Abstract
As of 15 August 2020, Coronavirus disease 2019 (COVID-19) has been reported in >21 million people world-wide and is responsible for more than 750,000 deaths. The occurrence of acute kidney injury (AKI) in patients hospitalized with COVID-19 has been reported to be as high as 43%. This is comparable to AKI in other forms of pneumonia requiring hospitalization, as well as in non-infectious conditions like cardiac surgery. The impact of AKI on COVID-19 outcomes is difficult to assess at present but, similar to other forms of sepsis, AKI is strongly associated with hospital mortality. Indeed, mortality is reported to be very low in COVID-19 patients without AKI. Given that AKI contributes to fluid and acid–base imbalances, compromises immune response and may impair resolution of inflammation, it seems likely that AKI contributes to mortality in these patients. The pathophysiologic mechanisms of AKI in COVID-19 are thought to be multifactorial including systemic immune and inflammatory responses induced by viral infection, systemic tissue hypoxia, reduced renal perfusion, endothelial damage and direct epithelial infection with Severe Acute Respiratory Syndrome Coronavirus 2. Mitochondria play a central role in the metabolic deregulation in the adaptive response to the systemic inflammation and are also found to be vital in response to both direct viral damage and tissue reperfusion. These stress conditions are associated with increased glycolysis and reduced fatty acid oxidation. Thus, there is a strong rationale to target AKI for therapy in COVID-19. Furthermore, many approaches that have been developed for other etiologies of AKI such as sepsis, inflammation and ischemia–reperfusion, have relevance in the treatment of COVID-19 AKI and could be rapidly pivoted to this new disease.
Keywords: AKI, COVID-19, mitochondria, mortality, sepsis
INTRODUCTION
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has rapidly spread around the world and has affected >11 million people by July 2020. The outbreak of infections with SARS-CoV-2 is termed Coronavirus Disease 2019 (COVID-19). Two other coronavirus infections, SARS in 2002–03 and Middle East Respiratory Syndrome in 2012, both caused severe respiratory syndrome in humans. All three of these emerging infectious diseases are caused by β-coronaviruses. Although COVID-19 is primarily a respiratory tract infection that may cause pneumonia and severe hypoxemia, other organs including the GI tract, heart and kidney are affected. Reporting is ongoing, but the incidence of acute kidney injury (AKI) secondary to COVID-19 (COV-AKI) is likely high, with period prevalence as high as 68% in critically ill patients in New York [1]. The majority of AKI cases are likely mild to moderate. However, dialysis rates may be as high as 30% and survival may be dramatically reduced when AKI occurs. Kidney failure appears to occur late in the course of disease, so there may be a window for treatment. Treatment currently consists mostly of preventive measures as no directed treatment for AKI is available. This makes AKI in general, and in the current COVID-19 pandemic in particular, an important condition to be addressed.
Similar to AKI in other settings [2], COV-AKI is likely to be of variable etiology. Most patients present with single-organ failure (severe hypoxemia). Thus, remote organ injury, including AKI can simply be a consequence of critical hypoxia. Late cardiac involvement reported in some series may also cause cardio-renal syndrome. Furthermore, critically ill patients with COVID-19 exhibit significant systemic hyperinflammation, and a small number may even develop a macrophage-activation syndrome-like phenotype with cytokine storm and high plasma ferritin. However, AKI might also result from direct infection of renal tubule epithelial cells (RTECs). A variety of epithelial cells express the angiotensin-converting enzyme 2 (ACE2) receptor and this receptor is used by β-coronaviruses to enter the cells [3, 4]. Evidence of direct infection of RTEC has been recently presented [5] and it seems quite likely to be one of the contributing processes in AKI given the involvement of other epithelia (lung, GI tract, etc.) and that the virus can be recovered from the urine at least from some individuals [6].
Thus, AKI may be a ‘typical’ complication of a novel disease. As such, the rationale for targeting AKI is the same as in other causes—it contributes to short-term morbidity and mortality, it can result in long-term complications including chronic kidney disease (CKD), and it dramatically increases both short- and long-term healthcare expenditures. Finally, the underlying mechanisms of COV-AKI likely include many of the same mechanisms in more common forms of AKI such as sepsis and major surgery. This review seeks to provide an overview of the current understanding of the epidemiology, characteristics and pathophysiology of COV-AKI in order to establish the rational for targeting AKI in severe COVID-19 infection with novel interventions. Importantly, no drugs have yet been approved to prevent or treat AKI, and there is concern whether we will be able to do so in the near future. Several trials of interventional agents have been conducted and nearly all have been negative. Nevertheless, several new compounds are being investigated and stronger preclinical data give us optimism that one or more will be successful.
ADDRESSABLE POPULATION
Estimates are still being formulated as to overall number of people who will become infected with SARS-CoV-2. In many countries (e.g. the UK, USA, Brazil), both incidence and mortality curves suggest a protracted pandemic with significant medical and societal consequences. There is intense debate about the number of asymptomatic persons and therefore what true incidence of COVID-19 is in various populations. However, asymptomatic or mild cases are not likely to be associated with AKI and therefore we can estimate AKI rates using hospitalized COVID-19 cases as the denominator. We recognize that this may be an underestimate, particularly in countries with limited hospital beds, but it provides a reasonable estimate of addressable population that may benefit from the development of drugs to prevent and/or treat COV-AKI. In the USA, estimates of confirmed cases of COVID-19 are still expected to double from 4 million as of mid-May to 8 million by the first of August. After this, it is unclear how the pandemic will play out given the seasonality of other coronaviruses. However, a conservative estimate would be that 4–8 million 'new' cases of COVID-19 would occur in the USA by next spring. A preventive therapy targeting COV-AKI could be appropriate for most, if not all, patients with more than mild symptoms. Finally, if only 15% of these develop AKI, which is probably a conservative estimate, we can estimate that 600 000–1 200 000 new cases will occur in the next 12 months. This is more than twice the total number of cardiac surgery cases per year in the USA. Accordingly, therapy for COV-AKI is a significant unmet need, both short-term and, possibly, long-term.
Importantly, estimates of the proportion of patients with COVID-19 developing AKI (Table 1) are still quite preliminary. Although some authors have used Kidney Disease Improving Global Outcomes (KDIGO) AKI criteria, most studies do not clearly describe AKI criteria or severity grade. The rates quoted for AKI in many manuscripts closely tracks the rates for renal replacement therapy (RRT), suggesting only the most severe AKI cases are counted. Given that RRT rates among critically ill AKI patients generally run between 15% and 25%, we can anticipate that AKI rates are 4–7 times greater than those reported [7]. Some of the patient data in the various publications in Table 1 are probably overlapping, since several papers are from the same hospitals from different periods of time. Initially, most data are from Wuhan, China and mostly represent patients who are hospitalized in the general ward and not in Intensive care unit (ICU). Rates of COV-AKI in hospitalized patients, in general, range from 0.5% to 27% in the studies from China and from 28% to 43% in the studies outside of China; the rates in the ICU are understandably highest: 29% in Chinese patients, and 19–78% in US patients (Table 1). The majority of AKI cases are mild to moderate with RRT requirement in 5–39% in critically ill patients (Table 1). An outlier for COV-AKI was the publication from Wang et al. which reported no AKI in 116 patients [8]. However, in follow-up publications in the same institution, 32% of deceased COVID-19 patients had kidney damage and 23% had AKI [9]. For reference, in Hong Kong, AKI developed in 7% of hospitalized patients with SARS [10].
