Abstract
Severe COVID-19 is a biphasic illness, with an initial viral replication phase, followed by a cascade of inflammatory events. Progression to severe disease is predominantly a function of the inflammatory cascade, rather than viral replication per se. This understanding can be effectively translated to changing our approach in managing the disease. The natural course of disease offers us separate windows of specific time intervals to administer either antiviral or immunomodulatory therapy. Instituting the right attack at the right time would maximize the benefit of treatment. This concept must also be factored into studies that assess the efficacy of antivirals and immunomodulatory agents against COVID-19.
Introduction
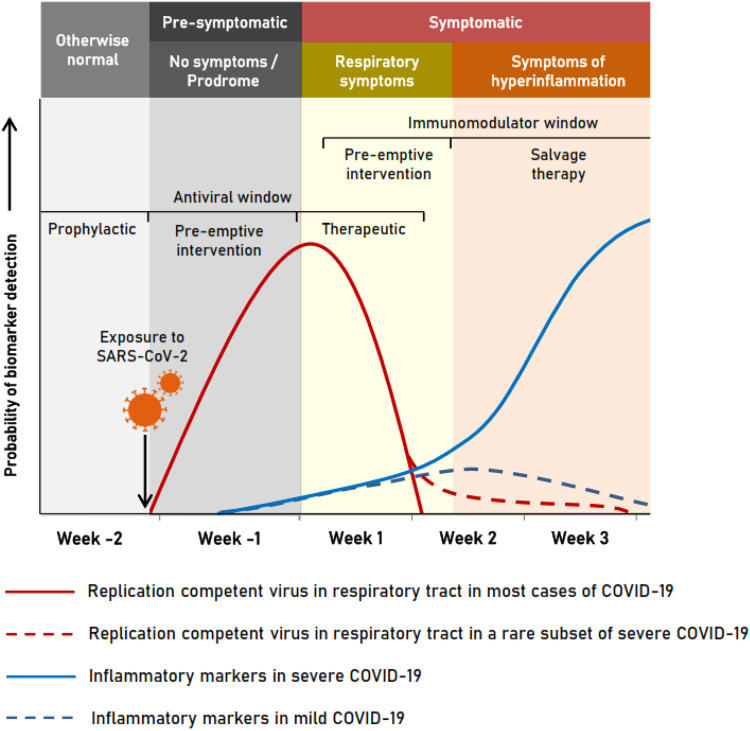
The dynamics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral replication and immune response in patients with coronavirus disease 2019 (COVID-19) continue to be elucidated. In the respiratory tract, viral replication occurs primarily during the first week of infection, when live virus can be isolated in culture, after which viral nucleic acid persists for variable durations in different anatomical sites.1 The vast majority of COVID-19 presents either as asymptomatic infections or as mild to moderate illness with fever and pulmonary and gastrointestinal symptoms, which resolve spontaneously or with minimal supportive care. Severe disease occurs only in a minority, due to exaggerated immune response, 5–7 days after symptom onset.2 However, the viral phase subsides quickly, after a brief overlap with the onset of the inflammatory phase, which either tapers down in mild illness or shoots up in serious disease.3,4 An analysis of the virus replication dynamics and course of inflammatory markers hints at the possibility of a virus prequel and an inflammatory sequel in serious disease, unraveling the window of opportunity to institute appropriate countermeasures to tackle the illness (Figure 1).
Figure 1.
