Abstract
As the coronavirus disease 2019 (COVID-19) pandemic has progressed, a large volume of literature has developed delineating the clinical manifestations of acute infection. Recent reports have also started to describe persistent symptoms extending beyond the period of initial illness or hospitalization. Anecdotes of different signs and symptoms occurring after acute infection have also arisen in the lay press. Here we describe the current existing medical literature on the emerging concept of postacute COVID-19 and suggest an approach to classifying different manifestations of the syndrome. We also review long-term clinical manifestations observed in patients who recovered from infection due to other epidemic coronaviruses and briefly discuss potential mechanisms driving the phenomenon of postacute COVID-19.
Keywords: COVID-19, definition, long COVID, postacute
Coronavirus disease 2019 (COVID-19) remains the major infectious disease concern of 2020. Acute infection due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can present as a spectrum of illness, ranging from asymptomatic infection to critical life-threatening disease [1]. Recently, clinicians have begun to observe prolonged sequelae of acute COVID-19. The purpose of this review is to describe the extant knowledge about the postacute phase of COVID-19. Because the United States and the world continue to experience substantial numbers of new COVID-19 cases daily, fully understanding postacute COVID-19 manifestations will be vital to providing long-term, evidence-based care for patients who recover from the infection.
OVERVIEW OF COVID-19 DEFINITIONS
For acute COVID-19, the widely accepted categorization for disease severity that currently exists was first published by the US National Institutes of Health in July 2020 [1]. Categories of acute COVID-19 include asymptomatic, mild, moderate, severe, and critical disease. Currently, there is no universally accepted time period that defines the beginning of the postacute period. Greenhalgh et al. propose that the postacute period for COVID-19 starts 3 weeks after symptom onset; they go on to define “chronic COVID-19” as persistent symptomatology extending beyond 12 weeks after initial symptoms [2]. This is a practical and useful clinical definition. First, from a clinical perspective, because the majority of infections due to SARS-CoV-2 are asymptomatic or mild, 3 weeks is a reasonable time frame to define recovery from a viral respiratory illness. Second, replication-competent virus has not been recovered after 10 days following symptom onset in mild to moderate cases or after 20 days even in severe or critical illness—hence, the clinical definition is congruent with the virological data [3]. Third, the median duration of polymerase chain reaction (PCR) positivity in symptomatic patients is 24 days [4], and the mean duration among asymptomatic patients is 24.5 days [5]. One specific modification we propose to the definitions as outlined by Greenhalgh et al. is that for patients who remain hospitalized at 3 weeks after symptom onset, the postacute period start when the patient is discharged from inpatient acute care.
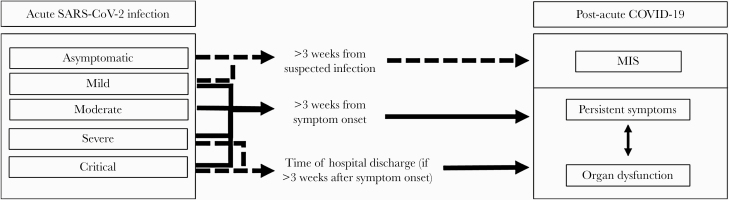
Currently, the medical literature on the topic of postacute COVID-19 includes reports of subjective postacute symptoms in patients who recovered from a wide spectrum of acute COVID-19, including mild infection through critical illness. The literature also describes various findings of objective organ pathology following acute infection. Therefore, to standardize our current understanding of postacute COVID-19, we propose dividing postacute manifestations into 3 categories, as represented in Figure 1: (1) residual symptoms that persist after recovery from acute infection; (2) organ dysfunction that persists after initial recovery; and (3) new symptoms or syndromes that develop after initial asymptomatic or mild infection. It is important to note that the first 2 categories outlined are not necessarily mutually exclusive. For example, dysfunction from 1 or multiple organ systems might result in certain symptoms patients experience; pulmonary pathology leading to persistent dyspnea or cough would be a prime example of this type of interplay.
