Abstract
Background
The combination of sofosbuvir and daclatasvir has a well-established safety profile and improves clinical outcomes in HCV patients. In silico and in vitro studies suggest that sofosbuvir/daclatasvir may show antiviral activity against SARS-CoV-2.
Methods
Three clinical trials comparing sofosbuvir/daclatasvir-based regimens with a comparator in hospitalized COVID-19 patients were combined in a meta-analysis. The primary outcomes measured were clinical recovery within 14 days of randomization, time to clinical recovery and all-cause mortality. A two-step approach was used to analyse individual-level patient data. The individual trial statistics were pooled using the random-effects inverse-variance model.
Results
Our search identified eight studies of which three met the inclusion criteria (n = 176 patients); two studies were randomized and one was non-randomized. Baseline characteristics were similar across treatment arms. Clinical recovery within 14 days of randomization was higher in the sofosbuvir/daclatasvir arms compared with control arms [risk ratio = 1.34 (95% CI = 1.05–1.71), P = 0.020]. Sofosbuvir/daclatasvir improves time to clinical recovery [HR = 2.04 (95% CI = 1.25–3.32), P = 0.004]. The pooled risk of all-cause mortality was significantly lower in the sofosbuvir/daclatasvir arms compared with control arms [risk ratio = 0.31 (95% CI = 0.12–0.78), P = 0.013].
Conclusions
Available evidence suggests that sofosbuvir/daclatasvir improves survival and clinical recovery in patients with moderate to severe COVID-19. However, the sample size for analysis was relatively small, one of the trials was not randomized and the designs were not standardized. These results need to be confirmed in larger randomized controlled trials.
Introduction
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a positive-sense RNA virus. Repurposing existing pharmaceuticals that are effective against viruses with similar replication mechanisms to SARS-CoV-2 is an attractive short-term treatment strategy. Current clinical trials investigating treatments, such as remdesivir,1 hydroxychloroquine,2 chloroquine and lopinavir/ritonavir,3 are yet to show clear survival benefits.
Sofosbuvir and daclatasvir are well tolerated and effective direct-acting antivirals (DAAs) against HCV.4 Sofosbuvir has broad antiviral activity against other viruses including Zika5 and Dengue.6 Evidence from some in silico and in vitro studies suggests that sofosbuvir/daclatasvir and ribavirin bind to SARS-CoV-2 RNA-dependent RNA polymerase (‘RdRp’).7–9 Molecular docking experiments predicts that sofosbuvir and ribavirin bind to SARS-CoV-2 with similar, high binding energies (−7.5 and −7.8 kcal/mol, respectively).10 However, sofosbuvir and daclatasvir11 showed less promise in another in vitro study with little antiviral activity against SARS-CoV-2. In another study, daclatasvir exhibited low binding affinity for SARS-CoV-2 main protease (molecular docking score = −45.44).12 Sofosbuvir/daclatasvir is widely available in generic formulations costing $6 per 14 day treatment course of 400/60 mg (India).13
Given the potential for sofosbuvir/daclatasvir as a therapeutic option for COVID-19, the combination has been evaluated in several small clinical trials. We, therefore, carried out this meta-analysis to determine whether sofosbuvir/daclatasvir-based regimens improve clinical outcomes of patients with moderate or severe COVID-19.
Methods
Search strategy and selection criteria
Trials were eligible for inclusion if they compared a sofosbuvir/daclatasvir-based regimen with a comparator for the treatment of hospitalized individuals diagnosed with COVID-19. Given the anticipated small number of trials, we included both randomized and non-randomized trials. We systematically identified trials by reviewing the following clinical trials databases (searched 1 June 2020): clinicaltrials.gov, Cochrane Central Register of Controlled Trials (CENTRAL), WHO International Clinical Trials Registry Platform (ICTRP), and the Iranian Registry of Clinical Trials (IRCT). For relevant and completed studies, we obtained individual patient data from trial investigators.
The primary outcomes were (i) clinical recovery within 14 days of randomization, (ii) time to clinical recovery and (iii) all-cause mortality from randomization to end of follow-up. Clinical recovery was defined as the point at which the individual was deemed eligible for discharge from hospital as per the study-specific criteria; in studies where recovery was not defined, clinical recovery was assumed to be the day of hospital discharge. Secondary outcomes were duration of hospitalization (days) and a composite outcome of ICU admission or requirement for invasive mechanical ventilation (IMV).