Table 1.
Incidence data on AKI in COVID-19 patients
| Author, Journal | Center | Period | Total, n | Population | AKI, n (%) | % RRT | Comorbid conditions |
|---|---|---|---|---|---|---|---|
| China | |||||||
| Chen et al. [11], Lancet | Jinyintan Hospital (Wuhan) | 1–20 January 2020 | 99 | Hospitalized | 3 (3) | 9; 39% in ICU, 0/76 non-ICU | CV disease 40%; DM 12% |
| Yang et al. [12], Lancet Respir Med | Jinyintan Hospital (Wuhan) | December 2019 to 26 January 2020 | 52 | Critically ill | 15 (29)a | 5 | Cardiac disease 10%; DM 17% |
| Zhou et al. [13], Lancet | Jinyintan Hospital and Wuhan Pulmonary Hospital (Wuhan) | Discharged or died by 31 January 2020 | 191 | Hospitalized | 28 (15)a | 5 | HTN 30%; DM 19%; CKD 1% |
| Wang et al. [14], JAMA | Zhongnan Hospital (Wuhan) | 21–30 January 2020 | 138 | Hospitalized | 5 (4)a; 3/36 (8) in ICU, 2/102 (2) non-ICU | 1.5 | HTN 31%; DM 10%; CKD 3% |
| Zhang et al. [15], MedRxiv | Zhongnan Hospital (Wuhan) | 1–10 February 2020 | 221 | Hospitalized | 10 (5)a | 2.3 | HTN 24%; DM 10%; CKD 3% |
| Diao et al. [16], MedRxiv | General Hospital of Theatre Command (Wuhan) | 17 January to 3 March 2020 | 85 | Hospitalized with renal function data | 23 (27) | Not reported | HTN 20%; DM 8%; CKD 6% |
| Cheng et al. [17], Kidney Int | Tongji Hospital (Wuhan) | 28 January to 11 February 2020 | 701 | Hospitalized | 36 (5)a; St.1: 2%, St.2: 1%, St.3: 2% | Not reported | ≥1 comorbidity: 43%; HTN 33%; DM 14%; CKD 2% |
| Wang et al. [8], Am J Nephrol | Renmin Hospital (Wuhan) | 14 January to 13 February 2020 | 116 | Hospitalized | 0a | 0 | HTN 37%; DM 16%; CKD 4% |
| Xiao et al. [18], MedRxiv | Hankou Hospital (Wuhan) | 5 January to 8 March 2020 | 287 | Hospitalized | 55 (19)a; St.1: 14%, St.2/3: 5% | Not reported | HTN 30%; DM 16%; CKD 2% |
| Guan et al. [6], N Engl J Med | 552 Hospitals (30 regions in China) | 11 December 2019 to 29 January 2020 | 1099 | Hospitalized | 6 (0.5)a | 0.8 | HTN 15%; DM 7%; CKD 1% |
| Cao et al. [19], MedRxiv | Shanghai Public Health Clinical Centre (Shanghai) | 20 January to 15 February 2020 | 198 | Hospitalized | 10 (5) | Not reported | HTN 21%; DM 8% |
| Liu et al. [20], Sci China Life Sci | Shenzhen Third People’s Hospital (Shenzhen) | 11–20 January to 3 March 2020 | 12 | Hospitalized | 2 (17) | Not reported | HTN 25%; DM 17%; CKD 17% |
| Wu et al. [21], Clin Infect Dis | 3 Hospitals (Jiangsu Province) | 22 January to 14 February 2020 | 80 | Hospitalized | 2 (2.5) | 1 | CV disease 31%; CKD 1% |
| Yang et al. [22], J Infect | 3 Hospitals (Wenzhou) | 17 January to 10 February 2020 | 149 | Hospitalized | 0 | Not reported | CV disease 19% |
| Outside China | |||||||
| Arentz et al. [23], JAMA | Evergreen Hospital (Seattle, USA) | 20 February to 5 March 2020 | 21 | Critically ill | 4 (19); defined as need for KRT | 4 (19) | ≥1 comorbidity: 86%; CKD 48%; ESKD 10% |
| ICNARC [24] | All ICUs (England, Wales, Northern Ireland; UK) | Until 8 May 2020 | 8250 | Critically ill | 1442 (17); defined as need for KRT | 1442 (17) | CV disease 0.4%; CKD 1.4% |
| Chan et al. [1], MedRxiv | Mount Sinai Hospital (New York, USA) | 27 February to 15 April 2020 | 3235 | Hospitalized | 1406 (43)a; 68% in ICU | 280 (20) | HTN 37%; DM 25%; CKD 10% |
| Hirsch et al. [25], Kidney Int | Northwell Health Hospital (New York, USA) | 1 March to 5 April 2020 | 5449 | Hospitalized | 1993 (37)a; St.1: 17%, St.2: 8%, St.3: 12% | 285 (5) | HTN 79%; DM 48%; Mean BMI 29 |
| Mohamed et al. [26], Kidney 360 | Ochsner Health Hospital (New Orleans, USA) | March 2020 | 575 | Hospitalized | 161 (28)a; 61% in ICU, 14% in non-ICU | 89 (15) | HTN 72%; DM 48%; CKD 29% |
| Argenziano et al. [27], BMJ | New York-Presbyterian / Columbia University Irving Medical Center (New York, USA) | 1 March to 5 April 2020 | 850 | Hospitalized | 288 (34); 78% in ICU | 117 (14); 35% in ICU | HTN 60%; DM 37%; CKD 14% |
KDIGO criteria used for defining AKI. Studies were not included which were clearly from same institution and period of time (to avoid double counting); however, the data from the first three studies from Jinyintan Hospital likely still does represent overlapping data. Source: http://www.nephjc.com/news/covidaki BMI, body mass index; DM, diabetes mellitus; ESKD, end-stage kidney disease; HTN, hypertension.
In general (non-COVID-19) populations, in-hospital rates of AKI are 5–15% of hospitalized patients, up to 60% in ICU admitted or sepsis patients [28]. Most reports of COVID-19 are from mixed hospitalized populations, but many of all hospitalized COVID-19 patients need critical care (26, 30 and 58% in the US studies) [1, 25, 26]. Early reports from the USA [23] in Seattle, Washington, found that for critically ill patients in the ICU the rate of AKI was 19%, defined using KDIGO (creatinine) criteria. However, severity categories were not specified and no data on dialysis rates were reported. Conversely, two larger series from the New York City area [1, 27] and New Orleans [26] reported that 61–78% of the COVID-19 patients on the ICU developed AKI, and of these up to 35% required dialysis, respectively. These numbers agree quite well with rates of AKI in patients admitted with community-acquired pneumonia not due to COVID-19 (mainly bacterial) [29]—∼33% overall and 60% for those going to ICU.
Underestimation of COV-AKI in early reports may be related to timing of AKI development. Initially, in the UK, only 19% of critically ill patients were reported to have AKI (April 10 report; n = 1684). However, in a follow-up report, the extent of kidney injury was seen to be much larger as 24% of all COVID-19 ICU patients required renal support (May 8 report; n = 8250), which was higher than reported for a historical control group (patients with non-COVID-19 viral pneumonia, 2017–19; n = 5367) in which 18% required renal support [24].