Figure 1.
Progression of acute SARS-CoV-2 infection to postacute COVID-19. The figure shows the various forms of progression fo acute SARS-CoV-2 infection (classified using NIH symptom severity criteria [1]) to the proposed categories of postacute COVID-19, which include (1) persistent symptoms; (2) organ dysfunction; and (3) MIS. There is likely a relationship between organ dysfunction and persistent symptoms that is not yet completely understood. Abbreviations: COVID-19, coronavirus disease 2019; MIS, multisystem inflammatory syndrome; NIH, National Institutes of Health; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
PERSISTENT SYMPTOMS IN SEVERE COVID-19
It appears that fatigue is one of the most frequently reported symptoms that patients experience after recovery from acute SARS-CoV-2 infection. Carfi et al. report persistent symptoms in 143 patients evaluated after hospitalization due to COVID-19 (Table 1) [6]. All patients in this study were PCR-negative in the follow-up period. They note that 87% of patients had at least 1 persistent symptom at a mean of 60 days after symptom onset, with the most common symptoms being fatigue (53.1%), dyspnea (43.4%), and arthralgias (27.3%). Importantly, a majority of those with persistent symptoms, 55%, had 3 or more symptoms, while 32% had 1 or 2 symptoms, suggesting a high overall symptom burden.
Table 1.
Summary of Publications Describing Residual and Persistent Symptoms of COVID-19
| Author,a Publication Date Country | No. of Patients Included | Median Age of Patients Included, y | Males, % | Study Mean Follow-up Time, d | Patient Locationb and Reported Symptoms, % |
|
|---|---|---|---|---|---|---|
| Carfi, July 9c Italy [6] | 143 | 56.5 | 63 | 60.3d | Outpatiente | |
| Fatigue | 53.1 | |||||
| Dyspnea | 43.4 | |||||
| Arthralgias | 27.3 | |||||
| Halpin, July 30c UK [7] |
100 (Ward 68; ICU 32) |
Ward 70.5 ICU 58.5 |
Ward 51.5 ICU 59.4 |
48f | Ward | |
| New fatigueg | 60.3 | |||||
| Breathlessnessg | 42.6 | |||||
| PTSDg | 23.5 | |||||
| Concentration & memory deficits | 33.8 | |||||
| Speech & swallow deficitsh | 42.6 | |||||
| ICU | ||||||
| New fatigueg | 72 | |||||
| Breathlessnessg | 65.6 | |||||
| PTSDg | 46.9 | |||||
| Concentration & memory deficits | 53.1 | |||||
| Speech & swallow deficitsh | 68.7 | |||||
| Tenforde, July 24c USA [9] |
274 | 42.5 | 48 | 14–21i | ED or outpatient clinics | |
| Cough | 43 | |||||
| Fatigue | 35 | |||||
| Dyspnea | 29 | |||||
| Garrigues, August 21c France [8] |
120 (Ward 96; ICU 24) |
Ward 64.1 ICU 59.6 |
Ward 58.3 ICU 79.2 |
110.9j | Ward |
Abbreviations: COVID-19, coronavirus disease 2019; ICU, intensive care unit; ward, general medical ward.
aFirst author.
bPatient location at the time study data were collected.
cPublication year 2020.
dFollow-up in days since initial symptoms.
ePatients previously hospitalized for COVID-19.
fFollow-up in days since hospital discharge.
gSymptom reported for any severity.
hSymptoms include difficulty swallowing, communicating, laryngeal sensitivity, and voice change.
iDays from test date.
jFollow-up in days since hospital admission.
