Abstract
Background
Emerging data suggest variability in susceptibility and outcome to coronavirus disease 2019 (COVID-19) infection. Identifying risk factors associated with infection and outcomes in cancer patients is necessary to develop healthcare recommendations.
Methods
We analyzed electronic health records of the US Veterans Affairs Healthcare System and assessed the prevalence of COVID-19 infection in cancer patients. We evaluated the proportion of cancer patients tested for COVID-19 who were positive, as well as outcome attributable to COVID-19, and stratified by clinical characteristics including demographics, comorbidities, cancer treatment, and cancer type. All statistical tests are 2-sided.
Results
Of 22 914 cancer patients tested for COVID-19, 1794 (7.8%) were positive. The prevalence of COVID-19 was similar across age. Higher prevalence was observed in African American (15.0%) compared with White (5.5%; P < .001) and in patients with hematologic malignancy compared with those with solid tumors (10.9% vs 7.8%; P < .001). Conversely, prevalence was lower in current smokers and patients who recently received cancer therapy (<6 months). The COVID-19–attributable mortality was 10.9%. Higher attributable mortality rates were observed in older patients, those with higher Charlson comorbidity score, and in certain cancer types. Recent (<6 months) or past treatment did not influence attributable mortality. Importantly, African American patients had 3.5-fold higher COVID-19–attributable hospitalization; however, they had similar attributable mortality as White patients.
Conclusion
Preexistence of cancer affects both susceptibility to COVID-19 infection and eventual outcome. The overall COVID-19–attributable mortality in cancer patients is affected by age, comorbidity, and specific cancer types; however, race or recent treatment including immunotherapy do not impact outcome.
The coronavirus disease 2019 (COVID-19) infection, first reported in China in December 2019 (1), has now spread worldwide affecting all demographics and regions. The emerging data suggest variability in susceptibility to the infection and ultimately outcome. A number of patient-related factors, socioeconomic conditions, racial and ethnic differences, and several comorbidities including obesity, diabetes, and cardiovascular diseases have been associated with higher susceptibility and/or risk of mortality (2-5). The relatively higher transmission rate and associated greater risk of adverse outcome have highlighted the need to understand the epidemiologic characteristics of COVID-19 prevalence, and the risk factors associated with poor outcome and death, to establish the best possible public health policies. Cancer patients are considered to be at higher risk of infection. This risk varies with functional status of the patient, cancer type, and/or treatment modalities used (6,7). Thus, along with reducing exposure to the virus, other prophylactic as well as cancer-related risk factors may need to be addressed to decrease susceptibility to COVID-19 infection or to mitigate related complications in cancer patients. Small epidemiologic studies mainly from China and the United States have also reported increased rates of death in cancer patients related to COVID-19 (8,9). These observations have informed some changes and reorganization of cancer care worldwide (10), but larger studies are needed to understand the comprehensive cancer-related issues with COVID-19 infection. Here, we investigated the prevalence and outcome of COVID-19 infection among cancer patients in a large cohort of patients from the nationwide Veterans Affairs (VA) Healthcare System.
Methods
Patients
This analysis was conducted using data from the VA Corporate Data Warehouse, which centralizes electronic health record data for patients seen at VA facilities nationwide. The study population is defined as veterans with cancer who were tested for COVID-19 at the VA. Cancer patients were identified as patients with at least 1 occurrence of an International Classification of Diseases (ICD) code for cancer between January 1, 2010, and May 4, 2020 (11). Nonmelanoma skin cancer and benign tumors were excluded. This study was performed under a protocol approved by the VA Boston Healthcare System Research and Development Committee.