Interestingly, and similar to the data from the UK, in China, Zhou et al. [13] found that severe COV-AKI (probably equivalent to dialysis) seems to develop at a median of 15 days (interquartile range 13–19.5 days), whereas Cheng et al. [17] (which used the more sensitive KDIGO criteria) mentions that most AKI developed within 7 days of admission. This is comparable to what was reported in New York where the time of AKI diagnosis was relative to initiation of mechanical ventilation [25]. The time from the first reported symptoms to initial intubation appears bimodal, with modes at 3 to 4 days and at 9 days after symptom onset [27]. This led us to speculate that severe AKI is not typical within the first days of hospitalization for COVID-19 and, unlike bacterial sepsis and cardiac surgery, there may be a window of opportunity to treat while still in the early stages.
PATHOPHYSIOLOGY OF COV-AKI
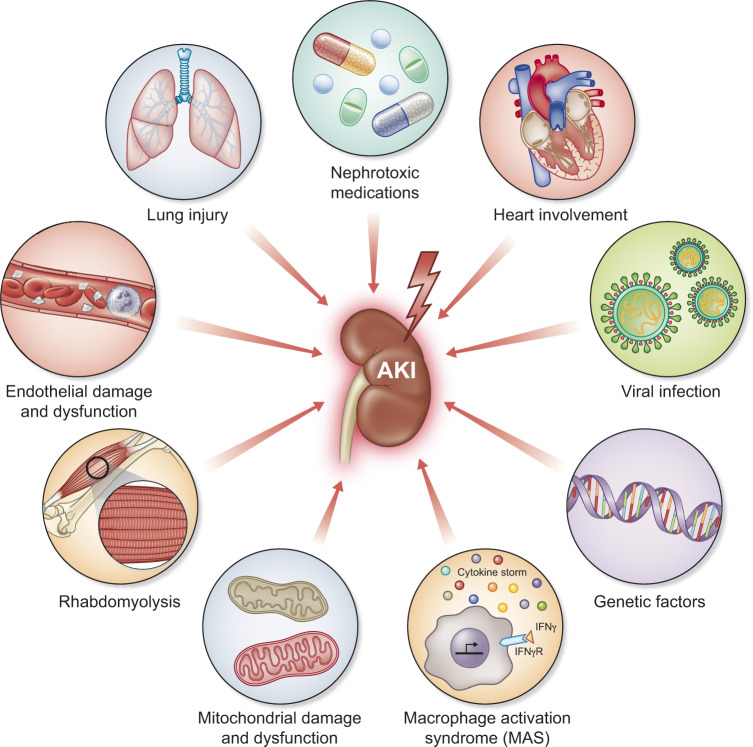
AKI is not a single disease but rather a loose collection of syndromes with a similar phenotype of rapid reduction in glomerular filtration rate [2]. Common causes of AKI include sepsis, cardiac surgery and cardio-renal syndrome. But even here multiple overlapping mechanisms are in play. Sepsis is a disease of systemic, uncontrolled inflammation in which multiple damage-associated molecular patterns (DAMPs) as well as pathogen-associated molecular patterns enter the bloodstream and are filtered at the glomerulus [30]. However, sepsis is also a disease characterized by use of multiple nephrotoxins (antibiotics, radiocontrast), high frequency of shock and oxidative stress. COV-AKI is likely to be of variable etiology as well (Figure 1). Severe hypoxemia, especially when coupled with dehydration from high fevers, may challenge the kidney even in patients early in their course. Once under medical care, fluid restriction and even diuretics, commonly used to manage acute respiratory distress syndrome (ARDS), combined with positive end-expiratory pressure on mechanical ventilation may also compromise renal perfusion even as it reverses hypoxemia. Later as the disease progresses, hyperinflammation and release of DAMPs from injured lung may cause tubular damage. A hypercoagulable state may injure renal microvasculature, but the clinical picture is unusual with normal platelets and no evidence of disseminated intravascular coagulation. Later still, cardiac involvement, reported in some series may also cause cardio-renal syndrome. As with other causes of sepsis, possibly related to underlying genetic predisposition, some patients with COVID-19, may develop macrophage-activation syndrome with cytokine storm and high plasma ferritin [31]. However, AKI in COVID-19 patients appears to be mainly due to acute tubular injury—similar to other forms of sepsis.
FIGURE 1.
Mechanisms of AKI in patients with COVID-19. Clockwise from the upper left corner: Lung injury releases DAMPs into circulation that are filtered at the glomerulus and bind with pattern recognition receptors on tubular epithelial cells. DAMPs also cause systemic inflammation which injures kidneys and other organs. Potentially nephrotoxic medications are commonly used in critically ill patients. Heart involvement is rare in COVID-19 but can have devastating effects on all organs including the kidney. High right heart pressures can occur from left heart failure but also from high positive end-expiratory pressures on mechanical ventilation. Direct viral infection of renal tubular epithelial cells has been reported. Genetic factors likely modify risk and disease presentation. Genetic variation in ACE receptor expression is one factor but also the APOL1 genotype is a known genetic risk factor for collapsing glomerulopathy that has been reported in some series. In a subset of patients with COVID-19, macrophage activation syndrome may occur resulting in uncontrolled inflammation and multiple organ failure. Mitochondrial damage and dysfunction can result from direct or indirect injury to the kidney. Rhabdomyolysis has been reported in some series resulting in AKI. Finally, endothelial damage and dysfunction appear commonly in COVID-19. Interestingly, disseminated intravascular coagulation is atypical and platelet counts remain normal in most patients suggesting that the kidney is not being damaged by thrombotic microagiopathy.
However, there is also evidence of direct viral involvement of the renal tubular epithelium. The SARS-CoV-2 virus binds with ACE2. ACE2 RNA expression in gastrointestinal organs (small intestine, duodenum) and kidney is much higher (nearly 100-fold) than that in the lung [32], which means that the kidney is susceptible to the viral infection and subsequent injury. There is particularly high expression in podocytes and proximal tubular epithelial cells seen in single-cell RNA sequencing data [33]. Thus, direct viral infection may contribute to injury mechanisms [34].
However, isolation of virus and/or viral RNA from urine has been uncommon, casting doubt on a direct effect of the virus in AKI in the majority of cases. There are reports that SARS-CoV-2 has been isolated from urine of patients in Guandong [35] and Wuhan [8]. A larger case series also noted the presence of the virus in the urine of one patient although it is not clear how many urine specimens were tested [6]. However, Wang et al. were unable to find the virus in 72 urine specimens [14], while virus shedding was fairly easily detected elsewhere: bronchoalveolar lavage (93%), followed by sputum (72%) and even feces (29%). Another report from Germany reports on detailed assessment of different body fluids over time in nine patients [36]. While they isolated virus from many other fluids, it was never detected in either blood or urine. However, it is unclear if any of these patients had AKI. Still, these data could suggest that direct viral effect on the kidney may be of less importance than AKI due to other causes, at least for most patients.