Another survey study from the United Kingdom evaluated 100 patients with COVID-19 at a mean of 48 days after hospital discharge [7]. The survey was conducted by telephone using a standardized screening tool administered to all participants. Out of 100 surveyed patients, 32 had required admission to the intensive care unit (ICU). The median age among patients admitted to a general hospital ward was 70.5 years, while in the ICU population it was 58.5 years. A slight majority of patients, 54%, were male. New fatigue was more common among patients admitted to the ICU (72%) than ward patients (60%). Although mild in severity, new or worsened breathlessness was more common among ICU patients (66%) than ward patients (43%). Almost half of patients admitted to the ICU also reported symptoms of post-traumatic stress disorder (PTSD). Finally, new or worsened concentration problems were reported by 16% of ward patients and 34% of ICU patients. A summary of these findings is included in Table 1.
In another single-center study from France, Garrigues et al. performed telephone surveys of 120 hospitalized patients (96 in general hospital wards; 24 in the ICU) at a mean follow-up (range) of 110.9 (100–121) days after admission [8]. The most frequently reported symptoms in this study were fatigue (55%), dyspnea (42%), loss of memory (34%), concentration issues (28%), and sleep disorders (30.8%). There was no statistically significant difference in symptomatology between patients admitted to the ICU and those admitted to general wards.
PERSISTENT SYMPTOMS IN MILD COVID-19
Postacute symptomatology does not appear to occur only in those recovering from more severe forms of COVID-19. A report published by the Centers for Disease Control and Prevention (CDC) described persistent symptoms in patients with predominantly mild COVID-19 [9]. This study included 274 patients from around the United States who underwent SARS-CoV-2 testing either in the emergency department or outpatient clinics. Study participants were contacted by telephone at a median of 16 days following testing and underwent an interview using a standardized questionnaire. The median age of 274 respondents was 42.5 years, and a slight majority (52%) were female. Out of 270 patients for whom data were available, the most commonly reported persistent symptoms at the time of telephone interview were cough (43%), fatigue (35%), and dyspnea (29%). A total of 95 (35%) patients reported not having returned to their baseline state of health. There was also a statistically significant association between the presence of underlying chronic medical conditions and not returning to baseline state of health, with 28% of those with 0 to 1 chronic condition reporting this, 46% of those with 2 chronic conditions, and 57% among those with 3 or more chronic conditions (P = .003). Other risk factors for not having returned to usual state of health included obesity (adjusted odds ratio [aOR], 2.31) and presence of an underlying psychiatric condition (aOR, 2.32). It should be noted that 19 of 262 patients (7%) in this study for whom data were available reported being hospitalized after initial testing.
PROLONGED ORGAN DYSFUNCTION
Along with symptomatology, the myriad effects of acute COVID-19 on different organ systems have been well described [10]. The persistence of organ dysfunction beyond the acute phase is now also beginning to be reported.
Pulmonary Sequelae
In the acute phase of infection with SARS-CoV-2, classifying disease severity relies predominantly on pulmonary pathology. A report of 72 314 patients from China noted that severe infection occurred in 10 123 (14%) patients and critical illness in 3616 (5%) [11]. Acute respiratory distress syndrome (ARDS) is another clinical manifestation of severe or critical COVID-19. In one of the first reports describing 138 hospitalized patients from Wuhan, China, ARDS occurred in 27 patients (19.6%) [12].
Regarding the postacute period, in a study of 110 hospitalized patients with acute COVID-19, pulmonary function tests (PFTs) were performed either on the day of hospital discharge or 1 day prior [13]. This testing noted abnormalities in the diffusing capacity of the lung for carbon monoxide (DLCO), with 51/110 patients (47.2%) having <80% of predicted capacity. Reduction in DLCO was more frequently seen among patients who had experienced more severe disease. A total of 30.4%, 42.4%, and 84.2% among those with mild illness, pneumonia, and severe pneumonia, respectively, had impairment in DLCO (P ≤ .05). A description of patients’ symptomatology was not provided in this study to correlate with objective findings. Additionally, a separate study of 13 patients with PFTs performed after clinical recovery from COVID-19 (on the day before hospital discharge) and at 6 weeks postdischarge showed a restrictive pattern in 10 of 13 patients with improvement after 6 weeks [14]. However, forced vital capacity was still lower than the lower limit of normal at 6 weeks postdischarge.