Study Variables and Outcomes
Patient demographics, COVID-19 laboratory tests, comorbidities, cancer type, cancer treatment, and outcomes were identified through structured data in the electronic health record. Patients were considered positive for COVID-19 if they had at least 1 positive test result and negative if all COVID-19 test results were negative. Based on manual review of laboratory test names, COVID-19 tests were identified using regular expressions matching either “.*covid.*” or “.*corona.*2019.*,” and excluding “.*not.covid.19.*.” COVID-19 tests that were cancelled or did not have a positive or negative result recorded were excluded from consideration. The Charlson comorbidity index and presence of individual comorbidities were derived from ICD codes in the year prior to each patient’s first COVID-19 test using the comorbidity package in R (12). Body mass index (BMI) was calculated from each patient’s most recently recorded weight and height prior to his or her first COVID-19 test. Smoking status was defined using health factors (13). The VA hospital each patient was tested in was extracted from structured data and mapped to geographical region using the mapping in the Supplementary Methods (available online). Cancer type was determined based on ICD codes in the study period, and patients may have multiple cancer types. Only cancer types with 200 or more patients are shown. The systemic therapies for cancer considered in this study were based on a list of approved cancer drugs tabulated by the National Cancer Institute (14). Patients were identified as being on active or recent treatment for cancer at the time of COVID-19 testing if they received systemic therapy for cancer in the 6 months prior to their first test for COVID-19, and only treatments in this time frame were considered to determine the type of therapy received. Regarding outcomes, hospitalization was defined as any inpatient visit after the patient’s first COVID-19 test, and intensive care unit (ICU) admission was defined as a subset of hospitalizations where the specialty ward is either “surgical ICU” or “medical ICU.” Respiratory support was determined based on the presence of a current procedural terminology (CPT) or ICD procedure code for intubation (CPT 94002, 94003, 94004, 94005, ICD-10 Z99.1, Z99.11, Z99.12) or mechanical ventilation (CPT 31500, ICD-10 J95.851) after the patient’s first COVID-19 test. Missing data existed for race, ethnicity, and smoking status and were coded as a separate level in all analyses.
Statistical Analysis
We evaluated the proportion of COVID-19–positive patients among those tested, along with 95% confidence intervals (CIs), and stratified by demographics, comorbidities, cancer treatment, and cancer type. We also evaluated the proportion of patients experiencing each outcome among COVID-19–positive and negative patients and defined the COVID-19 attributable risk of each outcome as the difference of these 2 proportions. We used χ2 tests to assess differences in proportion. Odds ratio as to COVID-19–positive status by cancer type was assessed using univariate and multivariate logistic regression, adjusting for race, ethnicity, and smoking status in the multivariate models. All statistical tests were 2-sided, and a P value of less than .05 was considered statistically significant.
Results
Susceptibility to COVID-19 Infection in Cancer Patients
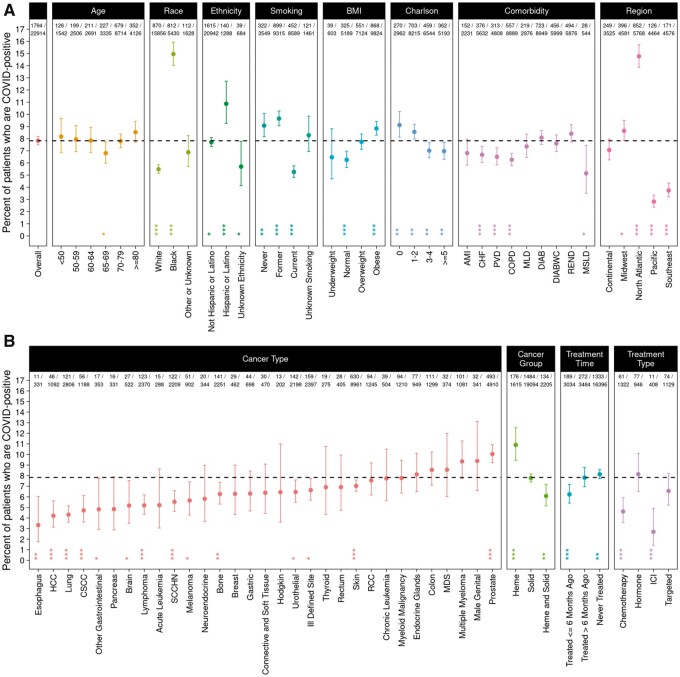
We identified 22 914 patients with history of cancer who were tested for COVID-19 infection on or before May 4, 2020, of whom 1794 patients (7.8%) reported positive (Figure 1, A). The prevalence of COVID-19 among those tested was similar across all ages (younger than 50 years to 80 years or older; P = .16), although older patients with cancer were tested for COVID-19 more frequently (Supplementary Table 1, available online). Importantly, there was a statistically significant difference in prevalence of infection across race and ethnicity, with 15.0% of African Americans but only 5.5% of White patients testing positive for COVID-19 (P < .001) and 10.9% of Hispanic or Latino patients vs 7.7% of non-Hispanic or non-Latino patients testing positive (P < .001). Higher prevalence was observed in patients with hematologic malignancy compared with those with solid tumors (10.9% vs 7.8%; P < .001). We also observed a statistically significantly lower prevalence in cancer patients who were current smokers compared with former smokers or those who never smoked (5.3% vs 9.5%; P < .001). Compared with the overall sample (7.8% positive), there was a small but statistically significant negative association between susceptibility to COVID-19 and concomitant congestive heart failure (6.7% positive; P < .001), peripheral vascular disease (6.5%; P < .001), chronic obstructive pulmonary disease (6.3%; P < .001), and moderate or severe liver disease (5.1%; P = .02). Higher BMI was associated with increased prevalence of infection (P < .001). During the period of observation, COVID-19 was much more frequent in the North Atlantic (14.8%) than other regions (7.1% Continental, 8.6% Midwest, 2.8% Pacific, 3.7% Southeast; P < .001). Differences in susceptibility across race persisted within each region and in individual VA hospital sites (Supplementary Tables 2 and 3, available online).