Evidence from histopathology support the role of the tubular epithelial cells as the primary target for cellular pathology in COV-AKI. Diao examined 85 patients who had kidney function details available and reported on the autopsies from 6 patients who died [16]. The kidney tissue, on light microscopy, mostly showed severe acute tubular necrosis with CD68+ macrophage infiltration of the tubulointerstitium. C5b-9 deposition on tubules was observed in all six cases although very little deposition was seen in glomeruli and capillaries. Additionally, immunohistochemistry demonstrated the presence of SARS-CoV-2 nucleocapsid protein in the kidneys, with the authors hypothesizing potential direct tubular injury from the virus. This is consistent with ACE2 expression in proximal tubule cells, as demonstrated in single-cell RNA sequencing data. In 12 consecutive COVID-19 patients who died, post-mortem examination showed viremia in 6 of 10 patients, and 5 of 12 patients demonstrated high viral RNA titers in the liver, kidney or heart; no data are given for the kidney specifically, and it is not reported if the patients had AKI, but this does indicate active viral replication in the organ [37]. Finally, Su et al. reported post-mortem renal histopathological analysis from 26 patients with COVID-19 in China [5]. Nine patients showed increased serum creatinine (SCr) and/or new-onset proteinuria. Electron microscopic examination showed clusters of coronavirus particles with distinctive spikes in the tubular epithelium and podocytes. Immunostaining with SARS-CoV nucleoprotein antibody was positive in tubules. These results provide direct evidence of the invasion of SARS-CoV-2 into kidney tissue. Of course, post-mortem analysis is biased toward the most severe patients, and does not exclude processes occurring after death, e.g. secondary contamination; these data do suggest a contribution of direct viral involvement in some patients.
Overall, the available evidence paints a picture that pathophysiology of COV-AKI is comparable to that of sepsis-associated AKI, which is caused by a multifactorial interplay of direct and indirect inflammation and cell death, framed by the concept that the clinical phenotype is predominantly the early expression of an adaptive response of the tubular cells to an injurious, inflammatory danger signal. The interplay between inflammation and microvascular dysfunction amplifies this signal. The resulting mitochondrial injury within tubular cells leads to downregulation and reprioritization of energy utilization, which favors individual cell survival processes (such as mitophagy and cell cycle arrest), at the expense of kidney function (i.e. tubular absorption and secretion of solutes) [30]. Importantly, if direct viral involvement is an important pathophysiological factor, data suggest this would exacerbate many of the same metabolic programs. Smallwood et al. have shown that viral infection, in adults or children, induces comparable metabolic reprogramming of the infected cells, impairing fatty acid oxidation (FAO) and oxidative phosphorylation while increasing glycolysis. Although this research focused on patient lungs during influenza infection [38], the effect in the kidneys is suspected to be similar. Treatment of patient cells with drugs that decreased glucose metabolism reversed this effect and also rescued mice from lethal influenza infection reducing viral load and improving respiratory distress [38]. These data suggest that the metabolic shift may be a central component of the reduced function of proximal tubular cells under a wide range of stress.
CLINICAL CONSEQUENCES OF COV-AKI
It is well known that AKI is associated with worse outcomes regardless of the etiology. For example, a multinational study of 1032 critically ill patients reported increased in-hospital mortality with all stages of AKI, with a hazard ratio (HR) increasing from 1.7 for AKI Stage 1 to 6.9 for AKI Stage 3 [7]. Even after adjusting for covariates, Stages 2 and 3 AKI were strongly associated with mortality. For long-term outcomes, AKI has been shown to more than double mortality risk compared to non-AKI survivors [39]. Lastly, the duration of AKI also matters: longer is associated with worse longer-term cardiovascular (CV) outcomes as well as risk of CKD [40].
From the 2005 SARS experience, the mortality rate was much higher in patients who developed AKI: 92% versus 8% in those who did not develop AKI [10]. Recent reports show an association between viral infections and increased rates of AKI, e.g. in the presence of human immunodeficiency virus (HIV) infection [41], and glomerulonephritis in the presence of HIV and hepatitis C virus [42]. In addition, mortality due to AKI in patients with influenza A (H1N1) viral infection was significantly increased [43]. From the current COVID-19 pandemic, the available data indicate a strong association between AKI and mortality (Table 2). In the NY cohorts, mortality was 35% in those with AKI compared with 6% in those without AKI [25] and 45% (41% for non-ICU, 52% for ICU) with AKI and 7% without AKI [1]. Notably, of those who developed AKI, only 30% survived and recovered kidney function. In survivors, 46% of patients still had persistent kidney dysfunction at the time of discharge [1].
Table 2.
AKI and mortality association
| References | n | AKI definition | Unadjusted association with death | Adjusted effect size of AKI with death | Covariates | Comments |
|---|---|---|---|---|---|---|
| Cheng et al. [17] | 701 | KDIGO |
St.1: HR = 3.5; St.2: HR = 6.2; St.3: HR = 9.8 |
St.1: HR = 1.9; St.2: HR = 3.5; St.3: HR = 4.7; proteinuria: HR = 6.8; hematuria: HR = 8.9; increased SCr: HR = 2.0 |
Age, sex, comorbidity., disease severity, lymphocyte count | Only 36 AKI events limits reliability of covariate adjustment |
| Zhou et al. [13] | 191 | Unclear | 27/28 (96%) with AKI died; 10/10 (100) with RRT died | Not reported | Not applicable | Did not have power to adjust to evaluate AKI as independent predictor of death. High mortality of AKI suggests biased selection of AKI patients toward severe phenotype |
| Hirsch et al. [25] | 5449 | KDIGO | 649/1993 (35%) with AKI died (6% in non-AKI); 157/285 (55%) with RRT died | Not reported | Not applicable | Predictors of AKI: older age, black race, DM, HTN, CV disease, mechanical ventilation and vasopressor use |
| Chan et al. [1] | 3235 | KDIGO | 45% with AKI died (7% in non-AKI) | Adjusted OR for death 9.6 in all AKI; 21 in ICU AKI | Age, sex, race, comorbidity, vital signs, lab values | AKI discharge in survivors: 43% did not recover from AKI at discharge |
| ICNARC [24] | 8250 | RRT | 71% with RRT died | SCr associated with increased risk of death | Not applicable | Update of 8 May 2020 |
| Mohamed et al. [26] | 575 | KDIGO | 50% with AKI died | Not reported | Not applicable | Predictors of RRT: higher BMI, lower age, higher ferritin, CRP, LDH |
CRP, C-reactive protein; LDH, lactic acid dehydrogenase.
Li et al. showed in a multicenter study in 193 COVID-19 patients that caution should be taken to signs of kidney dysfunction regardless of the past disease history [32]. On hospital admission, a large fraction of patients had subclinical signs of kidney dysfunctions that not yet constituted AKI, e.g. 59% with proteinuria, 44% with hematuria, 14% with increased levels of blood urea nitrogen and 10% with increased levels of SCr, although mild but worse than that in cases with other pneumonia. Three US studies found that proteinuria (69–85%) and hematuria (64–75%) are extremely common in COVID-19 [1, 25, 26].
A univariate Cox regression analysis showed that proteinuria, hematuria and elevated levels of blood urea nitrogen and SCr were significantly associated with the death of COVID-19 patients, respectively. Importantly, the Cox regression analysis also suggested that patients with COVID-19 who developed AKI had a ∼5.3 times mortality risk of those without AKI, much higher than that of co-morbid chronic illnesses (∼1.5 times risk of those without comorbid chronic illnesses). Similarly, Chan et al. reported adjusted odds ratios (ORs) for mortality in AKI versus non-AKI of 9.6 in the general ward population and 20.9 for patients in the ICU [1].
