ABSTRACT
Introduction
Coronavirus Disease 2019 (COVID-19) is spreading all over the world. Health systems around the globe have to deal with decreased capabilities and exhausted resources because of the surge of patients. The need to identify COVID-19 patients to achieve a timely opportunity to treat and isolate them is an ongoing challenge for health care professionals everywhere. A lack of testing capabilities forces clinicians to make the crucial initial decision on the basis of clinical findings and routine diagnostic laboratory test. This article reviews the current literature and presents a new adapted protocol for diagnosing and triaging COVID-19 patients. A special emphasis lies on the stepwise approach guiding the medical provider to a triage decision that is suitable for the individual patient and the situation of the local medical treatment facility.
Materials and Methods
On March 30, 2020, a PubMed based literature research on COVID-19 following the preferred reporting items for systematic reviews and meta-analyses guidelines was performed. A diagnostic and triage tool for COVID-19 was designed based on the major findings in the reviewed literature.
Results
After a selection process, focusing on the topics “epidemiology,” “clinical characteristics,” and “diagnostic tools,” 119 out of a total amount of 1,241 publications were selected to get an overview of the growing evidence.
Conclusions
The designed Early Recognition and Triage Tool enables the medical provider to use the applicable modules of the protocol for capabilities of the local setting to get the most appropriate diagnostic and triage done. The tool should give guidance for the initial approach until specific testing for the COVID-19 virus is available.
INTRODUCTION
The SARS-Coronavirus-2 (SARS-CoV-2) emerged in Wuhan province, China, in December 2019. This novel virus has since demonstrated global transmission and is the most significant pandemic of the last century. The clinical manifestations of the virus are referred to as Coronavirus Disease 2019 (COVID-19). COVID-19 is a huge challenge for health care systems worldwide. In the early stages of this pandemic, the diagnostic capabilities for COVID-19 were limited. Although the situation improved in well-established health systems, there remains a global paucity of testing capability. In order for appropriate quarantine of high risk individuals and isolation of those with COVID-19, a valid and reliable testing capability is ideal. If tests are unavailable, then other indicators of disease must be used for diagnosis in order to limit community spread.
The deployed military environment poses a challenging problem set when it comes to limiting disease propagation. In deployed military settings, it is not a possibility to work from home and community living is the standard with shared rooms, bathrooms, and dining facilities. It is not feasible to pause the mission. Although prevention measures in the deployed military setting are key to limiting exposure to the SARS-CoV-2, the most extreme prevention measures such as “lockdown” or “stay home” are not pragmatic in the deployed setting because the mission must continue. Additionally, like many areas of the world, testing capabilities can be limited; therefore, innovative strategies must be employed to ensure prevention and maintenance of a healthy population in order to facilitate mission success. Furthermore, medical capabilities and capacity in the deployed environment are well poised to manage trauma, most deployed military treatment facilities (MTFs) are not equipped to care for a large number of patients resulting from a medical pandemic. Determination of the disease presence is also necessary to preserve medical resources, which could quickly be overwhelmed in the deployed environment, especially given the natures of the SARS-CoV-2 with asymptomatic transmission resulting in patients that urgently need medical attendance. Given these challenges, the authors developed the Early Recognition and Triage Tool (ERECT Tool) to provide medical staff with practical guidance that is adapted to the special circumstances of this virus.
MATERIALS AND METHODS
The medical experts of a multinational MTF in Afghanistan developed a standardized and straightforward approach for identification and triage of COVID-19 patients at medical installations with limited resources. Data were aggregated from current medical intelligence reports of the German military health system and literature that could be found on relevant databases like PubMed, online archives of medical journals like “Lancet,” and open access literature databases of scientific publishers like “Springer Nature.” After an initial review of the current literature on March 30, 2020, a structured search according preferred reporting items for systematic reviews and meta-analyses guidelines in PubMed was conducted.
All types of publications listed with the key words “COVID-19” and “SARS-CoV-2” (original articles, editorials, correspondence, short reports, etc.) were eligible for the aim to seek evidence about COVID-19. This resulted in a total amount of 1,241 publications.
The result of the literature search was independently reviewed concerning the topics “epidemiology,” “clinical characteristics,” and “diagnostic tools” by two senior consultants and selected for further use.