Figure 1.
COVID-19 prevalence among cancer patients. Percent of COVID-19–positive patients among cancer patients tested for COVID-19 is shown with Panel A showing overall data and data stratified by age, race, ethnicity, smoking, BMI, Charlson score, comorbidity, and region. Panel B shows data stratified by cancer type, cancer group, treatment time, and treatment type. The dashed line indicates the overall percent positive (7.8%). The 2 rows at top show the number of COVID-19–positive patients and the total number of cancer patients tested. In addition to the percent positive in each group, a 95% confidence interval and P value are shown, based on a χ2 test for difference in proportions (2-sided). *P < .05; **P < .01; ***P < .001. AMI = acute myocardial infarction; BMI = body mass index; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; CSCC = cutaneous squamous cell carcinoma; DIAB = diabetes without complications; DIABWC = diabetes with complications; HCC = hepatocellular carcinoma; Heme = hematological malignancy; ICI = immune checkpoint inhibitor; MDS = myelodysplastic syndromes; MLD = mild liver disease; MSLD = moderate or severe liver disease; PVD = peripheral vascular disease; RCC = renal cell carcinoma; REND = renal disease; SCCHN = squamous cell carcinoma of the head and neck; Solid = solid tumor.
We further investigated cancer types and prevalence of COVID-19 (Figure 1, B) and observed higher prevalence in patients with hematologic malignancy (10.9%) in comparison with patients with solid tumors (7.8%; P < .001). Among specific cancer types, we observed statistically significantly higher frequency of COVID-19 among patients with prostate cancer (10.0%; P < .001), whereas we observed statistically significantly lower frequency among patients with esophagus (3.3%; P = .003), hepatocellular carcinoma (4.2%; P < .001), lung (4.3%; P < .001), cutaneous squamous cell carcinoma (4.7%; P < .001), lymphoma (5.2%; P < .001), squamous cell head and neck cancer (SCCHN) (5.5%; P < .001), bone cancer (6.3%; P = .004), and urothelial cancer (6.5%; P = .01), among others. To understand the relationship of race and cancer type, we assessed the odds of COVID-19 infection by cancer type, adjusting for race, ethnicity, and smoking status (Supplementary Table 4, available online). Although the univariate analysis showed that patients with prostate cancer had a higher risk of COVID-19 infection, this was no longer the case after adjustment, suggesting the higher prevalence observed in prostate cancer may be affected by race, ethnicity, and/or smoking status. On the other hand, patients with esophageal cancer, hepatocellular carcinoma, lung cancer, lymphoma, SCCHN, bone cancer, melanoma, and other cancers continued to have a lower risk of COVID-19 infection even after adjusting for these factors.
Next, we evaluated the impact of active cancer treatment on susceptibility to COVID-19 infection and observed that patients receiving cancer therapy within the last 6 months had statistically significantly lower prevalence (6.2%) of COVID-19 compared with those who received therapy more than 6 months ago (7.8%) or never received therapy (8.1%; P = .002). Moreover, compared with the overall cohort (7.8%), patients receiving immune checkpoint inhibitors (2.7%; P < .001) or chemotherapy (4.6%; P < .001) within the last 6 months had statistically significantly lower prevalence of COVID-19, whereas patients on hormonal therapy (8.1%; P = .76) or targeted therapy (6.6%; P = .11) did not have statistically significantly different prevalence.