In 701 Chinese patients with COVID-19, Cox proportional hazard regression confirmed that elevated baseline SCr (HR = 2.1), elevated baseline blood urea nitrogen (HR = 4.0), AKI Stage 1 (HR = 1.9), Stage 2 (HR = 3.5), Stage 3 (HR = 4.4) were independent risk factors for in-hospital death after adjusting for age, sex, disease severity, comorbidity and leukocyte count (Table 2) [17]. Of the patients with COV-AKI, a relatively high proportion was reported to have moderate to severe AKI (in the NY cohort 47% Stage 1, 22% Stage 2, 31% Stage 3) indicating a higher than normal risk for mortality in patients with AKI because of its relatively increased severity. Particularly, patients requiring RRT were reported to have high mortality (55% in NY, 71% in the UK) [25, 24] and high proportions of ICU patients with AKI needed RRT (50% [1]; 73% [26]).
While the long-term consequences of COV-AKI will not be known for some time, increased rates of CKD (de novo or worsening of underlying disease), infections and CV events with reduced survival can be expected as seen in sepsis-AKI. Recent evidence also suggests that even milder forms of AKI are also associated with increased risk of hospital mortality [29, 44]. Although the reasons for this increased mortality are not fully understood, there is a compelling argument that patients who develop AKI are at an additional increased risk of death that is in part attributable to AKI itself. However, translation of these clear epidemiological observations to clinical practice has proven complex, and current best management of AKI remains based on minimizing risk and providing best supportive care [45]. This dichotomy between increased recognition of the importance of AKI and the lack of significant developments in its therapy highlights the urgent need to develop new treatments to improve outcomes [46]. This seems especially pertinent in patients with COVID-19, for which there also is no curative therapy to prevent and/or treat AKI. Another important reason to target AKI is that it makes fluid management more difficult and careful fluid management is central to management of ARDS. Although there are some important differences between the lung injury in COVID-19 and other forms of ARDS, fluid overload significantly worsens gas exchange and may contribute to lung injury [47]. The resulting hypoxia may further impair kidney function leading to a downward spiral. At the same time reversing hypovolemia with fluids risks worsening gas exchange and pharmacological interventions may still fail in the setting of this complex interplay of renal perfusion and pulmonary function. Finally, AKI may impair clearance of inflammation and is associated with immune dysfunction [48].
DRUG TARGETS
A number of compounds targeting various biological mechanisms have been examined to treat or prevent AKI in a variety of settings. Earlier publications have discussed several potential therapies, including anti-inflammatory agents (e.g. AB-103), antioxidants (e.g. iron chelators, heme arginate), vasodilators (e.g. levosimendan) and repair agents (e.g. ANG-3777) [49, 50]. Unfortunately, in more recent years, several clinical trials investigating these targets have not shown clinical benefit: bone morphogenetic protein-7 agonism (THR-184) [51]; human allogeneic mesenchymal stem cells (AC-607) [52]; alpha melanocortin 1 receptor agonism (ABT-719/AP214/ZP1480) [53]. However, a number of new clinical studies have been initiated. Below novel promising targets currently in clinical development are considered with respect to their potential to be used in COV-AKI.
Mitochondrial dysfunction
As acute tubular injury seems to be a prominent factor in development of COV-AKI, and given the role of systemic hypoxia in this disease, metabolic improvement is thought to be essential to preserving tubular cell integrity and function. Similar to other causes of AKI (e.g. sepsis and cardiac surgery), the role of mitochondria in the metabolic change in sepsis and (viral) infection and its contribution to development of AKI [30, 38, 54], illustrates the possibility of benefit from agents that target FAO as discussed above. Persistent mitochondrial dysfunction occurs within damaged proximal tubular cells after AKI and may contribute to the sustained injury observed in these renal regions [55, 56].
An example of a mitochondria-oriented drug target is the peroxisome proliferator-activated receptor δ (PPARδ) nuclear receptor. This receptor controls genes that regulate mitochondria levels and function, promoting FAO and mitochondrial expansion as well as decreasing inflammation and fibrosis [57, 58]. ASP1128 (Astellas Pharma) is a selective small-molecule modulator of the PPAR δ receptor. This compound restores defective FAO and decreases glucose metabolism in human kidney cells and, in multiple rat models of AKI, improves proximal tubular injury, improves mitochondrial function and decreases fibrosis, thereby, leading to improved kidney function. In an ischemia/reperfusion injury (IRI) model in rats, the effect was seen on plasma creatinine, blood urea nitrogen, estimated GFR and cystatin-C, but also on biomarkers like tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor binding protein 7, fatty acid binding protein 1 and neutrophil gelatinase-associated lipocalin [59]. In preclinical pharmacology studies, ASP1128 increased the gene expression of selected PPARδ target genes that are involved in facilitating fatty acid metabolism in parallel with enhancing mitochondrial FAO rates in whole blood and tubular cells. A randomized Phase 2 trial of ASP1128 is underway to investigate efficacy in subjects at high risk of AKI after CV surgery (NCT03941483).
Another approach to target mitochondrial dysfunction is nicotinamide adenine dinucleotide (NAD+) conservation. This is a potential target to thwart mitochondrial dysfunction and cellular damage in acute inflammatory conditions. NAD+ plays an important role in not only oxidation–reduction reactions in cells but also as a signaling molecule, playing a key role in mitochondrial function via participation in pyruvate dehydrogenase, tricarboxylic acid cycle and oxidative phosphorylation. It plays a fundamental role in health through the regulation of energy biogenesis, redox homeostasis, cell metabolism and the arbitration of cell survival via linkages to apoptosis and autophagic pathways. NAD+ is a (co-)substrate for various enzymes, which are potential targets for drug treatments [60].
Two novel approaches have been put forth to conserve or replace NAD+ in the kidney [61]. First, in AKI, the bottleneck enzyme in the de novo biosynthesis pathway, quinolinate phosphoribosyltransferase (QPRT), was shown to defend renal NAD+ and mediate resistance to AKI in a study by Poyan Mehr et al. [62]. These investigators even conducted a small clinical trial with oral nicotinamide in patients undergoing cardiac surgery and reported a dose-related increase in NAD+ metabolites and lower rates of AKI. Metabolomics suggested an elevated urinary quinolinate/tryptophan ratio (uQ/T) as an indicator of reduced QPRT and uQ/T predicted AKI in patients. This is important as it directly identifies patients who are most likely to respond to this specific treatment. In a second approach, another group has been targeting α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase (ACMSD), an enzyme that controls cellular NAD+ levels, which can improve mitochondrial function, enhance sirtuin activity and protect against AKI (and liver injury) [63]. These investigators also characterized two novel inhibitors of ACMSD, one showing relative selectivity for the kidney.
Modulating inflammation and facilitating repair
For many years, AKI was viewed as a problem of glomerular hemodynamics, and interventions were focused almost exclusively on increasing renal blood flow leading to years of stagnation [64]. However, in recent years inflammation has become a major target for AKI drug development. While strong anti-inflammatory agents such as corticosteroids have not shown benefit in coronary artery bypass grafting-AKI, retrospective analyses suggest a subset can benefit [65]. Other anti-inflammatory agents including cyclosporine have shown some activity in preclinical AKI models [66]; however, immunosuppressive drugs are generally not considered favorable in COVID-19. Three novel agents show promise in sepsis-associated AKI, and thus may merit investigation in COV-AKI.