Because we tried to find evidence for a diagnostic and categorization tool, topics as “therapy strategies” and “rehabilitation” were not taken into account.
The literature that was chosen by both reviewers to be eligible was accepted, the literature that was refused by both reviewers was excluded, and the literature where the reviewers initially disagreed was reviewed together and then finally included or excluded.
In this first step, 1,079 publications were excluded because they did not meet the required topics.
From the remaining 162 articles, 21 had to be excluded because they were double listed. Seventeen documents were excluded because of lacking language skills of the authors: 16 documents were written in Chinese and 1 in Czech. In a last step, all results without available full texts were excluded, which sorted out 5 further documents. 119 publications were eligible for further use (Fig. 1).
FIGURE 1.
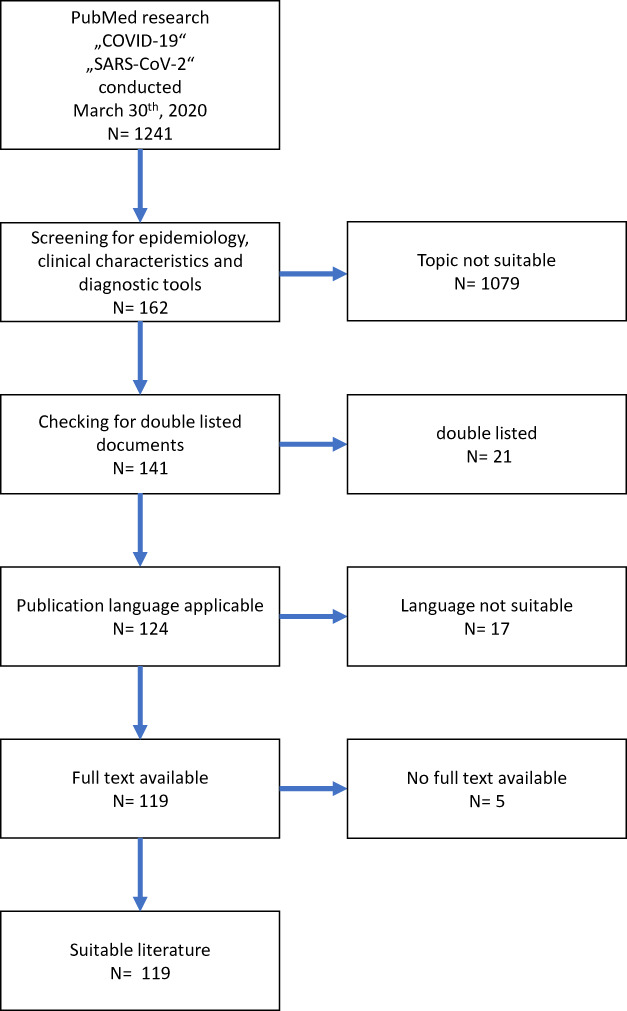
Selection process after initial literature research on March 30, 2020.
RESULTS
The findings were discussed in our international and interdisciplinary group of medical experts and put into a flow chart that shows the measures that found the greatest consent. The flow chart, which we named ERECT Tool is in our opinion a well-suited tool to be used by MTF with the same range of diagnostic capabilities. (Fig. 2) In a further step, we tried to simplify the flow chart in order to make it suitable for even smaller MTF with less diagnostic capabilities. (Fig. 3)
FIGURE 2.