Cardiac Sequelae
In the previously discussed early report of 138 hospitalized patients from Wuhan, China, cardiac manifestations were described in acute COVID-19, with acute cardiac injury occurring in 10 patients (7.2%), shock in 12 patients (8.7%), and arrhythmias in 23 patients (16.7%) [12]. The median hypersensitive troponin I (normal range <26.2 pg/mL) was 6.4 pg/mL in all hospitalized patients and 11.0 pg/mL in ICU patients.
With respect to postacute cardiac manifestations, in a matched prospective cohort study of cardiac magnetic resonance (CMR) imaging from Germany, Puntmann et al. assessed 100 patients with recently recovered COVID-19 (classified as a minimum of 2 weeks from original diagnosis with resolution of respiratory symptoms and negative PCR testing) [15]. The median time from COVID-19 diagnosis to CMR evaluation (range) was 71 (64–92) days. Compared with risk factor–matched control patients, participants who had recovered from COVID-19 had lower left ventricular ejection fraction (57% compared with 62%; P < .001) and were more likely to have detectable high-sensitive troponin T (>3 pg/mL; 71% compared with 31%; P < .001). Irrespective of preexisting conditions, 78% of recently recovered COVID-19 patients had ongoing cardiac involvement noted on CMR, such as myocardial inflammation, regional scarring, and pericardial enhancement. The majority of patients in this study had asymptomatic (n = 18) or mild to moderate (n = 49) symptoms, while the remaining one-third required hospitalization. Of note, 36 (36%) patients reported ongoing shortness of breath and general exhaustion at the time of evaluation, with 25 noting symptoms during less-than-ordinary daily activity. Four of these 25 (16%) patients had been previously hospitalized. It is difficult to know, however, whether these subjective symptoms were due to cardiac abnormalities or concomitant pulmonary pathology.
Thromboembolic Sequelae
Acute SARS-CoV-2 infection, especially in its severe form, has been shown to be associated with an increased risk for venous thromboembolism (VTE) [16]. The long-term risk of VTE, based on current knowledge, is less well defined. A retrospective observational cohort study of 163 patients, of whom 42 (26%) required ICU admission, had a 30-day postdischarge cumulative incidence of VTE of 0.6% (95% CI, 0.2%–4.6%) [17]. The cumulative incidence of all thrombosis (including pulmonary embolism, left ventricular thrombus, central retinal artery occlusion, thrombosis of arterio-venous dialysis fistula, and ischemic stroke) was 2.5% (95% CI, 0.8%–7.6%). The 30-day cumulative rates of major bleeding and clinically relevant nonmajor bleeding (using the International Society of Thrombosis and Haemostasis definitions for these conditions [18]) were 0.7% and 2.9%, respectively. These rates are comparable to the postdischarge hospital-acquired VTE (HA-VTE) and bleeding rates, 0.3%–2.5% and 0.7%–2.0%, respectively, that have been observed in patients with comparable forms of non-COVID-19 acute medical illness [17, 19, 20].
In another study of 102 patients, Engelen et al. report a low rate of VTE (<1%) at a mean of 44 days after hospitalization due to COVID-19 [21]. This finding is consistent with a large review of HA-VTE in patients with COVID-19 discharged from King’s College Hospital in the United Kingdom [22]. HA-VTE in this study was defined as VTE diagnosed in the hospital at least 48 hours after admission, or postoperatively, and occurring up to 90 days postdischarge. A total of 1877 patients discharged after hospitalization for COVID-19 were included in the analysis. There were 84 HA-VTE episodes that occurred during the study period. Overall, 9/84 (11%) occurred after discharge, at a median of 8 days. Therefore, the authors calculated the frequency of discharged patients who developed HA-VTE to be 4.8 per 1000 discharges. By comparison, the rate was found to be 3.1 per 1000 discharges in 2019, which occurred within 42 days of discharge. The odds ratio of HA-VTE associated with COVID-19 compared with rates observed in 2019 before COVID-19 was 1.6 (95% CI, 0.8–3.1) and was not statistically significant.