Alkaline phosphatase (AP) is an endogenous enzyme that exerts detoxifying effects through dephosphorylation of various compounds, including bacterial endotoxins and pro-inflammatory mediators such as extracellular adenosine triphosphate. In animal model of sepsis, treatment with AP attenuated systemic inflammation and organ dysfunction and improved survival rates. In two small clinical trials, administration of bovine AP significantly improved kidney function in patients with sepsis [67, 68]. Based on these results, a human recombinant form of AP was developed and studied in a randomized, double-blind, placebo-controlled, dose-finding, adaptive Phase 2a/2b study in 301 adult patients admitted to the intensive care unit with a diagnosis of sepsis and AKI [69]. In this study, recombinant AP did not significantly improve short-term kidney function. However, there was a significant improvement in long-term outcomes, most notably all-cause mortality: all-cause mortality at Day 28 was 14% in the highest dose group and 27% in the placebo group; this effect persisted to Day 90, 17% versus 29%.
Increased apoptosis plays a role in the inflammatory pathways involved in the development of sepsis-AKI [70]. It can be triggered by a myriad of stimuli including DNA damage, energy failure, growth factor deprivation and endoplasmic reticulum stress, all of which occur as a consequence of sepsis [30]. P53 is a stress–response gene activated by DNA damage, hypoxia, oxidative stress and other conditions, leading to the induction of cell cycle arrest, cell senescence or apoptosis. In acute settings, the temporary inhibition of p53 may mitigate apoptosis, which may allow time for repair of cellular damage, thereby preserving tissue and organ integrity and function. Systemic treatment with double-stranded RNA oligonucleotide designed to temporarily inhibit the expression of p53, via activation of the RNA interference pathway significantly ameliorated AKI in preclinical models [71]. In a randomized controlled clinical trial evaluating the efficacy and safety of a single dose of QPI-1002, a p53 siRNA, in CV surgery patients at high risk of AKI (NCT02610283), treatment resulted in a 26% relative risk reduction in AKI (50% placebo versus 37% QPI-1002; P = 0.02). An international Ph3 trial of QPI-002 Phase 3 for prevention of major adverse kidney events (MAKEs) in subjects at high risk for AKI following cardiac surgery is currently ongoing (NCT03510897).
Finally, a small molecule histone deacetylase-8 inhibitor, UPHD186, has been found to result in significantly improved survival and kidney histology by promoting proliferation and inhibiting fibrosis in a mouse cecal ligation and puncture model [72]. Interestingly, the best results were seen when therapy was started 96 h after inducing sepsis, and after the acute inflammation in the kidney had subsided. When the same treatment was started at 48 h, kidney injury was worse, accompanied by decreasing mononuclear cell infiltration into the kidney, skewing cells into a pro-inflammatory phenotype, and increased pro-fibrotic gene expression. These findings not only demonstrate a new potential drug target, but also introduce a timing dimension for therapeutic interventions such that delayed treatment with UPHD186 may enhance renal histologic repair. These effects may be expected to occur with other interventions that promote cell expansion and as such the timing of administration in the clinic may be extremely important.
CLINICAL TRIAL DESIGN CONSIDERATIONS
Progress in developing an effective treatment for AKI has not only been complicated by insufficient drug candidates, but also by the complexity of clinical trial design. In sepsis, the majority of patients developing moderate to severe AKI (KDIGO Stages 2 and 3) already have AKI when they present to medical attention [73], which limits the window for early therapeutic intervention. This does not appear to be the case for COV-AKI. While AKI is common at presentation or soon after admission, it is usually mild and may reverse rapidly with correction of hypoxemia and fluid replacement. Unfortunately, AKI that does not reverse may then progress over the course of several days, ultimately requiring dialysis. The challenge for COVID-19 AKI clinical trials is to use this window to identify patients with AKI who will progress and to identify them early enough so that therapies can be effective. Enrolling patients too early will result in many spontaneously resolving and thus diluting any effect of the intervention; while treatment too late will risk missing the window where treatment can still be effective.
It is important to note that, while we do not have sufficient data on COVID-19 AKI patients yet, recovery from sepsis-AKI, even partial recovery, appears to confer significant benefit such that 1-year survival is indistinguishable from sepsis patients without AKI [73]. Thus, for drugs that can improve recovery and severity of AKI, even when given for established AKI, the clinical benefits can be great. For these agents, inclusion criteria could include patients with newly developed Stages 2 and 3 AKI. Additionally, patients with mild (Stage 1) AKI may also be included when they present with a positive biomarker for tubular stress or injury. Koyner et al. found that patients with a positive urinary [TIMP-2]•[IGFBP7] who developed AKI (Stage 1 or greater) were at significantly increased risk for death or dialysis over the following year [74]. The composite endpoint known as MAKEs, which includes death, dialysis and persistent kidney dysfunction (defined typically as two times or more from the baseline creatinine) has been successfully used in large pragmatic studies in AKI [75, 76]. In this way, patients with COVID-19 who have a positive urinary [TIMP-2]•[IGFBP7], and develop, or even present with any stage of AKI, could be enrolled and the primary endpoint could be MAKE at 30, 60 or even 90 days.
Trial designs that require the interventional agent to be administered before AKI manifests can be more challenging but even here use of biomarkers can be helpful. Patients with sepsis who have a positive urinary [TIMP-2]•[IGFBP7] that remains elevated despite fluid resuscitation are at dramatically increased risk for dying or progressing to Stage 3 AKI within 7 days [77]. Thus, the design here can be to use a screening biomarker test first and a follow-up 6 h later. Persistently positive patients could then be randomized.
Because COVID-19 is a novel disease, caveats exist regarding our understanding of its pathophysiology that may complicate the development of new compounds targeted to effectively treat this disease or manage its complications. For COV-AKI, these include a lack of preclinical evidence in an applicable COV-AKI model, as available evidence may only cover the condition indirectly, and specific clinical factors (variable AKI onset, multifactorial AKI etiology, ongoing infectious processes and concurrent respiratory failure) may confound outcomes if not appropriately managed in the study design.
HORIZON OF THERAPIES FOR COV-AKI
An obvious concern with the development of interventions for COV-AKI is that it is unknown how long the disease will be with us. Ideally, the COVID-19 pandemic will be overcome or suppressed successfully in the near future. SARS-CoV-1 has disappeared with no new cases of disease reported in humans after the initial outbreak. However, the scale of the COVID-19 pandemic makes it likely that human disease will persist somewhere for many years—hopefully in much lower numbers. Also, a ‘second wave’ or even seasonal patterns of recurrent COVID-19 epidemics may be encountered in the future. A safe and effective vaccine is not available and will probably take several years to be implemented on a large scale, and ‘herd immunity’ likely will not be sufficient to prevent major outbreaks in the near term. Furthermore, COVID-19 is the third β-corona virus disease so far, so other epidemics may emerge with similar pathogens that may induce AKI in a similar way and to a similar extent. Therefore, while no treatment of COVID-19 is available currently, agents that can be shown to be effective for major disease-related complications like AKI will continue to have a need for some time. Importantly, treating COVID-19 patients now with promising AKI compounds could be an opportunity to improve outcomes in the current COVID-19 patients, even if it is in a clinical trial setting.