Outcome of COVID-19 Infection in Cancer Patients
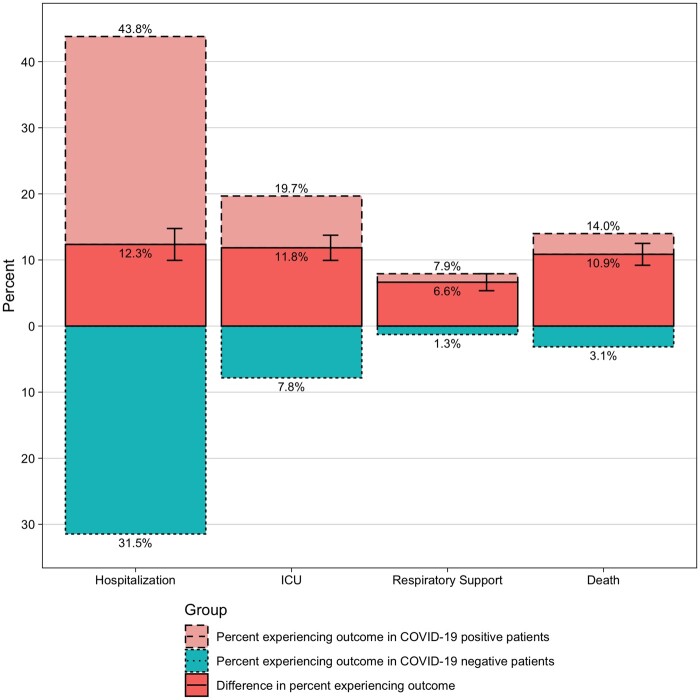
We next evaluated the impact of COVID-19 infection on outcome in cancer patients and calculated the difference between frequency in positive vs negative as the COVID-19 attributable contribution. Overall, compared with COVID-19–negative cancer patients, COVID-19–positive cancer patients have higher frequency of hospitalizations (31.5% vs 43.8%, respectively; an excess of 12.3% COVID-19 attributable), ICU admissions (7.8% vs 19.7%, respectively; 11.8% COVID-19 attributable), and respiratory support (1.3% vs 7.9%, respectively; 6.6% COVID-19 attributable). COVID-19–attributable death was 10.9%, with 14.0% death in COVID-19–positive compared with 3.1% in COVID-19–negative patients (Figure 2).
Figure 2.
Outcome of cancer patients with COVID-19. Percent of patients experiencing hospitalization, ICU visits, respiratory support, and death in COVID-19–positive (light red) and –negative (light blue) cancer patients are represented. The COVID-19–attributable risk of experiencing each outcome (ie, the difference of the percent experiencing each outcome in COVID-19–positive compared with negative patients is also shown; dark red), along with 95% confidence intervals. ICU = intensive care unit.
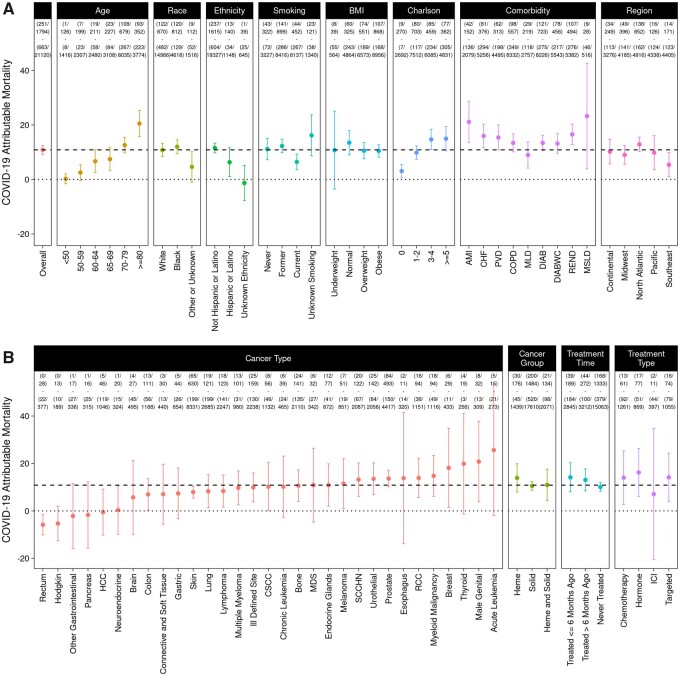
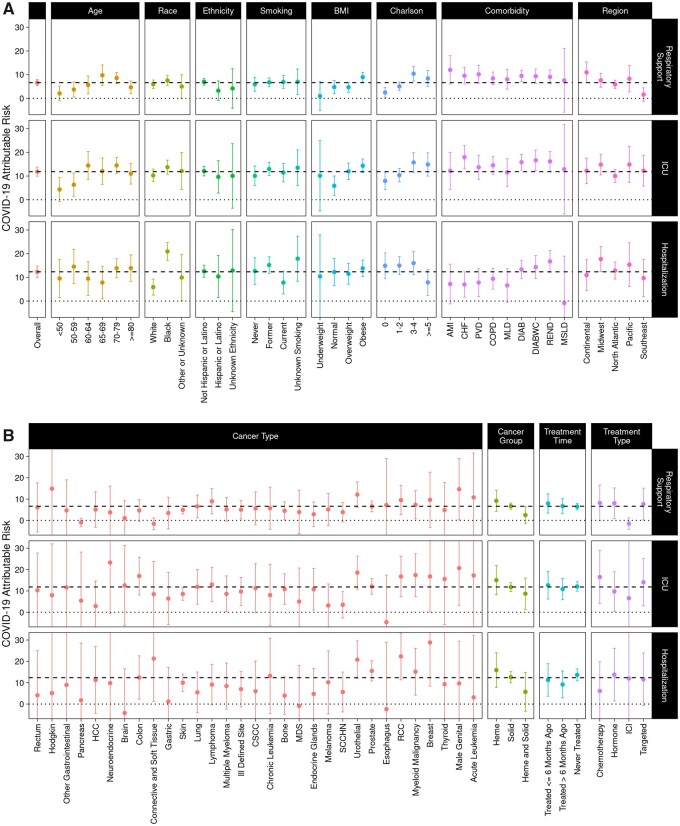
To further assess the impact of patient and disease-related features on COVID-19–attributable outcomes, we evaluated each of the demographic and comorbidity strata described above (Figure 3, A and Figure 4, A). In general, outcomes occur more frequently among COVID-19–positive cancer patients; however, the difference attributable to COVID-19 infection varies widely across strata. COVID-19–attributable mortality is strongly associated with age, ranging from 0.2% among patients younger than 50 years old to 20.5% among patients 80 years or older (P < .001). Presence of other comorbidities is also associated with increased COVID-19–attributable death, ranging from 