: re-emergence of coronavirus (review). Int J Mol Med. 2020;45:1631–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang XH, Deng W, Tong Z, et al. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp Med. 2007;57:450–459. [PubMed] [Google Scholar]
- 32. Prabakaran P, Xiao X, Dimitrov DS. A model of the ACE2 structure and function as a SARS-CoV receptor. Biochem Biophys Res Commun. 2004;314:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li F, Li W, Farzan M, et al. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. [DOI] [PubMed] [Google Scholar]
- 34. Chen Y, Guo Y, Pan Y, et al. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020;525:135–140. [Online ahead of print] doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heurich A, Hofmann-Winkler H, Gierer S, et al. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88:1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verdecchia P, Cavallini C, Spanevello A, et al. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haga S, Nagata N, Okamura T, et al. TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds. Antiviral Res. 2010;85:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu L, Wei Q, Alvarez X, et al. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J Virol. 2011;85:4025–4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. To KK, Tsang OT, Chik-Yan Yip C, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71:841–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Holshue ML, DeBolt C, Lindquist S, et al. ; Washington State 2019-nCoV Case Investigation Team First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu Y, Guo C, Tang L, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fiorino G, Allocca M, Furfaro F, et al. Inflammatory bowel disease care in the COVID-19 pandemic era: the Humanitas, Milan experience. J Crohns Colitis. 2020;14:1330–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bai X, Yang H, Qian J. COVID-19 outbreak and inflammatory bowel disease management: a questionnaire survey from realistic practice. J Crohns Colitis. 2020, jjaa064. [Online ahead of print] doi: 10.1093/ecco-jcc/jjaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Magro F, Abreu C, Rahier JF. The daily impact of COVID-19 in gastroenterology. United Eur Gastroent J. 2020;. 8:520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Su S, Shen J, Zhu L, et al. Involvement of digestive system in COVID-19: manifestations, pathology, management and challenges. Therap Adv Gastroenterol. 2020;13:1756284820934626. doi: 10.1177/1756284820934626. eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Norsa L, Indriolo A, Sansotta N, et al. Uneventful course in IBD patients during SARS-CoV-2 outbreak in northern Italy. Gastroenterology. 2020;159:371–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mazza S, Sorce A, Peyvandi F, et al. A fatal case of COVID-19 pneumonia occurring in a patient with severe acute ulcerative colitis. Gut. 2020;69:1148–1149. [DOI] [PubMed] [Google Scholar]
- 51. Turner D, Huang Y, Martin-de-Carpi J, et al. COVID-19 and paediatric inflammatory bowel diseases: global experience and provisional guidance (March 2020) from the Paediatric IBD Porto group of ESPGHAN. J Pediatr Gastroenterol Nutr. 2020;70:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Burgueño JF, Reich A, Hazime H, et al. Expression of SARS-CoV-2 entry molecules ACE2 and TMPRSS2 in the gut of patients with IBD. Inflamm Bowel Dis. 2020;26:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Krzysztof NJ, Christoffer LJ, Rahul K, et al. Age, inflammation and disease location are critical determinants of intestinal expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in inflammatory bowel disease. Gastroenterology. 2020;159:1151–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Magro F, Dias CC, Morato M. Aminosalicylates and COVID-19: facts or coincidences? Gastroenterology. 2020:S0016-5085(20)34785-5. [Online ahead of print] doi: 10.1053/j.gastro.2020.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sommerstein R, Kochen MM, Messerli FH, et al. Coronavirus disease 2019 (COVID-19): do angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J Am Heart Assoc. 2020;9:e016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mancia G, Rea F, Ludergnani M, et al. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. 2020;323:1769–1770. [DOI] [PubMed] [Google Scholar]
- 59. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020;81:537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang H, Penninger JM, Li Y, et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Monteleone G, Ardizzone S. Are patients with inflammatory bowel disease at increased risk for Covid-19 infection? J Crohns Colitis. 2020;14:1334–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Khan A, Benthin C, Zeno B, et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zou Z, Yan Y, Shu Y, et al. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 2014;5:3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vaduganathan M, Vardeny O, Michel T, et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Farre R, Fiorani M, Abdu Rahiman S, et al. Intestinal permeability, inflammation and the role of nutrients. Nutrients. 2020;12:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kałużna-Czaplińska J, Gątarek P, Chirumbolo S, et al. How important is tryptophan in human health? Crit Rev Food Sci Nutr. 2019;5:802–810 [DOI] [PubMed] [Google Scholar]
- 68. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–724. [DOI] [PubMed] [Google Scholar]
- 69. Kowalczuk S, Bröer A, Tietze N, et al. A protein complex in the brush-border membrane explains a Hartnup disorder allele. Faseb J. 2008;22: 2880–2887. [DOI] [PubMed] [Google Scholar]
- 70. Camargo SM, Singer D, Makrides V, et al. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with Hartnup mutations. Gastroenterology. 2009;136:872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nikolaus S, Schulte B, Al-Massad N, et al. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology. 2017;153:1504–1516.e2. [DOI] [PubMed] [Google Scholar]
- 72. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. [DOI] [PubMed] [Google Scholar]
- 73. Lin Q, Keller RS, Weaver B, et al. Interaction of ACE2 and integrin beta1 in failing human heart. Biochim Biophys Acta. 2004;1689:175–178. [DOI] [PubMed] [Google Scholar]
- 74. Clarke NE, Fisher MJ, Porter KE, et al. Angiotensin converting enzyme (ACE) and ACE2 bind integrins and ACE2 regulates integrin signalling. PLoS One. 2012;7:e34747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Choy MY, Richman PI, Horton MA, et al. Expression of the VLA family of integrins in human intestine. J Pathol. 1990;160:35–40. [DOI] [PubMed] [Google Scholar]
- 76. Dotan I, Allez M, Danese S, et al. The role of integrins in the pathogenesis of inflammatory bowel disease: approved and investigational anti-integrin therapies. Med Res Rev. 2020;40:245–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Danese S. Role of the vascular and lymphatic endothelium in the pathogenesis of inflammatory bowel disease: “brothers in arms.” Gut. 2011;60:998–1008. [DOI] [PubMed] [Google Scholar]
- 78. Sun H, Kuk W, Rivera-Nieves J, et al. β7 integrin inhibition can increase intestinal inflammation by impairing homing of CD25hiFoxP3+ regulatory T cells. Cell Mol Gastroenterol Hepatol. 2020;9:369–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rogler G. Mechanism of action of vedolizumab: do we really understand it? Gut. 2019;68:4–5. [DOI] [PubMed] [Google Scholar]
- 80. Cahill CM, Rogers JT, Walker WA. The role of phosphoinositide 3-kinase signaling in intestinal inflammation. J Signal Transduct. 2012;2012:358476. [DOI] [PMC free article] [PubMed] [Google Scholar]