Data analysis
Statistical analyses for all outcomes were conducted with individual patient data, on the ITT population. The analysis used a two-step method for all outcomes, first producing trial-specific estimates and then combining these to provide a pooled estimate of effect using standard meta-analysis techniques. Treatment effects were expressed as risk ratios (RRs) for binary outcomes and mean differences for continuous outcomes. For the survival outcome (time to recovery) we estimated (i) the cause-specific HRs for recovery using Cox proportional hazards models and (ii) the subdistribution HR (SHR) using the Fine and Gray14 competing risks model to account for death as a competing risk. For each outcome we pooled the individual trial statistics using the random-effects inverse-variance model; a continuity correction of 0.5 was applied to studies with zero cells. Heterogeneity was evaluated by I2. We planned a sensitivity analysis for the primary outcomes excluding non-randomized trials to account for the risk of bias. A second sensitivity analysis for the primary binary outcomes imputed the worst outcome for any individual randomized but not included in the ITT analyses of the respective studies (i.e. no clinical recovery and death). In a final sensitivity analysis to study the effects of non-random treatment assignment, we estimated the average treatment effect of sofosbuvir/daclatasvir on the binary outcomes (clinical recovery and death), using the inverse probability weighting (IPW) estimator and adjusting for age, sex and comorbidities (diabetes, hypertension and chronic pulmonary disease). The significance threshold was set at 5% (two-sided) and all analyses were conducted using Stata (version 14.2, StataCorp) and RStudio (version 3.5.3, R Foundation).
Results
Overall, eight studies were considered for inclusion; five did not meet the entry criteria [three were ongoing and use sofosbuvir either alone or in combination with alternative DAAs (velpatasvir or ledipasvir) and two were ongoing and enrolled outpatient populations]. Three studies met the inclusion criteria and all provided data for individual patients [n = 176 (92 intervention patients and 84 control patients)].15–17 All studies were conducted in Iran; two were randomized15,16 and one was non-randomized.17 Two assessed patients with severe disease and one assessed mild/moderate disease. The intervention arms in each trial received (i) sofosbuvir/daclatasvir+standard of care (SOC) (hydroxychloroquine ± lopinavir/ritonavir), (ii) sofosbuvir/daclatasvir+ribavirin and (iii) sofosbuvir/daclatasvir+hydroxychloroquine. The control groups received SOC at the time of the trial, which was (i) hydroxychloroquine ± lopinavir/ritonavir, (ii) hydroxychloroquine+lopinavir/ritonavir ± ribavirin and (iii) hydroxychloroquine+lopinavir/ritonavir+ribavirin, respectively (Table 1). Age and sex were generally balanced between arms. There was a tendency for a higher frequency of comorbidities in the control arms (however, this was not significant); vitals and laboratory findings were balanced (Table 2).
Table 1.
Characteristics of included studies
| Trial ID | Location | Design | n | Intervention | Control | Key inclusion criteria | Primary outcome |
|---|---|---|---|---|---|---|---|
| IRCT20200324 046850N217 | Abadan, Iran | non-randomized (single centre) | 62 | SOF/DCV +HCQ (n = 35) | HCQ+LPV/r +RBV (n = 27) |
(i) real-time PCR confirmed or abnormal chest CT (ii) hospitalized (iii) severe disease (O2 sat <94% or respiratory rate >24/min or decreased consciousness) |
time to hospital discharge |
| IRCT20200328 046886N115 | Sari, Iran | randomized (single centre) | 48 | SOF/DCV+RBV (n = 24) | HCQ+LPV/r±RBV (n = 24) |
(i) real-time PCR confirmed or abnormal chest CT (ii) hospitalized (iii) mild/moderate disease [fever (≥37.8°C) and at least one of respiratory rate <24/min, O2 sat >94%] (iv) ≤8 days since symptom onset |
duration of hospitalization |
| IRCT20200128 046294N216 | Tehran, Iran | randomized (multi centre) | 66 | SOF/DCV +HCQ±LPV/r (n = 33) | HCQ±LPV/r (n = 33) |
(i) real-time PCR confirmed and abnormal chest CT (ii) hospitalized (iii) severe disease [fever (≥37.8°C) and at least one of respiratory rate >24/min, O2 sat <94% or PaO2/FiO2 ratio <300 mgHg] (iv) ≤8 days since symptom onset |
clinical recovery within 14 days |
DCV, daclatasvir; HCQ, hydroxychloroquine; LPV/r, lopinavir/ritonavir; O2 sat, O2 saturation; RBV, ribavirin; SOF, sofosbuvir.
Table 2.