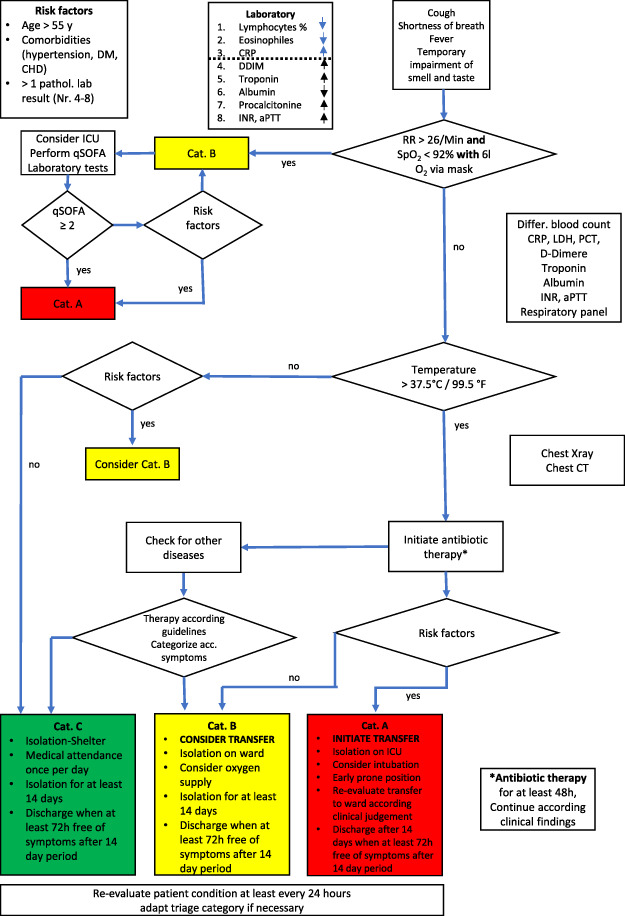
Early Recognition and Triage Tool including all modules of diagnostic capabilities.
FIGURE 3.
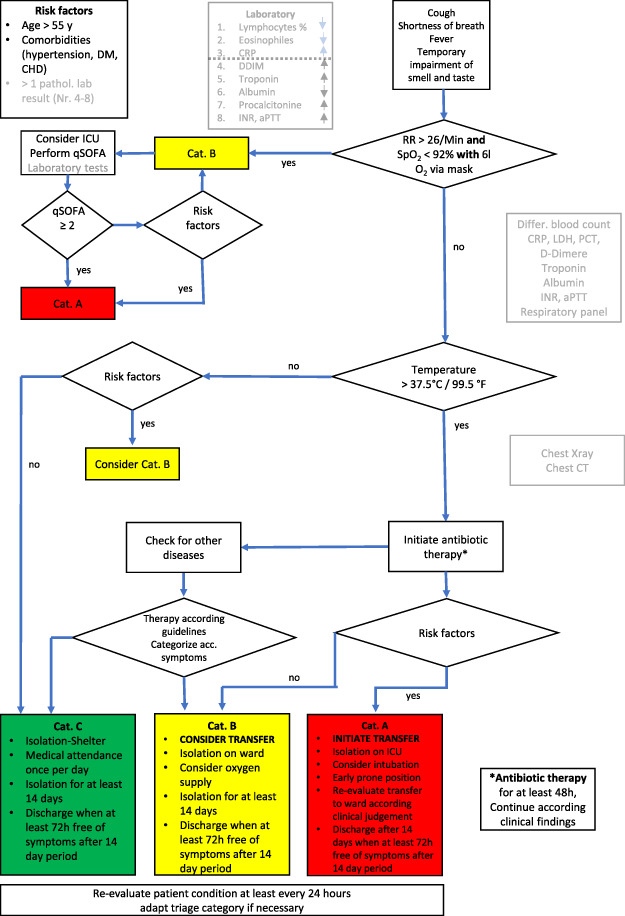
Early Recognition and Triage Tool reduced to the minimum of medical capabilities.
To implement the screening process we took the originally published process from a fever clinic in China1,2 and the Technical University Munich.3 Then we analyzed the available publications for criteria that are common clinical findings during the early phase of a CoV-SARS-2 infection.
These symptoms were set into a context with updated reports concerning main laboratory findings and other diagnostic results like chest computed tomography (CT) or conventional X-ray in order to make the process more reliable. In the next step, we tried to identify risk factors related to a severe clinical course of COVID-19, assuming that those patients would probably need a higher level of medical attendance. The risk factors identified could be preexisting conditions like age and comorbidities but also laboratory results that have shown to be predictive for a severe course of COVID-19.
The intent of the identified bundle of clinical and lab characteristics enables the medical personnel categorize patients in a comprehensive and decision matrix
At the end we tried to discriminate people, that present with flu-like symptoms not evoked by CoV-SARS-2 from those who likely to have COVID-19, in order to give them the suitable therapy for their specific disease.
DISCUSSION
Health care systems globally have been challenged by the COVID-19 pandemic. Even in countries with well-established health care infrastructure, the surge of the spreading COVID-19 pandemic is stretching the capabilities to the maximum and beyond.
Although the testing capabilities, for example, in Europe and the USA have been extended and an increasing number of patients can be tested every day, the situation is different for third world countries and many other parts of the world where the health systems are much more limited.