The rationale for testing agents being developed for other forms of AKI in COVID-19 is bidirectional. An effective therapy for COV-AKI will likely translate to similar conditions, especially sepsis-AKI and cardiac surgery-AKI. Sepsis is the leading clinical condition associated with AKI in the hospital and in the ICU [29, 78, 79]. Like AKI, sepsis is a disease of the elderly and is significantly associated with risk of death in hospital [80]. As a consequence, sepsis-AKI is an important condition with a very significant risk of death. Internationally, sepsis is found in close to half of severe-AKI patients in ICU [78, 81]. In a large US analysis of healthcare databases across seven states, AKI occurred in 22% of patients with diagnostic codes for severe sepsis and was associated with 38% mortality [80]. In Europe, a 51% incidence of AKI has been reported in patients with sepsis with an associated ICU mortality of 41% [82]. Thus, sepsis is likely to be at least a contributing factor to many AKI cases and drugs that work for COV-AKI will likely be effective for this syndrome.
CONCLUSIONS
COV-AKI shares many similarities with AKI arising from other forms of sepsis and even with conditions like cardiac surgery. Although direct viral invasion of the kidney is a unique feature, the implications may not be. The same cells, tubular epithelium, are affected and the secondary effects of intrarenal inflammation and mitochondrial dysfunction may still apply. Critically, the effects of AKI on outcomes in COVID-19 appear identical to other forms of sepsis and thus, we strongly advocate for the development of therapies for COV-AKI both to prevent subsequent CKD but also to improve outcomes from COVID-19 in general. Finally, several candidate therapies already exist for COV-AKI and testing their potential efficacy is encouraged. As appropriate, their use can be guided by currently available biomarkers. However, clinical studies should be designed carefully to avoid inclusion of low-risk cases or to treat too late, when effectiveness will be compromised.
CONFLICT OF INTEREST STATEMENT
J.A.K. discloses grant and consulting fees from AM Pharma, Astellas, Astute, bioMerieux, Atox Bio, Klotho, Mallinckrodt, Novartis and TES Pharma. O.vT. and G.M. are both employees of Astellas, developers of ASP1128.
REFERENCES
- 1. Chan L, Chaudhary K, Saha A. et al. Acute kidney injury in hospitalized patients with COVID-19. medRxiv 2020. doi:10.1101/2020.05.04.20090944 [Google Scholar]
- 2. Kellum JA, Prowle JR.. Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol 2018; 14: 217–230 [DOI] [PubMed] [Google Scholar]
- 3. Tai W, He L, Zhang X. et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol 2020; 17: 613–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ren X, Glende J, Al-Falah M. et al. Analysis of ACE2 in polarized epithelial cells: surface expression and function as receptor for severe acute respiratory syndrome-associated coronavirus. J Gen Virol 2006; 87: 1691–1695 [DOI] [PubMed] [Google Scholar]
- 5. Su H, Yang M, Wan C. et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020; 98: 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan WJ, Ni ZY, Hu Y. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoste EA, Bagshaw SM, Bellomo R. et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015; 41: 1411–1423 [DOI] [PubMed] [Google Scholar]
- 8. Wang L, Li X, Chen H. et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol 2020; 51: 343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi Q. Clinical characteristics of 101 COVID-19 non survivors Wuhan, China: a retrospective study. MedRxiv 2020; doi: 10.1101/2020.03.04.20031039 [DOI] [Google Scholar]
- 10. Chu KH, Tsang WK, Tang CS. et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int 2005; 67: 698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen N, Zhou M, Dong X. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 2020; 395: 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou F, Yu T, Du R. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: P1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang D, Hu B, Hu C. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang G, Hu C, Luo L et al. Clinical features and outcomes of 221 patients with COVID-19 in Wuhan, China. MedRxiv 2020; doi: 10.1101/2020.03.02.20030452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diao B. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv 2020; doi: 10.1101/2020.03.04.20031120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng Y, Luo R, Wang K. et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020; 97: 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xiao G., Huh, Wu F. et alAcute kidney injury in patients hospitalized withCOVID-19 in Wuhan. China: a Single-Center Retrospective Observational Study.MedRxiv 2020; 10.1101/2020.04.06.20055194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao M, Zhang D, Wang Y. et alClinical Features of Patients Infected with the 2019 Novel Coronavirus (COVID-19) in Shanghai, China. MedRxiv 2020; 10.1101/2020.03.04.20030395 [DOI] [Google Scholar]
- 20. Liu Y, Yang Y, Zhang C. et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020; 63: 364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu J, Liu J, Zhao X. et al. Clinical Characteristics of Imported Cases of Coronavirus Disease 2019 (COVID-19) in Jiangsu Province: a Multicenter Descriptive Study. Clin Infect Dis 2020; 71: 706–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect 2020; 80: 388–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arentz M, Yim E, Klaff L. et al. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA 2020; 323: 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ICNARC. ICNARC report on COVID-19 in critical care 8 May, 2020https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports
- 25. Hirsch JS, Ng JH, Ross DW. , et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020; 98: 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mohamed MMBL, Torres-Ortiz I, Walker AE. et al. AKI with COVID-19 in NOLA. Kidney3602020; doi: 10.34067/KID.0002652020
- 27. Argenziano MG, Bruce SL, Slater CL. et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ 2020; 369: m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ronco C, Bellomo R, Kellum JA.. Acute kidney injury. Lancet 2019; 394: 1949–1964 [DOI] [PubMed] [Google Scholar]
- 29. Murugan R, Karajala-Subramanyam V, Lee M. et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int 2010; 77: 527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gomez H, Ince C, De Backer D. et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 2014; 41: 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McGonagle D, Sharif K, O’Regan A. et al. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev 2020; 19: 102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Z. Caution on kidney dysfunctions of COVID-19 patients. medRxiv 2020. doi.org/10.1101/2020.02.08.20021212 [Google Scholar]
- 33. Pan XW, Xu D, Zhang H. et al. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med 2020; 46: 1114–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agis-Balboa RC, Fischer A.. Generating new neurons to circumvent your fears: the role of IGF signaling. Cell Mol Life Sci 2014; 71: 21–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peng L, Liu J, Xu W. et al. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J Med Virol 2020; doi:10.1002/jmv.25936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wolfel R, Corman VM, Guggemos W. et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581: 465–469 [DOI] [PubMed] [Google Scholar]
- 37. Wichmann D, Sperhake JP, Lutgehetmann M. et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med 2020; M20-2003. doi:10.7326/M20-2003. [DOI] [PubMed] [Google Scholar]