Window of opportunity for managing COVID-19. Initial viraemia subsides early in the disease, followed by a rise of inflammatory markers, which subsides in mild illness but spikes in severe illness. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Using antivirals in the right window
Live virus has been isolated in culture only in the first week after symptom onset in mild to moderate illness, despite the persistence of viral RNA.5,6 Antivirals that showed excellent activity against SARS-CoV-2 in laboratory conditions have given disappointing results when used in severe disease. The classical example is of the novel antiviral remdesivir, which was considered for treating SARS-CoV-2 after an excellent antiviral effect was demonstrated when given as prophylaxis or within 12 h of Middle Eastern respiratory syndrome coronavirus (‘MERS-CoV’) infection in animal models.7 However, the randomized controlled trial (RCT) completed in 237 severe COVID-19 patients by Wang et al.,8 in which the median time to initiation of remdesivir from date of symptom onset was 10 days (IQR = 9–12 days), showed no clinical benefit. In a much larger RCT, completed by Beigel et al.9 in 1062 moderately ill patients, which only showed modest reduction in time to recovery, the median time to initiation of remdesivir from date of symptom onset was 9 days (IQR = 6–12 days). The antiviral combination lopinavir/ritonavir also faced a similar fate when used in severe COVID-19 patients.10 This trend was even evident with convalescent plasma therapy (CPT), where passive transfer of neutralizing antibodies did not reduce mortality in severe COVID-19 when compared with standard treatment in a small RCT of 103 patients, probably because the median time to institution of CPT was well beyond 3 weeks from symptom onset.11 In contrast, there is accumulating evidence about the plausible benefits of early administration of CPT.12
Consistent failure of antiviral strategies in serious illness shown by several studies reiterates that the virus is not directly involved in serious disease and employing measures to control viral replication at this time is futile, as pointed out by other authors as well.13 Siddiqi and Mehra3 have rightly pointed out the importance of using antivirals as early as possible in order to reap maximum benefit. The median time taken from the onset of symptoms to a patient presenting at a healthcare facility was found to be 5 days (range = 1–24 days).14 This is a serious practical difficulty when enrolling subjects for therapeutic trials, as the antiviral window may close by the eighth day (Figure 1).
Capitalizing on the immunomodulatory window
Beyond the replicative stage, inflammatory biomarkers may indicate the transition from viral replication to a hyperimmune-response phase in severe disease.3 Various pro-inflammatory cytokine levels are elevated, such as ILs (IL-1b, IL-6, IL-8 and IL-17), granulocyte colony-stimulating factor (‘G-CSF’), GM-CSF, macrophage inflammatory protein-1α (‘MIP-1α’), TNF-α, complement-5 (‘C5’) and others, reflecting the ongoing cytokine storm and subsequent multi-organ dysfunction and hypercoagulability, as evidenced by raised D-dimer along with C-reactive protein (‘CRP’). Decreased counts of CD4 and CD8 T cells, B cells and natural killer (‘NK’) cells along with raised neutrophil count elevate the neutrophil/lymphocyte ratio, which correlates with disease severity. Based on these and other factors, different scoring systems have been developed to predict the probability of patients progressing to severe disease.15 Such a score may be used as a predictor of the immunomodulatory window (Figure 1).
The results of the RECOVERY trial of dexamethasone show that immunosuppressive therapy improved clinical outcome only in patients requiring oxygen or ventilator support and not in others with milder illness, which suggests the need for identifying individuals requiring immunomodulatory therapy early.16 Another immunomodulatory drug commonly in use is tocilizumab, an IL-6 antagonist. A recent meta-analysis suggests that there can be a mortality benefit by using tocilizumab in severe COVID-19. These results are, however, based on observational studies, many of which had used co-medications, including glucocorticoids. Tocilizumab is also part of the RECOVERY trial, the results of which may throw light on its utility in relation to disease severity and timing of therapy.17
Overlapping antiviral and immunomodulatory windows
In a small proportion of severe COVID-19, replication-competent virus has been isolated in culture for well beyond 7–8 days, although the probability falls to below 5% beyond 15.2 days.18 In such cases, there would possibly be a benefit in combining antivirals with immunomodulators (Figure 1). This theory also requires validation in well-designed RCTs.