In the first weeks of the COVID-19 pandemic in military installations around the globe, collecting specimen for SARS-CoV-2 tests was basically possible but the logistical effort to get the test done and the period to get the test results lasted several days.
Infectious diseases can spread easily in communities of people that live and work together in crowded spaces. For instance, in refugee camps and for inhabitants of slums that may be in the crowded accommodation tents or shacks, the drinking water supply point or purely because of the overcrowded space itself. In military, installations might happen in the accommodation as well as in the gym or dining facilities.
Public health issues like social distancing, mask wearing, sneezing and coughing etiquette, and personal hygiene are useful tools,4 but in those populations mentioned above, they might not be as effective as in other locations. Furthermore, especially in refugee camps or slums, many people may have an impaired immune system because of a long-term malnutrition which might deteriorate the situation.
For these reasons, the health care providers have to prepare for a high impact of an infectious disease outbreak that will exhaust their capabilities very easily. One approach to spare medical resources is to “flatten the curve” of new infections, once the outbreak occurred. This means to make every effort to push the reproduction number of the virus spread as low as possible.8 Efforts to make this effective are resource intensive, and challenging to employ in resources constrained environments.
Throughout the world researchers report that the COVID-19 appears in different degrees of severity. About 81% of the infected present only with mild or even no symptoms but may spread the virus unwillingly to those in their direct environment. About 14% of the infected people need medical attendance and 5% are developing severe symptoms and may need intensive care capabilities.5
On March 30, when we performed the data research there was no evidence about the impact of COVID-19 on special communities like people in slums, refugee camps, or military personnel in field camps. That is why there was no possibility to get a reliable estimation on an evidence-based calculation model. To get at least some numbers for medical resource management, the published data from China was used, knowing that the population characteristics of China and a military installation are not completely comparable.
Regardless the calculated numbers, the impact on well-established health care systems showed that our a priori limited medical resources could be easily stretched to the maximum.
Overwhelmed health care systems are associated with a poorer outcome and a higher case-fatality rate.6 One reason is the lack of capabilities in these systems because of which they cannot face patients presenting with complications like acute kidney failure or acute respiratory distress syndrome and deliver renal replacement therapy or extra-corporeal membrane oxygenation7 so that the patients die because of these complications.
For COVID-19, the reported reproduction number ranged from 3 up to 57 what means that one infected person infected 3 to 57 persons in his environment. This led to an exponential growth rate of the infection numbers.9–11 Early and liberal testing, consequent isolation of infected persons as well as social distancing are actions that are proven to reduce the reproduction number and thereby slowing down the outbreak within the affected community.12–14 However, in order to utilize this strategy, there must be testing or other tools, such as the clinical tool introduced here, to facilitate diagnosis. In the context of COVID-19 where the majority infected people have no or only mild symptoms, this aim is even harder to achieve, especially when the testing capability is limited by long transport times of the samples or other reasons.
The consequence that arises is the need for a reliable screening process to identify potentially COVID-19-positive persons in order to isolate them from (the rest of) the community as soon as possible without having a quickly available test result. On the other hand, the screening process should be able to discriminate other diseases beside COVID-19 to take into account that diagnostic capabilities and in general medical resources are limited.
In addition, the screening tool could give a guideline for health care providers on how to initiate further treatment in order to use the existing capabilities in the best possible way.
Several authors that are working on COVID-19 have described clinical characteristics that are common during the course of the disease. Fever (92%), dry cough (73%), and shortness of breath (36.7%) are the most common findings.15
Further on even in the initial phase, symptomatic patients report with a temporary impairment of smelling and tasting.16 These data were published from the King’s College London on a website without a peer review process.
People presenting with shortness of breath may already suffer from a “silent hypoxia.” That is why it is important to check the peripheral oxygen saturation early and to take it into account for the further categorization as a postponed admission may lead to a poorer outcome.17
Other clinical characteristics like gastrointestinal symptoms like nausea or diarrhea that are reported in about 17.3%, respectively, 12.9% of COVID-19 cases15 are in our opinion not eligible for the primary screening process, although health care providers should recognize these symptoms as well to use them to gain a complete picture of the individual’s situation.