Neurologic Sequelae
The susceptibility of certain tissues and organ systems to SARS-CoV 2 might result in other postacute COVID-19 symptoms that have yet to be described. For example, SARS-CoV-2 exhibits neurotropic properties, and ~36% of patients with COVID-19 develop neurologic signs and symptoms, such as headaches, encephalopathy, and paresthesias [23]. Cases of confirmed SARS-CoV-2 viral encephalitis have also been documented [23, 24]. It is conceivable that there may be long-term persistent effects on the nervous system because of these acute manifestations. However, such information has not yet been reported in the medical literature.
Renal Sequelae
Acute kidney injury (AKI) has been well described as a potential complication in patients hospitalized due to severe COVID-19, with rates in the United States as high as 37%–40% [25, 26]. Less is known regarding long-term recovery of kidney function after the acute period. Ng et al. report outcomes in 9657 patients admitted with COVID-19 during March and April 2020 [27]. They found that AKI, both with and without need for renal replacement therapy (RRT), was significantly associated with risk of death. They also note that among the 108 patients with AKI who did require RRT and survived, 33 (31%) patients remained on dialysis at the time of discharge, suggesting prolonged kidney dysfunction despite resolution of acute COVID-19 infection. Among patients with AKI who did not require RRT, a substantial proportion (36.9%) continued to have kidney dysfunction at the time of hospital discharge. In the pre-COVID-19 era, hospital-associated AKI was shown to be associated with increased mortality and increased risk of de novo chronic kidney disease [28].
Potential Rehabilitation Requirements
In the previously described UK study that surveyed 100 patients with COVID-19 at a mean of 48 days after hospital discharge, speech, swallow, and nutritional needs were also assessed [7]. Dysphagia was observed in 12.5% (4 of 32 patients) of ICU patients compared with 5.9% (4 of 68 patients) of ward patients, laryngeal sensitivity in 25% of ICU patients compared with 11.8% of ward patients, and voice changes in 25% of ICU patients compared with 17.6% of ward patients. Overall, the full extent and implications of COVID-19 from the standpoint of long-term physical, occupational, and speech therapy remain uncertain. A consensus statement regarding rehabilitation has been put forth by an expert panel from the Defense Medical Rehabilitation Centre in the United Kingdom. After review of the available literature at the time, including about the other epidemic coronaviruses SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), a recommendation was made for consideration of cardiac and pulmonary rehabilitation programs for patients who recover from COVID-19 [29].
POSTACUTE COVID-19-RELATED CLINICAL SYNDROMES
A unique aspect of SARS-CoV-2 infection that has been reported is a condition termed multisystem inflammatory syndrome (MIS). Initially described in children and adolescents, MIS-C is thought to occur many weeks after initial mild or asymptomatic SARS-CoV-2 infection [30]. Thus, MIS-C can be thought of as part of the range of clinical situations that can potentially occur in the postinfectious or postacute period.
In April 2020, an initial report described an inflammatory condition resembling Kawasaki disease (KD) in 8 children [31]. Since then, numerous reports of children with the condition have appeared in the literature [30, 32–34]. The working case definition for MIS-C as put forth by the CDC has multiple components: (1) age <21 years with fever, laboratory findings of inflammation, and severe illness affecting multiple organ systems; (2) no alternative diagnosis; and (3) evidence of SARS-CoV-2 infection as demonstrated by molecular, serological, or antigen testing (or exposure to a suspected or confirmed COVID-19 case within the 4 weeks before presentation) [35].
In the largest series to date describing MIS-C in 570 patients across the United States, approximately three-quarters had serological evidence of SARS-CoV-2 infection; slightly less than half had a negative PCR test for SARS-CoV-2 [36]. Additionally, among 147 patients with a positive PCR test but without positive serology, 137 actually had unknown serological test results. These data strongly suggest that MIS-C is an inflammatory condition with overlap with KD that most often occurs in the postinfectious period.