Precautions, hurdles and directions
Since the exact predisposing factor for progression to severe disease is unknown, using this approach of antiviral window would mean that every infection would require therapy, translating into antivirals being used in a large number, to treat a few. Choosing the right target populations that are most vulnerable would seem to be the right strategy. Drawing parallels with influenza, it would seem that post-exposure prophylaxis among high-risk contacts may also be a possible strategy to arrest community transmission, if we want to reduce reckless antiviral usage and subsequent antiviral resistance, at least until a safe and effective vaccine becomes available. If a large-scale administration is considered, we must find the most potent and practically feasible antiviral agent. Studies must be performed to assess the efficacy of all possible antiviral options in early treatment and prophylaxis, much like the use of oseltamivir for influenza, and observe if treating at this stage prevents the progression to the inflammatory phase and severe disease. Including virus culture from a specimen at the start of the study could indicate if the antiviral timing was appropriate in analysis.
Initiation of immunomodulation guided by biomarkers and scoring systems could play a key role in attenuating serious illness. Studies must be performed to investigate the reliability of biomarkers and scoring systems in early prediction, so that the window of opportunity is not missed. Considering this scenario, studies to find out the most efficient, safe and cost-effective immunomodulatory agent are the need of the hour.
Conclusions
To summarize, we draw four conclusions. First, antivirals may not help when started late. Second, ongoing trials need to focus on their inclusion criteria with regard to time since illness onset and, possibly, separately analyse those with demonstrable viable virus at the start of the trial to find out the true potential of the antivirals. Third, we need to develop a strategy to use the best antiviral early in the viral-replicative phase of the disease, thereby preventing progression to the inflammatory phase and severe disease. Finally, scoring systems or biomarkers need to be investigated to detect the ‘hyperinflammatory tip-off’ early and accurately, to utilize the pre-emptive window for initiating immunomodulatory therapy.
Acknowledgements
We extend our sincere thanks to Kei Miyakawa for his guidance and inputs.
Transparency declarations
None to declare.
References
- 1. Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA 2020; 323: 2249–51. [DOI] [PubMed] [Google Scholar]
- 2. Johnson RM, Vinetz JM. Dexamethasone in the management of covid-19. BMJ 2020; 370: m2648. [DOI] [PubMed] [Google Scholar]
- 3. Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant 2020; 39: 405–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Velavan TP, Meyer CG. Mild versus severe COVID-19: laboratory markers. Int J Infect Dis 2020; 95: 304–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wölfel R, Corman VM, Guggemos W. et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581: 465–9. [DOI] [PubMed] [Google Scholar]
- 6. Bullard J, Dust K, Funk D. et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis 2020; doi:10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Wit E, Feldmann F, Cronin J. et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci USA 2020; 117: 6771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Zhang D, Du G. et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395: 1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beigel JH, Tomashek KM, Dodd LE. et al. Remdesivir for the treatment of Covid-19—Final report. N Engl J Med 2020; doi:10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao B, Wang Y, Wen D. et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; 382: 1787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li L, Zhang W, Hu Y. et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA 2020; 324: 460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joyner MJ, Senefeld JW, Klassen SA. et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. medRxiv 2020; doi:10.1101/2020.08.12.20169359. [Google Scholar]
- 13. Schiffer JT, Johnston C, Wald A. et al. An early test-and-treat strategy for severe acute respiratory syndrome coronavirus 2. Open Forum Infect Dis 2020; 7: ofaa232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee TH, Lin RJ, Lin RTP. et al. Testing for SARS-CoV-2: can we stop at two? Clin Infect Dis 2020; doi:10.1093/cid/ciaa459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang C, Qin L, Li K. et al. A novel scoring system for prediction of disease severity in COVID-19. Front Cell Infect Microbiol 2020; 10: 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. RECOVERY Collaborative Group: Horby P, Lim WS, Emberson JR. et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med 2020; doi:10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malgie J, Schoones JW, Pijls BG. Decreased mortality in COVID-19 patients treated with tocilizumab: a rapid systematic review and meta-analysis of observational studies. Clin Infect Dis 2020; doi:10.1093/cid/ciaa1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Kampen JJA, van de Vijver DAMC, Fraaij PLA. et al. Shedding of infectious virus in hospitalized patients with coronavirus disease-2019 (COVID-19): duration and key determinants. medRxiv 2020; doi:10.1101/2020.06.08.20125310. [DOI] [PMC free article] [PubMed] [Google Scholar]