3.1% among patients with a Charlson score of 0 to 15.0% among patients with a Charlson score of 5 or higher (P < .001). COVID-19–attributable ICU admissions were more common in obese patients (14.3%) than in patients with normal BMI (5.9%; P < .001), as is attributable respiratory support (8.9% vs 4.8%; P = .01); however, attributable mortality was not higher (10.5% in obese, 13.5% in normal; P = .22). Interestingly, COVID-19–attributable mortality was lower in current smokers (6.5%) compared with former or never smokers (12.0%; P =.002).
Figure 3.
COVID-19–attributable mortality among cancer patients. COVID-19–attributable mortality defined as the difference in the percent mortality in COVID-19–positive compared with negative patients is shown, along with 95% confidence intervals. Panel A shows overall data and data stratified by age, race, ethnicity, smoking, BMI, Charlson score, comorbidity, and region. Panel B shows data stratified by cancer type, cancer group, treatment time, and treatment type. The dashed line shows the COVID-19–attributable mortality in the overall cohort (14.4%), and the dotted line marks 0, the point where there is no COVID-19–attributable mortality. The 4 rows at top show the number of COVID-19–positive patients who died, the total number of COVID-19–positive patients, the number of COVID-19–negative patients who died, and the total number of COVID-19–negative patients. AMI = acute myocardial infarction; BMI = body mass index; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; CSCC = cutaneous squamous cell carcinoma; DIAB = diabetes without complications; DIABWC = diabetes with complications; HCC = hepatocellular carcinoma; Heme = hematological malignancy; ICI = immune checkpoint inhibitor; ICU = intensive care unit; MDS = myelodysplastic syndromes; MLD = mild liver disease; MSLD = moderate or severe liver disease; PVD = peripheral vascular disease; RCC = renal cell carcinoma; REND = renal disease; SCCHN = squamous cell carcinoma of the head and neck; Solid = solid tumor.
Figure 4.