Combined baseline characteristics from the three clinical trials and outcomes in the ITT population
| Sofosbuvir/daclatasvir (n = 92) | Control (n = 84) | Pooled effect (95% CI)b | |
|---|---|---|---|
| Age (years), median (IQR) | 57 (42–68) | 61 (48–71) | |
| Male, n (%) | 48 (52) | 35 (42) | |
| Coexisting conditions, n (%) | |||
| diabetes | 31 (34) | 32 (38) | |
| hypertension | 24 (26) | 29 (35) | |
| chronic pulmonary disease | 8 (9) | 11 (13) | |
| Vitals on admission, median (IQR)a | |||
| O2 saturation (%) | 92 (90–93) | 91 (90–92) | |
| respiratory rate (breaths/min) | 22 (20–26) | 22 (20–26) | |
| Laboratory findings, median (IQR) | |||
| haemoglobin (g/dL) | 12 (11–14) | 12 (11–13) | |
| WBCs (×109/L) | 7 (5–9) | 8 (6–12) | |
| lymphocyte count (×109/L) | 14 (10–25) | 15 (7–25) | |
| AST (U/L) | 28 (21–43) | 32 (20–50) | |
| ALT (U/L) | 28 (16–35) | 27 (20–40) | |
| creatinine (mg/dL) | 1 (0.8–1.2) | 1 (0.8–1.2) | |
| Primary outcomes | |||
| clinical recovery within 14 days | 86/92 (93%) | 57/84 (68%) | 1.34 (1.05–1.71)c |
| time to clinical recovery (days), median (IQR) | 6 (5–8) | 8 (6–19) | 2.04 (1.25–3.32)d |
| all-cause mortality | 5/92 (5%) | 17/84 (20%) | 0.31 (0.12–0.78)c |
| Secondary outcomes | |||
| duration of hospitalization (days), median (IQR) | 6 (5–7) | 8 (6–11) | −0.56 (−0.86 to −0.26)e |
| ICU admission and/or IMV | 9 (10%) | 24 (29%) | 0.35 (0.18–0.69)c |
Percentages are calculated from non-missing values.
Vitals on admission were not available for the Sari study.
Pooled estimate from two-step meta-analysis (random effects).
RR (binary outcomes).
Cause-specific HR (time-to-event outcome).
Mean difference (continuous outcomes).
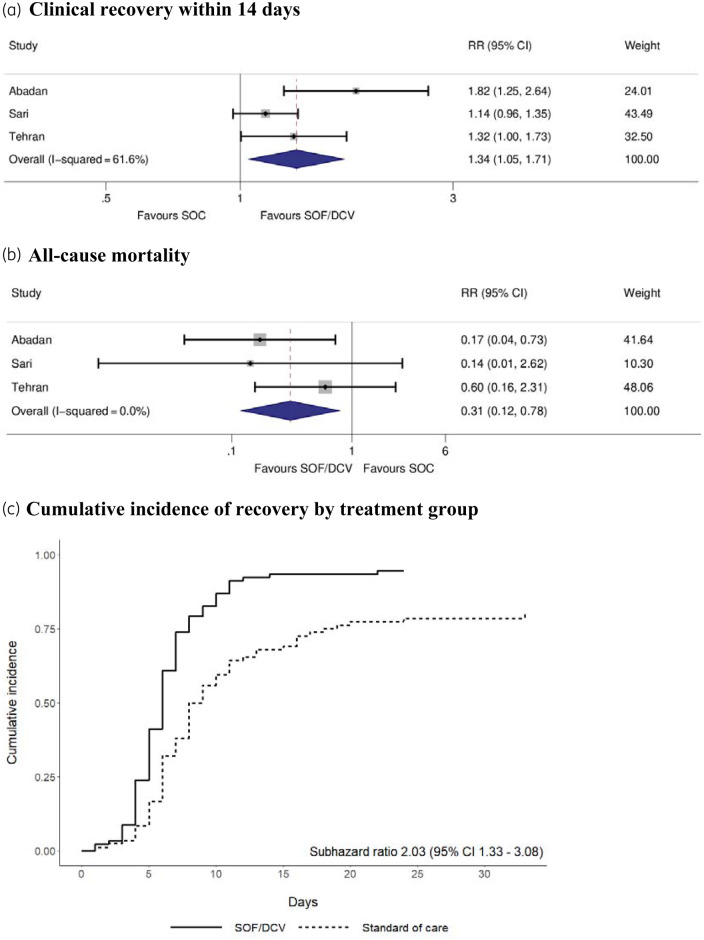
Overall, 86 (93%) of 92 patients in the sofosbuvir/daclatasvir arms and 57 (68%) of 84 patients in the control arms achieved clinical recovery within 14 days of randomization [RR = 1.34 (95% CI = 1.05–1.71), P = 0.020, I2 = 62%; Figure 1a]. Figure 1(c) shows the cumulative incidence of recovery by treatment group. The combined recovery-specific HR was 2.04 (95% CI = 1.25–3.32) (P = 0.004, I2 = 52%); similar results were observed in the competing risk analysis [SHR = 2.03 (95% CI = 1.33–3.08), P = 0.001, I2 = 51%]. Considering all-cause mortality, 5/92 (5%) in the sofosbuvir/daclatasvir arms and 17/84 (20%) in the control arms died whilst enrolled in a trial. The pooled risk of all-cause mortality was significantly lower in the sofosbuvir/daclatasvir arms compared with control arms [RR = 0.31 (95% CI = 0.12–0.78), P = 0.013, I2 = 0%; Figure 1b]. There were significant between-group differences in favour of sofosbuvir/daclatasvir for the secondary outcomes, duration of hospitalization and the composite of ICU admission and/or requirement for IMV (Table 1).