In the described process, the next step is early laboratory testing. Beside a testing for SARS-CoV-2 and (if available) other respiratory tract pathogens that may help differentiating the origin of the presented clinical symptoms, the additional testing for a broader spectrum of routine parameters can also help to identify certain constellations that are reported to be a typical sign of COVID-19.18
Furthermore, other laboratory findings may help to identify patients that might have to expect a more severe course of the ongoing disease.15,18
The recommended laboratory tests are written in a separate block beside the pathway on the algorithm, giving the health care professionals for the daily routine more freedom within the algorithm.
The next step after the primary assessment of the patient is to evaluate the body core temperature. Lai et al. and Zhang et al. reported that fever was associated in up to 92% of all cases with COVID-19.7,15
If a patient presents without fever, it is more likely that he or she is not COVID-19 positive. To make the whole process more sensitive and to decrease the potential number of missed persons with undetected COVID-19, laboratory testing can help out. If the laboratory results are not showing findings like a relative lymphocytopenia, an elevated CRP, or a decreased number of eosinophils, a serious COVID-19-infection is less likely. Further diagnostic efforts do not have to be taken in order to save resources.
If there are any clinical characteristics that were pathognomonic for COVID-19 infection, this type of guidance would not be necessary; however, with COIVD-19, diagnostics are needed.
The next piece of the diagnostic puzzle is radiologic imaging.
Zhao et al. and Chun et al. describe chest CT as primary approach because the “bilateral ground-glass opacity” is an early sign of COVID-19.19–21
For MTFs that do not have the capability to perform chest CT, conventional chest X-ray may also show early signs of a bilateral peripheral virus-associated pneumonia.22
The evidence about chest ultrasound in COVID-19 pneumonia is very limited. The diagnostic value in this specific context seems to be potentially there but might be very depending on the skills and experience of the single provider. For that reason, we did not include chest ultrasound in the protocol.23
After having finished this part of the flow chart, the multiple steps approach leads the way to or away from the diagnosis COVID-19. If the COVID-19 suspicion has been ruled out at that point, medical providers must look for other causes of the presented airway disease and treat this according to the applicable clinical practice guideline. In that case, the specific symptoms may help to categorize the patient for further treatment options.
If using the algorithm does not rule out the COVID-19 and their remains suspicion of pneumonia, then empiric antibiotic therapy should be initiated for at least 48 hours. This should fight a possible bacterial superinfection and prevent a deterioration of the patient’s health status. If the inflammatory signs during repeated laboratory tests (e.g. leucocytes, CRP, procalcitonin (PCT)) remain low or negative, the antibiotic treatment can be stopped.
The next step in the proposed flow chart focuses on the COVID-19-associated risk factors as they may have a prognostic value to predict a severe course of the whole inflammation process and probably predict the need for intensive medical care in the near future for the specific patient.
Several studies found age, hypertension, diabetes mellitus, and cardiovascular diseases as COVID-19-related risk factors for a more severe course of this disease.17,24,25 Other entities like active smoking, lung diseases such as chronic obstructive pulmonary disease (COPD), and asthma are not risk factors for the infection and not yet identified as obvious factors affecting the progression of COVID-19 but are still discussed.15,24,26,27
Beside certain underlying diseases some laboratory findings are also reported to be associated with a poor outcome of the patients. The most often reported pathologic laboratory findings are hypalbuminemia, elevated d-dimer, a prolonged activated Patrial Thromboplastin Time (aPTT), and prothrombin time. Elevated values for Troponin T/I and Muscle Brain isoenzyme of creatine kinase (CK-MB) and lactate dehydrogenase (LDH) were also found.17,18,25
Although the previous diseases can be easily evaluated by every health care provider, the additional laboratory results that are related to a higher severity and poor outcome are usually not available in resource constrained health care systems or in deployed environments with the exception of the larger MTFs. Therefore, we assumed that the described risk factors as a bundle could be used for a predictive risk assumption and a tool to categorize the patient to the applicable level of care. Lastly, it must be remembered to re-evaluate, reassess and if necessary re-categorize patients that have been already seen frequently. This is necessary to optimize resources in case of clinical improvement and to adjust the therapy in case of clinical deterioration.
Figure 2 outlines the minimum of steps needed and takes into account that many smaller MTF have no possibility to perform laboratory testing directly at their location and also radiologic imaging is lacking. After ruling in or ruling out a COVID-19 suspicion with only the help of clinical symptoms and medical history, this reduced flow chart results in suggestions how to deal with these patients with the assumed capabilities.