- 38. Smallwood HS, Duan S, Morfouace M. et al. Targeting metabolic reprogramming by influenza infection for therapeutic intervention. Cell Rep 2017; 19: 1640–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coca SG, Yusuf B, Shlipak MG. et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009; 53: 961–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mehta S, Chauhan K, Patel A. et al. The prognostic importance of duration of AKI: a systematic review and meta-analysis. BMC Nephrol 2018; 19: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Banda J, Chenga N, Nambaya S. et al. Predictors of acute kidney injury and mortality in intensive care unit at a teaching tertiary hospital_ID. Indian J Crit Care Med 2020; 24: 116–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kupin WL. Viral-associated GN: hepatitis C and HIV. Clin J Am Soc Nephrol 2017; 12: 1337–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dalbhi SA, Alshahrani HA, Almadi A. et al. Prevalence and mortality due to acute kidney injuries in patients with influenza A (H1N1) viral infection: a systemic narrative review. Int J Health Sci (Qassim) 2019; 13: 56–62 [PMC free article] [PubMed] [Google Scholar]
- 44. Sileanu FE, Murugan R, Lucko N. et al. AKI in low-risk versus high-risk patients in intensive care. Clin J Am Soc Nephrol 2015; 10: 187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pickkers P, Ostermann M, Joannidis M. et al. The intensive care medicine agenda on acute kidney injury. Intensive Care Med 2017; 43: 1198–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barasch J, Zager R, Bonventre JV.. Acute kidney injury: a problem of definition. Lancet 2017; 389: 779–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bellomo R, Kellum JA, Ronco C. et al. Acute kidney injury in sepsis. Intensive Care Med 2017; 43: 816–828 [DOI] [PubMed] [Google Scholar]
- 48. Singbartl K, Bishop JV, Wen X. et al. Differential effects of kidney-lung cross-talk during acute kidney injury and bacterial pneumonia. Kidney Int 2011; 80: 633–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gallagher KM, O’neill S, Harrison EM. et al. Recent early clinical drug development for acute kidney injury. Expert Opin Investig Drugs 2017; 26: 141–154 [DOI] [PubMed] [Google Scholar]
- 50. Benoit SW, Devarajan P.. Acute kidney injury: emerging pharmacotherapies in current clinical trials. Pediatr Nephrol 2018; 33: 779–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Himmelfarb J, Chertow GM, McCullough PA. et al. Perioperative THR-184 and AKI after cardiac surgery. J Am Soc Nephrol 2018; 29: 670–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Swaminathan M, Stafford-Smith M, Chertow GM. et al. Allogeneic mesenchymal stem cells for treatment of AKI after cardiac surgery. J Am Soc Nephrol 2018; 29: 260–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McCullough PA, Bennett-Guerrero E, Chawla LS. et al. ABT-719 for the prevention of acute kidney injury in patients undergoing high-risk cardiac surgery: a randomized phase 2b clinical trial. J Am Heart Assoc 2016; 5: e003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jin K, Ma Y, Manrique-Caballero CL. et al. Activation of AMP-activated protein kinase during sepsis/inflammation improves survival by preserving cellular metabolic fitness. FASEB J 2020; 34: 7036–7057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hall AM, Schuh CD.. Mitochondria as therapeutic targets in acute kidney injury. Curr Opin Nephrol Hypertens 2016; 25: 355–362 [DOI] [PubMed] [Google Scholar]
- 56. Ishimoto Y, Inagi R.. Mitochondria: a therapeutic target in acute kidney injury. Nephrol Dial Transplant 2016; 31: 1062–1069 [DOI] [PubMed] [Google Scholar]
- 57. Feng YZ, Nikolic N, Bakke SS. et al. PPARdelta activation in human myotubes increases mitochondrial fatty acid oxidative capacity and reduces glucose utilization by a switch in substrate preference. Arch Physiol Biochem 2014; 120: 12–21 [DOI] [PubMed] [Google Scholar]
- 58. Sahebkar A, Chew GT, Watts GF.. New peroxisome proliferator-activated receptor agonists: potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease. Expert Opin Pharmacother 2014; 15: 493–503 [DOI] [PubMed] [Google Scholar]
- 59. Bracken CPK, Tozzo E.. Modulation of PPARδ with MTB-2 post-reperfusion attenuates IR-induced AKI injury biomarkers and histopathology in rats. J Am Soc Nephrol 2017; 28: 167 (Abstract TH-PO243) [Google Scholar]
- 60. Verdin E. NAD(+) in aging, metabolism, and neurodegeneration. Science 2015; 350: 1208–1213 [DOI] [PubMed] [Google Scholar]
- 61. Kellum JA, Fuhrman DY.. The handwriting is on the wall: there will soon be a drug for AKI. Nat Rev Nephrol 2019; 15: 65–66 [DOI] [PubMed] [Google Scholar]
- 62. Poyan Mehr A, Tran MT, Ralto KM. et al. De novo NAD(+) biosynthetic impairment in acute kidney injury in humans. Nat Med 2018; 24: 1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Katsyuba E, Mottis A, Zietak M. et al. De novo NAD(+) synthesis enhances mitochondrial function and improves health. Nature 2018; 563: 354–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kellum JA. Impaired renal blood flow and the ‘spicy food’ hypothesis of acute kidney injury. Crit Care Med 2011; 39: 901–903 [DOI] [PubMed] [Google Scholar]
- 65. Parikh CR, Schaub JA.. Acute kidney injury: steroids for prevention of AKI after cardiopulmonary bypass. Nat Rev Nephrol 2015; 11: 509–510 [DOI] [PubMed] [Google Scholar]
- 66. Wen X, Peng Z, Li Y. et al. One dose of cyclosporine A is protective at initiation of folic acid-induced acute kidney injury in mice. Nephrol Dial Transplant 2012; 27: 3100–3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Heemskerk S, Masereeuw R, Moesker O. et al. Alkaline phosphatase treatment improves renal function in severe sepsis or septic shock patients. Crit Care Med 2009; 37: 417–423e411 [DOI] [PubMed] [Google Scholar]
- 68. Pickkers P, Heemskerk S, Schouten J. et al. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Crit Care 2012; 16: R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pickkers P, Mehta RL, Murray PT. et al. ; for the STOP-AKI Investigators. Effect of human recombinant alkaline phosphatase on 7-day creatinine clearance in patients with sepsis-associated acute kidney injury: a randomized clinical trial. JAMA 2018; 320: 1998–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Poston JT, Koyner JL.. Sepsis associated acute kidney injury. BMJ 2019; 364: k4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Molitoris BA, Dagher PC, Sandoval RM. et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol 2009; 20: 1754–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wen X, Li S, Frank A. et al. Time-dependent effects of histone deacetylase inhibition in sepsis-associated acute kidney injury. Intensive Care Med Exp 2020; 8: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kellum JA, Chawla LS, Keener C. et al. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med 2016; 193: 281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Koyner JL, Shaw AD, Chawla LS. et al. Tissue inhibitor metalloproteinase-2 (TIMP-2)IGF-binding protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol 2015; 26: 1747–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Semler MW, Self WH, Wanderer JP. et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med 2018; 378: 829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Self WH, Semler MW, Wanderer JP. et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med 2018; 378: 819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fiorentino MX Z, Smith A.. Serial measurement of cell-cycle arrest biomarkers [TIMP-2]x[IGFBP7] and risk for progression to death, dialysis or severe acute kidney injury in patients with septic shock. Am J Respir Crit Care Med 2020; doi: 10.1164/rccm.201906-1197OC. Online ahead of print.PMID: 32584598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Uchino S, Kellum JA, Bellomo R. et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005; 294: 813–818 [DOI] [PubMed] [Google Scholar]
- 79. Singbartl K, Kellum JA.. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int 2012; 81: 819–825 [DOI] [PubMed] [Google Scholar]
- 80. Angus DC, Linde-Zwirble WT, Lidicker J. et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29: 1303–1310 [DOI] [PubMed] [Google Scholar]
- 81. Bouchard J, Acharya A, Cerda J. et al. A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol 2015; 10: 1324–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Takasu O, Gaut JP, Watanabe E. et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 2013; 187: 509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]