Recently, a detailed report from the CDC described MIS occurring in 16 adult patients as opposed to children and adolescents [37]. In the 11 patients in whom SARS-CoV-2 antibodies were checked, all 11 had positive serology. Also, 6 of 16 patients had negative PCR test results. These findings suggest that MIS-A is also a postacute or postinfectious phenomenon.
POSTACUTE CLINICAL SYNDROMES IN OTHER EPIDEMIC CORONAVIRUSES
Postacute symptoms have been observed in a variety of infections, such as Epstein-Barr virus, dengue fever, tick-borne encephalitis, influenza, West Nile virus, Chikungunya, Zika virus, and Ross River virus, among others [38]. With regard to epidemic coronaviruses specifically, Tansey et al. assessed health outcomes in patients who had recovered from SARS-CoV up to 1 year after discharge and found that more than half of patients were continuing to experience fatigue and sleep disturbances [39]. Another 4-year follow-up study of SARS-CoV patients found that almost 50% still suffered from chronic fatigue, and over one-quarter qualified for a diagnosis of myalgia encephalitis/chronic fatigue syndrome (ME/CFS) [40]. In 2015, the National Academy of Medicine published proposed diagnostic criteria for ME/CFS [41]. Summarizing, these criteria state that a patient diagnosed with ME/CFS should have the following clinical findings: (1) substantial impairment in the ability to engage in pre-illness activities for more than 6 months, along with profound fatigue not alleviated by rest; (2) postexertional malaise; and (3) unrefreshing sleep. Additionally, patients meeting criteria for ME/CFS should also have either cognitive impairment or orthostatic intolerance.
Prolonged organ dysfunction has also been observed in patients who recovered from SARS-CoV and MERS-CoV. Ahmed et al. performed a comprehensive review and meta-analysis of long-term clinical outcomes in survivors of SARS-CoV and MERS-CoV after hospitalization and admission to ICU [42]. They found that a reduction in DLCO was present in 11%–45% of patients at 12 months. Approximately one-third of patients also had persistent symptoms of PTSD, depression, and anxiety beyond 6 months following recovery.
In addition to being associated with pulmonary complications, MERS-CoV is also believed to be potentially neuroinvasive. A study by Kim et al. showed that ~20% of patients with MERS developed delayed neurological symptoms 2–3 weeks after typical respiratory symptoms [23, 43]. Neurological findings included encephalopathy, paralysis, ischemic stroke, Guillain-Barre syndrome, and neuropathy [23]. Ultimately, our understanding of prolonged manifestations of different infectious diseases will likely become more robust with ongoing study of postacute COVID-19.
POTENTIAL MECHANISMS OF POSTACUTE COVID-19
Considering the broad spectrum of clinical presentation of COVID-19, the etiology of postacute COVID-19 symptoms is likely multifactorial. For example, in SARS-CoV, the virus’s potential for direct neuro-invasion was thought to lead to persistent neuropsychiatric sequelae [44]. Other proposed mechanisms for persistent sequelae of SARS-CoV and SARS-CoV-2 infections include the well-described virus-induced “cytokine storm” and dysregulated immune response [45]. Additionally, it is important to note that replication-competent virus is rarely recovered beyond 20 days after symptom onset [3]; this suggests that persistent symptoms are driven primarily by immunological phenomena. Another possibility is that lingering virus continues to be present in immunologically privileged sites within the body, where it can be difficult for the immune system to eradicate. Finally, in a postmortem study of histologic features of peripheral lung tissue, features of severe endothelial injury along with diffuse thrombosis with microangiopathy were observed [46]. Therefore, endothelial injury and ongoing dysfunction might also play a role in postacute symptomatology and organ dysfunction.