COVID-19–attributable hospitalizations, ICU admissions, and respiratory support among cancer patients. COVID-19–attributable hospitalizations, ICU admissions, and respiratory support, defined as the difference in the percent of COVID-19–positive patients experiencing each outcome minus the percent of COVID-19–negative patients experiencing each outcome, are shown. Panel A shows overall data and data stratified by age, race, ethnicity, smoking, BMI, Charlson score, comorbidity, and region. Panel B shows data stratified by cancer type, cancer group, treatment time, and treatment type. 95% confidence intervals are shown. The dashed line shows the COVID-19–attributable contribution in the overall cohort, and the dotted line marks 0, the point where there is no COVID-19–attributable contribution. AMI = acute myocardial infarction; BMI = body mass index; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; CSCC = cutaneous squamous-cell carcinoma; DIAB = diabetes without complications; DIABWC = diabetes with complications; HCC = hepatocellular carcinoma; Heme = hematological malignancy; ICI = immune checkpoint inhibitor; ICU = intensive care unit; MDS = myelodysplastic syndromes; MLD = mild liver disease; MSLD = moderate or severe liver disease; PVD = peripheral vascular disease; RCC = renal cell carcinoma; REND = renal disease; SCCHN = squamous cell carcinoma of the head and neck; Solid = solid tumor.
African Americans have increased COVID-19–attributable hospitalization (20.9%) compared with White patients (5.9%), a 3.5-fold difference (P < .001) (Figure 4; Supplementary Table 5, available online). However, despite the higher COVID-19 prevalence as well as increased COVID-19–attributable hospitalization, we do not observe a statistically significant difference in any of the other more serious COVID-19–attributable outcomes, including mortality (12.0% in African Americans vs 10.8% in Whites; P = .50), respiratory support (6.0% in African Americans vs 7.6% in Whites; P = .23), or ICU admissions (13.7% in African Americans vs 10.2% in Whites; P = .08). Similarly, we do not observe a statistically significant difference in COVID-19–attributable outcomes for Hispanic or Latino compared with non-Hispanic or non-Latino (Supplementary Table 6, available online).
Outcomes were variable based on type of cancer (Figure 3, B and Figure 4, B). COVID-19–attributable deaths were observed in most but not all cancer types, with the highest attributable mortality in acute leukemia (25.6%), male genital (20.8%), and thyroid cancer (19.9%). COVID-19–attributable mortality is similar in patients recently treated for cancer (≤6 months ago, 14.2%) compared with those treated more than 6 months ago (13.1%) or never treated with systemic therapy (10.1%; P = .25). There is a suggestive relationship between type of treatment and outcome attributable to COVID-19 infection. In patients receiving immune checkpoint inhibitor (ICI) within the last 6 months, COVID-19–attributable mortality (7.1%) was less than half that observed in patients receiving chemotherapy (14.0%), hormone therapy (16.2%), or targeted therapy (14.1%), although statistical significance was not reached because of fewer observed deaths (7.1% in ICI vs 14.7% in not ICI; P = .53).
Discussion
We investigated a large cohort of cancer patients evaluated for COVID-19 at the VA Healthcare System from across the United States to identify risk factors for COVID-19 susceptibility and outcomes in cancer patients. The overall frequency of COVID-19 positivity was 7.8% of tested patients. As of May 16, 2020, according to the US Center for Disease Control, the national positivity rate for COVID-19 infection in the United States among patients aged 18 years and older is 13.5% with 126 627 positive patients out of 928 366 tested patients (15). Thus, the prevalence of COVID-19 among cancer patients appeared to be lower than in the general population. However, the real prevalence of COVID-19 remains uncertain, as a substantial number of patients are not or have not been tested, particularly in the context of asymptomatic disease. Nevertheless, the observed lower prevalence of COVID-19 in cancer patients might be explained either by higher access to testing within the VA Healthcare System and/or higher rates of testing in cancer patients in general.
Perhaps the most striking observation in our data is the statistically significant racial and ethnic disparity in prevalence of COVID-19 infection. African Americans (15.0%) and Hispanic (10.9%) cancer patients had statistically significantly higher rates of COVID-19 infection in comparison with White cancer patients (5.5%) and had higher rates of hospitalization. The biological and/or social basis for this observation, also recently reported in the general population (16-18), remains unclear. Overall, the observation of this disparity across all the regions of the country and site by site (Supplementary Tables 2 and 3, available online) rules out the possible role of regional conditions in explaining this difference. COVID-19 was more frequent among patients with prostate cancer, which is also more frequent in African Americans patients (19), although after adjusting for race, ethnicity, and smoking status, this difference is no longer observed. The possible explanations requiring further investigation include socioeconomic factors or genetic predisposition. Importantly, although African Americans cancer patients were more frequently admitted to the hospital, with equal access to care in the VA Healthcare System, similar mortality rate was observed between African Americans and White cancer patients. A similar trend was also observed in Hispanic and Latino populations. These results suggest distinct factors modulating COVID-19 susceptibility and the related outcome.