The presented protocol has been worked out on the basis of the current literature and represents the current evidence on COVID-19 focusing on early recognition and triage. There has been no evaluation of this concept with retrospective data by the authors. The published literature that had been taken into account for its development is based on a high number of patients seen by scientists dealing with them. A prospective study that proofs this concept and that evaluates sensitivity and specificity is needed to validate the proposed paradigm of care.
CONCLUSION
The COVID-19 pandemic is a challenging task for health systems all over the world. Under certain circumstances, the capabilities of the dedicated MTFs are a priori limited and tests or at least test results for SARS-CoV-2 are not available on short notice. Ideally every health care system would have access to diagnostic testing to aid in early diagnosis of COVID-19. In scenarios where testing is not available or resource constrained, this clinical algorithm provides a framework to aid providers in clinical diagnosis. Additionally, given the high false-negative rate of tests, this protocol can act as an adjunct to testing strategies.
Therefore, in order to provide health care providers tools to detect COVID-19 positive patients with a reasonable accuracy, we developed the presented flow chart. The specific aim was to create a guiding protocol that can easily be adopt to the local capabilities by adding or removing diagnostic components. Further studies are necessary to proof the value and reliability of this tool that comprises the essence of the current evidence on COVID-19.
Contributor Information
CDR Dr. Christoph Jänig, Role 2E, MN MedCoy TAAC N, Mazar-e-Sharif, Afghanistan; Department for Anesthesiology and Intensive Care Medicine, Bundeswehr Central Hospital, 56072 Koblenz, Germany.
COL Jennifer M Gurney, U.S. Army Institute of Surgical Research, Joint Trauma System, TX 78234, USA; NATO Role 2E Commander, Kabul, Afghanistan.
LCOL Dr. Roger Froklage, Role 2E, MN MedCoy TAAC N, Mazar-e-Sharif, Afghanistan; Department for Anesthesiology, Pain Medicine and Palliative Care, Elisabeth-TweeSteden Ziekenhuis, 5042 AD Tilburg, The Netherlands.
MAJ Robin Groth, Role 2E, MN MedCoy TAAC N, Mazar-e-Sharif, Afghanistan; Department for Anesthesiology and Intensive Care Medicine, Bundeswehr Central Hospital, 56072 Koblenz, Germany.
SGM Christine Wirth, Role 2E, MN MedCoy TAAC N, Mazar-e-Sharif, Afghanistan; Department for Anesthesiology and Intensive Care Medicine, Bundeswehr Central Hospital, 56072 Koblenz, Germany.
COL Dr. Hendrik van de Krol, Role 2E, MN MedCoy TAAC N, Mazar-e-Sharif, Afghanistan; Department for Trauma Surgery, RadboudUMC, 6525 GA Nijmegen, The Netherlands.
COL Willi Schmidbauer, Department for Anesthesiology and Intensive Care Medicine, Bundeswehr Central Hospital, 56072 Koblenz, Germany.
COL Dr. Christoph Güsgen, Role 2E, MN MedCoy TAAC N, Mazar-e-Sharif, Afghanistan; Department for General, Visceral and Thoracic Surgery, Bundeswehr Central Hospital, 56072 Koblenz, Germany.