Post-ICU syndrome, another well-described condition that includes symptoms of persistent cognitive dysfunction, acquired weakness, fatigue, dyspnea, and intrusive memories after hospital discharge, might also be a contributing factor for patients with postacute COVID-19 symptoms whose hospitalization included ICU care [47]. Much remains to be learned about the interplay of all these potential factors and their relative contribution to persistent symptoms and organ dysfunction following acute COVID-19. The immunological origins of MIS are also a subject of ongoing research.
CONCLUSIONS
Overall, the available data suggest that a subset of patients who recover from acute SARS-CoV-2 infection will have longer-term sequelae from the disease, due to persistent symptomatology or prolonged organ dysfunction, or potentially through the development of new syndromes, such as MIS, that occur after initial asymptomatic or mild infection.
The full spectrum of the duration and severity of postacute COVID-19 is currently unknown. It is quite possible that with the significant number of patients who have already contracted COVID-19 and ongoing disease transmission, postacute COVID-19 symptomatology and organ dysfunction could be an important area of resource utilization in the future. In the rapidly evolving era of the COVID-19 pandemic, consensus is also needed regarding how to classify manifestations in the postacute period. Here, we have reviewed the existing literature on postacute COVID-19 symptomatology, prolonged organ dysfunction, and the likely postinfectious inflammatory condition MIS, a rare complication of acute SARS-CoV-2 infection. Additionally, we have proposed a potential approach to categorizing postacute COVID-19 into these 3 domains (Figure 1), recognizing the potential interplay between organ pathology and symptomatology. The overall prevalence of postacute COVID-19 symptoms and its association with prolonged organ dysfunction remain unclear. Further studies are needed to better understand postacute COVID-19 and to help inform the multidisciplinary approach that will likely be needed to diagnose and treat this heterogeneous condition.
Acknowledgments
The authors wish to thank Dr. Biykem Bozkurt for helpful discussions regarding the concepts reviewed in this manuscript.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This manuscript does not include factors necessitating patient consent.
References
- 1. National Institutes of Health. COVID-19 treatment guidelines Updated 17 July 2020. Available at: https://www.covid19treatmentguidelines.nih.gov/overview/management-of-covid-19/. Accessed 7 October 2020.
- 2. Greenhalgh T, Knight M, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ 2020; 370:m3026. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Duration of isolation and precautions for adults with COVID-19. Updated 16 August 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Accessed 31 August 2020.
- 4. Xiao AT, Tong YX, Zhang S. Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients. Clin Infect Dis 2020; 71:2249–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noh JY, Yoon JG, Seong H, et al. Asymptomatic infection and atypical manifestations of COVID-19: comparison of viral shedding duration. J Infect. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324:603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halpin SJ, McIvor C, Whyatt G, et al. Post-discharge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation [published online ahead of print July 30, 2020]. J Medical Virol 2020. doi: 10.1002/jmv.26368 [DOI] [PubMed] [Google Scholar]
- 8. Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19 [published online ahead of print July 30, 2020]. J Infect 2020; 81:e4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March–June 2020. Morbid Mortal Week Rep 2020; 69:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 324:782–93. [DOI] [PubMed] [Google Scholar]
- 11. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–42. [DOI] [PubMed] [Google Scholar]
- 12. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Resp J 2020; 55:2001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fumagalli A, Misuraca C, Bianchi A, et al. Pulmonary function in patients surviving to COVID-19 pneumonia [published online ahead of print July 28, 2020]. Infection 2020; doi: 10.1007/s15010-020-01474-9 [DOI] [Google Scholar]
- 15. Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; 5:1265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klok FA, Kruip MJ, Van der Meer NJ, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Res 2020; 191:145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patell R, Bogue T, Koshy AG, et al. Post-discharge thrombosis and hemorrhage in patients with COVID-19. Blood 2020; 136:1342–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–4. [DOI] [PubMed] [Google Scholar]