The statistically significantly reduced prevalence of COVID-19 infection in cancer patients with current smoking history, compared with those who have quit smoking or never smoked, is intriguing. This observation is also confirmed by observed reduced frequency of COVID-19 infection among patients with lung (4.3%; P < .001), SCCHN (5.5%; P < .001), and urothelial (6.5%; P = .01) cancers, all malignancies associated with smoking. These differences might also be related to race or ethnicity (Supplementary Tables 5 and 6, available online) or to socioeconomic factors. An earlier preliminary report has also suggested association of active smoking with lower rates of COVID-19 infections (20). This negative association requires further investigation. The role of nicotine, local epithelial cell changes, or inflammatory environment may play a role (21,22). We did not observe a substantial impact of cardiopulmonary and other comorbidities as well as age of patients on rate of infection (Figure 1), which may suggest that preexistence of cancer could outweigh the other comorbidities in regard to susceptibility to COVID-19. Moreover, it is possible that sick patients and patients under active cancer treatment may have been more cautious and thus less exposed to COVID-19, and this may affect the vulnerability. Although we are unable to directly quantify severity of cancer illness in our dataset, we included 2 proxies for severity of disease that are available: 1) whether or not each patient was recently treated for cancer (≤6 months ago), treated more than 6 months ago, or never treated with systemic therapy, which serves to some extent as a proxy for severity, and 2) the Charlson score, which reflects severity of overall health condition prior to COVID-19 diagnosis. Our analysis reveals statistically significantly lower rate of infection in patients treated within 6 months ago, although no difference in COVID-19–attributable mortality or other outcomes and higher rates of infection in patients with higher Charlson score were observed.
Outcome of cancer patients infected by COVID-19 was characterized by higher rates of mortality. Although the frequency of COVID-19 positivity was lower in patients receiving cancer-related therapy within 6 months of the COVID-19 infection, the overall mortality was not statistically significantly different in comparison with no treatment or treatment prior to the 6-months groups. These mortality rates are higher than the mortality rates reported in the global population (8,23) and confirm higher vulnerability of cancer patients to COVID-19 infection. Therapies including conventional chemotherapy, targeted therapies with small molecules, or monoclonal antibodies were associated with higher mortality rates in this study. ICI treatment was associated with a lower rate of infection; however, only a small number of patients are in this group suggesting both caution in interpreting this data and also a need for further focused investigation in patients receiving ICI therapy and with COVID-19 positivity. The lower mortality rate in patients receiving this treatment confirms the recent report about the impact of this therapy on COVID-19 outcome (24). Higher rates of mortality were observed in elderly patients, patients with higher Charlson score, underweight patients, and patients with comorbidities, mainly cardiovascular and renal disease (Figure 3). Although hematological malignancies were associated with higher rates of infection, similar COVID-19–attributable mortality was observed in comparison to solid malignancies (Figure 3; Supplementary Table 7, available online). Fatality rate related to each cancer type was variable with breast, male genital, acute leukemia, and thyroid cancer being associated with the highest rate of COVID-19–related mortality (Figure 3).
Our study has several limitations. First, the veteran population is primarily male, and hence, the study represents various trends and associations whose interpretations are restricted to male. Second, patients tested outside the VA system would not be included in this analysis. However, this number is likely to be very small because most patients who get cancer care in the VA do return for their healthcare needs to the VA. Third, it is possible that analyses are confounded by indication for testing. However, we believe that our comparison of those who tested positive to those who tested negative remains relevant, especially because COVID-19 testing criteria at VA hospitals was informed by centralized guidance from the VA Central Office, increasing consistency of testing criteria nationally (25).
In conclusion, the presence of cancer changes the susceptibility to COVID-19 infection and affects overall outcome. The overall disease behavior is modulated by both patient-related and cancer-related factors, which needs to be considered in development of COVID-19 preventative strategies as well as modulation of cancer therapies to optimize the patient care. Importantly, having equal access to care is an important component to improving overall outcome.
Funding
This work was supported by the VA Office of Research and Development, Cooperative Studies Program (NRF, NVD, MTB), the VA Merit Review Award 1I01BX001584 (NCM), and NIH grants P01-155258–07 and P50-100707 (NCM).