Figure 1.
Primary outcomes in the ITT population. (a and b) Forest plots for the relative risk of clinical recovery within 14 days and all-cause mortality, respectively. (c) Cumulative incidence of clinical recovery by treatment group and the SHR. All analyses were conducted including the three trials in the ITT population. DCV, daclatasvir; SOF, sofosbuvir. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
In sensitivity analyses, excluding non-randomized trials, the pooled RR for clinical recovery was 1.19 (95% CI = 1.03–1.37) (P = 0.019). The cause-specific HR for recovery was 1.63 (95% CI = 1.10–2.43) (P = 0.016). The difference in all-cause mortality was no longer significant [RR = 0.47 (95% CI = 0.14–1.58), P = 0.221]. In the second sensitivity analysis, one trial excluded four randomized individuals from the ITT population for not meeting inclusion criteria (two per arm). Imputing these as failures, the RR for clinical recovery was 1.34 (95% CI = 1.04–1.72) (P = 0.023) and for all-cause mortality was 0.35 (95% CI = 0.12–1.05) (P = 0.060). In the final sensitivity analysis, using the IPW estimator, the effect of sofosbuvir/daclatasvir on clinical recovery remained significant [average treatment effect (ATE) = 22.1% (95% CI = 0.9%–43.3%), P = 0.041]; however, the effect on all-cause mortality was not significant [ATE = −12.0% (95% CI = −25.3%–1.3%), P = 0.078].
Discussion
Our meta-analysis showed significant differences in clinical recovery and all-cause mortality in favour of sofosbuvir/daclatasvir regimens over the three trials included. The effect of sofosbuvir/daclatasvir on clinical recovery was sustained in sensitivity analyses.
This meta-analysis has several key limitations. Primarily, the number of studies and the overall population size are small. Caution should be taken in interpreting the results, which are subject to bias and potentially influential sampling error. Further, the meta-analysis can only be as reliable as the effect captured in the primary studies and cannot overcome problems inherent in the design and execution of individual trials. We included a non-randomized study and as such treatment effects may be confounded. The included studies were not fully blinded and therefore investigator bias is a possibility; however, managing a placebo-controlled clinical trial during a fast-moving pandemic remains challenging. Furthermore, the comparator arms across the included studies varied, reflecting the change in national guidelines in Iran during the course of the trials. Primary outcomes over studies were not uniform and no viral outcomes were measured. Viral load is an important parameter to demonstrate an effective antiviral therapy. Future studies should include viral load kinetics as a measure of clinical progress.
Ribavirin was administered in the treatment arm in one study.15 Ribavirin has predicted binding affinity for SARS-CoV-210 and therefore it is difficult to determine whether sofosbuvir/daclatasvir or ribavirin has a superior clinical benefit. Furthermore, a combination of antivirals may act synergistically against COVID-19. Hung et al.18 demonstrated that a triple combination of lopinavir/ritonavir, IFN-β-1b and ribavirin had a shorter time to negative nasopharyngeal swab than lopinavir/ritonavir alone.
In a sensitivity analysis excluding non-randomized trials and in methods adjusting for non-random treatment assignment, all-cause mortality was no longer significant. In the excluded non-randomized study, participants were allocated to either a sofosbuvir/daclatasvir or ribavirin arm.17 A retrospective cohort study, investigating ribavirin for the treatment of SARS, demonstrated that ribavirin is associated with greater adverse events.19 These adverse events may have contributed to the increased mortality measured in the ribavirin arm of this particular study.