- 19. Goldhaber SZ, Leizorovicz A, Kakkar AK, et al. ; ADOPT Trial Investigators Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med 2011; 365:2167–77. [DOI] [PubMed] [Google Scholar]
- 20. Gibson CM, Halaby R, Korjian S, et al. ; APEX Investigators The safety and efficacy of full- versus reduced-dose betrixaban in the Acute Medically Ill VTE (Venous Thromboembolism) Prevention With Extended-Duration Betrixaban (APEX) trial. Am Heart J 2017; 185:93–100. [DOI] [PubMed] [Google Scholar]
- 21. Engelen MM, Vanassche T, Balthazar T, et al. Incidence of venous thromboembolism in patients discharged after COVID-19 hospitalisation [abstract]. Res Pract Thromb Haemost 2020; 4(Suppl 1). [Google Scholar]
- 22. Roberts LN, Whyte MB, Georgiou L, et al. Post-discharge venous thromboembolism following hospital admission with COVID-19. Blood 2020; 136:1347–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun 2020; 87:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pilotto A, Masciocchi S, Volonghi I, et al. ENCOVID Study Group, clinical presentation and outcomes of SARS-CoV-2 related encephalitis: the ENCOVID multicentre study [published online ahead of print September 28, 2020]. J Infect Dis 2020; doi: 10.1093/infdis/jiaa609 [DOI] [Google Scholar]
- 25. Hirsch JS, Ng JH, Ross DW, et al. ; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020; 98:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chan L, Chaudhary K, Saha A, et al. AKI in hospitalized patients with COVID-19 [published online ahead of print September 3, 2020]. J Am Soc Nephrol 2020; doi: 10.1681/ASN.2020050615 [DOI] [Google Scholar]
- 27. Ng JH, Hirsch JS, Hazzan A, et al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury [published online ahead of print September 19, 2020]. Am J Kidney Dis 2020; doi: 10.1053/j.ajkd.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bucaloiu ID, Kirchner HL, Norfolk ER, et al. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 2012; 81:477–85. [DOI] [PubMed] [Google Scholar]
- 29. Barker-Davies RM, O’Sullivan O, Senaratne KPP, et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med 2020; 54:949–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dufort EM, Koumans EH, Chow EJ, et al. ; New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020; 383:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395:1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020; 324:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020; 395:1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in US children and adolescents. N Eng J Med 2020; 383:334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Centers for Disease Control and Prevention. Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C) Available at: https://www.cdc.gov/mis-c/hcp/. Accessed 31 August 2020.
- 36. Godfred-Cato S, Bryant B, Leung J, et al. COVID-19–associated multisystem inflammatory syndrome in children—United States, March–July 2020. Morbid Mortal Week Rep 2020; 69:1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bamrah Morris S, Schwartz N, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection - United Kingdom and United States, March-August 2020. Morbid Mortal Week Rep 2020; 69:1450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Islam MF, Cotler J, Jason LA. Post-viral fatigue and COVID-19: lessons from past epidemics. Fatigue Biomed Health Behav 2020; 8:61–9. [Google Scholar]
- 39. Tansey CM, Louie M, Loeb M, et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med 2007; 167:1312–20. [DOI] [PubMed] [Google Scholar]
- 40. Lam MH, Wing YK, Yu MW, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med 2009; 169:2142–7. [DOI] [PubMed] [Google Scholar]
- 41. Institute of Medicine (US), Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome -- Redefining an illness: Report guide for clinicians. 2005. Available at: https://www.nap.edu/resource/19012/MECFScliniciansguide.pdf [Google Scholar]
- 42. Ahmed H, Patel K, Greenwood DC, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehab Med 2020; 52:jrm00063. [DOI] [PubMed] [Google Scholar]
- 43. Kim JE, Heo JH, Kim HO, et al. Neurological complications during treatment of Middle East respiratory syndrome. J Clin Neurol 2017; 13:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun 2020; 87:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Broderick G, Fuite J, Kreitz A, et al. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav Immun 2010; 24:1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ackermann M, Verleden S, Kueknel M, et al. Pulmonary vascular endothlialitis, thombosis, and angiogenesis in Coivd-19. NEJM 2020; 383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40:502–9. [DOI] [PubMed] [Google Scholar]