FUNDING
None declared.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Wang H, Wang S, Yu K: COVID-19 infection epidemic: the medical management strategies in Heilongjiang province, China. Critical Care 2020; 24: 107 Published online March 18, 2020, doi: 10.1186/s13054-020-2832-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang J, Zhou L, Yang Y, et al. : Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respiratory Medicine 2020; 8(3): E11-12. doi: 10.1016/S2213-2600(20)30071-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lenzen-Schulte: Rasche Triage symptomatischer Patienten in der Notaufnahme. Deutsches Ärzteblatt 2020; 117(14): A715-6. [Google Scholar]
- 4. CDC—stop the spread of germs. Accessed April 4, 2020 https://www.cdc.gov/coronavirus/2019-ncov/downloads/stop-the-spread-of-germs.pdf
- 5. Johnson HC, Gossner CM, Colzani E, et al. : Potential scenarios for the progression of COVID-19 epidemic in the European Union and the European Economic Area. Euro Surveill 2020; 25(9): pii=2000202. doi: 10.2807/1560-7917.ES.2020.25.9.2000202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ji Y, Ma Z, Peppelenbosch MP, et al. : Potential association between COVID-19 mortality and healthcare resource availability. Lancet Global Health 2020; 8(4): E480. doi: 10.1016/S2214-109X(20)30068-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lai CC, Liu YH, Wang CY, et al. : Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microb Immunol Infect 2020; 53(3): 404-12. doi: 10.1016/j.jmii.2020.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan X, Ojcius DM, Gao T, et al. : Lessons learned from the 2019-nCoV epidemic on prevention of future infectious diseases. Microbes Infect 2020; 22(2): 86-91. doi: 10.1016/j.micinf.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li T: Diagnosis and clinical management of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection: an operational recommendation of Peking Union Medical College Hospital (V2.0). Emerg Microbes Infect 2020; 9(1): 582-5. doi: 10.1080/22221751.2020.1735265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lai CC, Wang CY, Wang YH, et al. : Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. J Antimicrob Agents. Published online March 12, 2020; 55(4). doi: 10.1016/j.ijantimicag.2020.105946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao S, Lin Q, Ran J, et al. : Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis 2020; 92(1): 214-7. doi: 10.1016/j.ijid.2020.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salzberger B, Glück T, Ehrenstein B: Successful containment of COVID-19: the WHO-report on the COVID-19 outbreak in China. Infection 2020; 48: 151-3. doi: 10.1007/s15010-020-01409-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilder-Smith A, Freedman DO: Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med 2020; 27(2): taaa020. doi: 10.1093/jtm/taaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salathé M, Althaus CL, Neher R, et al. : COVID-19 epidemic in Switzerland: on the importance of testing, contact tracing and isolation. Swiss Med Wkly 2020; 150: w20225. doi: 10.4414/smw.2020.20225 [DOI] [PubMed] [Google Scholar]
- 15. Zhang JJ, Dong X, Cao Y, et al. : Clinical characteristics of 140 patients infected with SARSCoV-2 in Wuhan, China. Allergy 2020; 75: 1730-41. doi: 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 16. Spector T (King’s College London) : Loss of smell and taste is strongest predictor of early COVID-19 infection. accessed April 4, 2020 https://neurosciencenews.com/covid-19-smell-taste-16056/
- 17. Deng Y, Liu W, Liu K, et al. : Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J. Published online March 20, 2020; 133(11): 1261-67. doi: 10.1097/CM9.0000000000000824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lippi G, Plebani M: Laboratory abnormalities in patients with COVID-19 infection. Clin Chem Lab Med 2020; 58(7): 1131-11. doi: 10.1515/cclm-2020-0198 [DOI] [PubMed] [Google Scholar]
- 19. Zhao W, Zhong Z, Xie X, et al. : Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. American Journal of Roentgenology 2020; 214(5): 1072-7. doi: 10.2214/AJR.20.22976 [DOI] [PubMed] [Google Scholar]
- 20. Chun SG, Zhi BL, Shuo Y, et al. : Imaging features of coronavirus disease 2019 (COVID-19): evaluationon thin-section CT. Acad Radiol. Published online March 6, 2020; 27(5): 609-13 doi: 10.1016/j.acra.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salehi S, Abedi A, Balakrishnan, et al. : Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. American Journal of Roentgenology 2020; 215: 87-93. doi: 10.2214/AJR.20.23034 [DOI] [PubMed] [Google Scholar]
- 22. Singhal T: A review of coronavirus disease-2019 (COVID-19). Ind J Pediatr 2020; 87(4): 281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poggiali E, Dacrema A, Bastoni D, et al. : Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID-19) pneumonia? Radiology 2020; 295:3, E6-E6. doi: 10.1148/radiol.2020200847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu W, Tao ZW, Wang L, et al. : Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chinese Medical Journal 2020; 133(9): 1032-8. doi: 10.1097/CM9.0000000000000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo T, Fan Y, Chen M, et al. : Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; 5(7): 811-8. 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vardavas CI, Nikitara K: COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis. 2020; 18(March): 20. doi: 10.18332/tid/119324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lippi G, Henry BM: Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Int Med 2020; 75: 107-8. doi: 10.1016/j.ejim.2020.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]