More detailed in vitro studies are needed to evaluate whether sofosbuvir or daclatasvir is the most active agent in the sofosbuvir/daclatasvir combination or if there is synergistic activity. Use of sofosbuvir/daclatasvir in combination with other antivirals, such as favipiravir or atazanavir/ritonavir, might further improve efficacy. There are guidelines from the US FDA on optimal trial design.20 The recent approval of remdesivir to treat COVID-19 was based on several randomized trials in over 2000 patients. Similar sample sizes may be needed to establish the evidence to support worldwide approval of sofosbuvir/daclatasvir. If the results from this meta-analysis were confirmed in larger randomized trials, sofosbuvir/daclatasvir would be an important treatment option. The oral dosing, established safety profile, low costs of production and large-scale manufacture of sofosbuvir/daclatasvir could allow rapid expansion to worldwide use in the treatment of COVID-19.
Funding
This analysis was funded by an unrestricted grant from the International Treatment Preparedness Coalition.
Transparency declarations
All authors were involved in the original analysis of the clinical trials.
References
- 1. Beigel JH, Tomashek KM, Dodd LE. et al. Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med 2020; doi:10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 2. Tang W, Cao Z, Han M. et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 2020; 369: m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cao B, Wang Y, Wen D. et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; doi:10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Merat S. SD1000: high sustained viral response rate in 1361 patients with hepatitis C genotypes 1, 2, 3, and 4 using a low-cost, fixed-dose combination tablet of generic sofosbuvir and daclatasvir: a multicenter, Phase III clinical trial. Clin Infect Dis 2020; 70: 2206–12. [DOI] [PubMed] [Google Scholar]
- 5. Bullard-Feibelman KM, Govero J, Zhu Z. et al. The FDA-approved drug sofosbuvir inhibits Zika virus infection. Antiviral Res 2017; 137: 134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu H-T, Colby-Germinario SP, Hassounah SA. et al. Evaluation of sofosbuvir (β-D-2′-deoxy-2′-α-fluoro-2′-β-C-methyluridine) as an inhibitor of Dengue virus replication. Sci Rep 2017; 7: 6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jácome R, Campillo-Balderas JA, Ponce de León S. et al. Sofosbuvir as a potential alternative to treat the SARS-CoV-2 epidemic. Sci Rep 2020; 10: 9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chien M, Anderson TK, Jockusch S. et al. Nucleotide analogues as inhibitors of SARS-CoV-2 polymerase. bioRxiv 2020; doi:10.1101/2020.03.18.997585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beck BR, Shin B, Choi Y. et al. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput Struct Biotechnol J 2020; 18: 784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elfiky AA. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci 2020; 253: 117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lo HS, Hui KPY, Lai H-M. et al. Simeprevir suppresses SARS-CoV-2 replication and synergizes with remdesivir. bioRxiv 2020; doi:10.1101/2020.05.26.116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alabboud M, Javadmanesh A. In silico study of various antiviral drugs, vitamins, and natural substances as potential binding compounds with SARS-CoV-2 main protease. DYSONA - Life Sci 2020; 1: 44–63. [Google Scholar]
- 13. Hill A, Wang J, Levi J. et al. Minimum costs to manufacture new treatments for COVID-19. J Virus Erad 2020; 6: 61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 15. Kasgari HA, Moradi S, Shabani AM. et al. Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID-19 patients with moderate disease compared with standard care: a single-centre, randomized controlled trial. J Antimicrob Chemother 2020; doi:10.1093/jac/dkaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sadeghi A, Asgari AA, Norouzi A. et al. Sofosbuvir and daclatasvir compared with standard of care in the treatment of patients admitted to hospital with moderate or severe coronavirus infection (COVID-19): a randomized controlled trial. J Antimicrob Chemother 2020; doi:10.1093/jac/dkaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eslami G, Mousaviasl S, Radmanesh E. et al. The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe COVID-19. J Antimicrob Chemother 2020; doi:10.1093/jac/dkaa331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hung IF-N, Lung K-C, Tso EY-K. et al. Triple combination of interferon β-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 2020; 395: 1695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muller MP, Dresser L, Raboud J. et al. Adverse events associated with high-dose ribavirin: evidence from the Toronto outbreak of severe acute respiratory syndrome. Pharmacotherapy 2007; 27: 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. US FDA. COVID-19 Guidance for Industry: Developing Drugs and Biological Products for Treatment or Prevention. Centre for Drug Evaluation and Research, 2020; 1–11. [Google Scholar]