Notes
Role of the funder: The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: NCM is consultant for BMS, Janssen, OncoPep, Amgen, Abbvie, and Takeda and on the board of directors for OncoPep. The remaining authors declare no competing financial interests.
Disclaimer: The views expressed are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Author contributions: NCM, NRF, JL, RES conceived the project; NCM, NRF, JL, RES, DPT, VN, CY, NVD, MTB analyzed the data; NRF, JL, VN, CY extracted data and generated figures; NCM, NRF, JL, RES wrote the manuscript which was reviewed and edited by the other co-authors.
Data Availability
The data underlying this article cannot be shared publicly because of VA policy. The data will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. doi:10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kumar A, Arora A, Sharma P, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14(4):535-545. doi:10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372-2374. doi:10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052.doi:10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flowers CR, Seidenfeld J, Bow EJ, et al. Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(6):794-810. doi:10.1200/J Clin Oncol.2012.45.8661. [DOI] [PubMed] [Google Scholar]
- 7. Elfaituri MK, Morsy S, Tawfik GM, et al. Incidence of infection-related mortality in cancer patients: trend and survival analysis. J Clin Oncol. 2019;37(suppl 15):e23095. doi:10.1200/J Clin Oncol.2019.37.15_suppl.e23095. [Google Scholar]
- 8. Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. doi:10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335-337. doi:10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van de Haar J, Hoes LR, Coles CE, et al. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26(5):665-671. doi:10.1038/s41591-020-0874-8. [DOI] [PubMed] [Google Scholar]
- 11. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gasparini A. Comorbidity: an R package for computing comorbidity scores. J Open Source Softw. 2018;3(23):648.doi:10.21105/joss.00648. [Google Scholar]
- 13. Melzer AC, Pinsker EA, Clothier B, et al. Validating the use of Veterans Affairs tobacco health factors for assessing change in smoking status: accuracy, availability, and approach. BMC Med Res Methodol. 2018;18(1):39.doi:10.1186/s12874-018-0501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cancer Institute. A to Z list of cancer drugs. https://www.cancer.gov/about-cancer/treatment/drugs. Accessed May 15, 2020.
- 15.Centers for Disease Control and Prevention. COVIDView: a weekly surveillance summary of U.S. COVID-19 activity. 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-05-22-2020.pdf. Accessed May 22, 2020.
- 16. Azar KMJ, Shen Z, Romanelli RJ, et al. Disparities in outcomes among COVID-19 patients in a large health care system In California. Health Aff (Millwood) 2020;39(7):1253-1262. doi:10.1377/hlthaff.2020.00598. [DOI] [PubMed] [Google Scholar]
- 17. Chowkwanyun M, Reed AL. Racial health disparities and Covid-19—caution and context. N Engl J Med. 2020;383(3):201-203. doi:10.1056/NEJMp2012910. [DOI] [PubMed] [Google Scholar]
- 18. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. doi:10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McGinley KF, Tay KJ, Moul JW. Prostate cancer in men of African origin. Nat Rev Urol. 2016;13(2):99-107. doi:10.1038/nrurol.2015.298. [DOI] [PubMed] [Google Scholar]
- 20. Rossato M, Russo L, Mazzocut S, Di Vincenzo A, Fioretto P, Vettor R. Current smoking is not associated with COVID-19. Eur Respir J. 2020;55(6):2001290. doi:10.1183/13993003.01290-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leung JM, Yang CX, Sin DD. COVID-19 and nicotine as a mediator of ACE-2. Eur Respir J. 2020;55(6):2001261.doi:10.1183/13993003.01261-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Russo P, Bonassi S, Giacconi R, Malavolta M, Tomino C, Maggi F. COVID-19 and smoking. is nicotine the hidden link? Eur Respir J. 2020;55(6):2001116.doi:10.1183/13993003.01116-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Coronavirus disease (COVID-2019) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. Accessed May 25, 2020.
- 24. Luo J, Rizvi H, Egger JV, Preeshagul IR, Wolchok JD, Hellmann MD. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10(8):1121-1128. doi:10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veterans Health Administration—Office of Emergency Management. COVID-19 response plan: incident-specific annex to the VHA high consequence infection (HCI) base plan. Version 16. https://www.va.gov/opa/docs/VHA_COVID_19_03232020_vF_1.pdf. Accessed March 23, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly because of VA policy. The data will be shared on reasonable request to the corresponding author.